Abstract
Hodgkin lymphoma (HL) is highly responsive to conventional chemotherapy (CT). Up to 90% of patients are cured with modern CT protocols which are often followed by radiotherapy. Patients with proven refractory or relapse after first-line therapy do significantly worse. High-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) is the standard of care for medically fit patients with relapsed HL. The results of ASCT, however, vary significantly depending on a number of prognostic factors—the most important of which are the time interval between first-line treatment and relapse, the clinical stage at relapse, and the sensitivity of the tumor to salvage treatments. Although results of ASCT have been improved with the introduction of brentuximab vedotin (BV) and checkpoint inhibitors, those patients who do not respond adequately to salvage CT do poorly. Consolidation strategies after ASCT can be helpful but there is still a significant proportion of patients that do poorly. Allogeneic stem cell transplantation (allo-SCT) is a well-accepted treatment strategy in this setting. Results of allo-SCT have improved over time and with the advent of haploidentical donors almost all potential candidates have a donor. The approval of both BV and checkpoint inhibitors such as nivolumab and pembrolizumab has given the clinicians the possibility to treat this difficult group of patients with highly effective therapies with a better toxicity profile than allo-SCT but with an unknown curative potential for the time being.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hodgkin lymphoma
- Autologous stem cell transplantation
- Allogeneic stem cell transplantation
- Graft-versus-host disease
- Brentuximab vedotin
- Checkpoint inhibitors
1 Introduction
Hodgkin lymphoma (HL) is highly responsive to conventional chemotherapy (CT). Close to 90% of patients even with advanced disease are cured with modern treatment which is often followed by radiation [1, 2]. Patients who are refractory or relapse after first-line therapy do significantly worse. High-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) is the standard of care for medically fit patients with relapsed HL [3, 4]. The results of ASCT, however, vary significantly depending on a number of prognostic factors—the most important of which are the time interval between first-line treatment and relapse, the clinical stage at relapse, and the sensitivity of the tumor to salvage CT [5,6,7,8,9]. The most recent analysis on prognostic factors indicates that accurate and reliable risk stratification in patients with relapsed/refractory HL who successfully undergo ASCT can be achieved with five easily available clinical risk factors: stage IV disease, time to treatment failure of ≤3 months, bulky disease ≥5 cm, ECOG status ≥1, and nonresponse to salvage treatment, either measured as achieving less than partial remission (PR) by CT scan or PET positivity [10]. In the setting of high-risk disease, consolidation therapy with brentuximab vedotin (BV) single dose up to 16 cycles after ASCT has demonstrated to significantly improve progression-free survival (PFS) in this subgroup of patients with the potential to avoid exposure to subsequent toxic therapies [11]. Despite all these efforts, a significant proportion of patients with relapsed or refractory HL fail to achieve a continuous complete remission (CR) after ASCT; these patients might be candidates for other treatment strategies such as allogeneic stem cell transplantation (allo-SCT).
2 Myeloablative Allogeneic Stem Cell Transplantation in Hodgkin Lymphoma: A Historical Perspective
The first reports on allo-SCT in patients with HL appeared in the mid-1980s [12, 13]. Two large registry-based studies published in 1996 gave disappointing results. Gajewski et al. analyzed 100 HL patients allografted from HLA identical siblings and reported to the International Bone Marrow Transplant Registry (IBMTR) [14]. A significant proportion of these patients was not in remission before transplant and had a poor performance status (PS) as well as active infections before transplantation. Almost 50% of the patients received total body irradiation (TBI)-containing regimens. The 3-year rates for overall survival (OS), disease-free survival (DFS), and the probability of relapse were 21%, 15%, and 65%, respectively. The major problems after transplantation were persistent or recurrent disease as well as respiratory complications, which accounted for 35–51% of deaths. Acute and/or chronic graft-versus-host disease (GVHD) did not significantly reduce the risk of relapse. At the same time, a case-matched analysis including 45 allografts and 45 autografts reported to the European Group for Blood and Marrow Transplantation (EBMT) was performed by Milpied et al. [15]. The matching criteria were sex, age at time of transplantation, stage of disease at diagnosis, bone marrow involvement at diagnosis and at transplantation, year of transplantation, disease status at time of transplantation, time from diagnosis to transplantation, and conditioning regimen with or without TBI. The 4-year actuarial probabilities of survival, PFS, relapse, and non-relapse mortality (NRM) were 25%, 15%, 61%, and 48% and 37%, 24%, 61%, and 27% after allo-SCT and ASCT, respectively. The toxic death rate at 4 years was significantly higher for allo-SCT patients (p = 0.04). Even for patients with sensitive disease at the time of transplantation, the 4-year actuarial probability of survival was 30% after allo-SCT and 64% after ASCT (p = 0.007). This difference was mainly due to a higher NRM rate after allo-SCT (65% vs. 12%, p = 0.005) that was basically associated with the development of acute GVHD after transplantation and/or concomitant infectious episodes. Although a GVHD ≥grade II was associated with a significantly lower risk of relapse, it was also associated with a lower OS rate.
A number of reports confirmed the registry data: allo-SCT resulted in lower relapse rates but significantly higher toxicity with no improvement over ASCT when PFS or OS were considered [16,17,18]. Although the poor results after myeloablative conditioning could at least partly be explained by the very poor risk features of many individuals included in these early studies, the high procedure-related morbidity and mortality prevented the widespread use of allo-SCT.
3 Reduced-Intensity Regimens
Given the high NRM seen in adults with HL following myeloablative allo-SCT, the use of reduced-intensity (RIC) or nonmyeloablative conditioning regimens was found to be an attractive therapeutic option. The goal of these therapies was to reduce regimen-related toxicity while still providing sufficient immunosuppression to facilitate donor engraftment and a subsequent graft versus lymphoma (GVL) effect. The aim of all these regimens was to shift the balance from the antilymphoma activity of the conditioning regimen to the immune cells transferred with the donor graft which may mediate a GVL response. The marked reduction in upfront toxicity of these regimens has extended the applicability of allo-SCT to older patients, those with comorbidities, and patients who had previously failed a prior ASCT (Fig. 21.1).
Number of allo-SCT reported to the EBMT Lymphoma database over time (EBMT Lymphoma database, with permission). (a) Evolution of numbers of allo-SCT over time (reduced intensity conditioning regimens (RIC) vs. myeloablative conditioning protocols (MAC)). (b) Allo-SCT activity over time: type of donors (HLA identical sibling donor (Hla-id sib) vs. matched unrelated donor (MUD) vs. haploidentical stem cell donor (Haplo-SCT) vs. cord blood transplants (CBT))
There are many reports detailing the outcomes of RIC transplants of patients with relapsed HL [19,20,21,22,23,24,25,26,27,28,29,30,31]. These results can be difficult to compare due to the difference in patient populations and conditioning regimens; however, in general, the NRM has been impressively reduced when compared to myeloablative conditioning regimens. This reduction in transplant mortality was confirmed by the lymphoma working party (LWP) of the EBMT which compared HL patients having standard myeloablative conditioning to those having RIC between 1997 and 2002 [19]. Transplant-related mortality was 48% at 3 years in the myeloablative group and 24% in the RIC group (p = 0.003).
Although RIC has allowed allo-SCT to be performed more safely, relapse is now the most common cause of treatment failure. Conditioning intensity/antilymphoma activity may be an important factor in determining relapse rates. This may be secondary to the requirement for a lengthy period of clinical remission to allow the incoming donor immune system to eradicate residual disease. An early GVL response is often delayed by the use of immunosuppressive drugs to prevent GVHD following T-cell-depleted transplantation or by the use of a T-cell-depleted graft which often necessitates the use of posttransplant donor lymphocyte infusions (DLI). Some of the truly nonmyeloablative (NMA) regimens have been associated with particularly high relapse rates [20, 21]. This concept of regimen intensity being important is also supported by the EBMT analysis which showed a 32% relapse rate following myeloablative conditioning compared to 58% with reduced-intensity regimens [19]. Furthermore, within the reduced-intensity group, there was a higher relapse and lower OS rate in patients who were conditioned with low-dose TBI which is one of the regimens with the least toxicity (p < 0.04). Other studies have also shown better outcomes using more intensive regimens such as the combination of fludarabine and melphalan when compared to less intensive regimens [22]; the BEAM-alemtuzumab regimen has also demonstrated good disease control [23]. Finally, overall results have improved over time: in an updated retrospective analysis of the LWP of the EBMT comparing RIC with myeloablative procedures [32], NRM was not different between MAC and RIC. Due to a higher relapse rate of RIC in front of MAC, both PFS and OS were better for those patients being allografted with myeloablative procedures than with RIC protocols.
There is mounting evidence that successful allogeneic transplantation for HL needs a combination of effective salvage CT and a moderately intensive pretransplant conditioning regimen to keep the disease under control for several months allowing the withdrawal of immunosuppression and/or the use of DLI to mount an effective GVL response.
4 Prognostic Factors of Long-Term Outcome for Allogeneic SCT
The introduction of RIC regimens in the allogeneic field allowed a significant reduction in the NRM associated with the procedure in HL patients [19]. The identification of independent prognostic factors better allowed to guide physicians in the choice of therapy for individual patients. However, the reported experience of RIC-allo in HL has been limited by the number of patients included [19,20,21,22,23,24,25,26,27,28,29,30,31], making it difficult to identify independent predictors of outcome. The largest prospective study published to date includes 78 patients with relapsed/refractory HL, most of them being treated with an allo-SCT due to a relapse after an ASCT [33].
The LWP of the EBMT performed a retrospective analysis comprising a population of 285 patients with relapsed or refractory HL treated with reduced-intensity allo-SCT in order to try to identify prognostic factors for long-term outcome [34]. Sixty patients died of NRM at a median of 91 days (range 1 day–20 months) following transplantation. The cumulative incidence estimates a NRM at 100 days and 1 and 3 years posttransplant were 10.9%, 19.5%, and 21.1%, respectively. In multivariate analysis, NRM was associated with PS, chemorefractory disease at transplantation, age greater than 45, and transplantation before 2002. Identifying poor PS, chemorefractory disease, and older age as adverse risk factors for NRM, patients with no adverse risk factors had a 3-year NRM rate of 12.5% compared with 46.2% for those with two or three risk factors. With a median follow-up of 26 months (range 3–94 months), 126 patients remained alive and 159 have died. The Kaplan-Meier estimates of OS and PFS at 1, 2, and 3 years were 67% and 52%, 43% and 39%, and 29% and 25% respectively. In multivariate analysis, patients in CR or with chemosensitive disease, those with a good PS, transplants other than sex-mismatched male recipients, and CMV−/− transplants had a significantly better OS. For PFS good PS, CR, or chemosensitive disease at transplantation and transplants other than male recipients from female donors was associated with a significantly better PFS in the multivariate analysis. Considering chemorefractory disease and poor PS as risk factors for a poor PFS and OS, patients with neither of these risk factors have a 3-year PFS and OS of 42% and 56% compared to 8% and 25% for patients with one or two of these risk factors. In an analysis restricted to patients who had relapsed after prior ASCT, relapse within 6 months of the autograft was associated with a significantly poorer disease progression rate (RR = 1.9 (1.2–3.1) p = 0.01) and PFS (RR = 1.9 (1.2–2.9) p = 0.003) following reduced-intensity allo-SCT. Reduced-intensity allo-SCT is an effective salvage strategy for patients with good risk features who relapse after ASCT (Fig. 21.2), and those outcomes are similar for both sibling and matched unrelated donor (MUD) transplants. Conversely for patients with chemorefractory disease or PS, the overall outcome is poor, and nowadays these patients should not be considered candidates to receive this treatment strategy.
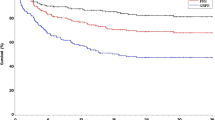
Long-term outcomes of haploidentical donor stem cell transplantation vs. HLA identical sibling donor and matched unrelated donors. (a) Cumulative incidence of nonrelapse mortality (NRM) in recipients of sibling donor (SIB), match unrelated donor (MUD), and haploidentical donor (HAPLO) transplantations (overall, P = 0.23). (b) Cumulative incidence of relapse and/or progression in recipients of SIB, MUD, and HAPLO transplantations (overall, P < 0.001). (c) Kaplan-Meier estimate of overall survival (OS) in recipients of SIB, MUD, and HAPLO transplantations (overall, P = 0.118). (d) Kaplan-Meier estimate of progression-free survival (PFS) in recipients of SIB, MUD, and HAPLO transplantations (overall, P = 0.086). (e) Kaplan-Meier estimate of combined incidence of extensive chronic graft-versus-host disease (cGVHD)-free and relapse-free survival in recipients of SIB, MUD, and HAPLO transplantations (overall, P = 0.04). (Martínez C et al. [35], with permission)
These results are in agreement with what was also published in smaller series of patients. The UK Cooperative Group reported that disease status before allo-SCT was the strongest prognostic factor for PFS and OS, the results being significantly better for those patients allografted in CR [25]. In the HDR-Allo trial [33], chemosensitivity was the most important prognostic factor (HR = 2.3; 95% CI = 1.3–3.1; P = 0.001) for PFS. Patients allografted in CR had the best outcome, with PFS rates at 1 and 4 years of 70% (95% CI = 67–73) and 50% (95% CI = 47–53), respectively. Refractory disease and a poor PS were associated with a significantly worse OS (HR = 1.9, 95% CI = 1.0–2.7, and P = 0.001 and HR = 2.5, 95% CI = 1.3–4.2, and P = 0.01, respectively) in the same study. Disease status was the strongest factor predicting for survival in virtually the rest of the retrospective analyses published in the literature [19,20,21,22,23,24, 26,27,28,29,30,31, 36].
5 Evidence for Graft Versus Hodgkin Lymphoma
Despite the theoretical reliance of reduced-intensity RIC transplantation on a GVL effect, there are relatively few studies which convincingly demonstrate this activity in patients with HL. Many of the myeloablative transplants done in adults had such a high NRM that it would have been almost impossible to see a GVL effect if one had existed. In the context of RIC transplantation, there is some evidence of a reduction in relapse in association with GVHD. Conversely, the apparent lack of impact of T-cell depletion on relapse risk is unexpected. This finding might simply be a function of the relatively small numbers of patients reported or it is possible that the in vivo monoclonal antibody used to facilitate T-cell depletion may have anti-Hodgkin lymphoma activity.
The most convincing evidence of GVL activity in HL comes from the use of DLI to treat patients who relapse following allo-SCT (Table 21.1). Response rates to DLI have been reported to be between 15% and 60%, with CR seen in around 30% of patients. Many of these patients had received concurrent CT or radiotherapy but responses have been seen to DLI alone and some of these have been durable. There appears to be a higher response rate in the two series coming from the UK [25, 38] and it is not known whether the high incidence of mixed chimerism seen in patients who received alemtuzumab promotes GVL responses as it does in some animal models. The optimal T-cell dose for GVL remains unclear, although many groups use an escalating dose schedule to try and reduce the risk of severe graft-versus-host disease. Unlike follicular lymphoma, there is preliminary evidence that in relapsed HL, GVL responses are unlikely in the absence of GVHD. However, when DLI are given for mixed chimerism, there appears to be a GVL effect that is independent of GVHD [38]. There are a number of factors that may increase the toxicity of DLI including: increasing age of the patient, HLA mismatching, use of unrelated donors, and short time interval from transplant to DLI infusion. Although the DLI responses are impressive in some patients, the majority of patients will not achieve long-term benefit from DLI and further study is needed to optimize this potential effect. Recent data indicate some potential benefit of the use of BV before DLI in order to exert some immunomodulatory effect that would enhance the effectiveness of donor lymphocytes [39] or to simply act as an effective antitumoral strategy [40].
6 Role of Allogeneic SCT in Autograft Failures
Allogeneic stem cell transplantation is considered an adequate treatment strategy for patients who relapse or progress after ASCT [41]. Nevertheless, the potential benefit of allo-SCT in front other non-transplant-based strategies has never been demonstrated in a prospective randomized clinical trial, and the evidence of a potential benefit of this therapy in front of others is based on our knowledge on small phase II prospective clinical trials [33] and single center or multicenter retrospective analysis [19,20,21,22,23,24,25,26,27,28,29,30,31, 36].
Although there are no randomized trials comparing the results of CT ± radiotherapy in patients who relapse post autograft, comparisons have been made with the outcomes of historical controls. The UK group identified a group of patients who had relapsed following a BEAM autograft, who were chemosensitive at relapse and had survived at least 12 months from relapse, and who would therefore have been eligible for a RIC transplant [42]. This was a highly selected group representing 44% of all relapses who were predicted to have the best survival. These conventionally treated patients were compared to more recently treated patients who received a reduced-intensity allograft. Despite the selection of a control group with a relatively good prognosis, both OS from time of diagnosis and time of autograft were significantly improved following allogeneic transplant, when compared to the historical control group. The estimated current PFS for the allografted patients was 34% at 5 years and 42% if in chemosensitive relapse at the time of transplant, suggesting the early promising results might translate into a favorable long-term outcome. A donor versus no donor comparison performed by Sarina et al. [43] indicated that, in patients relapsing after ASCT, if there was a donor available and they were able to proceed to allo-CST, both PFS and OS were significantly better than in the non-allografted population of patients, thus suggesting that allo-SCT was partially able to overcome the negative impact of disease relapse after the autologous procedure. Nowadays, the role of allo-SCT in this setting is increasingly being challenged, at least in some subgroups of patients, by the advent of new drugs: BV and checkpoint inhibitors (see Sect. 21.9 of this chapter).
7 Moving Allogeneic Stem Cell Transplantation to Earlier Stages of the Disease
The more recent investigation of a response-adjusted transplantation algorithm identifies a potential strategy for evaluation of allo-SCT in those deemed to be at high risk of failure of ASCT, targeting the intensification to those who have residual FDG-avid disease following salvage therapy [44]. The 3-year PFS of 68% in this high-risk group was encouraging, with 80% current PFS following DLI. These results constituted the basis for a phase II prospective clinical trial (CRUK-PAIReD, EUDRACT-2008-004956-60) already closed for recruitment that analyzes long-term outcome of relapsed/refractory HL patients that do not achieve a metabolic CR with first-line salvage chemotherapy and undergo an allo-SCT with BEAM protocol as conditioning regimen and the use of Campath-1H as GVHD prophylaxis. Final results of this trial have not been published in full so far. However, the lack of clear evidence of the potential benefit of allo-SCT as first transplant as well as the incapacity to be able to identify a subgroup of patients mostly benefiting from this approach together with the introduction of new drugs in the treatment armamentarium of these patients renders the role of allo-SCT quite blurred in this setting.
8 Role of Allogeneic SCT in the Pediatric Population
Information regarding the role of allo-SCT for HL in the pediatric population is very limited. Children undergoing allogeneic transplantation have been occasionally included in series of adult patients [16,17,18,19], whereas exclusively pediatric series were limited to fewer than ten patients [45].
The most extensive analysis of allo-SCT in the pediatric population comes from the LWP of the EBMT, and it comprises a group of 91 children and adolescents 18 years or younger treated with an allograft (myeloablative, n = 40; reduced intensity, n = 51) for relapsed or refractory HL [46]. NRM at 1 year was 21%, with comparable results after RIC or myeloablative allo-SCT. Probabilities of relapse at 2 and 5 years were 36% and 44%, respectively. Reduced-intensity conditioning allo-SCT was associated with an increased relapse risk compared with myeloablative transplantation, which was most apparent beginning 9 months after allo-SCT (p = 0.01). PFS was 40% and 30% and OS was 54% and 45% at 2 and 5 years, respectively. Beyond 9 months, PFS after RIC allograft was lower compared with myeloablative protocols (p = 0.02). The development of GVHD did not have any impact on PFS after allo-SCT. Of note, the 26 patients with sensitive disease and good PS who underwent transplantation between 2002 and 2005 showed a PFS of 60% (95% CI = 33–87%) and OS of 83% (95% CI = 67–98%), respectively, at 3 years. Fifteen of these patients (58% of the group) had previously failed ASCT. This retrospective analysis in a pediatric population of patients again raises the question of the exact dose intensity needed in HL patients. Because relapse now is the major problem after allogeneic transplantation for HL in pediatric as well as in adult patients, it may be wise to use myeloablative or “intermediate” conditioning at least in those children and adolescents who have a good PS. Nowadays, the improvement in first-line therapies also in the pediatric population as well as the introduction of BV [47] and eventually checkpoint inhibitors in this setting [48] has significantly reduced the need to allo-SCT in this specific group of patients.
9 Alternative Donor Transplants
In Europe and North America, only around a third of patients will have an HLA-matched sibling donor; therefore the use of alternative donors is essential to expand the number of patients eligible for the procedure. The advent of molecular techniques has improved the accuracy of tissue typing reports but the associated increase in HLA polymorphism has made finding an exact molecularly matched donor more difficult. However, the increase in unrelated donor numbers, the availability of cord blood, and the development of efficacious GVHD prophylaxis in haploidentical transplantation have significantly allowed a rise in the number of alternative donor transplants to be performed.
The transplant outcomes using unrelated donors appear similar to those reported using sibling donors [19, 25, 36, 49]. Not surprisingly, rates of GVHD may be higher and many groups have used T-cell depletion strategies with either alemtuzumab or ATG to reduce the incidence of this complication. Interestingly, unrelated donor transplants in patients with HL appear to have a similar overall survival and PFS to sibling donor transplants [19, 25]. Therefore, consideration of an unrelated allogeneic transplant is an adequate alternative for patients that do not have a HLA identical sibling donor [41].
The published experience with cord blood donors in HL is much more limited, but some retrospective analyses indicate that it may be feasible [50, 51]. A Eurocord-Netcord study showed a 30% PFS at 1 year in patients with relapsed HL [52]. A more recent retrospective analysis from Eurocord and EBMT Lymphoma and Cellular Therapy & Immunobiology Working Party that included 113 patients [53] demonstrates a 4-year PFS and OS of 26% and 46%, respectively, with significantly better results in those patients undergoing transplant in CR. A recently published French study showed that use of a cord blood donor was associated with inferior survival [37]. Because of the questionable results of cord blood transplants in terms of relapse rate after the procedure and high NRM but mostly because of the widespread use of haploidentical donors, the use of cord blood as stem cell source in patients with relapsed/refractory HL is almost nonexistent.
Finally, the introduction of posttransplantation cyclophosphamide (PT-Cy) for GVHD prophylaxis following NMA conditioning regimen has ameliorated survival and toxicity rates of haploidentical transplantation in hematologic malignancies [54]. In 2008, Burroughs et al. compared the outcome of NMA allo-SCT for 90 patients with relapsed HL based on donor cell source (38 matched related, 24 unrelated, 28 HLA-haploidentical related donors). Interestingly, the authors found no significant differences in grade III–IV aGVHD or cGVHD among the three groups, confirming a role for selective depletion of alloreactive T cells induced by PT-Cy in reducing the risk of GVHD in haploidentical transplants. Moreover, they reported no differences in 2-year OS (58% vs. 53% vs. 58%) with better 2-year PFS rates (51% vs. 23% vs. 29%) and lower 2-year cumulative incidence (CI) of relapse/progression (40% vs. 56% vs. 63%) in HLA-haploidentical related compared to matched related and unrelated recipients [21]. Subsequently, other groups reproduced promising outcomes for haploidentical allo-SCT, with reasonable grade II–IV aGVHD rates (range 23–39%) and low incidence of cGVHD (range 9–19%) [55,56,57]. Recently, the French Society of Bone Marrow Transplantation reported a significantly higher probability of GVHD-free relapse-free survival (GRFS) in HL patients who underwent allo-SCT with RIC or NMA conditioning from a haploidentical related donor, when compared with mismatched unrelated and cord blood donors (52% vs. 31% vs. 22%), indicating that haploidentical donors may be a valuable stem cell source in the absence of an HLA-matched donor [58]. Thereafter, the largest retrospective series of 709 adult HL patients recently published by the LWP of the EBMT reported similar survival outcomes in PT-Cy-based haploidentical allo-SCT compared with HLA-matched related and unrelated allo-SCT (1-year CI of NRM 17% vs. 13% vs. 21%; 2-year CI of relapse/progression 39% vs. 49% vs. 32%; 2-year OS 67% vs. 71% vs. 62%; 2-year PFS 43% vs. 38% vs. 45%), with a risk of chronic GVHD lower than that observed in matched unrelated transplants (1-year CI 26% vs. 41%), confirming the significant role of haploidentical allo-SCT in HL patients six [35] (Fig. 21.2).
10 Role of Allogeneic Stem Cell Transplantation in the Era of New Drugs
Brentuximab vedotin (BV) is an antibody-drug conjugate that selectively delivers monomethyl auristatin E, an antimicrotubule agent, into CD30-expressing cells. In phase I studies, BV demonstrated significant activity with a favorable safety profile in patients with relapsed/refractory CD30-positive lymphomas. The interesting results seen in the phase I trial lead to a phase II that evaluated the efficacy and safety of BV. The drug was given at doses of 1.8 mg/kg by intravenous infusion every 3 weeks up to a maximum number of 16 cycles in 102 patients with relapsed or refractory HL after ASCT [59]. Overall response rate (ORR) was 75% with a CR in 34% of patients. The median PFS for all patients was 5.6 months, and the median duration of response for those in CR 40.5 months. After a median observation of 3 years, 31 patients were alive and free of documented progressive disease. The drug was quite well tolerated: the most common treatment-related adverse events were peripheral sensory neuropathy, nausea, fatigue, neutropenia, and diarrhea. Subsequently, the use of BV has been anticipated in the clinical setting of selected high-risk HL patients with primary refractory, initial remission duration shorter than 1 year, and extranodal or advanced-stage disease at relapse. In these patients BV was administered as consolidation after ASCT for up to 16 cycles, with a significant improvement in PFS and no additional toxicities other than peripheral sensory neuropathy as compared to patients who received no consolidation (42.9 vs. 24.1 months) [11]. This observation prompted the approval of BV by FDA and EMA in patients at high risk of relapse or progression after ASCT. At 5-year follow-up the survival advantage of consolidation BV has been confirmed with 5-year PFS of 59% vs. 41% [60].
BV has also been used in the pre-allo-SCT setting, as a “bridge to allo,” and in the post-allogeneic setting to treat patients with relapsed/progressive disease after the allogeneic procedure. In 2012 Chen et al. [61] published their experience on 18 patients with multiply relapsed HL undergoing a RIC/allo-SCT after being treated with BV as salvage therapy. NRM and acute and chronic GVHD preferred incidence after the allogeneic procedure were not significantly different from what was previously described. With a median follow-up of only 12 months, PFS was 100%. In a retrospective analysis comparing outcomes after allo-SCT in relapsed/refractory HL patients, Chen et al. [62] also showed that the administration of BV as a bridge to transplant significantly increased the percentage of patients achieving a CR. Indeed, disease status at transplantation is a known significant prognostic factor for both long-term OS and GRFS [28]. Recently the LWP of the EBMT compared the outcomes of 210 patients who received BV prior to allo-SCT with that of 218 patients who did not receive BV. Differently from previous reports, in multivariate analysis pre-allo-SCT BV had no impact on aGVHD, NRM, CI of relapse, PFS, or OS, but significantly reduced the risk of cGVHD (hazard ratio = 0.64). Nevertheless, it must be noted that, while there were no differences between the two groups in disease status prior to ASCT, the population who received BV as pre-allo-SCT salvage was more heavily pretreated (median previous lines of treatment 4 vs. 3 of patients who did not receive BV). This might indicate that BV has a role in inducing favorable disease responses in otherwise refractory patients, therefore improving allo-SCT outcome [63] (Fig. 21.3). Moreover, the role of BV after allo-SCT has been reported in a recent registry study published by the LWP of the EBMT demonstrating encouraging results with ORR 76% (CR 29%) and with 34% of patients alive and in CR after a median follow-up of 33-month outcome [64].
Effect of pretransplant BV as last salvage therapy before allo-SCT on posttransplant outcomes. (a) Cumulative incidence of relapse; (b) cumulative incidence of non-relapse mortality; (c) progression-free survival; (d) overall survival. BV brentuximab vedotin, NRM non-relapse mortality, SCT allogeneic stem cell transplant (Bazarbachi A et al. [63], with permission)
The increasing anticipate use of BV before ASCT in clinical trials and the introduction of anti-programmed death 1 (PD-1) checkpoint inhibitors in the post-ASCT setting will most certainly change the treatment paradigm of these patients, either avoiding the allogeneic procedure in some patients or by increasing the group of potential candidates to allo-SCT. Recently, the PD-1 blocking antibodies nivolumab and pembrolizumab were shown to have significant therapeutic activity with an acceptable safety profile in patients with R/R classical HL. Based on the results of the phase II studies Checkmate 205 and Keynote 087, nivolumab and pembrolizumab have been approved by EMA and FDA for patients who failed ASCT and pre- and/or post-ASCT BV [65, 66]. The role of allo-SCT in cHL has become less clear after nivolumab and pembrolizumab became available. A recent retrospective analysis of 39 patients who underwent allo-SCT after a median of 62 days following anti-PD-1 therapy showed encouraging results with 1-year PFS and OS of 76% and 89%, respectively. However, a high rate of GVHD was reported, especially acute (1-year incidence of grade 2–4 aGVHD, grade 4 aGVHD, and cGVHD were 44%, 13%, and 41%, respectively), with four treatment-related deaths (three acute GVHD and one hepatic VOD) [67]. In the extended follow-up analysis of CheckMate 205 Trial, 44 over 243 patients proceeded to allo-SCT after a median time of 49 days from last nivolumab administration. Six-month PFS and OS estimates were 82% and 87%, respectively, with 13% of transplant-related mortality and four over five deaths from acute GVHD [66] (Fig. 21.4). Interestingly, in both studies no clear correlation has been identified between time from last anti-PD-1 administration to allo-SCT and onset of GVHD or NRM. Although the follow-up is limited, these studies indicate that allo-SCT after PD-1 therapy is feasible, but with an increased risk of toxicity. Nevertheless, since anti-PD-1 inhibitors have a very favorable toxicity profile, some responding patients could not benefit from anti-PD-1 discontinuation to proceed to a highly more toxic procedure as allo-SCT; thus the area of uncertainty is growing, making clinical decisions very difficult, especially for patients in CR [68, 69].
Results of allo-SCT after nivolumab treatment in patients with relapsed/refractory HL. Cumulative incidence of (a) transplant-related mortality (TRM) and disease progression, (b) acute graft-versus-host disease (aGVHD) and chronic graft-versus-host disease (cGVHD), and (c) overall survival (OS) and progression-free survival (PFS) after allogeneic hematopoietic cell transplantation (allo-HCT). Cumulative incidence (95% CI) at 100 days and 6 months for TRM, disease progression, and GVHD and median (95% CI) PFS and OS are shown. Death was considered a competing risk to GVHD, and posttransplant disease progression was considered a competing event to TRM. G grade, NA not available, NE not estimable. (Armand P et al. [66], with permission)
Recently, a retrospective multicenter study was conducted in 31 lymphoma patients undergoing anti-PD-1 treatment for relapse after allo-SCT. The majority of patients in the study (29 over 31) had HL, of which 27 had already received at least one salvage treatment after allo-SCT and before PD-1 blockade. Response rates were very promising, with ORR 77% (15 CRs and 8 PRs). After a median follow-up of 428 days from the first anti-PD-1 administration, 68% of patients were alive, while eight patients died because of GVHD (26%). Overall, 17 patients (55%) developed GVHD (six acute, four overlap, and seven chronic), after a median of 1–2 anti-PD-1 doses. GVHD was acute grade 3–4 or chronic severe in nine patients and was frequently steroid refractory, with the majority of patients requiring two or more systemic therapies and only two patients achieving CR. Interestingly, 12 over 17 patients had already experienced GVHD. Among these, six patients had active chronic GVHD at time of anti-PD-1 administration, three of which developed GVHD worsening after PD-1 blockade [70]. Similarly, in a French series, prior history of GVHD was reported in all GVHD cases occurred after anti-PD-1 administration. Moreover, median time from allo-SCT to PD-1 blockade was significantly shorter in patients who presented PD-1-related GVHD compared to GVHD-free patients (8 vs. 28 months) suggesting a role for anti-PD-1 blockade in triggering of GVHD [71]. These two retrospective studies and other reports infer that PD-1 blockade is feasible and highly effective also in the context of relapse after allo-SCT, although frequently complicated by severe and refractory GVHD [72,73,74,75,76,77]. Either in “bridge to allo-SCT” or in post-allo-SCT salvage contexts, further and larger studies are needed to clarify the combined role of PD-1 blockade, conditioning chemotherapy and GVHD prophylaxis (ATG, posttransplant cyclophosphamide, etc.) in the development of GVHD, and to optimize the management of these complications.
References
Horning SJ, Hoppe RT, Breslin S, Bartlett NL, Brown BW, Rosenberg SA (2002) Stanford V and radiotherapy for locally extensive and advanced Hodgkin disease: mature results of a prospective clinical trial. J Clin Oncol 20:630–637
Diehl V, Franklin J, Pfreundschuh M et al (2003) Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin disease. N Engl J Med 348:2386–2395
Linch DC, Winfield D, Goldstone AH et al (1993) Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin disease: results of a BNLI randomised trial. Lancet 341:1050–1054
Schmitz N, Pfistner B, Sextro M et al (2002) Aggressive conventional chemotherapy compared with high dose chemotherapy with autologous haematopoietic stem cell transplantation for relapsed chemosensitive Hodgkin disease: a randomised trial. Lancet 359:2065–2071
Sureda A, Arranz R, Iriondo A et al (2001) Autologous stem cell transplantation for Hodgkin disease: results and prognostic factors in 494 patients from the Grupo Español de Linfomas/Transplante Autólogo de Médula Ósea Spanish cooperative group. J Clin Oncol 19:1395–1404
Lazarus HM, Loberiza FR, Zhang MJ et al (2001) Autotransplants for Hodgkin disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR). Bone Marrow Transplant 27:387–396
Horning SJ, Chao NJ, Negrin RS et al (1997) High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin disease: analysis of the Stanford University results and prognostic indices. Blood 89:801–813
Brice P, Bouabdallah R, Moreau P et al (1997) Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin disease: analysis of 280 patients from the French registry. Bone Marrow Transplant 20:21–26
Sweetenham JW, Taghipour G, Milligan D et al (1997) High-dose therapy and autologous stem cell rescue for patients with Hodgkin disease in first relapse after chemotherapy: results from the EBMT. Bone Marrow Transplant 20:745–752
Bröckelmann PJ, Müller H, Casasnovas O et al (2017) Risk factors and a prognostic score for survival after autologous stem-cell transplantation for relapsed or refractory Hodgkin lymphoma. Ann Oncol 28:1352–1358
Moskowitz CH, Nademanee A, Masszi T et al (2015) Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 385:1853–1862
Appelbaum FR, Sullivan KM, Thomas ED et al (1985) Allogeneic marrow transplantation in the treatment of MOPP-resistant Hodgkin disease. J Clin Oncol 3:1490–1494
Phillips GL, Reece DE, Barnett MJ et al (1989) Allogeneic marrow transplantation for refractory Hodgkin’s disease. J Clin Oncol 7:1039–1045
Gajewski JL, Phillips GL, Sobocinski KA et al (1996) Bone marrow transplants from HLA-identical siblings in advanced Hodgkin disease. J Clin Oncol 14:572–578
Milpied N, Fielding AK, Pearce RM, Ernst P, Goldstone AH (1996) Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed Hodgkin disease. J Clin Oncol 14:1291–1296
Anderson JE, Litzow MR, Appelbaum FR et al (1993) Allogeneic, syngeneic, and autologous marrow transplantation for Hodgkin disease: the 21-year Seattle experience. J Clin Oncol 11:2342–2350
Jones RJ, Ambinder RF, Piantadosi S, Santos GW (1991) Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood 77:649–653
Akpek G, Ambinder RF, Piantadosi S et al (2001) Long-term results of blood and marrow transplantation for Hodgkin disease. J Clin Oncol 19:4314–4321
Sureda A, Robinson S, Canals C et al (2008) Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin lymphoma: an analysis from the lymphoma working Party of the European Group for blood and marrow transplantation. J Clin Oncol 26:455–462
Corradini P, Zallio F, Mariotti J et al (2005) Effect of age and previous autologous transplantation on nonrelapse mortality and survival in patients treated with reduced-intensity conditioning and allografting for advanced hematologic malignancies. J Clin Oncol 23:6690–6698
Burroughs LM, O’Donnell PV, Sandmaier BM et al (2008) Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Boil Bone Marrow Transplant 14:1279–1287
Anderlini P, Saliba R, Acholonu S et al (2005) Reduced-intensity allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s disease: low transplant-related mortality and impact of intensity of conditioning regimen. Bone Marrow Transplant 35:943–951
Faulkner RD, Craddock C, Byrne JL et al (2004) BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood 103:428–434
Robinson SP, Goldstone AH, Mackinnon S et al (2002) Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced intensity allogeneic progenitor cell transplantation: an analysis from the lymphoma working Party of the European Group for blood and bone marrow transplantation. Blood 100:4310–4316
Peggs KS, Hunter A, Chopra R et al (2005) Clinical evidence of a graft-versus lymphoma effect after reduced intensity allogeneic transplantation. Lancet 365:1906–1908
Alvarez I, Sureda A, Caballero D et al (2006) Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed Hodgkin lymphoma: results of a Spanish prospective cooperative protocol. Biol Bone Marrow Transplant 12:172–183
Gaudio F, Mazza P, Carella AM et al (2019) Outcomes of reduced intensity conditioning allogeneic hematopoietic stem cell transplantation for Hodgkin lymphomas: a retrospective multicenter experience by the rete Ematologica Pugliese (REP). Clin Lymphoma Myeloma Leuk 19:35–40
Spina F, Radice T, De Philippis C et al (2019) Allogeneic transplantation for relapsed and refractory Hodgkin lymphoma: long-term outcomes and graft-versus-host disease-free/relapse-free survival. Leuk Lymphoma 60:101–109
Giaccone L, Festuccia M, Zallio F et al (2017) Long-term follow-up of allogeneic stem cell transplantation in relapsed/refractory Hodgkin lymphoma. Bone Marrow Transplant 52:1208–1211
Anderlini P, Saliba RM, Ledesma C et al (2016) Gemcitabine, Fludarabine, and Melphalan for reduced-intensity conditioning and allogeneic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 22:1333–1337
Rashidi A, Ebadi M, Cashen AF (2016) Allogeneic hematopoietic stem cell transplantation in Hodgkin lymphoma: a systematic review and meta-analysis. Bone Marrow Transplant 51:521–528
Genadieva-Stavrik S, Boumendil A, Dreger P et al (2016) Myeloablative versus reduced intensity allogeneic stem cell transplantation for relapsed/refractory Hodgkin’s lymphoma in recent years: a retrospective analysis of the lymphoma working Party of the European Group for blood and marrow transplantation. Ann Oncol 27:2251–2257
Sureda A, Canals C, Arranz R et al (2012) Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study–a prospective clinical trial by the Grupo Español de Linfomas/Trasplante de Médula Osea (GEL/TAMO) and the lymphoma working Party of the European Group for blood and marrow transplantation. Haematologica 97:310–317
Robinson SP, Sureda A, Canals C et al (2009) Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin’s lymphoma: identification of prognostic factors predicting outcome. Haematologica 94:230–238
Martínez C, Gayoso J, Canals C et al (2017) Post-transplantation cyclophosphamide-based Haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for Hodgkin lymphoma: a registry study of the lymphoma working Party of the European Society for blood and marrow transplantation. J Clin Oncol 35:3425–3432
Anderlini P, Saliba R, Acholonu S et al (2008) Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma: the updated M.D. Anderson Cancer center experience. Haematologica 93:257–264
Marcais A, Porcher R, Robin M et al (2013) Impact of disease status and stem cell source on the results of reduced intensity conditioning transplant for Hodgkin’s lymphoma: a retrospective study from the French Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC). Haematologica 98:1467–1475
Peggs KS, Kayani I, Edwards N et al (2011) Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin’s lymphoma. J Clin Oncol 29:971–978
Theurich S, Malcher J, Wennhold K et al (2013) Brentuximab vedotin combined with donor lymphocyte infusions for early relapse of Hodgkin lymphoma after allogeneic stem-cell transplantation induces tumor-specific immunity and sustained clinical remission. J Clin Oncol 31:e59–e63
Tsirigotis P, Danylesko I, Gkirkas K et al (2016) Brentuximab vedotin in combination with or without donor lymphocyte infusion for patients with Hodgkin lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant 51:1313–1317
Duarte RF, Labopin M, Bader P et al (2019) Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant 54(10):1525–1552
Thomson KJ, Peggs KS, Smith P et al (2008) Superiority of reduced-intensity allogeneic transplantation over conventional treatment for relapse of Hodgkin’s lymphoma following autologous stem cell transplantation. Bone Marrow Transplant 41:765–770
Sarina B, Castagna L, Farina L et al (2010) Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood 115:3671–3677
Thomson KJ, Kayani I, Ardeshna K et al (2013) A response-adjusted PET-based transplantation strategy in primary resistant and relapsed Hodgkin lymphoma. Leukemia 27:1419–1422
Claviez A, Klingebiel T, Beyer J et al (2004) Allogeneic peripheral blood stem cell transplantation following fludarabine-based conditioning in six children with advanced Hodgkin disease. Ann Hematol 83:237–241
Claviez A, Canals C, Dierickx D et al (2009) Allogeneic hematopoietic stem cell transplantation in children and adolescents with recurrent and refractory Hodgkin lymphoma: an analysis of the European Group for Blood and Marrow Transplantation. Blood 114:2060–2067
Locatelli F, Mauz-Koerholz C, Neville K et al (2018) Brentuximab vedotin for paediatric relapsed or refractory Hodgkin’s lymphoma and anaplastic large-cell lymphoma: a multicentre, open-label, phase 1/2 study. Lancet Haematol 5:e450–e461
Foran AE, Nadel HR, Lee AF, Savage KJ, Deyell RJ (2017) Nivolumab in the treatment of refractory pediatric Hodgkin lymphoma. J Pediatr Hematol Oncol 39:e263–e266
Devetten MP, Hari PN, Carreras J et al (2009) Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Bone Marrow Transplant 15:109–117
Majhail NS, Weisdorf DJ, Wagner JE et al (2006) Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced Hodgkin lymphoma. Blood 107:3804–3807
Rodrigues CA, Sanz G, Brunstein CG et al (2009) Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol 27:256–2636
Paviglianiti A, Tozatto Maio K et al (2018) Outcomes of advanced Hodgkin lymphoma after umbilical cord blood transplantation: a Eurocord and EBMT lymphoma and Cellular Therapy & Immunobiology Working Party Study. Biol Blood Marrow Transplant 24:2265–2270
Rodrigues CA, Rocha V, Dreger P et al (2014) Alternative donor hematopoietic stem cell transplantation for mature lymphoid malignancies after reduced-intensity conditioning regimen: similar outcomes with umbilical cord blood and unrelated donor peripheral blood. Haematologica 99:370–377
Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ (2001) Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood 98:3456–3464
Raiola A, Dominietto A, Varaldo R et al (2014) Unmanipulated haploidentical BMT following non-myeloablative conditioning and post-transplantation CY for advanced Hodgkin’s lymphoma. Bone Marrow Transplant 49:190–194
Castagna L, Bramanti S, Devillier R et al (2017) Haploidentical transplantation with post-infusion cyclophosphamide in advanced Hodgkin lymphoma. Bone Marrow Transplant 52:797
Gayoso J, Balsalobre P, Pascual MJ et al (2016) Busulfan-based reduced intensity conditioning regimens for haploidentical transplantation in relapsed/refractory Hodgkin lymphoma: Spanish multicenter experience. Bone Marrow Transplant 51:1307–1312
Gauthier J, Castagna L, Garnier F et al (2017) Reduced-intensity and non-myeloablative allogeneic stem cell transplantation from alternative HLA-mismatched donors for Hodgkin lymphoma: a study by the French Society of Bone Marrow Transplantation and Cellular Therapy. Bone Marrow Transplant 52:689–696
Younes A, Gopal AK, Smith SE et al (2012) Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 30:2183–2189
Moskowitz CH, Walewski J, Nademanee A et al (2018) Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood 132:2639–2642
Chen R, Palmer JM, Thomas SH et al (2012) Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood 119:6379–6381
Chen R, Palmer J, Tsai NC et al (2013) Brentuximab vedotin improves HCT-CI, CR status, and peri-transplant toxicity in patients with relapsed/refractory Hodgkin lymphoma heading to RIC Allo-HCT. Blood 122:3374
Bazarbachi A, Boumendil A, Finel H et al (2018) Brentuximab vedotin prior to allogeneic stem cell transplantation in Hodgkin lymphoma: a report from the EBMT lymphoma working party. Br J Haematol 181:86–96
Bazarbachi A, Boumendil A, Finel H et al (2019) Brentuximab vedotin for recurrent Hodgkin lymphoma after allogeneic hematopoietic stem cell transplantation: a report from the EBMT lymphoma working party. Cancer 125:90–99
Chen R, Zinzani PL, Fanale MA et al (2017) KEYNOTE-087. Phase II study of the efficacy and safety of Pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 35:2125–2132
Armand P, Engert A, Younes A et al (2018) Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol 36:1428–1439
Merryman RW, Kim HT, Zinzani PL et al (2017) Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood 129:1380–1388
Herbaux C, Merryman R, Devine S et al (2018) Recommendations for managing PD-1 blockade in the context of allogeneic HCT in Hodgkin lymphoma: taming a necessary evil. Blood 132:9–16
Zinzani PL, Santoro A, Chiti A et al (2018) Italian expert panel consensus statement on the optimal use of PD-1 blockade therapy in classical Hodgkin lymphoma. Leuk Lymphoma 15:1–10
Haverkos BM, Abbott D, Hamadani M et al (2017) PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood 130:221–228
Herbaux C, Gauthier J, Brice P et al (2017) Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood 129:2471–2478
Angenendt L, Schliemann C, Lutz M et al (2016) Nivolumab in a patient with refractory Hodgkin’s lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant 51:443–445
Yared JA, Hardy N, Singh Z et al (2016) Major clinical response to nivolumab in relapsed/refractory Hodgkin lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant 51:850–852
Villasboas JC, Ansell SM, Witzig TE (2016) Targeting the PD-1 pathway in patients with relapsed classic Hodgkin lymphoma following allogeneic stem cell transplant is safe and effective. Oncotarget 7:13260–13264
Covut F, Pinto R, Cooper BW et al (2017) Nivolumab before and after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 52:1054–1056
Singh AK, Porrata LF, Aljitawi O et al (2016) Fatal GvHD induced by PD-1 inhibitor pembrolizumab in a patient with Hodgkin’s lymphoma. Bone Marrow Transplant 51:1268–1270
El Cheikh J, Massoud R, Abudalle I et al (2017) Nivolumab salvage therapy before or after allogeneic stem cell transplantation in Hodgkin lymphoma. Bone Marrow Transplant 52:1074–1077
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sureda, A., Pennisi, M., Corradini, P. (2020). Allogeneic Transplantation for Relapsed Hodgkin Lymphoma. In: Engert, A., Younes, A. (eds) Hodgkin Lymphoma. Hematologic Malignancies. Springer, Cham. https://doi.org/10.1007/978-3-030-32482-7_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-32482-7_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32481-0
Online ISBN: 978-3-030-32482-7
eBook Packages: MedicineMedicine (R0)