Abstract
Purines and pyrimidines are fundamental signaling molecules in controlling the survival and proliferation of astrocytes, as well as in mediating cell-to-cell communication between glial cells and neurons in the healthy brain. The malignant transformation of astrocytes towards progressively more aggressive brain tumours (from astrocytoma to anaplastic glioblastoma) leads to modifications in both the survival and cell death pathways which overall confer a growth advantage to malignant cells and resistance to many cytotoxic stimuli. It has been demonstrated, however, that, in astrocytomas, several purinergic (in particular adenosinergic) pathways controlling cell survival and death are still effective and, in some cases, even enhanced, providing invaluable targets for purine-based chemotherapy, that still represents an appropriate pharmacological approach to brain tumours. In this chapter, the current knowledge on both receptor-mediated and receptor-independent adenosine pathways in astrocytomas will be reviewed, with a particular emphasis on the most promising targets which could be translated from in vitro studies to in vivo pharmacology. Additionally, we have included new original data from our laboratory demonstrating a key involvement of MAP kinases in the cytostastic and cytotoxic effects exerted by an adenosine analogue, 2-CdA, which with the name of Cladribine is already clinically utilized in haematological malignancies. Here we show that 2-CdA can activate multiple intracellular pathways leading to cell cycle block and cell death by apoptosis of a human astrocytoma cell line that bears several pro-survival genetic mutations. Although in vivo data are still lacking, our results suggest that adenosine analogues could therefore be exploited to overcome resistance to chemotherapy of brain tumours.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- P1 receptors
- A3 ligands
- Astrocytoma
- Cladribine
- 2-Chloro-adenosine
- Caspase-2
- Caspase-9
- p53 mutations
- CD39
- CD73
- Matrix metalloproteinases
- Equilibrative nucleoside transporters
- MAP kinases
2.1 Introduction
Due to their universal role in cell growth, differentiation and death, purinergic mechanisms are intimately involved in the regulation of tumour growth, with both positive and negative influence on cancer growth, progression and metastatisation depending on the cell type, the involvement of specific extracellular receptors and/or intracellular apoptotic pathways (for a recent commentary, see Sek et al. 2018). Historically, the first evidence highlighting adenosine (Ado) as a regulator of cancer cell growth dates back to the 1940s, when purine derivatives started to be used as cytotoxic agents in hematologic tumours. It was then hypothesized these actions be due to impaired intracellular synthesis of nucleic acids and the involvement of specific P1 receptors for extracellular Ado was demonstrated only relatively recently. Here, we summarize the available evidence implicating Ado and its receptors in the regulation of brain astrocytomas, with special emphasis on the translation of these findings to the therapy of this form of cancer. It is worth mentioning that, apart from the A3 receptor subtype, which has been the object of extensive studies as a new target for chemotherapy of brain tumours (see Sect. 2.4.2) and from the studies on the intracellular effects of Ado in astrocytoma cells (see Sect. 2.5), in many papers astrocytoma cell lines have been utilized merely as in vitro models of astrocytes to test the presence and the effects of Ado-mediated signaling. This has to be taken into consideration when translating data from these studies to primary normal astrocytes, which can bear significant differences with respect to malignantly transformed cells.
2.2 Adenosine Metabolism and P1 Adenosine Receptors
Ado is considered rather a neuromodulator than a true neurotransmitter, since it is neither stored as such in synaptic vesicles nor released following membrane depolarization (however, see also Melani et al. 2012). It has been estimated that basal extracellular Ado concentration is in the range of 30–200 nM (Fredholm et al. 2011), and it can rapidly increase through two different mechanisms: enzymatic hydrolysis of extracellular ATP by the ecto-5′-nucleotidases CD39 and CD73 or the export via membrane bi-directional equilibrative transporters (ENTs) following its intracellular production (see also below, Fig. 2.1). Since ATP is released not only from neurons through synaptic vesicles, but also from various secretory cells (e.g., platelets, macrophages, endocrine cells; Lazarowski et al. 2011) as well as from damaged or dying cells, a delayed increase in Ado concentrations is always detected following ATP release, and, in general, Ado-mediated effects modulate the excitatory ATP effects in a compensatory inhibitory way.
Ado metabolism, P1 Ado receptors and associated signaling pathways
Ado can be generated intracellularly after SAH or ATP hydrolysis, and then transported extracellularly through ENT. Extracellular Ado concentrations can also increase thanks to ATP hydrolysis by ectoNTPDases. Low physiological Ado concentrations can activate the A1AR and A2AAR subtypes, whereas high micromolar concentrations (that can be reached following ischemic or traumatic events) are needed to recruit the A2BAR and A3AR subtypes. The classical signalling pathways activated by the four P1 receptor subtypes are shown, together with the A3AR-mediated pathways that have been more recently identified in glioma cells. Ado can also be deaminated to Inosine (Ino) by ADA either extracellularly or intracellularly, and finally metabolized to uric acid. See text for details (Sects. 2.2 and 2.4.2)
In the extracellular space, Ado can activate its membrane receptors, collectively referred to as the P1 receptors. Four subtypes of G protein-coupled Ado receptors have been identified, namely the A1 adenosine receptor (AR), A2AAR, A2BAR and A3AR subtypes (Alexander et al. 2017). Ado possesses a high affinity towards the A1AR and A2AAR subtypes, which can therefore be activated at low physiological Ado concentrations; conversely, only high Ado concentrations can effectively recruit the A2BAR and A3AR subtypes, which can thus play a key role upon pathological conditions (see also below). The A1AR and A3AR subtypes are either coupled to Gi proteins, which negatively regulate adenylyl cyclase activity (Fredholm et al. 2011), or Gq proteins (Alexander et al. 2017; Abbracchio et al. 1995a), leading to the hydrolysis of phosphatidylinositol to generate inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 in turn activates its receptors on the endoplasmic reticulum and stimulates the release of calcium from the intracellular stores, whereas DAG can activate protein kinase C (PKC) and additional second messenger pathways (Fig. 2.1). Especially in the brain and heart tissues, the A1AR subtype can also directly couple to K+ channels through the recruitment of a Go; the opening of these channels leads to the intracellular elevation of K+ concentrations and to membrane hyperpolarization. This latter action is at the basis of the bradycardic and anti-epileptic actions of Ado (Chen et al. 2013). The A3AR subtype can also recruit various intracellular pathways, which will be discussed in more details in Sect. 2.4.2. Conversely, the A2AAR and A2BAR subtypes are coupled to Gs and, consequently, to the activation of adenylyl cyclase and the generation of cAMP (Fredholm et al. 2011). The intracellular positive coupling to adenylyl cyclase is at the basis of the functional antagonism between the A2AAR and the D2 dopamine receptors in the striatum, since the latter inhibits adenylyl cyclase functions through Gi. Alterations in this functional cross-talk between the Ado and dopamine systems plays a crucial role in the development of various neurodegenerative disorders, such as Parkinson’s disease, as elegantly reviewed elsewhere (Nazario et al. 2017).
Ado is finally taken up by specific membrane transporters, which can be subdivided into equilibrative (bidirectional) or concentrative (i.e., working against concentration gradient, Pastor-Anglada and Pérez-Torras 2018), and phosphorylated in the cytosol by adenosine kinase (AK). Since this enzyme can operate only in the presence of high oxygen concentrations, Ado concentrations will remain high upon hypoxic conditions (such as following an ischemic event, at any site of inflammation or within a tumour mass, Uribe et al. 2017), when no ATP can be generated; therefore, Ado-mediated actions play an important role also upon pathological conditions. Intracellular Ado can also derive from the intracellular hydrolysis of ATP or of S-adenosyl-homocysteine (SAH). The latter represents the final step of the so-called “methyl cycle”, which is crucially involved in the synthesis of nucleic acids and in the methylation of proteins (Fig. 2.1). Ado can be also deaminated to inosine (Ino) by Ado deaminase (ADA), and further terminally metabolized to uric acid. Ado deamination and metabolism to uric acid can also occur extracellularly thanks to the presence of ecto-ADA enzymes on the cell surface (Fig. 2.1).
2.3 A Role for Ecto-5′-Nucleotidases CD39 and CD73 in Gliomas?
As mentioned above, Ado can be produced extracellularly from adenine nucleotides by two types of membrane-bound enzymes: CD39, the product of the ectonucleoside triphosphate diphosphohydrolase 1 gene (ENTPD1), which is responsible for the conversion of ATP to ADP and AMP, and CD73, the product of NT5E gene, which converts AMP to adenosine. Several papers have been recently published indicating that the scavenging of extracellular ATP and the production of adenosine have direct effects on tumour growth, spread of metastases, vascular regulation, as well as on the adhesion, migration and homing of both cancer cells and activated immune cells mediating anti-tumour effects (for details, see Allard et al. 2017).
Wang and coworkers have shown that CD73 expression inhibits anti-tumour immune responses and that, conversely, CD73-null mice bearing transplanted non-CNS tumours display a survival benefit when compared to wild type mice (Wang et al. 2011). Twelve days after tumour inoculation, higher numbers of CD8+ immune T cells are found in CD73-null mice. Authors examined in detail the role of CD73 in modulating the interaction of tumour infiltrating regulatory CD4+ T cells (tumour Tregs), that can promote metastatic tumour growth, with effector T cells. Importantly, CD73-null effector T cells did not show any changes in mediating anti-tumour responses. CD4+ tumour Tregs directly inhibited CD8+ T cell functions, such as interferon-gamma (IFN-γ) production, and consequently CD8+ T cell-mediated anti-tumour effects in a CD73-dependent manner. This study therefore shows that both local and systemic production of Ado by CD73 (in tandem with CD39) results in decreased effector functions of CD8+ T cells and in a reduction of their homing to tumours. This occurs by several means, including both decreased expression of adhesion molecules, direct in vivo modulation of anti-tumour responses and/or possibly induction of apoptosis (in this respect, see also Sect. 2.4). These data suggest that both cancer cells and host-derived cells cooperatively mediate tumour immune evasion, and that this occurs in a CD73-dependent manner.
Highly relevant to the present review, overexpression of CD73 has also emerged as a component of glioma cell adhesion and tumour cell-extracellular matrix interactions (Cappellari et al. 2012, see also below). In both C6 and U138MG glioma cells, treatment with 1 μM α,β-methylene ADP (APCP), a competitive CD73 inhibitor, caused a 30% reduction in glioma cell proliferation. In addition, 100 μM Ado increased cell proliferation by 36%, and treatment with Ado plus inhibitors of its uptake produced an additional and significant increase in cell proliferation. The inhibitory effect on cell proliferation caused by APCP was reverted by co-treatment with Ado uptake inhibitors. AMP (1 mM and 3 mM) decreased U138MG glioma cell proliferation by 29% and 42%, respectively (Bavaresco et al. 2008). Taken together, these results suggest the participation of CD73 in cell proliferation, and that this process is dependent upon (i) the enzyme’s production of Ado, acting here as a proliferative factor, and, (ii) removal of AMP, a toxic molecule for gliomas. These findings would therefore suggest CD73 as a new target for anti-glioma therapies. Interestingly, data on newly diagnosed glioma patients have demonstrated that CD39 and CD73 act in synergy to promote local adenosinergic immune suppression. Downregulation of CD73 is accompanied by a better prognosis for glioblastoma patients (Xu et al. 2013). These data have been confirmed in the only available in vivo study in rats with implanted C6 glioma cells, where chronic exposure to methotrexate led to the up-regulation of CD73, which in turn increased local Ado concentrations and subsequent immune suppression (Figueiró et al. 2016). However, other reports indicate that extracellular adenine nucleotides inhibit C6 glioma cell growth via Ado, which is produced by ecto-nucleotidases including CD73 at the extracellular space and then incorporated into cells by the equilibrative nucleoside transporters ENT2 (Ohkubo et al. 2007). Intracellular AMP accumulation by AK after Ado uptake would then induce C6 cell growth inhibition through pyrimidine starvation. It has to be underlined that these contrasting data have been obtained on cell lines in vitro. Therefore, at variance from other non-central nervous system (CNS) tumours (Wang et al. 2011; Allard et al. 2017), elucidation of the exact role of CD39 and CD73 in gliomas still awaits additional and more detailed direct in vivo evaluation in tumour-bearing animals and in patients where factors other than cancer cell proliferation (i.e., tumour cells’ adhesion, migration and invasiveness as well as immune cell-mediated responses) can be determined (see also Conclusions).
2.4 Receptor-Mediated Effects of Adenosine on Glioma Cell Growth and Survival
The interest in the role of the purinergic system in general, and of Ado in particular, in modulating the growth and survival of various types of tumour cells has grown since the 1990s, based on the demonstration of high extracellular purine concentrations within the tumour mass. For example, concentration of Ado in the extracellular fluid of glioma tissue has been directly measured in 21 patients undergoing surgical removal of the tumour and it has been reported to be in the low micromolar range (Melani et al. 2003), meaning high enough to stimulate all the four P1 receptor subtypes which have been all identified in C6 glioma cells, functionally coupled to the modulation of adenylate cyclase activity. Quantitative real time PCR allowed to rank their expression as follows: A1AR = A3AR > A2AAR > A2BAR (Castillo et al. 2007). Moreover, functional ENTs (Sinclair et al. 2000) and ecto-5′-nucleotidase/CD73 (Cappellari et al. 2012) have been also found expressed by glioma cells (see above), thus suggesting that the whole machinery controlling purine metabolism is present and functional in these cells, and therefore cell survival and properties could be deeply influenced by intra- and extracellular Ado concentrations. Indeed, in both C6 glioma and U138MG glioblastoma cells the activity of ecto-5′-nucleotidase/CD73 increased in parallel with cell proliferation to a maximum when cells reached confluence (Bavaresco et al. 2008). This means that ATP hydrolysis to Ado will progressively augment with the growing of the tumour mass, and that Ado-mediated effects on cell growth and proliferation might become progressively more important.
Many studies have been performed on in vitro or in vivo models of cancer of different origins (such as melanoma, leukemia, prostate carcinoma etc.), or on P1 receptor-transfected cells, and they have reported different, and often opposite, effects on cell survival exerted by the various Ado receptor subtypes (Gessi et al. 2011). These contradictory effects are probably due to the various experimental models, leading to a consequent coupling of P1 receptor subtypes to different intracellular pathways controlling cell survival and proliferation depending upon the cell type, but also to the different experimental settings (e.g., hypoxic vs normoxic), and to the concentration of the agonists utilized (see Sect. 2.4.2).
2.4.1 A1AR-, A2AAR-, and A2BAR-Mediated Effects on Glioma Cells
Based on studies performed on solid tumour cells or on transfected cells, both the A1AR and the A2AAR subtypes can exert both pro-survival and anti-apopotic actions, by recruiting different intracellular signaling pathways (Gessi et al. 2011). Interestingly, Ado has been shown to reduce glioblastoma cell invasion through the A1AR expressed by microglia cells, thus confirming the complexity of purinergic signaling acting on multiple cell types, including the immune system where P1 receptors play a fundamental role (reviewed in Gessi et al. 2011). It is interesting to point out that both the A2AAR and the A2BAR subtypes have been crucially involved in the positive regulation of angiogenesis, by promoting the survival and proliferation of endothelial cells in synergy with VEGF (reviewed in Gessi et al. 2011), and their activation could therefore indirectly boost tumour growth by improving the supply of oxygen and nutrients.
A significant upregulation of the A1AR has been identified in the peritumour area in animals injected with F98 or C6 glioma cells by 3D imaging reconstruction of the tumour mass (Dehnhardt et al. 2007). This upregulation was dependent upon the tumour volume. No significant changes, but rather a tendency to decrease, were instead detected within the tumour itself, possibly due to induction of cell death by necrosis. Although the functional significance of this increase in A1AR is not known, monitoring A1AR expression and receptor presence around tumours could represent a valuable diagnostic and prognostic marker for glioblastoma progression. In fact, this receptor system has been studied by examining the binding of the radioactive molecule 18F-CPFPX (a selective A1 antagonist) by positron emission tomography (PET) in vivo and described as being up-regulated during the cerebral response to glioma invasion in primary human glioblastoma multiforme (Bauer et al. 2005).
Specific down-regulation of the A1AR and a parallel up-regulation of the A2AR subtypes have been observed in C6 glioma cells following induction of hypoxia (Castillo et al. 2008). This effect was mimicked by Ado itself, therefore suggesting that high extracellular Ado concentrations that are generated as a consequence of hypoxic conditions (as observed within a tumour mass; see also below) can auto-regulate Ado-dependent signaling. These data further confirm that the results on receptor recruitment and activation can significantly vary depending upon the experimental setting utilized, and can even become the opposite when shifting from in vitro to in vivo conditions.
While most of the immunosuppressive actions of Ado in the tumour environment are mediated by the A2AARs (see above, Sect. 2.3; Gessi et al. 2011), the only paper dealing with a putative role for the A2AAR in controlling glioma survival shows a protective role for this receptor, at least under the experimental conditions utilized. In fact, authors have demonstrated that Ado, at concentrations ranging from 125 to 500 μM, significantly protected C6 glioma cells from NO-dependent cell death induced by the incubation with a mix of cytokines (Isakovic et al. 2008). In this study, the possible involvement of the A2AAR (although the Ado concentrations utilized here are extremely high) and of the A2BAR has been only postulated, based on the inability of an inhibitor of ENTs to prevent Ado-mediated effects and on previous data demonstrating that overexpression of the A2AAR and inhibition of AK (which in turn increases extracellular Ado concentrations) in C6 glioma cells significantly inhibited NF-κB activation and cytokine-induced iNOS upregulation (Lee et al. 2005; Sands et al. 2004).
It is worth mentioning that A2AAR agonists transiently increase blood-brain barrier permeability, and this could represent a novel pharmacological approach to boost drug delivery to the brain. In this respect, Regadenoson, the only moderately selective A2AAR agonist approved for human use, was tested in glioblastoma patients in an attempt to raise the concentration of the anticancer drug temozolomide in the brain interstitium (reviewed in Jacobson et al. 2019).
Functional A2BARs were identified in human astrocytoma ADF cells, (a cell line directly derived from a cancer patient), which underwent desensitization following prolonged agonist exposure due to phophorylation at threonine residues (Trincavelli et al. 2004). Interestingly, the pro-inflammatory cytokine TNFα potentiated A2BAR coupling and inhibited its desensitization, so that only in its presence could the A2 receptor agonist NECA promote A2BAR-mediated elongation of numerous thin cellular processes (Trincavelli et al. 2004). These morphological changes generally reflect the commitment of astrocytes towards a cell differentiation program, which could be also accompanied by an increased resistance to cell death (see below, Sect. 2.4.2). It should be noted, however, that in cancer cells any stimulus promoting differentiation would conflict with their intrinsic tendency towards uncontrolled proliferation. These contradictory messages could therefore promote the decision of the cell to undergo apoptotic cell death. Thus, activation of the A2BAR in the presence of pro-inflammatory cytokines could prove useful in glioma therapy.
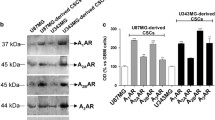
In this respect, more recent data from the same group have shown that activation of both A1ARs and A2BARs expressed by glioblastoma multiforme cancer stem cells (CSCs; the cell population within a tumour mass endowed with high proliferative and malignancy potential and likely responsible for tumour spreading and metastatization; Eramo et al. 2006) exerts anti-proliferative/pro-differentiative roles and can sensitize CSCs to temozolomide cytotoxicity (Daniele et al. 2014). Thus, recruiting Ado receptors on CSCs represents an innovative option to overcome cell resistance and to boost the effects of chemotherapy.
Moreover, in human U373 glioblastoma multiforme cells activation of the A2BAR recruited a series of intracellular pathways, including p38 MAP kinase and PKC delta and epsilon, which in turn promoted IL6 synthesis and release (Fiebich et al. 2005). Since this cytokine has been associated to the development of a malignant and aggressive phenotype in various types of cancer, such as breast carcinoma, caution should be kept in evaluating the final outcome of A2BAR activation in brain tumours, and to discriminate effects on tumour cells from effects on CSCs. More experimental data are therefore needed to clearly determine the role of this receptor subtype in modulating the survival and proliferation of glioma cells.
2.4.2 The A3 Receptor Subtype as a New Pharmacological Target for Innovative Chemotherapy Approaches to Gliomas
The A3AR subtype is the only member of the P1 receptor family that was cloned before its pharmacological characterization. As knowledge on its structure were accumulating, it appeared that this receptor would have been difficult to characterize, mostly based on the low level of interspecies homology, reflecting in highly different pharmacological profiles, especially in terms of antagonist binding and affinities (reviewed in Gessi et al. 2008). Moreover, also receptor distribution showed significant differences among species, with high expression detected in the lung, spleen, testis, brain, heart and liver (Gessi et al. 2008). Interestingly, very high levels of A3AR protein were found in a variety of cancer cell lines, including astrocytoma and glioblastoma, suggesting a role for this receptor as a tumour marker (Gessi et al. 2008) or as a target for new chemotherapy approaches (see below).
The analysis of receptor dynamics in ADF astrocytoma cells has led to discover a fast desensitization response to agonist stimulation, followed by agonist-mediated internalization and recycling to the cell surface within 120 min. A more prolonged exposure to a selective A3AR agonist (Cl-IB-MECA) led to a significant down-regulation of receptor expression, with slow recovery (Trincavelli et al. 2002). This rapid desensitization kinetics is possibly due to the presence of a higher number of serine and threonine residues at the receptor C-terminal region with respect to A1AR and A2AR subtypes (Palmer and Stiles 2000).
Apart from the “classical” second messenger systems (see above, Sect. 2.2), the A3AR can also couple to the activation of the small G protein RhoA, which in turn stimulates phospholipase D (PLD) (Gessi et al. 2008), and to the MAP kinase signaling pathways through the release of the βγ subunit from pertussis toxin-sensitive Gi proteins (Schulte and Fredholm 2003), or to the PI3K/Akt pathway (see Fig. 2.1). Concerning the latter, inhibition of PKA and PKB/Akt was demonstrated in melanoma cells upon activation of the A3AR; this in turn maintained glycogen synthase kinase 3β (GSK-3β) in its active form, with a consequent deregulation of the Wnt pathway, increase in β-catenin degradation, and finally inhibition of tumour cell proliferation (Fishman et al. 2002). These observations, together with the high expression of the A3AR in tumour cells and with its pro-apoptotic effects on various lymphoma, melanoma and prostate cell lines in vitro and in mouse xenografts in vivo (Fishman et al. 2002), have led to the hypothesis that A3AR agonists might be useful as chemotherapic agents. Clinical studies are currently ongoing on the use of CF102 (Cl-IB-MECA) in patients with advanced hepatocellular carcinoma (Fredholm et al. 2011). Taken together, these results suggest an inhibitory action of the A3AR on cancer cell growth and proliferation.
The opposite, however, seems to hold true for glioblastoma cells. In fact, the selective A3AR antagonist, MRS1220, blocked Ado-mediated glioma proliferation (Morrone et al. 2003). Upon hypoxic conditions, activation of the A3AR upregulated the transcription factor HIF-1α, leading to increase VEGF production, with consequent angiogenesis (Merighi et al. 2006). Hypoxia-induced chemoresistance of human glioblastoma cells was also demonstrated to depend upon the activation of the A3AR linked to the PKB/Akt pathway, which in turn mediated the phosphorylation and consequent inactivation of the pro-apopototic member of the Bcl2 protein family Bad, leading to cell survival (Merighi et al. 2007). Indeed, upregulation of matrix metalloproteinase-9 (MMP-9) expression and increased tumour migration was also observed upon activation of the A3AR in human U87MG glioblastoma cells under normoxic conditions (Gessi et al. 2010). MMPs degrade the extracellular matrix and consequently promote migration of glioma cells towards brain areas protected by an intact blood-brain barrier. Unfortunately, no differences between the migratory biology of transformed and normal brain cells have been identified, which renders this potential target difficult to be exploited (Westphal and Lamszus 2011). Based on the above-mentioned evidence, inhibition of A3ARs with selective antagonists could therefore represent an appealing therapeutic approach to solid tumours in general (which are characterized by an hypoxic core) and to gliomas in particular, not only to inhibit MMPs upregulation, but also to affect cancer cell growth and properties at various molecular levels, thus increasing the possibility of a more effective chemotherapy. In this respect, possible lead compounds could emerge from the development of a series of pyrazol[3,4-d]pyrimidines derivatives with drug-like physicochemical properties, which demonstrated a high affinity and selectivity for the A3AR and were able to potently inhibit Cl-IB-MECA- and IB-MECA-stimulated glioblastoma cell growth in vitro (Taliani et al. 2010). In line with the pro-survival role of the A3AR in glioma cells, our group has demonstrated that in human astrocytoma ADF cells, nanomolar Cl-IB-MECA concentrations produced clear morphological changes (i.e., emission of numerous long filaments, Fig. 2.2b and higher magnification in Fig. 2.2c), which were accompanied by the appearance of stress fibers, a typical hallmark of cytoskeletal rearrangement, and by the redistribution of the anti-apoptotic protein Bcl-XL towards cell processes (Abbracchio et al. 1997), through the specific involvement of the small G protein Rho (Abbracchio et al. 2001). Overall, these changes increased the ability of the cell to adhere to the culture substrate, which in turn reflected in a reduced sensitivity to cell death, thus confirming that the A3AR could contribute to improve cancer cell survival.
Morphological changes induced by nanomolar concentrations of the A3AR agonist Cl-IB-MECA in human astrocytoma ADF cells
Scanning electron micrographs of ADF astrocytoma cells grown for 72 h under Control conditions (a) or in the presence of 100 nM Cl-IB-MECA (b, c). A typical bipolar shape with a relatively low number of cell protrusions was observed in Control cells, whereas exposure to nanomolar Cl-IB-MECA induced marked morphological changes with an increased of the number and length of cellular processes. Original magnification: (a, b) 1,000x; (c) 3,300x. Numbers on micrograph represent numbering in the original manuscript from which the figure is reproduced (Abbracchio et al. 1997) with permission from Elsevier
An opposite effect was, however, exerted by high micromolar Cl-IB-MECA concentrations, since a significant cytotoxicity, reduction of cell number and induction of apoptosis were detected both in C6 glioma (Appel et al. 2001) and in human astrocytoma ADF cells (Abbracchio et al. 1998) upon treatment with this selective A3AR agonist. These data were in line with previous results showing a dual opposite effect on ischemia-induced tissue damage exerted by either acute or chronic A3AR agonist administration (reviewed in Jacobson 1998), suggesting an opposite outcome on cell survival depending upon the “intensity” of receptor activation. Although the selective recruitment of the A3AR subtype by such high agonist concentrations could be questioned, these apparent discrepancies in A3AR-mediated effects on cell survival are in line with its enigmatic (and yet-to-be fully clarified) role as “Dr. Jekill and Mr. Hyde” (Gessi et al. 2008).
A further confirmation of the possible anti-proliferative role of the A3AR in glioma comes from a study showing a significant upregulation of the mRNAs encoding for the A3AR and for ENTPDase/CD73 upon exposure of C6 and U138-MG glioma cells to indomethacin (Bernardi et al. 2007). Increase in CD73 mRNA was accompanied by a significantly higher ratio of ATP hydrolysis; although actual Ado concentration was not measured in this paper, it can be envisaged that it reaches values high enough to activate the A3AR subtype. Indomethacin also significantly inhibited glioma cell proliferation, and this effect was reverted by the A3AR agonist MRS1220, but not by antagonists acting at other P1 receptors (Bernardi et al. 2007), thus suggesting a specific involvement of this receptor subtype in indomethacin-mediated effects.
Finally, it should be also noted that A3ARs can modulate the immune system by activating NK cells and, possibly, NK-mediated disruption of tumour cells. Conversely, Ado has been demonstrated to exert an immunosuppressive role through the A3AR, which could interfere with the recognition of tumour cells by T killer cells, within the environment of a solid tumour (Gessi et al. 2008, see also above Sect. 2.3). Therefore, this double immunomodulatory effect can positively or negatively influence tumour growth, progression, and metastatisation depending upon the cell environment and the type of malignancy.
2.5 Receptor-Independent Effects of Adenosine Analogues in Glioma Cell Growth and Survival
Apart from the more recently identified receptor-mediated effects, the intracellular cytotoxic actions of purine and pyrimidine derivatives have been known since the 1940s, and are at the basis of their use as chemotherapic agents and anti-metabolites. For example, 2-chloro-2′-deoxyadenosine (2-CdA) is utilized as one of the drug of choice in hairy cell leukemia and in other haematological malignancies, with the name of Cladribine (Kreitman and Arons 2018). The molecular bases of nucleoside analogue cytotoxicity were initially mainly ascribed to their ability to alter intracellular purine and pyrimidine pools, leading to impaired synthesis of nucleic acids, or to directly interfere with the enzymes involved in DNA synthesis, thanks to their structural similarities with endogenous nucleosides (Dighiero 1996). In the 1990, however, it was demonstrated that nucleoside analogues could also activate specific pathways of apoptotic cell death. For example, in haemathological disorders, Cladribine was demonstrated to recruit both the extrinsic caspase-8-dependent (Nomura et al. 2000), and the intrinsic mitochondrial pathway of cell death, with the involvement of caspase-9 (Genini et al. 2000). The actions of nucleoside analogues on CNS cancer cells have not, however, been properly investigated.
The first clues of a possible cytotoxic role for Ado analogues on astrocytes came from the demonstration that exposure of primary rat astrocytes to 2-chloro-adenosine (2-CA), a non-selective agonist of P1 receptors, induced a dramatic reduction of cell number (Abbracchio et al. 1994), due to the induction of cell death by apopotosis (Abbracchio et al. 1995b; Ceruti et al. 1997). To our surprise, Ado receptor antagonists did not inhibit 2-CA-mediated effects, thus suggesting an intracellular site of action for the Ado analogue. These results prompted us to analyze the possible cytotoxic effects of 2-CA on human astrocytoma cells (ADF cells, see above), in comparison with 2-CdA, in order to verify the possible use of Ado analogues as anti-tumour agents also in solid brain tumours. First of all, we demonstrated a high cytotoxic activity exerted by the two Ado analogues on ADF cells, due to the induction of apoptosis (Fig. 2.3, Ceruti et al. 2000, Ceruti et al. 2003a). 2-CdA resulted to be more potent than 2-CA in inducing ADF cell death, with significant effects already detected at 5 μM concentration. Both molecules exerted their effects after their intracellular phosphorylation to the corresponding nucleotide derivatives, which occurred through the recruitment of two independent biochemical pathways, as demonstrated by the complete inhibition of 2-CA-mediated actions by Itub (an inhibitor of AK) and of 2-CdA-induced effects by dCyd, which competes for the active site of deoxy-cytidine kinase (Fig. 2.3, Ceruti et al. 2000, Ceruti et al. 2003a). Only in the case of Cladribine was a cell cycle block demonstrated to precede induction of apoptosis (see below for details), further confirming that, although closely chemically related, these two compounds indeed recruited diverging pathways of death (Ceruti et al. 2000). Nevertheless, the two Ado analogues converged on a common and rather new pathway of apoptosis; in fact, both activated an atypical caspase pathway in ADF cells, with the recruitment of caspase-2 as initiator caspase, followed by the contemporary consequent activation of caspase-8 and caspase-9, and of caspase-3 as the main effector caspase (Ceruti et al. 2003b). This was the first demonstration of the possible activation of a caspase-2-dependent pathway of cell death in astrocytoma cells, which was later demonstrated also following gamma-irradiation and overexpression of the pro-apoptotic molecule Smac/Diablo (Giagkousiklidis et al. 2005).
Different intracellular pathways of apoptotic cell death are activated by 2-CA and 2-CdA in human astrocytoma ADF cells
2-CA and 2-CdA are prodrugs, and they have to be intracellularly phosphorylated/activated by two distinct kinases to exert their toxic effects. This has been demonstrated by blocking 2-CA- and 2-CdA-mediated effects with 2 specific kinase inhibitors: Itub (acting on adenosine kinase) and dCyd (acting on deoxy-cytidine kinase), in the case of 2-CA and 2-CdA, respectively. Thus, the active cytotoxic species are represented by the corresponding 2-chloronucleotide or deoxy-nucleotide derivatives, which drive cell death by apoptosis through complex intracellular pathways (see text for details and Fig. 2.6). Apoptosis has been demonstrated by: (1) staining of cells floating in the culture supernatants with the chromatin fluorescent dye Hoechst 33258 (see pictures), and (2) flow-cytometric analysis of PI-stained cultures (see histograms on the right). The former technique demonstrated chromatin condensation and nuclear fragmentation in a highly significant percentage of detached cells (see micrographs and numbers below), whereas the appearance of hypodiploid DNA peak after a 48-h exposure to either adenosine analogue was shown by flow-cytometry (see numbers on histograms). Numbers represent the percentage of apoptotic cell death. For more details, see text. (Reproduced from Ceruti et al. 2003a with permission from John Wiley and Sons)
In the attempt to better disclose the biochemical pathways contributing to 2-CA and 2-CdA toxicity in ADF cells, we have more recently performed a detailed time-course of effects exerted on cell cycle progression. As previously mentioned, we already showed a G2/M cell cycle block after a 7-h exposure to 2-CdA followed by changing of culture medium and analysis at 48 h (Ceruti et al. 2000). Cytofluorimetric analysis at early time points (i.e., at the end of 7-h 2-CdA exposure) indeed showed an actual G1/S block of the cell cycle, which was accompanied by the inhibition of pRb phosphorylation, a typical G1 block-related event (Gire and Dulic 2015). As expected, dCyd fully prevents 2-CdA-induced effects (Fig. 2.4a). Additionally, Affymetrix® microarray analysis of RNA extracted from Control cultures and cultures exposed to 2-CdA for 7 h demonstrated the differential expression of a high number of genes related to cell cycle block, with the highest number of differentially expressed genes found under the terms “regulation of cell cycle” (18 genes) and “regulation of MAP kinase pathways” (13 genes). The most representative differentially expressed genes, including transcription factors and the cell cycle blocker p21 (Gire and Dulic 2015), are listed in Table 2.1. Our data demonstrate that short exposure to 2-CdA block astrocytoma cells at G1/S phases; if the drug is removed, cells recover from the block and proceed synchronously together along the cell cycle (and are therefore found in the G2/M phases at 48 h; Ceruti et al. 2000). If exposure to the drug is prolonged, blocked cells undergo cell death by apoptosis by activating multiple intracellular pathways (see below, Fig. 2.6).
A short exposure to 2-CdA promotes G1/S cell cycle block of ADF human astrocytoma cells, accompanied by pRb and p53 phosphorylation
(a): representative flow-cytometric analysis of DNA content after staining of cells with PI showing an increased percentage of cells in G1/S phase after a short (i.e., 7-h) exposure to 100 μM 2-CdA. Histograms on the right show the mean percentage of cells in each cell cycle phase of 12 replicates/condition. ∗p < 0.05 with respect to C and 2-CdA + dCyd; #p < 0.05 with respect to 2-CdA alone, one-way ANOVA, Scheffe’s F-test. Western blotting analysis below histograms show the reduced pRb phosphorylation upon 2-CdA exposure. (b): time-dependent Ser15 phosphorylation of p53 induced by exposure to 2-CdA. Total p53 is shown as control for protein loading on gel. 2-CA has no effect in agreement with the lack of cell cycle block (Ceruti et al. 2000). A representative filter is shown; similar results have been obtained in 5 independent experiments performed in triplicate. 2-CdA-induced cell cycle block, reduction of pRb phosphorylation and induction of p53 phosphorylation were all blocked by the contemporary exposure to 100 μM dCyd (see also Fig. 2.3)
It is well known that tumorigenesis is linked to the acquisition of several genetic defects, which render malignant cells resistant to the normal growth inhibitory and apoptotic signals, with a parallel deregulation of proliferation leading to out-of-control expansion of damaged cells (Kelly and Strasser 2011). In this respect, we have detected various genetic alterations borne by ADF cells. First of all, we identified a single mutation in the p53 protein (Ceruti et al. 2006), a G-to-A nucleotide substitution in position 797 of the p53 coding sequence, with a consequent single amino acid change (G-to-E) at position 266 of the p53 protein, belonging to the DNA-binding domain. This mutation leads to an inactive p53 isoform (Ceruti et al. 2006), meaning that ADF cells cannot activate the cell death pathways that depend upon the transcriptional activity of p53. Nevertheless, at early times (from 15′ to 7 h) after exposure of ADF cells to 2-CdA we detected by Western blotting analysis a rapid and time-dependent phosphorylation of p53 at Ser15, which was inhibited by dCyd (Fig. 2.4b). This suggests that, despite the presence of a mutated protein, 2-CdA can still recruit p53-mediated pathways, which could contribute to cell cycle arrest and cell death, possibly by non-trascriptional mechanisms. 2-CA had no effect on p53 phosphorylation, in agreement with its inability to alter cell cycle progression (Ceruti et al. 2000) and confirming that, although very similar, these two purine analogues activate distinct pathways of cytotoxicity in astrocytoma cells.
Next, we evaluated the possible involvement in the observed effects of MAP kinases, which are implicated not only in controlling cell proliferation but are actively recruited during apoptotic cell death (Sun et al. 2015; Dhanasekaran and Reddy 2017). Microarray data already suggested the involvement of these signaling pathways in 2-CdA-mediated effects, as shown by the differential expression of several genes associated with the MAP kinase pathways (Table 2.1). Thus, we exposed ADF cells to 2-CdA for 1–7 h and evaluated the phosphorylation/activation of JNK1/2, p38 and ERK1/2 on cell extracts by Western blotting and specific antibodies. A shown in Fig. 2.5a, all the three members of the MAP kinase family were activated by 2-CdA starting from 3 h. To directly link MAP kinase activation to cell cycle block, we exposed ADF cells for 7 h to either 100 μM 2-CdA alone or in combination with selective MAP kinase inhibitors, namely PD98059 (50 μM), SB203580 (25 μM) and SP600125 (20 μM) which are known to block ERK1/2, p38 and JNK1/2 signaling pathways, respectively. Only SP600125 inhibited 2-CdA-induced phosphorylation of p53 and G1/S cell cycle block (Fig. 2.5b). Thus, in agreement with literature data (Tomicic et al. 2015) our data demonstrate the recruitment of the JNK1/2 pathway by 2-CdA to exert its cytostatic effects on astrocytoma cells. Additionally, we also utilized in combination with 2-CdA inhibitors of other intracellular signaling pathways that are directly related to cell cycle block and cell death, namely NU7026 (10 μM), LY294002 (50 μM) and Caffeine (10 mM) acting on DNA-PK, PI3K/Akt and ATM-ATR, respectively. In agreement with their only partial inhibition of Ser15 p53 phosphorylation (see Western blotting filters in Fig. 2.5c), NU7026 and Caffeine were able to partially prevent 2-CdA-induced cell cycle block (histograms in Fig. 2.5c). This suggests that the PI3K/Akt and ATM-ATR signaling pathways are recruited by 2-CdA along with JNK1/ 2 to alter astrocytoma cell proliferation.
Time-dependent activation of MAPkinases and specific involvement of JNK1/2 in the cell cycle block induced after a 7-h exposure to 2-CdA
(a): cultures were grown under Control conditions (C) or exposed to 100 μM 2-CdA for the indicated time points. Cells were collected, lysed and the phosphorylation/activation of MAPkinases was evaluated by Western blotting analysis with specific antibodies. Arrows indicate the expected molecular weight for each enzyme. One representative experiment out of five run in triplicate is shown. (b): 2-CdA-induced Ser15 phosphorylation of p53 in the presence of selective inhibitors of the three classes of MAP kinases or (c): inhibitors of PI3K/Akt (LY294002), DNA-PK (NU7026) and ATM-ATR (Caffeine), analysed by Western blotting. Total p53 is shown as control for protein loading on gel. A representative filter is shown; similar results have been obtained in 5 independent experiments performed in triplicate. Histograms on the right show the percentage of cells in each phase of the cell cycle after incubation of cultures for 7 h with 2-CdA alone or in the presence of the indicated inhibitors. Data are the mean ± S.E.M. of three independent experiments run in triplicate. ∗p < 0.05 with respect to C, #p < 0.05 with respect to 2-CdA alone and ^p < 0.05 with respect to both C and 2-CdA alone, one-way ANOVA, Scheffe’s F-test
We already demonstrated that ADF cells are insensitive to apoptotic triggers (e.g., betulinic acid, potassium cyanide, and 2-deoxy-ribose) that recruit the” classical” intrinsic pathway of cell death, e.g. the mitochondria/cytochrome C/caspase-9 pathway (Ceruti et al. 2005). This is possibly due to the expression of a mutated form of caspase-9, bearing a single C-to-T nucleotide substitution in position 83 of the coding sequence, leading to a single amino acid substitution (A-to-V) in position 28 of the caspase-9 protein. This mutation occurs in the caspase pro-domain region, which allows the proper assembly of the apoptosome complex (Bratton and Salvesen 2010); it can be anticipated that a highly ramified amino acid as valine substituting for alanine will negatively influence the generation of the proper protein-to-protein interactions. Moreover, ADF cells also express a truncated splice variant of caspase-9, the so-called caspase-9β, which lacks a relevant part of the large subunit of the protein, including the catalytic site (Ceruti et al. 2005). Caspase-9β is therefore inactive and it is generally believed to behave as a dominant negative of the full-length caspase-9. Based on these observations, we therefore postulated that ADF cells couldn’t efficiently activate the intrinsic pathway of cell death, which could explain their resistance to several known cytotoxic agents.
Despite the presence of the above-mentioned multiple genetic alterations of different cell death pathways; both 2-CA and 2-CdA have proved able to induce massive ADF cell death by recruiting caspase-2 and, in the case of Cladribine, also by interfering with the cell cycle (see above, Ceruti et al. 2000, 2003b). This unveils the cytotoxic potential of these derivatives also in the presence of resistance to various pharmacological approaches, and suggests the use of Ado derivatives as second line drugs in astrocytoma refractory to currently utilized chemotherapic regimens. Interestingly, our new data show that apoptotic death of human ADF astrocytoma cells by a prolonged (24 h) exposure to 2-CdA also involves MAPkinases, specifically JNK1/2 (which is also involved in the induction of cell cycle block; see above) and ERK1/2, as demonstrated by the partial protection exerted by selective inhibitors of these two signaling pathways (Fig. 2.6). Other pathways which are recruited by 2-CdA at early time points, i.e. PI3K/Akt, DNA-PK, ATM-ATR and p38, are not necessary to promote cell death by longer incubation (Fig. 2.6). These data highlight for the first time the crucial role played by members of the MAP kinase family of enzymes in the cytotoxic activity of deoxyadenosine derivatives on astrocytoma cells, and confirm the ability of 2-CdA to activate multiple and parallel pathways of cytotoxicity in astrocytoma cells which might have important future applications to overcome cell resistance to “classic” chemotherapy approaches.
Involvement of ERK1/2 and JNK1/2 in 2-CdA-induced apoptotic death of ADF cells
Left: representative flow cytometry histograms of PI-stained nuclei of ADF cells grown under Control conditions or in the presence of 100 μM 2-CdA for 24 h. The position of the hypodiploid DNA peak is reported along with the percentage of apoptotic cells. Right: histograms show the percentage of apoptotic cells in cultures grown for 24 h in the presence of 2-CdA alone or in combination with inhibitors of several signaling pathways (see Fig. 2.5 for inhibitor concentrations). Data are the mean ± S.E.M. of five independent experiments run in triplicate. ∗p < 0.05 with respect to C and ^p < 0.05 with respect to both C and 2-CdA alone, one-way ANOVA, Scheffe’s F-test
It is also important to note that both 2-CA and 2-CdA were ineffective in inducing cell death on primary neuronal cultures when utilized at concentrations which were already almost maximally effective on ADF cells (Fig. 2.7, Ceruti et al. 2000). Although the mechanism at the basis of this selective sparing of neuronal cells (despite the marked apoptotic effect observed on cancerous glial cells) has not been clarified, it could represent an important additional therapeutic advantage in the case of in vivo administration of these drugs. Moreover, a specific carrier for Ado at the level of the blood-brain barrier has been demonstrated (Pardridge et al. 1994), and since 2-CA and Ado utilize the same ENT to permeate cells, it may be hypothesized that 2-CA may easily cross the blood-brain barrier. Concerning 2-CdA, concentrations in cerebrospinal fluid of leukemic patients have been demonstrated to represent 25% of plasma concentrations (Liliemark 1997), suggesting a high tropism toward nervous tissues. Moreover, an early disruption of the blood-brain barrier is known to occur in several CNS pathologies that would favour accumulation of these antitumour agents in brain tissues.
Selective sparing of primary neurons by concentrations of 2-CA and 2-CdA inducing significant cytotoxicity in human astrocytoma ADF cells
ADF cells and primary cortical neurons were exposed to increasing 2-CA (upper panel) or 2-CdA concentrations (lower panel) for 48 h. At the end of the incubation period the percentage of cell death was evaluated by the ability of cultures to metabolize the MTT dye, a typical feature of healthy cells. Data are expressed as percentage of the MTT metabolizing activity in the corresponding control cultures and represent the mean ± S.E.M. of five independent experiments run in triplicate. ∗p < 0.05 with respect to corresponding control, one way ANOVA, Scheffe’s F-test. (Reproduced from Ceruti et al. 2000 with permission from John Wiley and Sons)
Despite the above-mentioned encouraging preclinical data, a few unsuccessful clinical studies have been performed on the use of Cladribine in malignant gliomas. A Phase II study performed on 7 patients with recurrent glioma has shown no significant effects (Rajkumar et al. 1999). A interventional Clinical Trial entitled “Chemotherapy Followed by Radiation Therapy in Treating Patients with Malignant Glioma” is available on the USA ClinicalTrials.gov website (#NCT00019071). The study has been completed, but neither publications nor results have been posted on the website or published, thus making it impossible to evaluate its outcome. No data are currently available on the possible effects exerted by Cladribine, which is now considered one of the drugs of choice in multiple sclerosis for its immunomodulatory activities (Holmøy et al. 2017), as a second-line drug on refractory gliomas (see above).
As already mentioned above, Ado itself has been demonstrated to have inhibitory effects on the growth of C6 glioma cells by activating an intracellular pathway involving its uptake through ENT2 followed by phosphorylation by AK (Ohkubo et al. 2007). Authors hypothesize the induction of pyrimidine starvation by excessive intracellular AMP concentrations, since extracellular uridine completely reversed Ado-mediated effects. These results suggest that, in glioma cells, not only can elevated extracellular Ado concentrations activate its membrane receptors (see Sect. 2.4 above), but that the nucleoside can also directly modulate cell proliferation through intracellular signaling pathways.
2.6 Conclusions and Future Perspectives
The ubiquitous distribution and heterogeneity of the various components of Ado-mediated signaling (i.e., metabolizing enzymes, various subtypes of membrane receptors and of membrane transporters, direct actions on intracellular enzymes involved in cell survival, etc.), and the multiplicity of effects described upon its recruitment, with sometimes opposite outcomes, have made it difficult to exploit this system as a new pharmacological target for brain tumours. Nevertheless, the data collected so far and reviewed in this article along with our new unpublished data presented here strongly suggest that the Ado system plays a fundamental role in controlling the proliferation, migration, and survival of glioma cells, where a marked hypoxic environment is generated. Not only Ado receptors, but also nucleotide metabolizing enzymes could represent important and druggable targets in the fight against brain tumours. Moreover, the ability of Ado analogues to recruit atypical intracellular pathways of death in tumours where the “classical” apoptotic triggers are useless and to selectively spare surrounding healthy cells would represent additional therapeutic advantages, which should be further explored. Contrasting results can often be reconciled by taking into consideration the various experimental settings, the different cell lines, and the agonist concentrations utilized. While many studies have been addressed to assess the potential of Ado analogues as anti-glioma agents in vitro, we feel that an accurate evaluation of these compounds in appropriate in vivo models is still missing. We therefore envisage that well-designed in vivo studies could shed light on the current inconsistencies between different results, and more clearly highlight the possible advantages of targeting the Ado system for the therapy of glioma.
Abbreviations
- 18F-CPFPX:
-
8-cyclopentyl-3-(3-18F-fluoropropyl)-1-propyl-xanthine
- 2-CA:
-
2-chloro-adenosine
- 2-CdA:
-
2-chloro-2′-deoxyadenosine
- 8-CPT:
-
8-cyclo-pentyl-theophylline
- ADA:
-
adenosine deaminase
- Ado:
-
adenosine
- AK:
-
adenosine kinase
- APCP:
-
α,β-methylene ADP
- CI-IB-MECA:
-
2-chloro-N6-(3-iodobenzyl)-N-methyl-5′-carbamoyladenosine
- DAG:
-
diacylglycerol
- dCyd:
-
2′-deoxycytidine
- ENT:
-
equilibrative nucleoside transporter
- GSK-3β:
-
glycogen synthase kinase 3β
- HIF-1α,:
-
hypoxia-inducible factor 1 α subunit
- IFNγ:
-
interferon-gamma
- Ino:
-
inosine
- IP3 :
-
inositol-1,4,5-trisphosphate
- ITub:
-
5-iodotubercidin
- MAP kinases:
-
mitogen-activated protein kinases
- MMP-9:
-
matrix metalloproteinase-9
- MRS1220:
-
N-(9-chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-2-phenylacetamide
- MRS1706:
-
N-(4-acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]acetamide
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NECA:
-
N-ethyl-carboxamide adenosine
- NTPDase:
-
nucleoside triphosphate diphosphohydrolase
- PET:
-
positron emission tomography
- PI:
-
propidium iodide
- PI3K:
-
phosphatidylinositol 3-kinase
- PKA:
-
protein kinase A
- PKB/Akt:
-
protein kinase B
- PKC:
-
protein kinase C
- PLC:
-
phospholipase C
- PLD:
-
phospholipase D
- pRb:
-
Retinoblastoma protein
- SAH:
-
S-adenosyl-homocysteine
- SAM:
-
S-adenosyl-methionine
- TNFα:
-
tumour necrosis factor alpha
- VEGF:
-
vascular endothelial growth factor
References
Abbracchio MP, Saffrey MJ, Höpker V, Burnstock G (1994) Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience 59:67–76. https://doi.org/10.1016/0306-4522(94)90099-X
Abbracchio MP, Brambilla R, Ceruti S, Kim HO, von Lubitz DK, Jacobson KA, Cattabeni F (1995a) G protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol 48:1038–1045
Abbracchio MP, Ceruti S, Barbieri D, Franceschi C, Malorni W, Biondo L, Burnstock G, Cattabeni F (1995b) A novel action for adenosine: apoptosis of astroglial cells in rat brain primary cultures. Biochem Biophys Res Commun 213:908–915. https://doi.org/10.1006/bbrc.1995.2215
Abbracchio MP, Rainaldi G, Giammarioli AM, Ceruti S, Brambilla R, Cattabeni F, Barbieri D, Franceschi C, Jacobson KA, Malorni W (1997) The A3 adenosine receptor mediates cell spreading, reorganization of actin cytoskeleton, and distribution of Bcl-XL: studies in human astroglioma cells. Biochem Biophys Res Commun 241:297–304. https://doi.org/10.1006/bbrc.1997.7705
Abbracchio MP, Ceruti S, Brambilla R, Barbieri D, Camurri A, Franceschi C, Giammarioli AM, Jacobson KA, Cattabeni F, Malorni W (1998) Adenosine A3 receptors and viability of astrocytes. Drug Dev Res 45:379–386. https://doi.org/10.1002/(SICI)1098-2299(199811/12)45:3/4<379::AID-DDR38>3.0.CO;2-Y
Abbracchio MP, Camurri A, Ceruti S, Cattabeni F, Falzano L, Giammarioli AM, Jacobson KA, Trincavelli L, Martini C, Malorni W, Fiorentini C (2001) The A3 adenosine receptor induces cytoskeleton rearrangement in human astrocytoma cells via a specific action on rho proteins. Ann N Y Acad Sci 939:63–73. https://doi.org/10.1111/j.1749-6632.2001.tb03613.x
Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, Collaborators CGTP (2017) The concise guide to PHARMACOLOGY 2017/18: G protein-coupled receptors. Br J Pharmacol 174(Suppl 1):S17–S129. https://doi.org/10.1111/bph.13882
Allard B, Longhi MS, Robson SC, Stagg J (2017) The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev 276(1):121–144. https://doi.org/10.1111/imr.12528
Appel E, Kazimirsky G, Ashkenazi E, Kim SG, Jacobson KA, Brodie C (2001) Roles of BCL-2 and caspase 3 in the adenosine A3 receptor-induced apoptosis. J Mol Neurosci 17:285–292. https://doi.org/10.1385/JMN:17:3:285
Bauer A, Langen KJ, Bidmon H, Holschbach MH, Weber S, Olson RA, Coenen HH, Zilles K (2005) 18F-Cyclopentyl-3(3-18F-Fluoropropyl)-1-propyl-xanthine PET identifies changes in cerebral A1 adenosine receptor density caused by glioma invasion. J Nucl Med 46:450–454
Bavaresco L, Bernardi A, Braganhol E, Cappellari AR, Rockenbach L, Farias PF, Wink MR, Delgado-Cañedo A, Battastini AM (2008) The role of ecto-5′-nucleotidase/CD73 in glioma cell line proliferation. Mol Cell Biochem 319:61–68. https://doi.org/10.1007/s11010-008-9877-3
Bernardi A, Bavaresco L, Wink MR, Jacques-Silva MC, Delgado-Cañedo A, Lenz G, Battastini AM (2007) Indomethacin stimulates activity and expression of ecto-5′-nucleotidase/CD73 in glioma cell lines. Eur J Pharmacol 569:8–15. https://doi.org/10.1016/j.ejphar.2007.04.058
Bratton SB, Salvesen GS (2010) Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci 123:3209–3214. https://doi.org/10.1242/jcs.073643
Cappellari AR, Vasques GJ, Bavaresco L, Braganhol E, Battastini AM (2012) Involvement of ecto-5′-nucleotidase/CD73 in U138MG glioma cell adhesion. Mol Cell Biochem 359(1–2):315–322. https://doi.org/10.1007/s11010-011-1025-9
Castillo CA, Albasanz JL, Fernández M, Martìn M (2007) Endogenous expression of adenosine A1, A2 and A3 receptors in rat C6 glioma cells. Neurochem Res 32:1056–1070. https://doi.org/10.1007/s11064-006-9273-x
Castillo CA, León D, Ruiz MA, Albasanz JL, Martín M (2008) Modulation of adenosine A1 and A2A receptors in C6 glioma cells during hypoxia: involvement of endogenous adenosine. J Neurochem 105:2315–2329. https://doi.org/10.1111/j.1471-4159.2008.05314.x
Ceruti S, Barbieri D, Veronese E, Cattabeni F, Cossarizza A, Giammarioli AM, Malorni W, Franceschi C, Abbracchio MP (1997) Different pathways of apoptosis revealed by 2-chloro-adenosine and deoxy-D-ribose in mammalian astroglial cells. J Neurosci Res 47:372–383. https://doi.org/10.1002/(SICI)1097-4547(19970215)47:4%3C372::AID-JNR2%3E3.0.CO;2-B
Ceruti S, Franceschi C, Barbieri D, Malorni W, Camurri A, Giammarioli AM, Ambrosini A, Racagni G, Cattabeni F, Abbracchio MP (2000) Apoptosis induced by 2-chloro-adenosine and 2-chloro-2′-deoxy-adenosine in a human astrocytoma cell line: differential mechanisms and possible clinical relevance. J Neurosci Res 60:388–400. https://doi.org/10.1002/(SICI)1097-4547(20000501)60:3<388::AID-JNR14>3.0.CO;2-V
Ceruti S, Mazzola A, Beltrami E, Passera D, Piantoni E, Cattabeni F, Abbracchio MP (2003a) Intracellular phosphorylation of chloro-adenosine analogs is a pre-requisite for activation of caspase-3 and induction of apoptosis in human astrocytoma cells. Drug Dev Res 58:396–404. https://doi.org/10.1002/ddr.10184
Ceruti S, Beltrami E, Matarrese P, Mazzola A, Cattabeni F, Malorni W, Abbracchio MP (2003b) A key role for caspase-2 and caspase-3 in the apoptosis induced by 2-chloro-2′-deoxy-adenosine (cladribine) and 2-chloro-adenosine in human astrocytoma cells. Mol Pharmacol 63:1437–1447. https://doi.org/10.1124/mol.63.6.1437
Ceruti S, Mazzola A, Abbracchio MP (2005) Resistance of human astrocytoma cells to apoptosis induced by mitochondria-damaging agents: possible implications for anticancer therapy. J Pharmacol Exp Ther 314:825–837. https://doi.org/10.1124/jpet.105.085340
Ceruti S, Mazzola A, Abbracchio MP (2006) Proteasome inhibitors potentiate etoposide-induced cell death in human astrocytoma cells bearing a mutated p53 isoform. J Pharmacol Exp Ther 319:1424–1434. https://doi.org/10.1124/jpet.106.109397
Chen JF, Eltzschig HK, Fredholm BB (2013) Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Discov 12(4):265–286. https://doi.org/10.1038/nrd3955
Daniele S, Zappelli E, Natali L, Martini C, Trincavelli ML (2014) Modulation of A1 and A2B adenosine receptor activity: a new strategy to sensitise glioblastoma stem cells to chemotherapy. Cell Death Dis 5:e1539. https://doi.org/10.1038/cddis.2014.487
Dehnhardt M, Palm C, Vieten A, Bauer A, Pietrzyk U (2007) Quantifying the A1AR distribution in peritumoural zones around experimental F98 and C6 rat brain tumours. J Neurooncol 85:49–63. https://doi.org/10.1007/s11060-007-9391-6
Dhanasekaran DN, Reddy EP (2017) JNK-signaling: a multiplexing hub in programmed cell death. Genes Cancer 8(9–10):682–694. https://doi.org/10.18632/genesandcancer.155
Dighiero G (1996) Adverse and beneficial immunological effects of purine nucleoside analogues. Hematol Cell Ther 38:S75–S81
Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, De Maria R (2006) Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ 13(7):1238–1241. https://doi.org/10.1038/sj.cdd.4401872
Fiebich BL, Akundi RS, Biber K, Hamke M, Schmidt C, Butcher RD, van Calker D, Willmroth F (2005) IL-6 expression induced by adenosine A2b receptor stimulation in U373 MG cells depends on p38 mitogen activated kinase and protein kinase C. Neurochem Int 46:501–512. https://doi.org/10.1016/j.neuint.2004.11.009
Figueiró F, de Oliveira CP, Bergamin LS, Rockenbach L, Mendes FB, Jandrey EH, Moritz CE, Pettenuzzo LF, Sévigny J, Guterres SS, Pohlmann AR, Battastini AM (2016) Methotrexate up-regulates ecto-5′-nucleotidase/CD73 and reduces the frequency of T lymphocytes in the glioblastoma microenvironment. Purinergic Signal 12(2):303–312. https://doi.org/10.1007/s11302-016-9505-8
Fishman P, Bar-Yehuda S, Madi L, Cohn I (2002) A3 adenosine receptor as a target for cancer therapy. Anti-Cancer Drugs 13:437–443
Fredholm BB, Ijzerman AP, Jacobson KA, Linden J, Müller CE (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors – an update. Pharm Rev 63:1–34. https://doi.org/10.1124/pr.110.003285
Genini D, Adachi S, Chao Q, Rose DW, Carrera CJ, Cottam HB, Carson DA, Leoni LM (2000) Deoxyadenosine analogs induce programmed cell death in chronic lymphocytic leukemia cells by damaging the DNA and by directly affecting the mitochondria. Blood 96:3537–3543
Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA (2008) The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther 117:123–140. https://doi.org/10.1016/j.pharmthera.2007.09.002
Gessi S, Sacchetto V, Fogli E, Merighi S, Varani K, Baraldi PG, Tabrizi MA, Leung E, Maclennan S, Borea PA (2010) Modulation of metalloproteinase-9 in U87MG glioblastoma cells by A3 adenosine receptors. Biochem Pharmacol 79:1483–1495. https://doi.org/10.1016/j.bcp.2010.01.009
Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA (2011) Adenosine receptors and cancer. Biochim Biophys Acta 1808(5):1400–1412. https://doi.org/10.1016/j.bbamem.2010.09.020
Giagkousiklidis S, Vogler M, Westhoff MA, Kasperczyk H, Debatin KM, Fulda S (2005) Sensitization for gamma-irradiation-induced apoptosis by second mitochondria-derived activator of caspase. Cancer Res 65:10502–10513. https://doi.org/10.1158/0008-5472.CAN-05-0866
Gire V, Dulic V (2015) Senescence from G2 arrest, revisited. Cell Cycle 14(3):297–304. https://doi.org/10.1080/15384101.2014.1000134
Holmøy T, Torkildsen Ø, Myhr KM (2017) An update on cladribine for relapsing-remitting multiple sclerosis. Expert Opin Pharmacother 18(15):1627–1635. https://doi.org/10.1080/14656566.2017.1372747
Isakovic A, Harhaji L, Dacevic M, Trajkovic V (2008) Adenosine rescues glioma cells from cytokine-induced death by interfering with the signalling network involved in nitric oxide production. Eur J Pharmacol 591:106–113. https://doi.org/10.1016/j.ejphar.2008.06.076
Jacobson KA (1998) Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol Sci 19:184–191. https://doi.org/10.1016/S0165-6147(98)01203-6
Jacobson KA, Tosh DK, Jain S, Gao ZG (2019) Historical and current adenosine receptor agonists in preclinical and clinical development. Front Cell Neurosci 13:124. https://doi.org/10.3389/fncel.2019.00124
Kelly GL, Strasser A (2011) The essential role of evasion from cell death in cancer. Adv Cancer Res 111:39–96. https://doi.org/10.1016/B978-0-12-385524-4.00002-7
Kreitman RJ, Arons E (2018) Update on hairy cell leukemia. Clin Adv Hematol Oncol 16(3):205–215
Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM (2011) Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol 61:221–261. https://doi.org/10.1016/B978-0-12-385526-8.00008-4
Lee JK, Won JS, Singh AK, Singh I (2005) Adenosine kinase inhibitor attenuates the expression of inducible nitric oxide synthase in glial cells. Neuropharmacology 48:151–160. https://doi.org/10.1016/j.neuropharm.2004.09.006
Liliemark J (1997) The clinical pharmacokinetics of cladribine. Clin Pharmacokinet 32:120–131. https://doi.org/10.2165/00003088-199732020-00003
Melani A, De Micheli E, Pinna G, Alfieri A, Corte LD, Pedata F (2003) Adenosine extracellular levels in human brain gliomas: an intraoperative microdialysis study. Neurosci Lett 346:93–96. https://doi.org/10.1016/S0304-3940(03)00596-2
Melani A, Corti F, Stephan H, Müller CE, Donati C, Bruni P, Vannucchi MG, Pedata F (2012) Ecto-ATPase inhibition: ATP and adenosine release under physiological and ischemic in vivo conditions in the rat striatum. Exp Neurol 233(1):193–204. https://doi.org/10.1016/j.expneurol.2011.09.036
Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, Maclennan S, Borea PA (2006) Adenosine modulates vascular endothelial growth factor expression via hypoxia-inducible factor-1 in human glioblastoma cells. Biochem Pharmacol 72:19–31. https://doi.org/10.1016/j.bcp.2006.03.020
Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, Maclennan S, Baraldi PG, Borea PA (2007) Hypoxia inhibits paclitaxel-induced apoptosis through adenosine-mediated phosphorylation of bad in glioblastoma cells. Mol Pharmacol 72:162–172. https://doi.org/10.1124/mol.106.031849
Morrone FB, Jacques-Silva MC, Horn AP, Bernardi A, Schwartsmann G, Rodnight R, Lenz G (2003) Extracellular nucleotides and nucleosides induce proliferation and increase nucleoside transport in human glioma cell lines. J Neuro-Oncol 64:211–208
Nazario LR, da Silva RS, Bonan CD (2017) Targeting adenosine signaling in Parkinson’s disease: from pharmacological to non-pharmacological approaches. Front Neurosci 11:6–58. https://doi.org/10.3389/fnins.2017.00658
Nomura Y, Inanami O, Takahashi K, Matsuda A, Kuwabara M (2000) 2-Chloro-2′-deoxyadenosine induces apoptosis through the Fas/Fas ligand pathway in human leukemia cell line MOLT-4. Leukemia 14:299–306
Ohkubo S, Nagata K, Nakahata N (2007) Adenosine uptake-dependent C6 cell growth inhibition. Eur J Pharmacol 577:35–43. https://doi.org/10.1016/j.ejphar.2007.08.025
Palmer TM, Stiles GL (2000) Identification of threonine residues controlling the agonist-dependent phosphorylation and desensitization of the rat A(3) adenosine receptor. Mol Pharmacol 57:539–545. https://doi.org/10.1124/mol.57.3.539
Pardridge WM, Yoshikawa T, Kang YS, Miller LP (1994) Blood-brain barrier transport and brain metabolism of adenosine and adenosine analogs. J Pharmacol Exp Ther 268:14–18
Pastor-Anglada M, Pérez-Torras S (2018) Who is who in adenosine transport. Front Pharmacol 9:627. https://doi.org/10.3389/fphar.2018.00627
Rajkumar SV, Burch PA, Nair S, Dinapoli RP, Scheithauer B, O’Fallon JR, Etzell PS, Leitch JM, Morton RF, Marks RS (1999) Phase II north central Cancer treatment group study of 2-chlorodeoxyadenosine in patients with recurrent glioma. Am J Clin Oncol 22:168–171
Sands WA, Martin AF, Strong EW, Palmer TM (2004) Specific inhibition of nuclear factor-kappaB-dependent inflammatory responses by cell type-specific mechanisms upon A2A adenosine receptor gene transfer. Mol Pharmacol 66:1147–1159. https://doi.org/10.1124/mol.104.001107
Schulte G, Fredholm BB (2003) Signaling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 5(9):813–827. https://doi.org/10.1016/S0898-6568(03)00058-5
Sek K, Mølck C, Stewart GD, Kats L, Darcy PK, Beavis PA (2018) Targeting adenosine receptor signalling in cancer immunotherapy. Int J Mol Sci 19(12):Pii: E3837. https://doi.org/10.3390/ijms19123837
Sinclair CJ, LaRivière CG, Young JD, Cass CE, Baldwin SA, Parkinson FE (2000) Purine uptake and release in rat C6 glioma cells: nucleoside transport and purine metabolism under ATP-depleting conditions. J Neurochem 75:1528–1538. https://doi.org/10.1046/j.1471-4159.2000.0751528.x
Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF (2015) Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 35(6):600–604. https://doi.org/10.3109/10799893.2015.1030412
Taliani S, La Motta C, Mugnaini L, Simorini F, Salerno S, Marini AM, Da Settimo F, Cosconati S, Cosimelli B, Greco G, Limongelli V, Marinelli L, Novellino E, Ciampi O, Daniele S, Trincavelli ML, Martini C (2010) Novel N2-substituted pyrazolo[3,4-d]pyrimidine adenosine A3 receptor antagonists: inhibition of A3-mediated human glioblastoma cell proliferation. J Med Chem 53:3954–3963. https://doi.org/10.1021/jm901785w
Tomicic MT, Meise R, Aasland D, Berte N, Kitzinger R, Krämer OH, Kaina B, Christmann M (2015) Apoptosis induced by temozolomide and nimustine in glioblastoma cells is supported by JNK/c-Jun-mediated induction of the BH3-only protein BIM. Oncotarget 6(32):33755–33768. https://doi.org/10.18632/oncotarget.5274
Trincavelli ML, Tuscano D, Marroni M, Falleni A, Gremigni V, Ceruti S, Abbracchio MP, Jacobson KA, Cattabeni F, Martini C (2002) A3 adenosine receptors in human astrocytoma cells: agonist-mediated desensitization, internalization, and down-regulation. Mol Pharmacol 62:1373–1384. https://doi.org/10.1124/mol.62.6.1373
Trincavelli ML, Marroni M, Tuscano D, Ceruti S, Mazzola A, Mitro N, Abbracchio MP, Martini C (2004) Regulation of A2B adenosine receptor functioning by tumour necrosis factor a in human astroglial cells. J Neurochem 91:1180–1190. https://doi.org/10.1111/j.1471-4159.2004.02793.x
Uribe D, Torres Á, Rocha JD, Niechi I, Oyarzún C, Sobrevia L, San Martín R, Quezada C (2017) Multidrug resistance in glioblastoma stem-like cells: role of the hypoxic microenvironment and adenosine signalling. Mol Asp Med 55:140–151. https://doi.org/10.1016/j.mam.2017.01.009
Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, Zhang B (2011) CD73 has distinct roles in non hematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest 121:2371–2382. https://doi.org/10.1172/JCI45559
Westphal M, Lamszus K (2011) The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci 12:495–508. https://doi.org/10.1038/nrn3060
Xu S, Shao QQ, Sun JT, Yang N, Xie Q, Wang DH, Huang QB, Huang B, Wang XY, Li XG, Qu X (2013) Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas. Neuro-Oncology 15(9):1160–1172. https://doi.org/10.1093/neuonc/not067
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ceruti, S., Abbracchio, M.P. (2020). Adenosine Signaling in Glioma Cells. In: Barańska, J. (eds) Glioma Signaling. Advances in Experimental Medicine and Biology, vol 1202. Springer, Cham. https://doi.org/10.1007/978-3-030-30651-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-30651-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30650-2
Online ISBN: 978-3-030-30651-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)