Abstract
The most important step in evaluating a patient with possible Hodgkin lymphoma is examination of an adequate biopsy by an experienced hematopathologist. Usually an excisional biopsy is best, but in some situations a generous cutting needle biopsy can be adequate. A careful history and physical remains a key part of the evaluation of a patient with Hodgkin lymphoma and can direct further evaluation. In general the most useful imaging study is the PET-CT scan. The purpose of the initial evaluation is to identify those patients with local disease for which treatment might be different, those with more widespread disease, and those with an unfavorable prognosis. The initial treatment decision is usually based on the assignment of the patient to one of these groups. It is increasingly frequent to use an interim re-evaluation of the patient after the first two cycles of therapy. In some cases the treatment might be modified based on these results. The goal of therapy is to have the patient in complete remission, usually as documented by a PET-CT scan, at the completion of the planned course of therapy. In the absence of clinical signs of recurrent lymphoma, no further imaging studies would need to be done after documentation of a complete remission.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hodgkin lymphoma
- Thrombotic thrombocytopenic purpura
- Superior vena cava syndrome
- Surveillance imaging
- Classical Hodgkin lymphoma
1 Presenting Manifestations
Hodgkin lymphoma can come to clinical attention in a variety of ways. These include symptoms caused by a growing mass and systemic symptoms that are presumably cytokine induced, and a diagnosis can be made incidentally as part of an evaluation for an unrelated problem. By far the most common presentation of Hodgkin lymphoma is the enlargement of lymph nodes that is typically painless and progressive. Although the most common place for lymph nodes to be found is in the neck and supraclavicular region, any lymph node-bearing area can be involved. Patients typically find enlarged nodes above the clavicle and seek medical attention when they do not regress, while physicians are relatively more likely to discover lymph nodes in other areas as part of a physical examination. Mediastinal lymphadenopathy is a particularly common finding in young women with Hodgkin lymphoma. This might be found incidentally on a chest X-ray or can be symptomatic. Although unusual, patients with Hodgkin lymphoma can present with superior vena cava syndrome, but chest pain, cough, and shortness of breath are more common symptoms caused by a large mediastinal mass. Lymphadenopathy found only below the diaphragm is more common in males and in elderly patients. Mesenteric lymphadenopathy is unusual in Hodgkin lymphoma. Retroperitoneal lymphadenopathy can be painful, but is more commonly asymptomatic and found on a staging evaluation or as part of the investigation to explain system symptoms such as fever, night sweats, or weight loss. Epitrochlear lymph node involvement is unusual in Hodgkin lymphoma.
Hodgkin lymphoma can involve essentially any organ in the body as either a site of presentation or by spread from lymphatic involvement. However, extranodal presentation of Hodgkin lymphoma is unusual. The most common sites to be involved are the spleen, liver, lungs, pleura, and bone marrow, although Hodgkin lymphoma confined to these sites is rare. Hodgkin lymphoma can rarely present in unusual extranodal sites. Primary CNS [1] and cutaneous [2] Hodgkin lymphoma are rare but well described. Perianal presentations are seen more commonly in patients with HIV infection. Gastrointestinal system, bone, genitourinary system, and other unusual sites are extremely rare but have been described. Bone involvement can be seen as an “ivory vertebrae,” i.e., a densely sclerotic vertebrae [3].
By far the most common systemic symptoms that occur as the presenting manifestations of Hodgkin lymphoma are fevers, night sweats, weight loss, pruritus, and fatigue. These occur in a minority of patients but can present diagnostic challenges. Hodgkin lymphoma is one of the illnesses that can cause fever of unknown origin. Occasionally the fevers of Hodgkin lymphoma occur intermittently with several days of fevers alternating with afebrile periods. This is the Pel-Ebstein fever [4, 5] that is rare, but typically occurs in the evening. Fevers from Hodgkin lymphoma can be prevented with nonsteroidal anti-inflammatory drugs such as naproxen [6].
The presence of drenching night sweats (i.e., as opposed to dampness of the head and neck) and unexplained weight loss are both characteristics of Hodgkin lymphoma and, along with fever, are associated with a poor prognosis. Pruritus can be the presenting manifestation of Hodgkin lymphoma. Such patients sometimes have severely excoriated skin and sometimes have been diagnosed as having neurodermatitis. Patients who present with refractory pruritus are often grateful to find the explanation of their symptoms which usually disappear with the initiation of therapy. As with other lymphomas, fatigue can be an important, although nonspecific, symptom and also usually improves with therapy. There are many unusual, but well-described, presentations for Hodgkin lymphoma. One rare but very characteristic presentation is alcohol-induced pain [7, 8]. The pain typically begins soon after drinking alcohol and occurs primarily in areas of involvement by lymphoma. The pain can be quite severe and last for variable periods of time. Patients with the symptom have often discontinued alcohol before the diagnosis of Hodgkin lymphoma, and to elicit the symptom often requires specific questioning by the physician.
Patients can present with Hodgkin lymphoma involving the skin, but cutaneous abnormalities are more often paraneoplastic phenomenon. These can include erythema nodosum [9]; ichthyosiform atrophy [10]; acrokeratosis paraneoplastica [11]; granulomatous slack skin [12]; nonspecific urticarial, vesicular, and bullous lesions [13]; and others.
A variety of other unusual presentations of Hodgkin lymphoma have been reported. Patients can present with nephrotic syndrome [14], symptoms of hypercalcemia [15,16,17], jaundice due to cholestasis without involvement of the liver by the lymphoma, and the “vanishing bile duct syndrome” [18, 19].
Hodgkin lymphoma very rarely presents with a primary tumor in the CNS causing the symptoms of a brain tumor characteristic of the site of involvement. Other neurological manifestations that can be present at the diagnosis of Hodgkin lymphoma involve a variety of paraneoplastic syndromes. These include paraneoplastic cerebellar degeneration [20], which typically presents with ataxia, dysarthria, nystagmus, and diplopia. The symptoms may precede the diagnosis of Hodgkin lymphoma by many months. Hodgkin lymphoma can, of course, present with spinal cord compression from retroperitoneal and osseous tumors. Other rare manifestations include limbic encephalitis (i.e., which presents with memory loss and amnesia), peripheral neuropathy, and others.
2 Physical Findings and Laboratory Abnormalities
By far the most common physical findings in Hodgkin lymphoma are enlarged lymph nodes that might be in any lymph node-bearing area. The lymph nodes are typically firm (i.e., “rubbery”) and vary from barely palpable to large masses. However, almost any aspect of the physical examination can be made abnormal by the presence of Hodgkin lymphoma. This might include icterus, involvement of Waldeyer’s ring, findings of superior vena cava syndrome, a sternal or suprasternal mass from tumor growing out of the mediastinum, findings of a pleural effusion or pericardial fusion, an intra-abdominal mass, hepatomegaly or splenomegaly, skin involvement, and, rarely, cutaneous or neurological abnormalities.
Almost any laboratory test can be abnormal at the time of diagnosis of Hodgkin lymphoma, but certain tests are characteristic and should be specifically evaluated. Patients can have leukocytosis or leukopenia. Neutrophilia and lymphopenia are sometimes seen and can confer a poor prognosis. Eosinophilia can be found incidentally before the diagnosis of Hodgkin lymphoma, and Hodgkin lymphoma should always be included in the differential diagnosis of unexplained eosinophilia [21]. In some cases, the explanation of the eosinophilia is related to production of interleukin-5 by the tumor cells [22, 23].
The most common hematological manifestation of Hodgkin lymphoma is anemia. The most usual explanation seems to be a normocytic anemia associated with the presence of the tumor that resolves after therapy. However, patients can also have autoimmune hemolytic anemia [24] and a microangiopathic hemolytic anemia as part of the syndrome of thrombotic thrombocytopenic purpura has been reported.
Patients can present with thrombocytopenia for a variety of reasons including hypersplenism and bone marrow involvement. However, idiopathic thrombocytopenic purpura can be a presenting manifestation of the disease [25].
Other rare hematological manifestations of Hodgkin lymphoma have included autoimmune neutropenia [26], hemophagocytic syndrome [27], coagulation factor deficiencies [28], and unexplained microcytosis [29].
Routine chemistry screening should be done in patients with Hodgkin lymphoma and might reveal renal or hepatic dysfunction, protein abnormalities, hypercalcemia, and hyperuricemia.
Elevated erythrocyte sedimentation rate and C-reactive protein are frequently seen and have been associated with a poor prognosis.
3 Pathologic Diagnosis: The Biopsy
The oncologist must be certain that the Hodgkin lymphoma diagnosis was based on an adequate biopsy specimen that was examined using appropriate morphologic and immunohistochemical criteria. Whole lymph node excision is preferable for pathologic examination. The pathologic diagnosis of Hodgkin lymphoma is fully discussed in Chap. 3.
The site of biopsy must be determined with the radiologist and surgeon. In general, the largest abnormal peripheral lymph node should be excised. If a fluorine-18-deoxyglucose positron emission tomography (FDG-PET) has been performed, the patient should be biopsied in the most avid site to avoid a partially necrotic zone.
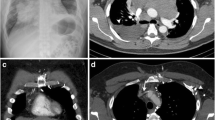
If there are only deep nodes, the following types of biopsy can be proposed. A thoracoscopic or laparoscopic approach under general anesthesia with, if necessary, preoperative localization to facilitate resection can be performed [30]. Image-guided core needle biopsy is increasingly used and has a rising success rate of more than 90% [31,32,33]. However, the method has the disadvantage of only permitting relatively small biopsies. In addition, this type of biopsy is capable of sampling several core specimens with a single biopsy tract. Large-volume cutting needles, ranging from 18 to 14 G, yield enough tissue for most immunochemistry stainings and even for RNA extraction from frozen tissue (Fig. 6.1). Fine-needle aspiration cytology should not be used for diagnosis of Hodgkin lymphoma, but may help in a screening procedure, before biopsy [34].
Several pathologic pitfalls or differential diagnoses should be kept in mind. Drugs such as phenytoin or antibiotics may cause histologic changes within lymph nodes that may mimic Hodgkin lymphoma, particularly the mixed cellularity subtype. Other benign conditions like infectious mononucleosis, lymphoid hyperplasia, or Castleman disease may produce lymphadenopathy with histologic features similar to those of Hodgkin lymphoma. In fact, the distinction between different diseases, including certain forms of non-Hodgkin lymphoma (NHL), has been made clearer, thanks to a better definition of the entities by the WHO classification. T-cell-rich large B-cell lymphoma is usually included in the differential diagnoses of both nodular lymphocyte-predominant Hodgkin lymphoma and classical Hodgkin lymphoma, while anaplastic CD30-positive NHL may display similar histology to that of classical Hodgkin lymphoma. Nevertheless, molecular studies require adequate material, sometimes including frozen tissue in difficult cases, and the role of the clinician is to make sure that the node to be analyzed is given to an experienced laboratory. If the clinical presentation of disease is not typical for the given pathologic diagnosis, then a review of the pathology by an expert hematopathologist should be considered or even a second biopsy.
4 Staging Systems for Hodgkin Lymphoma
The initial clinical evaluation and staging of patients with Hodgkin lymphoma serve to confirm the Hodgkin lymphoma diagnosis, determine the extent and distribution of disease, evaluate the patient’s fitness for standard treatments, and provide prognostic information (Table 6.1).
Several staging systems were developed very early and modified according to the progress made in imaging and treatment of the disease. The Ann Arbor staging was developed in the 1970s, when radiotherapy was the main curative treatment option, and was based on the tendency of Hodgkin lymphoma to spread to contiguous lymph nodes [35].
Since the Ann Arbor staging, several significant changes in the management of Hodgkin lymphoma have taken place. The Cotswolds modification of the Ann Arbor staging system was introduced in 1989 to approve the use of CT scanning for the detection of intra-abdominal disease, to formalize a definition of disease bulk, and to provide guidelines for evaluating the response to treatment [36]. The current standard is the Lugano classification which addresses both staging and re-staging (Table 6.1) [37].
A prognostic factor score for advanced Hodgkin lymphoma treated by chemotherapy has been worked out, based mostly on biological parameters, including serum albumin <4 g/dL, hemoglobin <10.5 g/dL, male sex, stage IV disease, age >45 year, white cell count >15,000 mm−3, and lymphocyte count <600 mm−3 [38].
These prognostic factors are used to define risk-adapted therapy. However, as combined modality treatment with modern chemotherapy has become the standard procedure for patients with early-stage disease, the risk of relapse is reduced, and some of these factors are no longer associated with a high risk of relapse. In addition, computed tomography (CT) and fluorine-18-deoxyglucose positron emission tomography (FDG-PET) are now routinely used for the staging and evaluation of the response to treatment. PET-CT provides reliable information on treatment efficacy.
5 Imaging Evaluation of the Extent of Disease
Thanks to the progress and availability of imaging techniques, it has been possible to improve the accuracy of clinical staging, so that invasive pathologic procedures are rarely necessary. At present, the established radiological technique for the diagnosis of Hodgkin lymphoma is FDG-PET [39].
FDG-PET is based on the increased glycolysis of cancer cells. This is visualized using the radioactive glucose analog FDG, which after phosphorylation is metabolically trapped within the cell. Thus, FDG-PET has become an established imaging modality to stage, restage, and monitor therapy and detect recurrent lymphoma. PET and CT, which, respectively, supply metabolic and anatomic information, are complementary, and interpretation of the PET portion of the study is more accurate when the results of PET correlate with those of CT [40, 41]. Therefore, integrated PET-CT systems were developed which are now the standard care [42]. If PET-CT is not available, an alternative imaging technique is computed tomography (CT) scan of the neck, chest, abdomen, and pelvis. In rare cases where it is desirable to avoid radiation exposure (such as pregnancy), MRI may be utilized as a substitute for CT imaging.
It is important that imaging results be interpreted within the framework of the known patterns of spread and other prognostic factors. A certain degree of variation in the size of mediastinal and hilar nodes is normal, but those measuring more than 10 mm on the shortest cross section can be considered abnormal. However, although clearly abnormal findings on CT scanning may be indicative of Hodgkin lymphoma, there is a risk of false positives, particularly in the abdomen, when interpreting these findings. Therefore, when lymph nodes in the 15–20 mm range are seen, uptake on FDG-PET-CT is indicative of involvement by lymphoma.
Substantial variations in stage assignment have nevertheless been demonstrated among patients with extranodal involvement, specifically regarding the distinction between stage IV and early-stage extranodal disease. Thus, even experienced oncologists vary in their stage assignment of patients with nearby but discontinuous extranodal involvement [43]. However, the involvement of two or more noncontiguous extranodal sites should typically be considered indicative of stage IV disease. The use of risk-adapted treatment with chemotherapy has reduced the importance of such factors.
The definition of bulk has varied considerably in the literature. For the mediastinum, one definition involved measuring the greatest transverse diameter of the mediastinal mass on a standard posteroanterior chest radiograph and dividing it by the maximal diameter of the chest wall at its pleural surfaces, usually at the level of the diaphragm or alternatively at the T5–T6 interspace (Cotswolds approach) [36]. A ratio exceeding one third (1:3) was considered bulky and a negative feature among patients treated with RT alone or chemotherapy alone. There are no widely accepted criteria for the definition of bulk using measurements obtained from CT scans: the Cotswolds Committee recommended that to constitute bulk, a nodal mass must be greater than 10 cm in diameter [36], whereas in some ongoing trials, bulk was defined as confluent nodal masses greater than 7 cm [44]. The Lugano criteria states that a single nodal mass, in contrast to multiple smaller nodes, of 10 cm or greater than a third of the transthoracic diameter at any level of thoracic vertebrae as determined by CT is the definition of bulky disease for HL. A chest X-ray is not required to determine bulk because of its high concordance with CT [37].
It appears that FDG-PET can largely eliminate the necessity for doing bone marrow biopsies in patients with Hodgkin lymphoma. One report of 454 patients found that no patients with a FDG-PET scan assigned stage of 1 or 2 had a positive bone marrow biopsy. The presence of focal skeletal FDG-PET scan lesions identified positive and negative bone marrow biopsies with a sensitivity and specificity of 85% and 86%. A negative FDG-PET scan for skeletal lesions had a 99% negative predictive value for the results of a bone marrow biopsy [37, 45].
6 Clinical Evaluation During Therapy
Clinical evaluation during treatment can be an important component of the individualization of treatment intensity. A rapid early response to initial therapy is increasingly recognized as a favorable prognostic factor among Hodgkin lymphoma patients. Response can be evaluated by FDG-PET-CT after two or three cycles of chemotherapy. Performing PET early during treatment has also proved to be prognostically important and has been incorporated into the response criteria. Thus, a meta-analysis demonstrated that for low- to intermediate-risk Hodgkin lymphoma patients, PET may be a good prognostic indicator after a few cycles of standard chemotherapy [46].
FDG-PET scans performed after two cycles of therapy are increasingly being utilized to guide subsequent treatment [47,48,49,50]. The results of interim FDG-PET scans have been used to shorten the duration of therapy, to complete a “standard” course of chemotherapy, to escalate treatment to more intensive chemotherapy in patients with a slow response, and to deescalate therapy in patients with an excellent response.
7 Definition of the Response to Treatment
FDG-PET scans have revolutionized determination of response to therapy in patients with Hodgkin lymphoma. The often called “Lugano criteria” have become the standard approach in determining treatment response [51]. A key to improving our ability to determine response to therapy was standardization of interpretation of PET scans. The so-called 5-point or Deauville scoring system is recommended in the Lugano criteria (Table 6.2) [37].
This system appears to have a high interobserver agreement. There has been debate about what should be the definition of a complete remission using the 5-point score. The consensus appears to be a 5-point score of 3 or less is the definition of a complete remission at the end of therapy. Some studies of interim PET scans, where the interim PET scan will be used to guide possible treatment changes, have chosen to use a more conservative 5-point score of 2 or less to identify an early complete remission [47]. Ongoing studies are evaluating other criteria, such as total metabolic tumor volume change, and SUV change over time which may have less variability between observers.
8 Complete Remission
The patient has no clinical, radiologic, or other evidence of Hodgkin lymphoma. Changes due to the effects of previous therapy (i.e., radiation fibrosis) may, however, be present.
The category (CRu) has been eliminated from the updated response criteria and now denotes patients whose remission status is unclear, because they display no clinical evidence of Hodgkin lymphoma, but some radiologic abnormality that persists at a site of previous disease. In this respect, it is generally recognized that imaging abnormalities may persist following treatment and do not necessarily signify active disease [52].
It must be borne in mind that after mediastinal RT, thymic rebound, reactive lymph node hyperplasia, or subclinical radiation pneumonitis may lead to abnormalities on FDG-PET [53]. To avoid false-positive interpretations, some authors recommend that FDG-PET re-evaluation should be delayed until 3 months after the completion of mediastinal RT, although the characteristic appearance of post-RT lung changes occurring before 3 months can usually be distinguished from lymphoma by experienced nuclear radiographers [42].
The inclusion of PET in the new response criteria and the removal of CRu have simplified the management of lymphoma patients by removing some of the limiting factors of CT, which include the size of lymph nodes that indicates involvement, the differentiation of unopacified bowel from lesions in the abdomen and pelvis, the inability to distinguish viable tumor from necrotic/fibrotic lesions after therapy, and the characterization of small lesions. A combined PET-CT scan with a Deauville score of 1, 2, or 3 is consistent with complete remission.
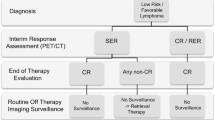
9 Follow-Up Management
The manner in which patients are evaluated after completing treatment may vary according to whether treatment was administered in a clinical trial or clinical practice and whether it was delivered with curative of palliative intent. In a clinical trial, the requirement of uniform reassessment may lead to follow-up studies that would not be routinely done in practice.
Follow-up should involve identifying relapse but also focus on identifying and dealing with long-term adverse effects of treatment. These can include secondary cancers, cardiac toxicity, thyroid disease, depression, and fertility issues [54].
Good clinical judgment, careful recording of history, and a thorough physical examination are the most important components of monitoring patients after treatment. A complete blood count, selected serum chemistry studies, and a sedimentation rate are frequently done with each visit. However, there is no evidence to support the need for regular surveillance CT scans. The patient or physician identifies the relapse in more than 80% of cases without imaging studies [55]. The most important potential reason to do surveillance imaging would be the detection of early relapse that allowed early institution of salvage therapy and increased survival. However, there is no evidence to support this hypothesis. One study of 241 patients that compared patients treated at different centers who did or did not do routine surveillance imaging found a 97% overall survival rate in patients who received routine surveillance imaging and a 96% 5-year survival rate in patients who were only followed clinically [56]. In both groups, salvage therapy was effective with only one patient in the routine surveillance imaging group dying of Hodgkin lymphoma. It was calculated that each relapse detected by surveillance imaging costs $629,615, with no benefit in eventual outcome. Similar results have been found in the use of surveillance imaging in pediatric Hodgkin lymphoma [57].
In addition to financial costs, surveillance imaging has other “side effects.” One study found that patients undergoing surveillance imaging had increased anxiety and fear associated with the images [58]. In addition, it is known that CT scans deliver a high level of radiation and are a significant cause of cancer [59, 60].
An alternative to using CT scans would be the use of FDG-PET scans as a potential tool for the detection of relapse. However, in a prospective study of 36 Hodgkin lymphoma patients, routine FDG-PET correctly identified all 5 relapses that followed treatment, but had a false-positive rate of 55% [61]. A more recent study using PET-CT scans showed a positive predictive value of only 28% for routine PET-CT scans for surveillance for relapse [62]. Given the observation that patients with cHL who are event-free at 2 years have an excellent outcome regardless of baseline prognostic factors, surveillance imaging beyond 2 years has not been demonstrated to have value [63].
10 Conclusion
The careful and accurate clinical evaluation of patients with Hodgkin lymphoma from presentation to follow-up in remission has a significant impact on treatment outcome. The ability to perform an excellent history and physical and knowledge regarding when, where, and how to perform laboratory evaluations, images, and biopsies are necessary for excellent care.
References
Gerstner ER, Abrey LE, Schiff D, Ferreri AJ, Lister A, Montoto S et al (2008) CNS Hodgkin lymphoma. Blood 112(5):1658–1661
Tassies D, Sierra J, Montserrat E, Marti R, Estrach T, Rozman C (1992) Specific cutaneous involvement in Hodgkin’s disease. Hematol Oncol 10(2):75–79
Granger W, Whitaker R (1967) Hodgkin’s disease in bone, with special reference to periosteal reaction. Br J Radiol 40(480):939–948
Pel PK (1887) Pseudoleukämie oder chronisches Rückfallsfieber? Symptomatol Sogenannten Pseudoleukämie II:644
Ebstein W (1887) Das chronische Rückfallsfieber, eine neue Infectionskrankheit. Berl Klin Wochenschr 24:565
Chang JC, Gross HM (1985) Neoplastic fever responds to the treatment of an adequate dose of naproxen. J Clin Oncol 3(4):552–558
Bichel J (1959) The alcohol-intolerance syndrome in Hodgkin’s disease. Acta Med Scand 164(2):105–112
James AH (1960) Hodgkin’s disease with and without alcohol-induced pain. A clinical and histological comparison. Q J Med 29:47–66
Simon S, Azevedo SJ, Byrnes JJ (1985) Erythema nodosum heralding recurrent Hodgkin’s disease. Cancer 56(6):1470–1472
Ronchese F, Gates DC (1956) Ichthyosiform atrophy of the skin in Hodgkin’s disease. N Engl J Med 255(6):287–289
Lucker GP, Steijlen PM (1995) Acrokeratosis paraneoplastica (Bazex syndrome) occurring with acquired ichthyosis in Hodgkin’s disease. Br J Dermatol 133(2):322–325
Noto G, Pravata G, Miceli S, Arico M (1994) Granulomatous slack skin: report of a case associated with Hodgkin’s disease and a review of the literature. Br J Dermatol 131(2):275–279
Milionis HJ, Elisaf MS (1998) Psoriasiform lesions as paraneoplastic manifestation in Hodgkin’s disease. Ann Oncol 9(4):449–452
Dabbs DJ, Striker LM, Mignon F, Striker G (1986) Glomerular lesions in lymphomas and leukemias. Am J Med 80(1):63–70
Rieke JW, Donaldson SS, Horning SJ (1989) Hypercalcemia and vitamin D metabolism in Hodgkin’s disease. Is there an underlying immunoregulatory relationship? Cancer 63(9):1700–1707
Seymour JF, Gagel RF (1993) Calcitriol: the major humoral mediator of hypercalcemia in Hodgkin’s disease and non-Hodgkin’s lymphomas. Blood 82(5):1383–1394
Laforga JB, Vierna J, Aranda FI (1994) Hypercalcaemia in Hodgkin’s disease related to prostaglandin synthesis. J Clin Pathol 47(6):567–568
Lieberman DA (1986) Intrahepatic cholestasis due to Hodgkin’s disease. An elusive diagnosis. J Clin Gastroenterol 8(3 Pt 1):304–307
Hubscher SG, Lumley MA, Elias E (1993) Vanishing bile duct syndrome: a possible mechanism for intrahepatic cholestasis in Hodgkin’s lymphoma. Hepatology 17(1):70–77
Hammack J, Kotanides H, Rosenblum MK, Posner JB (1992) Paraneoplastic cerebellar degeneration. II. Clinical and immunologic findings in 21 patients with Hodgkin’s disease. Neurology 42(10):1938–1943
Reid TJ III, Mullaney M, Burrell LM, Redmond J III, Mangan KF (1994) Pure red cell aplasia after chemotherapy for Hodgkin’s lymphoma: in vitro evidence for T cell mediated suppression of erythropoiesis and response to sequential cyclosporin and erythropoietin. Am J Hematol 46(1):48–53
Samoszuk M, Nansen L (1990) Detection of interleukin-5 messenger RNA in reed-Sternberg cells of Hodgkin’s disease with eosinophilia. Blood 75(1):13–16
Di Biagio E, Sanchez-Borges M, Desenne JJ, Suarez-Chacon R, Somoza R, Acquatella G (1996) Eosinophilia in Hodgkin’s disease: a role for interleukin 5. Int Arch Allergy Immunol 110(3):244–251
Bjorkholm M, Holm G, Merk K (1982) Cyclic autoimmune hemolytic anemia as a presenting manifestation of splenic Hodgkin’s disease. Cancer 49(8):1702–1704
Kirshner JJ, Zamkoff KW, Gottlieb AJ (1980) Idiopathic thrombocytopenic purpura and Hodgkin’s disease: report of two cases and a review of the literature. Am J Med Sci 280(1):21–28
Heyman MR, Walsh TJ (1987) Autoimmune neutropenia and Hodgkin’s disease. Cancer 59(11):1903–1905
Kojima H, Takei N, Mukai Y, Hasegawa Y, Suzukawa K, Nagata M et al (2003) Hemophagocytic syndrome as the primary clinical symptom of Hodgkin’s disease. Ann Hematol 82(1):53–56
Slease RB, Schumacher HR (1977) Deficiency of coagulation factors VII and XII in a patient with Hodgkin’s disease. Arch Intern Med 137(11):1633–1635
Shoho AR, Go RS, Tefferi A (2000) 22-Year-old woman with severe microcytic anemia. Mayo Clin Proc 75(8):861–864
De Kerviler E, Gossot D, Frija J (1996) Localization techniques for the thoracoscopic resection of pulmonary nodules. Int Surg 81(3):241–244
de Kerviler E, Guermazi A, Zagdanski AM, Meignin V, Gossot D, Oksenhendler E et al (2000) Image-guided core-needle biopsy in patients with suspected or recurrent lymphomas. Cancer 89(3):647–652
Picardi M, Gennarelli N, Ciancia R, De Renzo A, Gargiulo G, Ciancia G et al (2004) Randomized comparison of power Doppler ultrasound-directed excisional biopsy with standard excisional biopsy for the characterization of lymphadenopathies in patients with suspected lymphoma. J Clin Oncol 22(18):3733–3740
Agid R, Sklair-Levy M, Bloom AI, Lieberman S, Polliack A, Ben-Yehuda D et al (2003) CT-guided biopsy with cutting-edge needle for the diagnosis of malignant lymphoma: experience of 267 biopsies. Clin Radiol 58(2):143–147
Landgren O, Porwit MacDonald A, Tani E, Czader M, Grimfors G, Skoog L et al (2004) A prospective comparison of fine-needle aspiration cytology and histopathology in the diagnosis and classification of lymphomas. Hematol J 5(1):69–76
Rosenberg SA, Boiron M, DeVita VT Jr, Johnson RE, Lee BJ, Ultmann JE et al (1971) Report of the committee on Hodgkin’s disease staging procedures. Cancer Res 31(11):1862–1863
Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC et al (1989) Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 7(11):1630–1636
Cheson BD, Fisher RI, Barrington SF, Cavaoli F, Schwarz LH, Zucca E, Lister TA (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3067
Hasenclever D, Diehl V (1998) A prognostic score for advanced Hodgkin’s disease. International prognostic factors project on advanced Hodgkin’s disease. N Engl J Med 339(21):1506–1514
Armitage JO (2005) Staging non-Hodgkin lymphoma. CA Cancer J Clin 55(6):368–376
Blodgett TM, Meltzer CC, Townsend DW (2007) PET/CT: form and function. Radiology 242(2):360–385
von Schulthess GK, Steinert HC, Hany TF (2006) Integrated PET/CT: current applications and future directions. Radiology 238(2):405–422
Kazama T, Faria SC, Varavithya V, Phongkitkarun S, Ito H, Macapinlac HA (2005) FDG PET in the evaluation of treatment for lymphoma: clinical usefulness and pitfalls. Radiographics 25(1):191–207
Connors JM, Klimo P (1984) Is it an E lesion or stage IV? An unsettled issue in Hodgkin’s disease staging. J Clin Oncol 2(12):1421–1423
Laskar S, Gupta T, Vimal S, Muckaden MA, Saikia TK, Pai SK et al (2004) Consolidation radiation after complete remission in Hodgkin’s disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: is there a need? J Clin Oncol 22(1):62–68
El-Galaly TC, d’Amore F, Mylam KJ et al (2012) Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naïve patients with Hodgkin lymphoma. J Clin Oncol 30(36):4508–4514
Terasawa T, Lau J, Bardet S, Couturier O, Hotta T, Hutchings M et al (2009) Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin’s lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol 27(11):1906–1914
Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, Wimperis J, Culligan D, Popova B, Smith P, McMillan A, Brownell A, Kruger A, Lister A, Hoskin P, O’Doherty M, Barrington S (2015) Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J M 372(17):1598–1607
Raemaekers JMM, Andre MPE, Federico M, Girinsky T, Oumedaly R, Brusamolino E, Brice P, Ferme C, van der Maazen R, Gotti M, Bouabdallah R, Sebban CJ, Lievens Y, Re A, Stamatoullas A, Morschhauser F, Lugtenburg PJ, Abruzzese E, Olivier P, Casasnovas RO, van Imhoff G, Raveloarivahy T, Bellei M, vad der Borght T, Bardet S, Versari A, Hutchings M, Meignan M, Fortpied C (2014) Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized DORTC/LYSA/FIL H10 trial. J Clin Oncol 32(12):1188–1194
Press OW, Li H, Schoder H, Straus DJ, Moskowitz CH, LeBlanc M, Rimsza LM, Bartlett NL, Evens AM, Mittra ES, LaCasce AN, Sweetenham JW, Barr PM, Fanale MA, Knopp MC, Noy A, Hsi ED, Cook JR, Lechowicz MJ, Gascoyne RD, Leonard JP, Kahl BS, Cheson BD, Fisher RI, Friedbert JW (2016) US intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: southwest oncology group S0816. J Clin Oncol 34(17):2020–2027
Johnson P, Federico M, Kirkwood A, Fossa A, Berkahn L, Carella A, d’Amore F, Englad G, Franceschetto A, Fulham M, Luminari S, O’Doherty M, Patrick P, Roberts T, Sidra G, Stevens L, Smith P, Trotman J, Viney Z, Radford J, Barrington S (2016) Adapted treatment guided by interim PET-CT in advanced Hodgkin’s lymphoma. N Engl J Med 374(25):2419–2429
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, Schwartz LH, Zucca EM, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O’Doherty MJ, Hustinx R, Biggi A, Cheson BD (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32(27):3048–3058
Radford JA, Cowan RA, Flanagan M, Dunn G, Crowther D, Johnson RJ et al (1988) The significance of residual mediastinal abnormality on the chest radiograph following treatment for Hodgkin’s disease. J Clin Oncol 6(6):940–946
Jerusalem G, Hustinx R, Beguin Y, Fillet G (2005) Positron emission tomography imaging for lymphoma. Curr Opin Oncol 17(5):441–445
Ng AK (2014) Current survivorship recommendations for patients with Hodgkin lymphoma: focus on late effects. Blood 124(23):3373–3379
Henry-Amar M, Friedman S, Hayat M, Somers R, Meerwaldt JH, Carde P et al (1991) The EORTC lymphoma cooperative group. Erythrocyte sedimentation rate predicts early relapse and survival in early-stage Hodgkin disease. Ann Intern Med 114(5):361–365
Pingali SR, Jewell SW, Havlat L et al (2014) Limited utility of routine surveillance imaging for classical Hodgkin lymphoma patients in first complete remission. Cancer 120:2122
Voss SD, Chen L, Constine LS et al (2012) Surveillance computed tomography imaging and detection of replace in intermediate – and advanced-stage pediatric Hodgkin’s lymphoma: a report from the Children’s oncology group. J Clin Oncol 30(21):2635–2640
Thompson CA, Charlson ME, Schenkein E et al (2010) Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Ann Oncol 21(11):2262–2266
Berrington de Gonzalez A, Mahesh M, Kim KP et al (2009) Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 169(22):2071–2077
Smith-Bindman R, Lipson J, Marcus R et al (2009) Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 169(22):2078–2086
Jerusalem G, Beguin Y, Fassotte MF et al (2003) Early detection of relapse by whole-body positron emission tomography in the follow-up of patients with Hodgkin’s disease. Ann Oncol 14(1):123–130
El-Galay TC, Mylam KJ, Brown P et al (2012) Positron emission tomography/computed tomography surveillance in patients with Hodgkin lymphoma in first remission has a low positive predictive value and high costs. Haematologica 97(6):931–936
Hapgood G, Zheng Y, Sehn LH, Villa D, Klasa R, Gerrie AS, Shenkier T, Scott DW, Gascoyne RD, Slack GW, Parsons C, Morris J, Pickles T, Connors JM, Savage KJ (2016) Evaluation of the risk of relapse in classical Hodgkin lymphoma at event-free survival time points and survival comparison with the general population in British Columbia. J Clin Oncol 34(21):2493–2500
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Armitage, J.O., Friedberg, J.W. (2020). Clinical Evaluation. In: Engert, A., Younes, A. (eds) Hodgkin Lymphoma. Hematologic Malignancies. Springer, Cham. https://doi.org/10.1007/978-3-030-32482-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-32482-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32481-0
Online ISBN: 978-3-030-32482-7
eBook Packages: MedicineMedicine (R0)