Abstract
In the present chapter, we summarize the knowledge of wild and cultivated potato species, diversity, a taxonomic update including group concept classification and description, species valid names, and a complete synonymy, distribution, and habitat. Likewise, the importance of reproductive characters, breeding barriers, interspecific hybridization, and gene flow, introgression, polyploidy in potato evolution and ecological adaptation, and conservation strategies is explained. Also a comprehensive taxonomy of all wild and cultivated potatoes, based on the integration of multiple evidences and phylogenetic relationships between taxa is discussed, providing a framework for further investigation of complex groups as well as rare endemic species. Hypothesis regarding patterns of species diversity and distributions, and adaptive mechanisms to different extreme environments are proposed. More recent genomic approaches are promissory not only to investigate wild potato genome evolution but also to detect alleles related to important agronomic traits. Germplasm of more wild species or potato landraces can be explored considering hypothesis of relationships. A taxonomic framework could be useful for harmonizing names and classification of potato collection among genebanks. The knowledge of species diversity and distribution patterns is fundamental for collecting strategies and the establishment of natural protected areas as well as agrobiodiversity zones, and for management and sustainable use of potato genetic resources.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Ancient American farmers domesticated potato species on the high plateaus of Andean Punas, and also in the lowlands of Southern Chile. The temperament and capacity of these communities, their knowledge of natural diversity and environment, led to the development of a rich culture, reflected in the diversity of their food resources, farming practices, and traditions (Zhang and Rodríguez 2015; De Haan et al. 2019). Andean farmers not only domesticated potatoes, but also more than 40 species for their subsistence (Parodi 1966; Popenoe et al. 1990) and generated ingenious cultivation methods on the slopes of the high mountains, which still last to this day. This legacy is one of America's great treasures bequeathed to the world, and potatoes are essentials for human food subsistence.
Potatoes were introduced to Europe in the mid-sixteenth century and then its cultivation spread to the whole world (Hawkes 1990; Ames and Spooner 2008). Nowadays, potato is one of the most important crops for human nutrition and health (Burgos et al. 2020), and it is the first tuberous species cultivated worldwide, with an average production of 378,201,964 Ton and a harvested area of 19,062,653 Ha (http://www.fao.org/faostat).
Cultivated potatoes were domesticated from its indigenous relatives. Wild species are native of America distributed from Southwestern United States (latitude 38 °N), Central and South America to Argentina, and adjacent mainland Chile (latitude 41 °S) (Fig. 4.1), with greatest species richness at latitude 21 °S in South America and a secondary center of speciation around 20 °N in the Central Mexican highlands (Hijmans and Spooner 2001; Hijmans et al. 2002). Landraces of cultivated potatoes are grown throughout mid to high (about 3000–3500 m) elevations in the Andes from Northern South America to Northwestern Argentina, and in lowland South-Central Chile, concentrated in the Chonos Archipelago (Fig. 4.2).
Distribution of cultivated species: S. tuberosum L. with two cultivar groups, the ‘Andigenum Group’ of upland Andean genotypes with diploid, triploid, and tetraploids, and the ‘Chilotanum Group’ of lowland tetraploid Chilean landraces, and other three Andean cultivated species S. ajanhuiri (diploid), S. juzepzukii (triploid) and S. curtilobum (pentaploid)
Traditionally, potatoes have been included in the genus Solanum section Petota Dumort., which comprises all wild tuber bearing species and the cultivated potato (S. tuberosum L.). A closely related group, Solanum section Etuberosum (Bukazov & Kameraz.) A. Child, includes three wild non-tuber bearing species but morphologically similar to potatoes (Contreras and Spooner 1999; Spooner et al. 2016).
The taxonomy of potatoes have been difficult to elucidate due to their great diversity along a wide geographic range, ecological adaptation to different habitat, great phenotypic variation that made difficult the interpretation of morphological characters, and also complicated by reproductive features such as interspecific hybridization, introgression, allopolyploidy, prezygotic and postzygotic mechanisms, also a unique mixture of sexual and asexual reproduction, and possible recent species divergence (Ghislain et al. 2006; Rodríguez et al. 2009; Spooner et al. 2004, 2014, 2016, 2019).
Besides the biological processes involved in potatoes evolution and ecological adaptations, early taxonomists who have worked on section Petota applied different concepts in their treatments, fundamentally in the criteria to delimitate taxa and number of species, hypotheses of their interrelationships, and interpretations of the hybrid origins of various taxa (Spooner and Van den Berg 1992; Spooner and Salas 2006; Rodríguez et al. 2010; Spooner et al. 2014, 2016, 2019).
More recently comprehensive monographs of section Petota and section Etuberosum (Spooner et al. 2004, 2016, 2019; Ovchinnikova et al. 2011) revised initial taxonomic treatments, incorporating studies of numerous herbarium specimens, including types, and cultivated representatives of all recognized species. In these comprehensive monographs, it is important to highlight the application of common taxonomic concepts based on phylogeny to elucidate potato diversity. Furthermore, the results of recent morphological and molecular phylogenetic studies have driven us to continuously reduce the number of potato species relative to early taxonomic treatments (Spooner et al. 2004, 2016, 2019; Ovchinnikova et al. 2011).
In the present chapter, we summarize the knowledge of potato species diversity, a taxonomic update including group concept classification and description, species valid names and a complete synonymy, distribution and habitat, as well as the importance of reproductive characters, breeding barriers, interspecific hybridization, gene flow, introgression, polyploidy in potato evolution and ecological adaptation. Additionally, a discussion is included about difficult groups for further taxonomical studies, and possible approaches to clarify and solve taxonomical controversies. The methods and issues for ex-situ conservation of potato genetic resources are also considered. Finally, the strategies for in situ conservation in natural and agroecological areas are discussed.
4.2 Potato Reproductive Characteristics
The reproductive characteristics of wild potato species and the evidences of natural hybridization phenomena have led botanists to have different taxonomical interpretations that are sometimes conflicting (Spooner and Van den Berg 1992; Spooner and Salas 2006; Spooner et al. 2016, 2019).
All species of section Etuberosum and Petota have the same basic chromosome number of x = 12. Ploidy refers to the number of chromosome sets in the genome, and Rybin (1929, 1933) first described the polyploid series in wild potatoes (2x, 3x, 4x, 5x, 6x). Ploidy assessment, summarized in Table 4.1, revealed that the majority (66%) of wild species are diploids (2x = 24), but there is also variation in species ploidy (Gavrilenco 2011). Hijmans et al. (2007) determined the geographical and environmental correlations of ploidy for the wild taxa of Solanum sect. Petota, documented multiple cytotypes in 21 wild species, and found that diploids occupy a larger geographical area, at the northern and southern edges of distribution, than polyploids that most frequently occur in small areas at ecological extremes where higher-level polyploids species occur in colder habitats and triploids in warmer and drier sites than diploid. In Table 4.1, ten diploid species have additional triploid populations with 36 chromosomes (3x), and one diploid species also present tetraploid populations with 48 chromosomes (4x); two of hybrid origin were found exclusively triploids (3x = 36); eleven exclusively tetraploids (4x = 48); one exclusively pentaploid (5x = 60) and six exclusively hexaploids (6x = 72). In few species, populations of diploids and hexaploids, triploids and tetraploids and tetraploids and hexaploids have been detected. Four species have populations with more than one even ploidy level (S. colombianum, S. andreanum, S. brevicaule and S. candolleanum) (Table 4.1). Triploid and pentaploid populations are generally highly sterile. Ploidy in cultivated potatoes has been also investigated in the S. tuberosum Andigenum Group, where no ecogeographical association for the ploidy variants and different habitat was found, while in S. tuberosum Chilotanum Group ploidy was related with extreme northern and southern distribution (Spooner et al. 2010).
Genome structure has been analyzed by Matsubayashi (1991) and Gavrilenko (2007, 2011) through various cytological techniques (see also Chap. 2). Additional approaches, like the analysis of orthologous GBSSI genes sequences, showed the first molecular evidence of allopolyploidy in potato (Spooner et al. 2008). Analysis of single-copy genes have been useful to understand genomic complexity, revealing patterns of hybrid origins and allele losses in potato polyploids (Rodriguez and Spooner 2009; Cai et al. 2012). Genome rearrangements in S. bulbocastanum, a wild potato species with B genome, were uncovered for the first time when its linkage map was compared with potato and tomato physical maps, and provided a promissory approach for investigation of genome-specific structural chromosome rearrangements between Solanum A and B genomes as well as for mapping of agronomical traits (Iorizzo et al. 2014; Mann et al. 2011; Aversano et al. 2015).
Wild potatoes are distributed along a wide geographic range in America, where physical and ecological barriers can prevent gene flow among species. Nevertheless, Camadro et al. (2004) argued that these external factors are not sufficient to explain maintenance of potato species integrity. These authors, based on the evidences of little genome differentiation in potatoes, and also taking into account the lack of interspecific crossing in several sympatric populations, proposed that internal barriers to hybridization may have played a fundamental role in wild potatoes evolution. Interspecific pollen-pistil incompatibility, nuclear-cytoplasmic male sterility, and seed endosperm development are major forces that strengthen geographic and ecological barriers, even though a certain amount of gene exchange could be possible, species remain separate in an evolutionary context (Camadro et al. 2004).
Most diploid potato species are self-incompatible due to a multiallelic S-locus with gametophytic expression (Cipar et al. 1964; Goldberg et al. 2010). The style produces a ribonuclease codified by the S-locus that prevents the normal growth of genetically matching pollen tubes (Dodds et al. 1996; Luu et al. 2000). The S locus has been mapped to chromosome 1 in potato germplasm (Gebhardt et al. 1991) and S-RNase genes have also been mapped in the same chromosome (Rivard et al. 1996). A dominant self-incompatibility inhibitor gene that allows self-pollination has been reported in wild diploid species and mapped to the distal end of chromosome 12 (Hosaka and Hanneman 1998). Interspecific pollen-pistil interaction has been explained by a genetic system of cross incompatibility or incongruity (CI), in which genes interact on a one-to-one basis to allow or prevent hybridization (Hogenboom 1973, 1979). Camadro and Peloquin (1981) proposed a genetic model to explain the isolation in tuber-bearing Solanum species and the maintenance of their genetic integrity, with dominant CI genes in styles that prevent fertilization by pollen carrying specific dominant complementary genes. These genetic systems developed during the evolution of sympatric species at the pollen-pistil level. Polyploid species are self-compatible due to a “competition interaction” that either reduce or suppress the incompatibility reaction that occurs in pollen grains carrying different S-alleles (Frankel and Galun 1977). Tetraploid and hexaploid species (with the exception of tetraploid forms of S. andreanum) are capable of self-fertilization (Hawkes 1990). When diploid self-incompatible potato species are induced to chromosome doubling produces self-compatible tetraploids (Stout and Chandler 1941; Ross 1986). Interestingly, in these tetraploids, pollen tube growth is inhibited when pollen is homozygous for S alleles, but not when it is heterozygous (Lewis 1943, 1947).
Male sterility of hybrid plants is an important post-zygotic isolating mechanism in natural potato species populations. Cytoplasmic-genetic male sterility occurs when dominant nuclear genes from the male parent interact with sensitive cytoplasm from the female parent (Hermundstad and Peloquin 1985; Tucci et al. 1996). Male sterility has been reported in several F1 hybrids derived from crosses involving various wild and cultivated species (Lamm 1941, 1953; Brown 1984; Hermundstand and Peloquin 1985; Tucci et al. 1996, Santini et al. 2000; Carputo et al. 2003a) and between cultivated potatoes (Grun 1973; Hanneman and Peloquin 1981). Genetic and environmental conditions can influence the expression of cytoplasmic-genetic male sterility (Hanneman and Peloquin 1981). Hybrids lacking sensitive cytoplasm or nuclear male sterility genes are male-fertile (Iwanaga et al. 1991; Tucci et al. 1996).
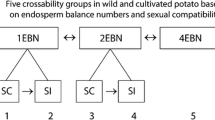
In Angiosperms the development of viable seed depends on double fertilization that generates a diploid embryo and triploid endosperm. The endosperm contains two genomes of the maternal parent and one genome of the paternal parent. Intraspecific, intraploidy crosses in potato typically produce viable seeds containing well-developed endosperm, on the contrary, in most interploidy crosses, seeds are inviable due to endosperm failure (Friedman 1998). Normal endosperm development in potato requires a 2:1 maternal: paternal ratio of a set of genes called endosperm balance factors (Johnston and Hanneman 1980, 1982, 1996). Viable seeds could be generated from crosses between plants that produce gametes with the same endosperm balance number (EBN), resulting in a 2:1 maternal:paternal ratio of endosperm balance factors after male gamete fusion with two nuclei of the central cell to produce triploid endosperm, and consequently allowed further development of a normal embryo. The EBN is an arbitrary value, which is not necessarily a direct indication of species ploidy, assigned to each Solanum species based on its behavior in crosses with EBN standards and on the assumption that the 2:1 ratio is essential for normal endosperm development (Hanneman 1994). The ploidy and endosperm balance number combinations in potato are 6x(4EBN), 4x(4EBN), 4x(2EBN), 2x(2EBN) and 2x(1EBN) (Table 4.1). However, endosperm development may also fail in some intraploidy, interspecific crosses, while some interploidy crosses could succeed. The nature of these endosperm balance factors is not yet known, but nuclear genetic models have been proposed (Ehlenfeldt and Hanneman 1988; Camadro and Masuelli 1995).
Wild and cultivated potatoes have meiotic mutants that result in the production of unreduced (2n) gametes (Carputo et al. 2003b). Some meiotic mutations produce 2n eggs (Stelly and Peloquin 1986; Werner and Peloquin 1991), while others produce 2n pollen (Quinn et al. 1974). The fusion of unreduced (2n) gametes during fertilization explain the occurrence of spontaneous polyploidization in wild plant populations (Harlan and de Wet 1975; Veilleux 1985; Bretagnolle and Thompson 1995). Unreduced gametes can be detected microscopically, since diploid pollen grains are larger than monoploid pollen grains (Quinn et al. 1974) and 2n eggs can be identified with stain-clearing techniques (Stelly et al. 1984). Unreduced gametes facilitate the evolution of polyploids by allowing interspecific hybridization across ploidy levels (Mason and Pires 2015). Nevertheless, triploid seeds resulting from the union of an n and a 2n gamete are generally inviable due to endosperm failure (Kohler et al. 2009). Camadro et al. (2004) pointed out the complementary role of EBN and unreduced gametes, not only because it facilitates interspecific gene introgression but also because it maintains the ploidy integrity of the two parental species. Den Nijs and Peloquin (1977) and more recently by Carputo et al. (2003b) proposed an evolutionary scenario for potatoes where n and 2n gametes link together all ploidy and EBN levels, thereby providing an opportunity for gene flow throughout sympatric species with different EBN and chromosome numbers (Camadro et al. 2004). Hawkes (1962) considered that introgression and interspecific hybridization that not led to speciation seems to be a common phenomenon throughout the range of section Petota. The lack of strong biological isolating mechanisms, morphologically intermediate characteristics in natural populations, and sympatry of many species suggest that much of the taxonomic confusion in section Petota is due most probably to frequent gene flow among the species (Spooner et al. 2019). Interestingly, Rabinowitz et al. (1990) documented high levels of gene flow between wild and cultivated species in Peru, supporting Ugent’s hypotheses who proposed that cultivated species were formed and genetically enriched by gene flow from the wild species (Ugent 1970). Traditional Andean farming systems incorporate natural hybrids between cultivated potatoes and the wild potato relatives growing in their surrounding fields (Brush et al. 1981). The unique reproductive characteristics of tuber-bearing potatoes allow to incorporate new genetic combinations by sexual reproduction, while asexual reproduction maintains adapted gene complexes (Spooner et al. 2019).
In natural potato populations, several biological internal barriers such us pollen-style interactions, male sterility, and endosperm failure, prevent the production of interspecific hybrids and maintain the integrity of sympatric species (Camadro et al. 2004). However, on the other side of an evolutionary perspective, the wide natural occurrence of unreduced gametes, self-incompatibility, and little genome differentiation among potato species favor hybridization between wild Solanum species (Erazzú et al. 2009; Masuelli et al. 2009; Ispizúa et al. 2015).
Potato wild relatives are critical natural resources that serve as a model system for genebank conservation (Jansky et al. 2013; Bethke et al. 2019). The understanding of several biological mechanisms allows the use of wild species in potato breeding. The introduction of diploid wild potato genes to the tetrahaploid cultivated species has been successfully carried out using various breeding methods and strategies such as haploid production (Peloquin et al. 1989a), the use of unreduced gametes (Mendiburu and Peloquin 1977, Peloquin et al. 1989b, 1996, 2008), the application of the balance of endosperm value to produce hybrids (Johnston and Hanneman 1980, 1982; Hanneman 1994), and the use of embryo rescue techniques and somatic fusion of protoplasts. Carputo and Frusciante (2011) highlighted the classical genetics and traditional approaches applied in potato crop improvement. New insights from genomic research provided promissory methods to explore a wide pool of genetic resources that include not only wild species but also landraces, and increase the efficiency of identifying and introgressing alleles rather than traits (Iorizzo et al. 2014; Mann et al. 2011; Potato Genome Sequencing Consortium 2011; Bethke et al. 2019; Ghislain and Douches 2020).
In the context of present knowledge, wild species have shown their enormous value as a source of traits of agronomic importance and resistances to biotic and abiotic factors for crop improvement (Ross 1986; Hanneman, 1999; Kuhl 2011; Watanabe 2015; Bonierbale et al. 2020; Ortiz 2020). Nevertheless, few wild species have been used in breeding. A better comprehension of wild potato diversity and ecological adaptation, taxonomy, and relationships, and the application of new promissory methods will also contribute to their utilization as models to understand genetic and genomic evolution as well as in cultivated potato improvement (Hardigan et al. 2017) (see other chapters).
4.3 Taxonomy of Potato Species (Section Petota) and Close Related Non Tuberous Species (Section Etuberosum)
Classical treatments of Bukasov (1978), Correll (1962), Gorbatenko (1989, 2006), Hawkes and Hjerting (1969, 1989), Ochoa (1990, 1999, 2001) and Hawkes (1990), proposed taxa delimitation mainly based on morphological species concept (Spooner and Van der Berg 1992). Hawkes (1990) also considered species intercrossability, and his taxonomic treatment of section Petota has been the most comprehensive and traditionally used, where he recognized 228 wild species and seven cultivated species, grouped into 21 Series. Taxonomic treatments differ in author’s concepts and interpretation of taxonomic rank used to establish species, botanical varieties or subspecies, hypotheses about species hybrid origin and introgression with other species, as well as the criteria to define the arrangement and number of taxonomical series, the number of species in each series, and the different affiliation of species to these series (Spooner and Van den Berg 1992; Spooner and Hetterscheid 2005; Spooner and Salas 2006).
It is interesting to compare taxonomic interpretations of two sister phylogenetic lineages of genus Solanum, potatoes and tomatoes, that separated earlier about eight Ma and later section Petota started diversifying around seven Ma (Särkinen et al. 2013). Prevalent taxonomical interpretation (Hawkes 1990) considers more than 200 wild potato species in contrast with the 13 species of tomatoes (Sect. Lycopersicon) and four species in the most related groups (Sect. Juglandifolia and Lycopersicoides) (Peralta et al. 2008). This enormous differences in the interpretation of diversity in sister groups can be explained by the unique reproductive characteristics, genetic structure, and ecological adaptations of potatoes in a wide geographic area. However, another non biological explanation are the concepts to circumscribe species and a complex nomenclature system initially used by potato taxonomists that cause an overestimation of natural diversity (Peralta et al. 2008).
A different philosophy and consistent application of a comprehensive criteria have driven to continuously reduce the number of potato species relative to early taxonomic treatments (Hijmans and Spooner 2001; Spooner and Salas 2006; Ames and Spooner 2010; Fajardo and Spooner 2011; Spooner et al. 2004, 2014, 2016, 2019). More recently comprehensive treatments of section Petota and section Etuberosum (Spooner et al. 2004, 2016, 2019) not only revised early taxonomical contribution, but also applied a taxonomical integrative approach using different sources of evidences mainly based on phylogeny to propose new group classification. These 3 monographs treated the complete diversity of tuber bearing and stoloniferous wild species in America, based on the analysis of numerous herbarium specimens, including types, and field assessment of cultivated representatives of all recognized species. Other relevant evidences of recent morphological, reproductive and cladistics molecular studies were integrated into these comprehensive treatments. A similar approach has been used to describe wild potato diversity for regional or country Floras (Spooner et al. 2009; Clausen et al. 2013).
The classification of potatoes based on a phylogenetic approach clarified evolutionary relationships, and the recognition of close related cluster of organisms by a parental pattern of ancestry and descent, and diagnosable distinct from other clusters (Cracraft 1989). Most methods for studying cladistics have been based on models of strictly branching cladogeny, however, in complex groups with possible reticulate evolution at chromosomal, genomic and species levels inference of relationships could fail when modeled by a bifurcating tree. Classification of wild potatoes is a difficult goal, since interpretation of relationships is complicated by introgression, interspecific hybridization, auto- and allopolyploidy, sexual incompatibility among many species, a mixture of sexual and asexual reproduction, possible recent species divergence, phenotypic plasticity, and consequent great morphological similarity among species (Spooner and van den Berg 1992; Spooner et al. 2014; Camadro et al. 2004; Camadro 2012). Further phylogenetic analysis using models of reticulated evolution in potatoes, a complex group with hybridization and introgression phenomena, could elucidated their relationships.
The relationships between Sect. Petota and Sect. Etuberosum have been a subjet of debate. Initially Juzepczuk and Bukasov (1929) included non-tuber-bearing species in ser. Etuberosa within Sect. Petota [then referred to as Sect. Tuberarium (Dunal) Bitter]. Morphological similarities of Solanum species in Sect. Petota and Sect. Etuberosum led to considered them as closest relatives. Nevertheless, several concordant molecular studies have clarified relationships among these Solanum sections, supporting tomatoes (Sect. Lycopersicon) and close related species (sects. Juglandifolia and Lycopersicoides) as a monophyletic sister clade to Sect. Petota, with Sect. Etuberosum sister to all the above (Spooner et al. 2016, 2019). These relationships have been corroborated by plastid phylogenies (Spooner et al. 1993; Olmstead and Palmer 1992, 1997; Bohs and Olmstead 1997, 1999; Olmstead et al. 1999), nuclear genes and conserved orthologous sequences phylogenies (Peralta and Spooner 2001; Rodríguez and Spooner 2009; Rodríguez et al. 2009) and recently by seven regions (five plastids and two nuclear) used to generate the Solanaceae mega-phylogeny (Särkinen et al. 2013). Further phylogenetic analysis of large data set could test the hypothesis of relationships between Sect. Petota and Sect. Etuberosum. Within genus Solanum, sects. Etuberosum, Petota, Juglandifolia, Lycopersicoides, and Lycopersicon are all members of a New World broader group of species informally named the Potato Clade (Tepe et al. 2016; Bohs 2005; Särkinen et al. 2013).
We summarized our actual comprehensive taxonomy of Sections Petota and Etuberosum, including species distributions and habitat, ploidy, and EBN numbers, and phenology (Table 4.1; Fig. 4.1), as well as a complete list of accepted names, synonyms (450), and the inclusion of species in non-formal groups (Tables 4.1 and 4.2). These provisional taxonomic groups are based on hypothesis of phylogenetic relationships among species that reflect the evolutionary history of potatoes, and pending from more data to elucidate interrelationships. Similar non-formal group systems of classification have been widely applied to Solanum by Whalen (1984), Bohs (1994, 2005), Knapp (1991, 2000, 2002, 2008, 2013), Peralta et al. (2008), and Spooner et al. (2004, 2016, 2019). These non-formal groups should not be confused with “Groups”, which are category taxonomic names for groups of cultivated plants (ICNCP 2016). Non-formal groups in section Petota are detailed in Tables 4.1 and 4.2, and the most widespread ones are represented in Fig. 4.1. Detailed morphological description of the accepted wild species, synonyms, taxonomic keys, illustrations, locality distributions, habitat, phenology, uses, and taxonomic characteristics, as well as a detailed explanation of non-formal groups, are found in the three monographic treatments of Spooner and collaborators (2004, 2016, 2019), information that has been also incorporated in the Solanaceae Source website (http://www.solanaceaesource.org).
SECT. ETUBEROSUM (Bukasov & Kameraz) A. Child: comprises three species confined to southern South America, erect to ascending herbs that possess thickened rhizomes, from which arise thin stolons but lacking tubers (Tables 4.1 and 4.2; Fig. 4.1).
SECT. PETOTA Dumortier: comprises 108 wild herbaceous species (Tables 4.1 and 4.2), erect to ascending, sometimes forming a rosette or semi-rosette, bearing tubers at the ends of stolons. A large geographical distribution of wild potato species from the southwestern United States (latitude 38 °N), Central and South America to Argentina, and adjacent mainland in Chile (39 °S) (Fig. 4.1) indicates a varied range of ecological diversity as well as adaptations to extreme climatic conditions; these species can be found from sea level at both Atlantic and Pacific oceans, and from high altitude deserts to rainforests (Hawkes 1990; Spooner and Hijmans 2001; Hijmans et al. 2002; Spooner et al. 2004, 2016, 2019). Some are widespread such as S. acaule, S. brevicaule and S. chacoense while others, with a restricted range and an endemic nature, are found in areas with specific ecological conditions. Solanum chacoense, S. palustre and S. commersonii are found at very low altitudes, frequently at sea level, while S. acaule, S. × aemulans, S. brevicaule and S. boliviense and S. candolleanum, reach more than 4000 m in the Andes. Solanum morelliforme is mainly restricted to Central Mexico, Guatemala, and Honduras but a single population has been identified 4000 km south in Bolivia. This is the only species in sect. Petota, growing in both Central and South America (Simon et al. 2011). Cultivated species present a more restricted geographical distribution, from northern South America and down to southern Chile (Fig. 4.2).
Wild potatoes are classified into 16 non-formal species groups (Spooner et al. 2004, 2016, 2019).
ACAULIA GROUP: three species and three nothospecies species with rosette to semi-rosette habit, in some cases erect and taller, typical flower pedicel with articulation appearing toward the distal end or no articulated (S. acaule), all polysomic polyploids, distributed in Mexico and Central America, and in South America from Ecuador to Argentina reaching the high puna plateau and possessing high frost tolerance (Vega and Bamberg 1995).
BULBOCASTANA GROUP: S. bulbocastanum and S. cardiophyllum are characterized by cream to light yellow corollas, diploids and triploids with and EBN = 1, distributed in Guatemala, Honduras, and Mexico (Spooner et al. 2004).
COMMERSONIANA GROUP: S. commersonii and S. malmeanum possess characteristic stellate corollas, diploids and triploids (S. commersonii) with EBN = 1, distributed in Argentina, Brasil, Paraguay, and Uruguay. Both are partially sympatric and are likely sister taxa (Spooner et al. 2016).
CONICIBACCATA GROUP: 19 species characterized by non-glossy parallel shaped leaves, with the distal-most lateral leaflet pairs diminishing toward the leaf base, and typical conical fruits (Spooner et al. 2019; Fajardo and Spooner 2011), diploids, tetraploids, and hexaploids (S. colombianum) with EBN = 2 or 4, distributed in Central America and South America from Venezuela to Bolivia.
IOPETALA GROUP: four species distributed in Mexico, polysomic polyploids with 6x(4EBN) crossability, and with no clear morphological characters uniting them, probably because they could have multiple origins (Spooner et al. 2004).
LONGIPEDICELLATA GROUP: two species and one nothospecies, with 4x(2EBN) crossability, but there are no clear specific morphological characters defining them, distributed from the Southwestern U.S.A to South Mexico.
MEGISTACROLOBA GROUP: four species, herbaceous low-growing rosette plants, with terminal leaflets much larger than the lateral leaflets and with the proximal leaflets reduced in size and often broadly decurrent on the rachis, diploids, and also triploids with EBN = 2, distributed in Peru, Bolivia, and northern Argentina.
MORELLIFORME GROUP: S. morelliforme and S. clarum, small plants with stellate corollas. Solanum clarum is present in Guatemala and Mexico, and S. morelliforme in Mexico to Guatemala but with a disjunct population in Bolivia (Spooner and Sytsma 1992).
PINNATISECTA GROUP: two species and two nothospecies, characterized by the presence of pinnatifid pseudostipules, diploids, distributed from southern U.S.A. to Mexico (Lara Cabrera and Spooner 2004).
PIURANA GROUP: 29 species, the majority with moniliform tubers and coriaceous, glabrous to subglabrous shiny or glossy leaves (Ames and Spooner 2010), diploids, also triploids, and tetraploids with EBN = 1, 2, or rare 4, distributed in Colombia, Ecuador, and Peru.
POLYADENIA GROUP: two species identified by their glandular leaves (type A trichrome) and strong odor, diploids, distributed in México (Lara Cabrera and Spooner 2004, Spooner et al. 2004).
STENOPYLLIDIA GROUP: three species with typical lunate pseudostipules and with triangular to conical fruits, diploids with EBN = 1, restricted to Mexico (Spooner et al. 2004).
STIPULOIDEA GROUP: S. stipuloideum and S. neocardenasii, diploids endemic to Bolivia, with typical white corollas (Spooner et al. 2016).
TRIFIDA GROUP: two endemic, diploid, Mexican species supported by cpDNA data (Spooner and Sytsma 1992) and AFLP data (Lara Cabrera and Spooner 2004).
TUBEROSUM GROUP: 21 species and two nothospecies, with dissected leaves, round fruits, and rotate-pentagonal corollas, diploids, triploids, tetraploids, and hexaploids with EBN = 1, 2 or 4, widely distributed in South America (Spooner et al. 2007). Also includes the cultivated potatoes (Table 4.3).
VERRUCOSA GROUP: S. verrucosum, distinguished by the corollas with the edges inrolled dorsally and often with berries with raised white dots, diploid species with 2EBN, widely distributed throughout Mexico.
Although provisional taxonomic groups attempt to reflect phylogenetic relationships in sect Petota, they are hypothesis that needs further investigations. In South America some taxa have been difficult to understand, like S. boliviense Dunal initially considered in Acaulia group (Spooner et al. 2016) but recently included in Megastricoloba group (Spooner et al. 2019), provisionally accepted here. Further research including more accessions representative of larger areas could help to solve this taxonomic puzzle. Also, with in Tuberosa group, additional studies are needed in complex taxa, fundamentally in two morphologically very similar species, S. candolleanum and S. brevicaule, to elucidate relationships and current hypothesis of potato domestication and cultivation origin (Spooner et al. 2005; Rodríguez et al. 2017). The taxonomy of S. brevicaule and related species has long been controversial (Correll 1962; Ugent 1970; Grun 1990), and several studies using morphological phenetics (Van den Berg et al. 1998; Alvarez et al. 2008), molecular marker data of RFLPs and RAPDs (Miller and Spooner 1999), and with AFLPs (Spooner et al. 2005) have been focusing on this group. All datasets distinguished S. candolleanum, distributed from Central Peru to Northernmost Bolivia, and S. brevicaule, distributed from northern Bolivia to northern Argentina, but with little to no support for the many names that were placed in synonymy (Table 4.2). Although Conicibaccata group have been recently revised, further studies are needed in S. colombianum and related taxa. Likewise, within Piurana a revision will be necessary to better define the members included in this group. Regarding endemism, new methodologies such environmental niche modeling, could be helpful to define collecting strategies as well as to identify threatened environment and antropic factors that may affect natural populations, like in the case of S. rhomboideilanceolatum (Castañeda Álvarez et al. 2015). These approaches can be also applied to explore and define new areas of distributions, as in the case of S. maglia, a wild species found in Chile but only one population in the mountains of Mendoza, Argentina. In this case, it will be interesting to test the hypothesis of ploidy in relation of habitat adaptation.
4.4 Cultivated Potatoes Origin, Domestication, Diversity and Taxonomy
South American indigenous farmers domesticated potatoes in the high plateaus of the Andean Punas and also in the lowlands of south-central Chile, where landrace cultivars are still highly diverse with an enormous variety of tuber shapes, sizes, and different colors of skin and internal tissues (INIA 2012; Fonseca et al. 2014; INIAF VDRA and MDRyT 2014; SPDA CCTA and INIA 2015; CIP 2015; MINAGRI 2017; PRODERN 2018; MINAM 2019).
The scarcity of direct botanical evidence has made difficult archeological research focused on potato species used by early American inhabitants and evidences of domestication. Ancient remains of potato have been found in archeological sites in Southern Chile, revealing that potato species have been consumed for at least 13,000 years (Ugent et al. 1987). Similarly, archeological rest indicated that about 10,000 years ago potato species have been used as food supply by native communities of Perú (Engel 1970). Fossilized tubers found in Casma Valley of Perú have been directly dated to 7800 cal B.P. (C14 calibrated date), and even though starch microremains resembled those of the domesticated potato may still represented a wild species (Ugent et al. 1982). Recently, an archeological study based on the microscopic analysis of starch granules found on ground stone tools in deposits dating between 10,900 and 10,100 cal B.P. at North Creek Shelter (Utah) documented the earliest use of wild potatoes in North America as important food source (Louderback and Pavlik 2017). These archeological findings evidence the early consumption of potato tubers at a time that precedes agriculture (Ugent et al. 1982; Hawkes 1990; Louderback and Pavlik 2017). The analysis of starch microremains recovered from groundstone tools found at Jiskairumoko, an ancient village in Perú, revealed an intensive exploitation of potatoes that took place between 3400 and 1600 B.P. during Late Archaic to Early Formative in the western Titicaca Basin (Rumold and Aldenderfer 2016). These archeological evidences, based on the consistency of ancient starch remains with those of cultivated potato, documented a time of transition from nomadism to sedentism and food production, and may be related to potato domestication and early cultivation in southern Perú (Rumold and Aldenderfer 2016).
Prevalent hypothesis for cultivated potato’s origins advocated multiple, independent domestications from a group of about 20 morphologically similar wild potato species, the “Solanum brevicaule complex” (Alvarez et al. 2008), distributed from southern Peru, northwestern Bolivia, and northern Argentina (Brücher 1964; Ugent 1970; Bukasov 1978; Hawkes 1990; Grun 1990; Ochoa 1990, 1999; Van den Berg et al. 1998; Huamán and Spooner 2002; Spooner et al. 2014). New insights from phylogenetic analysis that include a wide sampling of 362 representatives of landraces, putative progenitors, and outgroups, supported a reduction in the number of species in the Solanum brevicaule complex and a monophyletic origin of landrace cultivars from a single species in a broad area of southern Peru (Spooner et al. 2005). Landraces developed by early Andean farmers were dispersed from Peru both north and south. Nowadays, potato landraces reveal great morphological, physiological, and genetic diversity, and are distributed throughout the Andes, from western Venezuela to northern Argentina, and in southern Chile (Spooner et al. 2010). Landrace potato populations in Mexico and Central America are recent, post-Columbian introductions (Ugent 1968).
Cultivated potatoes were first introduced from America in the Canary Islands in 1567 (Hawkes and Francisco Ortega 1993; Spooner et al. 2005; Ríos et al. 2007), and soon arrived in Spain in 1573 (Hawkes 1990; Hawkes and Francisco Ortega 1993; Romans 2005). The first botanical description of potato in Europe was made by Caspar Bauhin in 1596, but the origin of the plant described was unknown (Hawkes 1990). In 1597 Gerard made the first description of the potato in English and a detailed illustration in The Herbals, although he mistakenly believed it came from Virginia in North America rather than South America. Later Carolus Clusius (1601) described potatoes and mentioned he received the tubers in 1588 from Phillippe de Sirvry who cultivated potatoes in Belgium and made the first drawing of potato in Europe indicating with his handwriting its common name and origin “Taratoufli Vienae, 26 januarii 1588, Papas Peruam Petri Cieca” (Parodi 1966). Evidences from early herbalist indicated that potatoes were cultivated since mid-XVI century in different European countries and rapidly disseminated worldwide. Regarding the origin of the first potatoes introduced in Europe, two hypotheses have been proposed: from lowland Chile (Juzepczuk and Bukasov 1929) or from the Andes (Salaman 1937; Salaman, and Hawkes 1949), being the Andean origin the most accepted. Ames and Spooner (2008) investigated these two competing hypothesis using historical herbarium potato specimens for a screening with a plastid DNA deletion marker. Interestingly, the first direct evidences from early preserved plants showed not only that potatoes of Andean origin predominated in Europe in the 1700s, but also that potatoes from Chile were introduced as early as 1811 in Europe, and became predominant long before the late blight epidemics begun in 1845 in potato crops causing high mortality and famine (Ames and Spooner 2008). Consequently, after the late blight epidemics, resistances from Chilean landraces were introduced into European potato cultivars.
Alphonse de Candolle, French-Swiss botanist, was a pioneer to investigate the origins of cultivated plants and crop geographic distribution. In his influential contribution, Origin of Cultivated Plants, De Candolle (1882) used evidences from different disciplines, presence of wild relatives, historical sources, linguistics (local names), archeology, and variation patterns, to determine the origin of cultivated plants. De Candolle (1882) was the first to name as distinct the Chilean populations of S. tuberosum as var. chiloense A.DC. Vavilov (1920, 1940), Russian geneticist and plant geographer, participated in over 100 collecting missions to explore the major agricultural centers worldwide, and built crop origin hypothesis. Vavilov and his Russian colleagues made several expeditions to Central and South America between 1925 and 1930, and generated a large potato collection that initiated the basic germplasm of the N. I. Vavilov Institute of Plant Industry in Saint Petersburg, Russia (Loskutov 1999). Some of the potato accessions are still maintained, as well as the herbarium specimens of the initial collections of high value to elucidate the taxonomy and nomenclature of cultivated potato (Ovchinnikova et al. 2011). Later, between 1938 and 1939 other important potato germplasm collections in South America were made by British Botanists Balls, Gourley and Hawkes (Hawkes 1944; 2004; Hawkes and Hjerting 1969, 1989). Germplasm derived from these initial collections is maintained at the Scottish Crop Research Institute (SCRI) in Dundee, United Kingdom, and specimens are mainly deposited at the Herbarium of Kew Botanical Gardens (KEW), and in many other collections worldwide (Ovchinnikova et al. 2011). Peruvian botanists C. Ochoa and A. Salas collected potatoes throughout South America that initiate the base of germplasm collections at the Universidad Nacional Agraria La Molina, Peru, and later the International Potato Center (Centro Internacional de la Papa, CIP). M. Cárdenas made early collections and descriptions of new potatoes from Bolivia (Cárdenas and Hawkes 1946), where National Agricultural and Forestry Institute (INIAF) maintains an important and diverse collection of cultivated potatoes (Cadima–Fuentes et al. 2013). In Argentina, several potato germplasm collections have been made by K. A. Okada, A. Clausen, and collaborators, which are maintained at INTA Potato Germplasm Bank in Balcarce (Clausen et al. 2010). In Chile, A. Contreras also collected and led germplasm collections that are maintained at the Chilean Germplasm Bank (Contreras 1987). David Spooner and collaborators from different countries also made important potato collections in North, Central and South America, which are maintained in CIP, US Potato Genebank, and other genebanks.
In order to organize large collections of cultivated potatoes, early Russian taxonomists applied a complex method to describe, named, and classified them, based on ploidy, ecogeography, and analysis of morphological and physiological characters. This system of nomenclature considered the homologous series of variation (Vavilov 1922), where geographical distribution and ecological types are major characters to define and name taxa (Juzepczuk and Bukasov 1929; Bukasov 1930; Juzepczuk 1937). Initially, Hawkes also applied this system to describe and name his potato collections, but later he simplified his classifications (Hawkes 2004). The application of this complex nomenclature system in initial cultivated potato collections, created numerous names, sometimes polynomials as well as many invalid names and a complicated classification by ranks. Recently, Ovchinnikova et al. (2011) clarified the nomenclature and taxonomy of cultivated potatoes, recognized 626 epithets associated with all taxa of cultivated potato and placed them in synonymy, and also made lectotype designations for names validly published (Ovchinnikova et al. 2011, Solanaceae Source website http://www.solanaceaesource.org). Four cultivated potato species are recognized: S. tuberosum L., S. ajanhuiri Juz. & Bukasov, S. curtilobum Juz. & Bukasov and S. juzepczukii Bukasov (Spooner et al. 2007), the three later species were formed by hybridization of S. tuberosum with more distantly related wild species of groups Acaulia and Megistacroloba (Rodríguez et al. 2010; Ovchinnikova et al. 2011) (Table 4.3).
The current taxonomical interpretation considers two main groups of landraces within Solanum tuberosum L, named according to the International Code of Nomenclature of Cultivated Plants (2016). The ‘Andigenum Group’ comprising diploid, triploid, or tetraploid, adapted to short-day flowering and tuberization. The ‘Chilotanum Group’ includes landraces from Southern Chile, mainly concentrated in the Chonos and Guaitecas Archipelagos, adapted to long-day flowering and tuberization (Huamán and Spooner 2002; Spooner et al. 2007; Ovchinnikova et al. 2011) (Table 4.3).
4.5 Methods and Issues for the Conservation of Potato Genetic Resources
The conservation of potato genetic resources implies two different strategies: the first one, ex situ conservation, is focused on the maintenance of potato genetic diversity outside its natural environment. The second, in situ strategy, comprise the conservation of ecosystems and natural habitats as well as the maintenance and recovery of viable populations of species in their natural surroundings, and in the case of domesticated or cultivated plant species conservation incorporate the surroundings where they have developed their distinctive properties (UNCED 1992; FAO 2009). These strategies have advantages and disadvantages, but the most remarkable characteristic is that both are complementary rather than exclusive. Nowadays it is accepted that a holistic approach is more effective in conservation programs of genetic resources.
The ex situ conservation of potato genetic resources is performed mainly in genebanks, and in a few cases in botanical gardens and museums. A fundamental goal of genebanks is to preserve germplasm and made it available for different purposes including research, breeding, agriculture production, industry, etc. (Ellis et al. 2020). The Second Report of the State of the Genetic Resources for Food and Agriculture (FAO 2010) registered 174 potato genebanks around the world and a total number 98,285 accessions, with possible material duplication, but it was estimated that 24,500–29,500 unique potato accessions are conserved worldwide. The same report showed that six genebanks hold 41% of the global potato accessions: The French National Institute for Agricultural Research (INRA) in France (11%), Vavilov Institute in Russia (9%), The International Potato Center (CIP) in Peru (8%), The Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) in Germany (5%), USDA-ARS in the USA (5%), and The National Institute of Agrobiological Sciences (NIAS) in Japan (3%) and other 20 genebanks hold over 1000 potato accessions each. These genebanks collectively held collections of 15% wild relatives of potato, 20% cultivated potato accessions, 16% research, and breeding materials, 14% advanced breeding lines, and 35% uncategorized accessions (Ellis et al. 2020). According to the last report of the Global Strategy for Ex Situ Conservation of Potato, Latin American genebanks contain principally native cultivars while those in Europe and North America contain modern cultivars, breeding materials, and wild relatives (Ellis et al. 2020). Castañeda-Álvarez et al. (2015) determined that 43.8% of wild potato species are under-represented in genebanks, some of them with no accessions available such as S. ayacuchense, S. neovavilovii, S. olmosense and S. salasianum. To improve the worldwide representation of wild potatoes conserved ex situ in genebanks, Instituto Nacional de Innovación Agraria (INIA, Peru) and CIP joined to collect more than 300 new accessions in Peru; two of them belong to S. ayacuchense (Zorrilla et al. 2019a). The type of biological sample preserved in genebanks varies depending on the biological status of the accessions, if they come from wild or cultivated species. In genebanks seeds are the most common biological sample for conservation of potato wild relatives, to assure the preservation of genetic diversity of the original population instead of individual characteristics. The most important variables, taken into account for adequate seed conservation, are population size, storage temperature, seed humidity, and seed quality as these variables can affect the expected viability of the seed accession lot. The number of regenerations, sexual multiplication, should be the minimum in order to preserve the accessions identity. Genebanks follow standard conservation methods recommended by Biodiversity International and other institutions (FAO-IPGRI, Engels, and Visser 2003; FAO 2014; CGIAR 2020). Seeds of cultivated and wild potatoes are orthodox (Holle 1988), meaning that seeds can be dried to low moisture contents and stored at cold temperature without damage during different periods of time (Roberts and Ellis 1984). For instance, seeds are stored at −18 °C for the maintenance as base collections or safety copy in the genebank Svalbard Global Seed Vault which preserves potato duplicated seeds as a “black box” for long term conservation (Ellis et al. 2020). In this case, the depositors are responsible for processing, packing, and shipping the seeds before storage; subsequent seed multiplication when needed, and distributing these seeds from their stocks under conditions similar to the International Treaty for Plant Genetic Resources for Food and Agriculture (ITPGRFA). The International Potato Center (CIP) in Perú stores potato seeds in the active collection at 4 °C, and in the base collection at −20 °C. The active collection is used for distributing germplasm and the base collection for the regeneration of new seed stocks. CIP has defined standard procedures for seed processing, packing, and storage of potato wild relatives, considering the extraction, cleaning of impurities, drying to 5% water seed content before entering the corresponding storage room (Salas et al. 2008). Similarly, the US potato genebank stores dried seeds with 5% of water content at −20 °C, while a subset of the most requested accessions is maintained at 4 °C, and another copy is sent to genebank Fort Collins Seed Vault for safety duplication (del Río A. pers. com.).
Seed regenerations are necessary to maintain seed stocks, and are performed when viability and/or the amount of seeds drop below the established minimum value. Indeed, regeneration is performed at CIP when viability drops below 85% and/or when the amount of seeds drops below 5000 units. The same criteria as in CIP, is taking into account for the regeneration of wild potato seeds at the Potato Genebank at Balcarce Agricultural Experimental Station of the National Institute of Agricultural Research (INTA) (Digilio A. pers. com.). At the US Potato Genebank, accessions are regenerated every 20 years (del Rio, pers.com.). Seed regeneration procedures define population size and type of crosses (open pollination, reciprocal sibling crosses, or mass pollination), depending on the species reproductive biology (cleistogamous, autogamous, exogamous, or alogamous). CIP has defined 25 plants as the minimum population size for regeneration; meanwhile, the US potato genebank uses 20 plants. Camadro (2012) recommended using 15–25 plants in controlled crosses during the multiplication process. The results of studies that assessed the effects of population size on genetic diversity during regeneration of the accessions, found that a sample of 25 to 30 plants is the optimal number to capture and maintain most of the alleles (Bamberg and del Río 2004), and similar criteria is used by the Potato Genebank at Balcarce. A concern in genebanks are the effects of accession multiplication, since populations derived from different regeneration events can differ significantly (Cadima-Fuentes 2014; Zorrilla et al. 2019b). Currently, the genetic diversity of Solanaceous crops from the principal genebanks is being studied within a similar objectives and approaches outlined in the G2PSOL initiative. As part of this project, the effects of sample size on the genetic diversity of conserved accessions in genebanks is an important issue expected to be solved.
A different strategy is used to preserve accessions of cultivated potatoes, botanical tubers named tuber-seeds are the main type of biological sample in genebanks for maintaining potato characteristics as a clone (asexually) where unique allelic combinations in the individual are preserved. Andean farmers traditionally have been used tuber-seeds as the principal method for conservation of their cultivated potatoes, since is the type of preservation that requires fewer resources in terms of equipment and supplies. Even though tubers conservation in genebanks is less demanding, only allows tuber conservation between 3 and 9 months at temperatures between 0 and 10 °C, depending of the dormancy and quality of the tuber before entering the storage room (JICA 2016). In the same way, the lowland tetraploid landraces, S. tuberosum L. Chilotanum Group, is conserved using botanical tubers at Institute of Agricultural Research of Chile and, Agricultural and Livestock Service of Chile (Muñoz et al. 2016).
Other methods, like in vitro slow growth have been used for the conservation of landraces, modern cultivars, and/or specific genotypes, and it eliminates the challenges of external biotic and abiotic factors. This procedure is based on tissue-culture conservation methodology, minimizing tissue growth so subcultures are reduced. Explants, under aseptic conditions, are introduced into glass tubes containing slow growth media and transferred into cultivated chamber where environmental variables, temperature, light intensity, and photoperiod, are regulated. In vitro conservation allows potato samples survive up to two years, depending on the composition of the media and environmental conditions. The culture media frequently used for potato in vitro conservation is Murashige and Skoog supplemented with sorbitol and sucrose, and samples are maintained at temperatures between 6 to 10 °C and low light intensity (Clausen et al. 2010; Bamberg et al. 2016; Muñoz et al. 2019). CIP’s potato method (2019) is currently the most efficient in vitro method for conserving potato accessions, which prolonged transference periods from once every 6–8 weeks up to two years. CIP currently maintains the largest in vitro potato collection with 8354 potato in vitro accessions, the majority of which (89.8%) are landraces or “papas nativas” originating mainly from the Andean region, with the remaining accessions being improved varieties and breeding lines (Ellis et al. 2020). In vitro slow growth conservation has been used by farmers of highland communities at the Potato Park in Cusco, Peru, to maintain their native potatoes tuber-seed clean of pest and diseases.
Cryopreservation is another methodology for long-term germplasm preservation where different types of explants are maintained at ultra-low temperatures in liquid nitrogen or liquid nitrogen vapor (Wang et al. 2020), and using osmo-protectant solutions in order to dehydrate the tissues and decrease the formation of ice crystals that would be lethal for cells. Potato seeds and meristems can be preserved and maintain its genetic stability by cryopreservation for longer periods of time compared to any other method (Digilio et al. 2018). This conservation strategy allows the storage of large quantities of samples in reduced spaces and at low costs, and is being employed in the largest genebanks with adequate infrastructure, equipment, trained personnel, and a continuous supply of liquid nitrogen. At CIP, the droplet PVS2 vitrification method preceded by a pre-culture treatment at 6 °C is used for application of cryopreservation for the long-term conservation of a wide diversity of potato genotypes (Panta et al. 2014, 2015) and has a recovery rate of 55–61% (Vollmer et al. 2019). A high-quality management of cryopreservation systems includes periodical viability reassessment, clear recovery criteria and the monitoring of success and contamination rates has been implemented (Vollmer et al. 2016, 2017). Currently, CGIAR in vitro genebanks unite the in vitro slow growth conservation and cryopreservation as part of the clonal crop conservation strategy, where the first is implemented to maintain for a medium-term the active collection, and the latter for the long term conservation of the base collection (Benson et al. 2011).
Another fundamental objective of genebanks is the characterization of genetic resources, in potato different molecular markers have been used to assess genetic variability, population structure, and taxa relationships, but single nucleotide polymorphisms (SNPs) are most frequently used due to their affordable cost and data quality, SNP arrays allow to assess about one million genome-wide SNP’s simultaneously in an individual assay, and because provide fundamental information not only for conservation but also for genetic analysis and breeding (Ellis et al. 2018).
DNA storage is another type of sample used for the conservation of genetic resources. However, only fragments of nucleic acids that can be later amplified, cloned, and inserted into another plant. This type of conservation is employed by a limited number of genebanks.
Herbarium specimens are another type of samples representative of potato genetic resources diversity. These collections kept voucher samples of the originally collected material and later regenerations. Herbarium specimens are important to document potato diversity and also permit the correct taxonomic characterization and identification of genebanks collections. Interestingly, DNA samples have been obtained from historical specimens using isolation techniques to determine potato species identity with molecular markers (Ames and Spooner 2008).
The maintenance of genetic resources in their natural environment where the species naturally evolved, is the strategy for in situ conservation. The main goal is to establish genetic reserves that guarantee the dynamic species evolutionary process, favoring the appearance of new allelic variants in natural populations that allow species adaptation in front of changing environments. The most effective method, in economic and political terms, is to implement Genetic Reserves inside existing Protected Areas (Maxted et al. 1997). In order to establish protected areas for in situ conservation, it is fundamental to know species taxonomy, distribution, phenology, genetic characteristics, plant demography, ecology, etc. as well as species diversity in the community of the Protected Areas (Dulloo et al. 2008). Clausen et al. (2018) identified 12 wild potato species growing in different Protected Areas of Argentina, and all these species were included in at least one protected area. More recently, studies of wild potato population species are in progress for the future establishment of a genetic reserves within Los Cardones National Park in Salta province (Kozub et al. 2019), in the Natural Reserve Villavicencio in Mendoza province (Marfil et al. 2015), and in the Natural Reserve Paititi in the southeast of Buenos Aires province (Garavano 2018). In the last area, the wild species S. commersonii could lose a high percentage of its distribution range due to agricultural activities. In Bolivia, Cadima-Fuentes et al. (2013), found that only 7 of the 21 Bolivian species were detected in parks and protected areas and recommended an increase in the inventories in these areas. In Peru, Sotomayor and Zorrilla (2019) are developing GIS-based studies for the identification of a network of areas with high diversity of wild potato species that need to be protected from threats such as urbanization, land-use change, mining, etc. One way to protect them is to become “Agrobiodiversity Zones”, community-owned territories recognized by the government as prioritized for in situ conservation activities. The establishment of on farm conservation zones not only focused on the conservation of cultivated landraces but also in the fundamental role of local communities in the processes of crop evolution (De Haan et al. 2016a, b, c). One of these initiatives is “The Potato Park”, recognized as Agrobiodiversity Zones by the Peruvian Minister of Agriculture and Irrigation (MINAGRI), based on their native, cultural and ecological wealth where indigenous peoples preserve their cultural traditions to manage and maintain genetic resources of their fields and ecosystems. The Potato Park located in the Cusco Inca Sacred valley in Calca province (Peru) comprise and extension of more than 7000 ha, where around 1330 potato landraces and other native Andean crops are cultivated and maintained in a local genebank, managed by four Quechua communities or “ayllus”. This example of in situ conservation is characterized by strong interactions between the crops, their wild relatives, and the farmers, promoting different strategies for conservation and sustainable use of these genetic resources (MINAM 2019). Currently, different initiatives and efforts, such as the Papa Andina experience are focused on sustainable agriculture-food system development considering the needs for nutrition security while promoting better crop management and productivity, and the optimization of the potato value chain (Devaux et al. 2020). At present different projects in Andean countries are focused on the conservation of their potato genetic resources.
The in situ strategy could be essential for the conservation of genetic resources, and considering different scenarios of climatic change the distribution of wild potato species is likely to diminish at low altitudes and wild species population could be affected or became extinct (Jarvis et al. 2008). Different approaches, such as environmental niche modeling methods, have been used to evaluate the conservation status 73 wild potatoes maintained in genebanks, revealing high priority for collecting 32 species (43.8%), while 20 species have medium priority for collection and only three species have good representation in ex situ collections (Castañeda-Álvarez et al. 2015). These studies not only support collecting strategies but also help to define zones for in situ conservation through the detection of areas where species are potentially threatened by several factors (urbanization, agriculture expansion, overgrazing, etc.). In front of uncertain scenarios, could be possible to securely preserve diversity in potato, and predict what diversity has been lost or is in imminent danger of being lost, and also estimated the actual economic value, as well as, potential future value of the potato diversity that is not securely conserved (Ellis et al. 2020). The understanding of the diversity and distribution of wild and cultivated potato also contributes to the development of policies on biosafety as it has occurred in Peru (MINAM 2019).
4.6 Conclusions
At present, there is a better understanding of the diversity, distribution, and genetic diversity of potatoes. A recent comprehensive taxonomy of all wild and cultivated potatoes, based on the integration of multiple evidences and phylogenetic relationships between taxa, proposed a framework for further investigation of complex groups as well as endemic species in restricted areas, which have been poorly collected.
Integrative taxonomy also provides hypothesis to study patterns of species diversity and distributions, physical and ecological isolation factors, biological barriers and physiological plasticity, and in particular the role of ploidy, gene flow, and interspecific hybridization in the adaptation of species to different and extreme environment.
Genetic and genomic studies have contributed to understand potato evolution. Recent investigations provide promissory approaches to compare and differentiate potato species genomes that help not only to elucidate the process of evolution, but also to detect alleles related to important agronomic traits. Few wild species have been used to improve cultivated potatoes, and these valuable resources can be incorporate into breeding plans.
Results from different disciplines demonstrated the value of complementary evidences from archaeological remains and phylogeny to support the hypothesis of a single origin of domestication and culture of potatoes occurred in southern Peru, and encouraged to continue research to elucidate the sources and areas of initial cultivation.
Genebanks worldwide guarantee the conservation of valuable potato genetic resources for food security. In addition, advances in ex situ conservation techniques like in vitro and cryoconservation made more efficient sample preservation reducing costs and maintenance space. A stable taxonomic framework could be useful for harmonizing potato nomenclature used in global genebanks and to identify unique and redundant material to promote an efficient conservation through collection homologation.
A better understanding of potato diversity is fundamental for the establishment of protected areas, where populations could be monitored to ensure their persistence in natural habitat or in local communities. Agrobiodiversity Zones are also fundamentals for preservation and sustainable use of potato genetic resources and for local communities’ alimentary subsistence.
References
Alefeld FGC (1866) Landwirthschaftliche flora. Weigant & Hempel, Berlin
Álvarez NMB, Peralta IE, Salas A, Spooner DM (2008) A morphological study of species boundaries of the wild potato Solanum brevicaule complex: replicated field trials in Peru. Plant Syst Evol 274:37–45
Ames M, Spooner DM (2008) DNA from herbarium specimens settles a controversy about origins of the European potato. Am J Botany 95:252–257
Ames M, Spooner DM (2010) Phylogeny of Solanum series Piurana and related species in Solanum section Petota based on five conserved ortholog sequences. Taxon 59:1091–1104
Aversano R, Contaldi F, Ercolano MR, Grosso V, Iorizzo M, Tatino F, Xumerle L, Dal Molin A, Avanzato C, Ferrarini A, Delledonne M, Sanseverino W, Cigliano RA, Capella-Gutierrez S, Gabaldón T, Frusciante L, Bradeen JM, Carputo D (2015) The Solanum commersonii genome sequence provides insights into adaptation to stress conditions and genome evolution of wild potato relatives. Plant Cell 27:954–968
Bauhin C (1596) Pinax Theatri Botanici, sive Index in Theophrasti, Dioscoridis, Plinii et botanicorum qui à seculo scripserunt opera. Basilea
Bamberg JB, Del Rio AH (2004) Genetic heterogeneity estimated by RAPD polymorphism of four tuber-bearing potato species differing by breeding system. Am J Potato Res 81(6):377–383
Bamberg JB, Martin MW, Abad J, Jenderek MM, Tanner J, Donnelly DJ, Novy RG (2016) In vitro technology at the US Potato Genebank. In Vitro Cell Dev Biol Plant 52(3):213–225
Benson EE, Harding K, Debouck D, Dumet D, Escobar R, Mafla G, Panis B, Panta A, Tay D, Van den Houwe I, Roux N (2011) Refinement and standardization of storage procedures for clonal crops Global Public Goods Phase 2: Part I. Project landscape and general status of clonal crop in vitro conservation technologies. System-wide Genetic Resources Programme, Rome, Italy
Brown CR (1984) Tetrad sterility: a cytoplasmic-genic male sterility attractive to bumble bees. In: Winiger FA, Stockli A (eds) Abstracts of the conference papers of the 9th Trienn Conf Europ Assoc Potato Res, Interlaken, Switzerland, 1–6 July 1984, pp 101–102
Brücher H (1964) El origen de la papa (Solanum tuberosum) Physis 24:439–452
Burgos G, Felde TZ, Andre C, Kubow S (2020) The Potato and Its Contribution to the Human Diet and Health. In: Campos H, Ortiz O (eds) The potato crop, Chap. 2, pp 37–74. https://doi.org/10.1007/978-3-030-28683-5_2
Bethke PC, Halterman DA, Jansky SH (2019) Potato Germplasm enhancement enters the genomics era. Agronomy 9: 575. https://doi.org/10.3390/agronomy9100575
Bohs L (1994) Cyphomandra (Solanaceae). Fl. Neotrop Monogr 63:1–175
Bohs L, Olmstead RG (1997) Phylogenetic relationships in Solanum (Solanaceae) based on ndhF sequences. Syst Bot 22:5–17
Bohs L, Olmstead RG (1999) Solanum phylogeny inferred from chloroplast DNA sequence phylogeny. In: Nee M, Symon DE, Lester RN, Jessop JP (eds) Solanaceae IV: advances in biology and utilization. Royal Botanic Gardens, Kew, pp 97–110
Bohs L (2005) Major clades in Solanum based on ndhF sequence data. In: Keating RC, Hollowell VC, Croat TB (eds) A Festschrift for William G. D’Arcy: A Legacy of a Taxonomist. Missouri Botanical Garden, St. Louis, pp 27–49
Bonierbale MW, Amoros WR, Salas E, de Jong W (2020) Potato breeding. In: Campos H, Ortiz O (eds) The potato crop, Chap. 6, pp 163–217. https://doi.org/10.1007/978-3-030-28683-5_6
Bretagnolle F, Thompson JD (1995) Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol 129:1–22
Brush SB, Carney HJ, Huamán Z (1981) Dynamics of Andean potato agriculture. Econ Bot 35:70–88
Bukasov SM (1930) The cultivated plants of Mexico, Guatemala and Colombia. Trudy Po Prikladnoj Botanike Genetike i Selekcii, Supplement 47(191–226):513–525
Bukasov SM (1978) Systematics of the potato. Trudy Po Prikladnoj Botanike Genetike i Selekcii 62:3–35
Cadima-Fuentes X, Van Zonneveld M, Scheldeman MX, Castañeda N, Patiño F, Beltrán M, Van Damme P (2013) Endemic wild potato (Solanum spp.) biodiversity status in Bolivia: reasons for conservation concerns. J Nat Conserv 22:113–131
Cadima-Fuentes X (2014) Conserving the genetic diversity of Bolivian wild potatoes Doctoral dissertation, PhD thesis, Wageningen University, Wageningen. http://library.wur.nl/WebQuery/clc/2075456
Cai D, Rodríguez F, Teng Y, Ané C, Bonierbale M, Mueller LA, Spooner DM (2012) Single copy nuclear gene analysis of polyploidy in wild potatoes (Solanum section Petota). BMC Evol Biol 12:70
Camadro EL, Peloquin SJ (1981) Cross-incompatibility between two sympatric polyploid Solanum species. Theor Appl Genet 60:65–70
Camadro EL, Masuelli RW (1995) A genetic model for the endosperm balance number (EBN) in the wild potato Solanum acaule Bitt. and two related diploid species. Sex Plant Reprod 8:283–288
Camadro EL, Carputo D, Peloquin SJ (2004) Substitutes for genome differentiation in tuber-bearing Solanum: interspecific pollen-pistil incompatibility, nuclear-cytoplasmic male sterility, and endosperm. Theor Appl Genet 109:1369–1376
Camadro EL (2012) Relevance of the genetic structure of natural populations, and sampling and classification approaches for conservation and use of wild crop relatives: potato as an example. Botany 90:1065–1072
Cárdenas M, Hawkes JG (1946) New or little-known wild potato species from Bolivia and Peru. J Linn Soc Bot 53:91–108
Carputo D, Frusciante L (2011) Classical genetics and traditional breeding. In: Bradeen J, Kole C (eds) Genetics, genomics and breeding of potato. Science Publishers, pp 20–40
Carputo D, Parisi M, Consiglio F, Iovene M, Caruso G, Monti L, Frusciante L (2003a) Aneuploid hybrids from 5x–4x crosses in potato: chromosome number, fertility, morphology and yield. Am J Potato Res 80:93–101
Carputo D, Frusciante L, Peloquin SJ (2003b) The role of 2n gametes and endosperm balance number in the origin and evolution of polyploids in the tuber-bearing Solanums. Genetics 163:287–294
Castañeda-Álvarez NP, de Haan S, Juárez H, Khoury CK, Achicanoy HA, Sosa CC, Bernau V, Salas A, Heider B, Simon R, Maxted N, Spooner DM (2015) Ex situ conservation priorities for the wild relatives of potato (Solanum L. Section Petota). PLos One 10(14):e0122599
CGIAR (2020) Crop genebank knowledge base. Strengthening capacity to manage genebanks. https://cropgenebank.sgrp.cgiar.org/index.php/procedures-mainmenu-242
CIP (2015) Catálogo de variedades de la papa andina de Chugay, La Libertad, Perú Centro Internacional de la papa, Asociación Pataz, Instituto Nacional de Innovación Agraria
CIP (2019) https://cipotato.org/genebankcip/. Accessed 15 Mar 2019
Cipar MS, Peloquin SJ, Hougas RW (1964) Variability in the expression of self-incompatibility in tuber-bearing diploid Solanum species. Am Potato J 41:155–162
Clausen AM, Ispizúa VN, Digilio A (2010) Native andean potato varieties in Argentina: conservation and evaluation of an endangered genetic resource. Am J Plant Sci Biotechnol. Plant Sci Biotechnol South America: Focus Argentina I. Vol. 3 Special Issue 1:72–82
Clausen AM, Peralta IE, Spooner DM (2013) Grupo VIII. Potato. In: Anton AM, Zuloaga FO (eds) Flora Argentina (Flora Vascular de la República Argentina) Vol 13, pp 264–289
Clausen AM, Ispizua VN, Atencio HM, Calandroni M, Digilio A (2018) Especies silvestres de papa (Solanum sect. Petota y sect. Etuberosum) identificadas en áreas protegidas de la Argentina. Bol Soc Argent Bot 53:67–75
Clusius C (1601) Rariorum plantarum historia: quae accesserint, proxima pagina docebit. Ioannem Moretum, Antwerp
Contreras A (1987) Germoplasma chileno de papas (Solanum spp.). Anales Simposio Recursos Fitogenéticos, Valdivia, 1984. Universidad Austral de Chile, Valdivia: International Board of Plant Genetic Resources, pp 43–75
Contreras A, Spooner DM (1999) Revision of Solanum section Etuberosum. In: Nee M, Symon DE, Lester RN, Jessop JP (eds) Solanaceae IV advances in biology and utilization. Royal Botanic Gardens, Kew, pp 227–245
Correll DS (1962) The potato and its wild relatives contributions from the Texas Research Foundation. Bot Stud 4:1–606
Cracraft J (1989) Speciation and its ontology: the empirical consequences of alternative species concepts for understanding patterns and processes of differentiation. In: Otte D, Endler JA (eds) Speciation and its consequences, a view for evolutionary biology and ecology, biology and philosophy, vol 2, pp 415–434
De Candolle AP (1882) Origine des plantes cultivées
De Haan S, Rodríguez F (2016a) Potato origin and production. In: Singh J, Kaur L (eds) Advances in potato chemistry and technology. Academic Press, pp 1–32
De Haan S, Polreich S, Rodríguez F, Juarez H, Plasencia F, Ccanto R, Alvarez C, Otondo A, Sainz H, Venegas C, Kalazich J (2016b) A long-term systematic monitoring framework for on-farm conserved potato landrace diversity. In: Maxted N, Ehsan Dulloo M, Ford-Lloyd BV (eds) Enhancing crop genepool use: Capturing wild relative and landrace diversity for crop improvement. CAB International, Oxfordshire, pp 289–296
De Haan S, Rodríguez F, Becerra LA, Polreich S, Scurrah M, Nuñez J, Juarez H, Plasencia F, Bernardo L, Meza K (2016c) Conservation dynamics of roots and tuber crops under on-farm management. Indian J Plant Genet Resour 29:289–291
De Haan S, Burgos G, Rodríguez F, Creed H, Liria M, Bonierbale M (2019) The nutritional role of potato varietal diversity in Andean food systems: a case study. Am J Potato Res. https://doi.org/10.1007/s12230-018-09707-2
Devaux A, Ordinola M, Horton D (eds) (2011) Innovation for development: the Papa Andina experience. International Potato Center (CIP)
Devaux A, Goffart JP, Petsakos A, Kromann P, Gatto M, Okello J, Suarez V, Hareau G (2020) Global food security, contributions from sustainable potato agri-food systems. In: Campos H, Ortiz O (eds) The potato crop, Chap. 1, pp 3–35. https://doi.org/10.1007/978-3-030-28683-5_1
Digilio A, Molina-García AD, Deladino L, Schneider Teixeira A (2018) Effective cryopreservation approach for the Andean potato shoot tip in vitro culture. Abstracts/cryobiology 85:172
Dodds PN, Clarke AE, Newbigin E (1996) A molecular perspective on pollination in flowering plants. Cell 85:141–144
Dulloo ME, Labokas J, Iriondo JM, Maxted N, Lane A, Laguna E, Jarvis A, Kell SP (2008) Genetic reserve location and design. In: Iriondo JM, Dulloo E, Maxted N (eds) Conserving plant genetic diversity in protected areas. CAB International Publishing, Wallingford, pp 23–64
Ehlenfeldt MK, Hanneman RE Jr (1988) Genetic control of endosperm balance number (EBN): three additive loci in a threshold-like system. Theor Appl Genet 75:825–832
Ellis D, Chavez O, Coombs J, Soto J, Gomez R, Douches D, Panta A, Silvestre R, Anglin NL (2018) Genetic identity in genebanks: application of the SolCap 12K SNP array in fingerprinting the global in trust potato collection. Genome 61:523–537
Ellis D, Salas A, Chavez O, Gomez R, Anglin N (2020) Ex Situ conservation of potato [Solanum Section Petota (Solanaceae)] genetic resources in genebanks. In: Campos H, Ortiz O (eds) The potato crop, Chap. 4, pp 109–138. https://doi.org/10.1007/978-3-030-28683-5_4
Engel F (1970) Exploration of the Chilca Canyon, Peru. Curr Anthropol 11:55–58
Engels JMM, Visser L (eds) (2003) A guide to effective management of germplasm collections. IPGRI. Handbooks for Genebanks No 6. IPGRI, Rome, Italy
Erazzú LE, Camadro EL, Clausen AM (2009) Persistence over time, overlapping distribution and molecular indications of interspecific hybridization in wild potato populations of Northwest Argentina. Euphytica 168:249–262
Fajardo D, Spooner DM (2011) Phylogenetic relationships of Solanum series Conicibaccata and related species in Solanum section Petota inferred from five conserved ortholog sequences. Syst Bot 36:163–170
FAO (2009) International treaty on plant genetic resources for food and agriculture. http://www.fao.org/3/a-i0510e.pdf
FAO (2010) The second report on the state of the world’s plant genetic resources for food and agriculture. FAO, Rome
FAO (2014) Genebank standards for plant genetic resources for food and agriculture, rev. FAO, Rome
FAO-IPGRI Technical Guidelines for the Safe Movement of Germplasm. https://cropgenebank.sgrp.cgiar.org/images/file/learning_space/potato_tech_guid_safe_move_germplasm.pdf
Fonseca C, Burgos G, Rodríguez F, Muñoa L, Ordinola M (2014) Catálogo de variedades de papa nativa con potencial para la seguridad alimentaria y nutricional de Apurímac y Huancavelica. Lima: Centro Internacional de la Papa
Frankel R, Galun E (1977) Pollination mechanisms, reproduction and plant breeding. Springer, Berlin Heidelberg New York
Friedman WE (1998) The evolution of double fertilization and endosperm: an “historical” perspective. Sex Plant Reprod 11:6–16
Garavano ME (2018) Estudio de Solanum commersonii Dunal en un ecosistema serrano del Sistema de Tandilia (Buenos Aires) para implementar su conservación in situ. MSc thesis, Universidad Nacional University de Mar del Plata, Argentina, library. http://intrabalc.inta.gob.ar/dbtw-wpd/advanced.htm
Gavrilenko T (2007) Potato cytogenetics. In: Vruegdenhil D (ed) Potato Biology and biotechnology: advances and perspectives. Elsevier, Amsterdam, pp 203–206
Gavrilenko T (2011) Application of molecular cytogenetics in fundamental and applied research of potato. In: Bradeen J, Kole C (eds) Genetics, Genomics and breeding of potato. Science Publishers, Enfield, pp 184–206
Gebhardt C, Ritter E, Barone A, Debener T, Walkemeier MN, Ganal MW, Tanksley SD, Salamini F (1991) RFLP maps of potato and their alignment with the homoeologous tomato genome. Theor Appl Genet 83:49–57
Gerard J (1597) The herball or generall historie of plantes (1st ed.). London
Ghislain M, Andrade D, Rodríguez F, Hijmans RJ, Spooner DM (2006) Genetic analysis of the cultivated potato Solanum tuberosum L. Phureja Group using RAPDs and nuclear SSRs. Theor Appl Genet 113(8):1515–1527
Ghislain M, Douches DS (2020) The genes and genomes of the potato. In: Campos H, Ortiz O (eds) The potato crop, Chap. 5, pp 139–162. https://doi.org/10.1007/978-3-030-28683-5
Goldberg EE, Kohn JR, Lande R, Robertson KA, Smith SA, Igic B (2010) Species selection maintains self-Incompatibility. Science 330:493–495
Gorbatenko LE (1989) Systematic conspectus of section Petota Dumort of the genus Solanum L. in South America (in Russian). Trudy Prik Bot 126:92–108
Gorbatenko LE (2006) Potato species of South America: ecology, geography, introduction, taxonomy, and breeding value. Russian Academy of Agricultural Sciences, State Scientific Centre of the Russian Federation, St. Petersburg
G2PSOL: http://www.g2psol.eu/image/users/432653/ftp/my_files/GPSOL_Leaflet.pdf?id=30388884
Grun P (1990) The evolution of cultivated potatoes. Econ Bot 44(3):39–55
Grun P (1973) Cytoplasmic sterilities that separate the Group Tuberosum cultivated potato from its putative tetraploid ancestor. Evolution 27:633–643
Hanneman RE Jr, Peloquin SJ (1981) Genetic-cytoplasmic male sterility in progeny of 4x–2x crosses in cultivated potatoes. Theor Appl Genet 59:53–55
Hanneman RE Jr (1994) Assignment of endosperm balance numbers to the tuber-bearing Solanums and their close non-tuber-bearing relatives. Euphytica 74:19–25
Hanneman RE Jr (1999) The reproductive biology of the potato and its implications for breeding. Potato Res 42:283–312
Hardigan MA, Parker F, Laimbeer E, Newton L, Crisovan E, Hamilton JP, Vaillancourt B, Wiegert-Rininger K, Wood JC, Douches DS, Farré EM, Veilleux R, Buella CR (2017) Genome diversity of tuber-bearing Solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato. Proc Nat Acad Sci USA 114(46):E9999–E10008. https://doi.org/10.1073/pnas.1714380114
Harlan J, De Wet J (1975) On Ö Winge and a prayer: the origins of polyploidy. Bot Rev 41:361–390
Hawkes JG (1944) Potato collecting expeditions in Mexico and South America. II. Systematic classification of the collections. Imperial Bureau of Plant Breeding and Genetics, Aberystwyth, pp 1–142
Hawkes JG (1962) Introgression in certain wild potato species. Euphytica 11:752–757
Hawkes JG (1990) The potato: evolution, biodiversity and genetic resources. Belhaven Press, London
Hawkes JG (2004) Hunting the wild potato in the South American Andes. Memories of the British Empire potato collecting expedition to South America 1938–1939. Nijmegen: Botanical and Experimental Garden, University of Nijmegen
Hawkes JG, Hjerting JP (1969) The potatoes of Argentina, Brazil, Paraguay and Uruguay. A biosystematic study. Oxford University Press, Oxford
Hawkes JG, Hjerting JP (1989) The potatoes of Bolivia, their breeding value and evolutionary relationships. Oxford University Press, Oxford, UK
Hawkes JG, Francisco-Ortega J (1993) The early history of the potato in Europe. Euphytica 70:1–7
Hermundstad SA, Peloquin SJ (1985) Germplasm enhancement with potato haploids. J Hered 76:463–467
Hijmans RJ, Spooner DM (2001) Geography of wild potato species. Am J Bot 88:2101–2112
Hijmans RJ, Spooner DM, Salas AR, Guarino L, De la Cruz J (2002) Atlas of wild potatoes. Systematic and ecogeographic studies on crop genepools. International Plant Genetic Resources Institute, Rome
Hijmans RJ, Gavrilenko T, Stephenson S, Bamberg J, Salas A, Spooner, (2007) Geographic and environmental range expansion through polyploidy in wild potatoes (Solanum section Petota). Global Ecol Biogeogr 16:485–495
Hogenboom NG (1973) A model for incongruity in intimate partner relationships. Euphytica 22:219–233
Hogenboom NG (1979) Incompatibility and incongruity in Lycopersicon. In: Hawkes JG, Lester RN, Skelding AD (eds) The biology and taxonomy of the Solanaceae. Academic Press, London, pp 435–444
Holle M (1988) Seed conservation of potato genetic resources – IBPGR standards: theoretical ideals and practical reality. Report of the 29th planning conference on strategies for the conservation of potato genetic resources, CIP, Lima, pp 115–128
Hosaka K, Hanneman RE Jr (1998) Genetics of self-compatibility in a self-incompatible wild diploid potato species Solanum chacoense.2. Localization of an S locus inhibitor (Sli) gene on the potato genome using DNA markers. Euphytica 103:265–271
Huamán Z, Spooner DM (2002) Reclassification of landrace populations of cultivated potatoes (Solanum sect. Petota). Amer J Bot 89:947–965
INIA (2012) Catálogo de nuevas variedades de papa: sabores y colores para el gusto peruano. INIA, CIP, Red LatinPapa
INIAF, VDRA and MDRyT (2014) Catálogo de accessiones de papa Solanum tuberosum subsp. Andigenum (Juz. & Bukasov) Hawkes del Banco de Germoplasma de tubérculos y raíces de Bolivia
ICNCP, International Code of Nomenclature for Cultivated Plants Ninth Edition (2016) Scripta Horticulturae 18
Iorizzo M, Gao L, Mann H, Traini A, Chiusano ML, Kilian A, Aversano R, Carputo D, Bradeen JM (2014) A DArT marker-based linkage map for wild potato Solanum bulbocastanum facilitates structural comparisons between Solanum A and B genomes. BMC Genet 15:123
Iovene M, Savarese S, Cardi T, Frusciante L, Scotti N, Simon P, Carputo D (2007) Nuclear and cytoplasmic genome composition of Solanum bulbocastanum (+) S. tuberosum somatic hybrids. Genome 50:443–450
Ispizúa VN, Camadro EL, Clausen AM (2015) Variation patterns among natural populations of wild potatoes at Inca Cueva (Jujuy, Argentina). Genet Resour Crop Evol 62:235–253. https://doi.org/10.1007/s10722-014-0149-7
Iwanaga M, Ortiz R, Cipar MS, Peloquin SJ (1991) A restorer gene for genetic-cytoplasmic male sterility in cultivated potatoes. Am Potato J 68:19–28
Jansky SH, Dempewolf H, Camadro EL, Simon R, Zimnoch-Guzowska E, Bisognin DA, Bonierbale M (2013) A case for crop wild relative preservation and use in potato. Crop Sci 53:746–754
Jarvis A, Lane A, Hijsmans RJ (2008) The effect of climate change on crop wild relatives. Agric Eco Syst Environm 126:13–23
JICA (2016) Potato seed tuber production techniques manual. https://www.jica.go.jp/nepal/english/office/others/c8h0vm0000bjww96-att/tm_4.pdf
Johnston SA, Hanneman RE Jr (1980) Support of the endosperm balance number hypothesis utilizing some tuber-bearing Solanum species. Am Potato J 57:7–14
Johnston SA, Hanneman RE Jr (1982) Manipulations of endosperm balance number overcome crossing barriers between diploid Solanum species. Science 217:446–448
Johnston SA, Hanneman RE Jr (1996) Genetic control of endosperm balance number (EBN) in the Solanaceae based on trisomic and mutation analysis. Genome 39:314–321
Johnston SA, den Nijs TM, Peloquin SJ, Hanneman RE Jr (1980) The significance of genic balance to endosperm development in interspecific crosses. Theor Appl Genet 57:5–9
Juzepczuk SW (1937) New species of the genus Solanum L. in the group Tuberarium Dun. Izvestiya Akademii Nauk SSSR 2:295–331
Juzepczuk SW, Bukasov SM (1929) A contribution to the question of the origin of the potato. Trudy Vsesoyuznogo S”zeda po Genetike i Selektsii Semenovodstvu i Plemennomu Zhivotnovodstvu 3: 593–611
Knapp S (1991) A revision of Solanum sessile species group (section Geminata pro parte: Solanaceae). Bot J Linn Soc 105:179–210
Knapp S (2000) A revision of Solanum thelopodium species group (section Anthoresis sensu Sheite, pro parte): Solanaceae. Bull Nat Hist Mus. London (bot) 30:13–30
Knapp S (2002) Solanum section Geminata (G.Don) Walpers (Solanaceae). Flora Neotropical Monograph 84:1–45
Knapp S (2008) A revision of Solanum havanense species group (section Geminata (G.Don)Walp pp) and new taxonomic additions to the Geminata clade (Solanum: Solanaceae). Ann Mo Bot Gard 95:405–458
Knapp S (2013) A revision of the Dulcamaroid clade of Solanum L. (Solanaceae). PhytoKeys 22:1–432
Kohler C, Scheid OM, Erilova A (2009) The impact of the triploid block on the origin and evolution of polyploid plants. Trends Genet 26:142–148
Kozub PC, Ibañez VN, Digilio A, Atencio HM, Garavano ME, Sánchez ME, Marfil CF (2019) Wild potato genetic reserves in protected areas: prospection notes from Los Cardones National Park, Salta, Argentina. Rev FCA UNCuyo 51:461–474
Kuhl JC (2011) Mapping and tagging of simply inherited traits. In: Bradeen J, Kole C (eds) Genetics, genomics and breeding of potato. Science Publishers, Enfield, pp 90–112
Lamm R (1941) Varying cytological behavior in reciprocal Solanum crosses. Hereditas 27:202–208
Lamm R (1953) Investigations on some tuber-bearing Solanum hybrids. Hereditas 39:97–112
Lara Cabrera SI, Spooner DM (2004) Taxonomy of North and Central American diploid wild potato (Solanum sect. Petota) species: AFLP data. Plant Syst Evol 248:129–142
Linnaeus C (1753) Species plantarum, 2 vol. Stockholm
Lewis D (1943) Physiology of incompatibility in plants. III. Autopolyploids. J Genet 45:171–185
Lewis D (1947) Competition and dominance of incompatibility alleles in diploid pollen. Heredity 1:85–108
Louderback LA, Pavlik BM (2017) Starch granule evidence for the earliest potato use in North America. Proc Natl Acad Sci USA 114(29):7606–7610
Loskutov IG (1999) Vavilov and his institute. A history of the world collection of plant genetic resources in Russia. International Plant Genetic Resources Institute, Rome
Luu DT, Qin X, Morse D, Cappadocia M (2000) S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature 407:649–651
Mann H, Iorizzo M, Gao L, D’Agostino N, Carputo D, Chiusano ML, Bradeen JM (2011) Molecular linkage maps: strategies, resources and achievements. In: Bradeen J, Kole C (eds) Genetics, genomics and breeding of potato. Science Publishers, Enfield, pp 68–86
Mason AS, Pires JC (2015) Unreduced gametes: Meiotic mishap or evolutionary mechanism? Trends Genet 31:5–10
Marfil CF, Hidalgo V, Masuelli RW (2015) In situ conservation of wild potato germplasm in Argentina: example and possibilities. Global Ecol Conserv 3:461–476
Masuelli RW, Camadro EL, Erazzu LE, Bedogni MC, Marfil CF (2009) Homoploid hybridization in the origin and evolution of wild diploid potato species. Plant Syst Evol 277:143–151
Matsubayashi M (1991) Phylogenetic relationships in the potato and its related species. In: Tsuchiya, Gupta PK (eds) Chromosome engineering in plants: genetics, breeding, evolution, part B. Amsterdam, Elsevier, pp 93–118
Maxted N, Hawkes JG, Ford-Lloyd BV, Williams JT (1997) A practical model for in situ genetic conservation. In: Maxted N, Hawkes JG, Ford-Lloyd BV, Williams JT (eds) Plant genetic conservation: the in situ approach. Chapman & Hall, London, pp 339–367
Mendiburu A, Peloquin SJ (1977) Bilateral sexual polyploidization in potatoes. Euphytica 26:573–583
Miller JT, Spooner DM (1999) Collapse of species boundaries in the Solanum brevicaule complex: molecular data. Plant Syst Evol 214:103–130
MINAM (2019) Línea de base de la diversidad genética de la papa peruana con fines de bioseguridad. Ministerio del Ambiente. Perú
Ministerio de Agricultura y Riego (MINAGRI), Grupo Yanapai, Instituto Nacional de Innovación Agrarias (INIA), Centro Internacional de la papa (2017) Catálogo de variedades de la papa andina del sureste del Departamento de Junín, Perú. Lima, Perú CIP
Muñoz M, Folch C, Rodríguez F, Kalazich J, Orena S, Santos J, Vargas R, Fahrenkrog A, Puga A (2016) Genotype number and allelic diversity overview in the national collection of Chilean potatoes. Potato Res 59:227. https://doi.org/10.1007/s11540-016-9329-5
Muñoz M, Díaz O, Reinún W, Winkler A, Quevedo R (2019) Slow growth in vitro culture for conservation of Chilotanum potato germplasm. Chil J Agric Res 79(1):26–35
den Nijs TPM, Peloquin SJ (1977) 2n gametes in potato species and their function in sexual polyploidization. Euphytica 26:585–600
Ochoa CM (1990) The potatoes of South America: Bolivia. Cambridge Univ. Press, Cambridge
Ochoa CM (1999) Las Papas de Sudamerica: Peru (parte I). Lima, Peru: International Potato Center
Ochoa CM (2001) Las Papas de Sudamérica: Bolivia. Volumen 127 de Travaux de l'Institut français d'études andines, Institut Français d'Études Andines, CIP Lima
Olmstead RG, Palmer JD (1992) A chloroplast DNA phylogeny of the Solanaceae: subfamilial relationships and character evolution. Ann Mo Bot Gard 79:346–360
Olmstead RG, Palmer JD (1997) Implications for the phylogeny, classification, and biogeography of Solanum from cpDNA restriction site variation. Syst Bot 22:19–29
Olmstead RG, Sweere JA, Spangler RE, Bohs L, Palmer JD (1999) Phylogeny and provisional classification of the Solanaceae based on chloroplast DNA. In: Nee M, Symon DE, Lester RN, Jessop JP (eds) Solanaceae IV: advances in biology and utilization. Royal Botanic Gardens, Kew, pp 111–137
Ortiz R (2020) Genomic-led potato breeding for increasing genetic gains: achievements and outlook. Crop Breed Genet Genom. 2:e200010. https://doi.org/10.20900/cbgg20200010
Ovchinnikova A, Krylova E, Gavrilenko T, Smekalova T, Zhuk M, Knapp S, Spooner DM (2011) Taxonomy of cultivated potatoes (Solanum section Petota: Solanaceae). Bot J Linn Soc 165:107–155
Panta A, Panis B, Ynouye C, Swennen R, Roca W (2014) Development of a PVS2 droplet vitrification method for potato cryopreservation. Cryo Letters 35:255–266
Panta A, Panis B, Ynouye C, Swennen R, Roca W, Tay D, Ellis D (2015) Improved cryopreservation method for the long-term conservation of the world potato germplasm collection. Plant Cell Tis Org Cult 120:117–125
Parodi LR (1966) La Agricultura aborigen argentina. Eudeba, Buenos Aires
Parque de la Papa, Cusco, Perú. https://parquedelapapa.org/
Peloquin SJ, Yerk GL, Werner JE (1989) Ploidy manipulations in potato. In: Adolph KW (ed) Chromosomes: Eukaryotic, prokaryotic and viral. CRC Press, Bosa Raton, FL, pp 167–178
Peloquin SJ, Yerk GL, Werner JE, Darmo E (1989) Potato breeding with haploids and 2n gametes. Genome 32:1000–1004
Peloquin SJ, Gabert AC, Ortiz R (1996) Nature of ‘pollinator’ effect in potato (Solanum tuberosum L.) haploid production. Ann Bot 77:539–542
Peloquin SJ, Boiteux LS, Simon PW, Jansky SH (2008) A chromosome-specific estimate of transmission of heterozygosity by 2n gametes in potato. J Hered 99:177–181
Pendinen G, Gavrilenko T, Jiang J, Spooner DM (2008) Allopolyploid speciation of the tetraploid Mexican potato species S. stoloniferum and S. hjertingii revealed by genomic in situ hybridization. Genome 51:714–720
Pendinen G, Spooner DM, Jiang J, Gavrilenko T (2012) Genomic in situ hybridization (GISH) reveals both autopolyploid and allopolyploid origins of different North and Central American hexaploid potato (Solanum section Petota) species. Genome 55:407–415
Peralta IE, Spooner DM (2001) Granule-bound starch synthase (GBSSI) gene phylogeny of wild tomatoes (Solanum L. section Lycopersicon [Mill.] Wettst. subsection Lycopersicon). Amer J Bot 88:1888–1902
Peralta IE, Spooner DM, Knapp S (2008) The taxonomy of tomatoes: a revision of wild tomatoes (Solanum section Lycopersicon) and their outgroup relatives in sections Juglandifolia and Lycopersicoides. Syst Bot Monogr 84:1–186 + 3 plates
Popenoe H, King SR, León J, Kalinowski LS (1990) Lost crops of the Incas. Little known plants of the Andes with promise for worldwide cultivation. In: Vietmeyer ND (ed) The National Academies Press, Washington, DC
Potato Genome Sequencing Consortium (2011) Genome sequence and analysis of the tuber crop potato. Nature 475:189–195
PRODERN (2018) La papa nativa en Apurímac, identificación participativa de variedades en los distritos de Huayana y Pomococha, Perú
Quinn A, Mok D, Peloquin SJ (1974) Distribution and significance of diplandroids among the diploid Solanums. Am Pot J 51:16–21
Rabinowitz D, Linder CR, Ortega R, Begazo D, Murguia H, Douches DS, Quiros CF (1990) High levels of interspecific hybridization between Solanum sparsipilum and S. stenotomum in experimental plots in the Andes. Am Pot J 67:73–81
Ríos D, Ghislain M, Rodriguez F, Spooner DM (2007) What is the origin of the European potato? Evidence from Canary Island landraces. Crop Sci 47:127–1280
Rivard SR, Cappadocia M, Landry BS (1996) A comparison of RFLP maps based on anther culture derived, selfed, and hybrid progenies of Solanum chacoense. Genome 39:611–621
Roberts EH, Ellis RH (1984) The implication of the deterioration of orthodox seeds during storage for genetic resources conservation. In: Holden JHW, Williams JT (eds) Crop genetic resources. George Allen and Unwin, London, pp 18–37
Rodríguez A, Spooner DM (1997) Chloroplast DNA analysis of Solanum bulbocastanum and S. cardiophyllum, and evidence for the distinctiveness of S. cardiophyllum subsp. ehrenbergii (sect. Petota). Syst Bot 22:31–43
Rodríguez F, Spooner DM (2009) Nitrate reductase phylogeny of potato (Solanum sect. Petota) genomes with emphasis on the origins of the polyploid species. Syst Bot 34:207–219
Rodríguez F, Wu F, Ané C, Tanksley S, Spooner DM (2009) Do potatoes and tomatoes have a single evolutionary history, and what proportion of the genome supports this history? BMC Evol Biol 9:191. https://doi.org/10.1186/1471-2148-9-191
Rodríguez F, Ghislain M, Clausen AM, Jansky SH, Spooner DM (2010) Hybrid origins of cultivated potatoes. Theor Appl Genet 121:1187–1198
Rodríguez F, Núñez J, Vowinkel A, Sanseverino W, Simon R, Spooner DM, Bonierbale M (2017) Insights on the origin of cultivated potatoes. Poster presented at The Plant and Animal Genome XXV Conference (PAG), San Diego. 14–18 January 2017
Romans A (2005) The potato book. Frances Lincoln, London
Ross H (1986) Potato breeding, problems and perspectives. Advances in Plant Breeding, Suppl. 13. Berlin and Hamberg: Paul Parey
Rumold CU, Aldenderfer MS (2016) Late archaic–early formative period microbotanical evidence for potato at Jiskairumoko in the Titicaca Basin of southern Peru. Proc Natl Acad Sci USA 13(48):13672–13677
Rybin VA (1929) Karyological investigation on some wild growing and indigenous cultivated potatoes of America. Trudy Po Prikladnoi Botanike, Genetike i Selektsii 20:655–720
Rybin VA (1933) Cytological investigation of the South American cultivated and wild potatoes, and its significance for plant breeding. Trudy po Prikladnoi Botanike, Genetike i Selektsii Ser. 2. Genet. Rast 2:3–100
Salaman RN (1937) The potato in its early home and its introduction into Europe. J Roy Hort Soc 62: p 61–67, 112–113, 156–162, 253–266
Salaman RN, Hawkes JG (1949) The character of the early European potato. Proc Linn Soc London 161:71–84
Salas A, Gaspar O, Rodríguez W, Vargas M, Centeno R, Tay D (2008). Guías para la regeneración de germoplasma: especies de papa silvestre. In: Dulloo ME, Thormann I, Jorge MA, Hanson J (eds) Crop specific regeneration guidelines [CD-ROM]. CGIAR System-wide Genetic Resource Programme (SGRP), Rome, Italy. 8 p. https://www.genebanks.org/resources/publications/potato-sp/UNCED, 1992. Convention on Biological Diversity, United Nations Conference on Enviromental and Development, UNCED, Genova
Santini M, Camadro EL, Marcellán ON, Erazzú LE (2000) Agronomic characterization of diploid hybrid families derived from crosses between haploids of the common potato and three wild Argentinean tuber-bearing species. Am J Potato Res 77:211–218
Särkinen T, Bohs L, Olmstead RG, Knapp (2013) A phylogenetic framework for evolutionary study of the Nightshades (Solanaceae): A Dated 1000-tip Tree BMC Evol Biol 13:214. https://doi.org/10.1186/1471-2148-13-214.
Simon R, Fuentes AF, Spooner DM (2011) Biogeographic implications of the striking discovery of a 4000 kilometer disjunct population of the wild potato Solanum morelliforme in South America. Syst Bot 36:1062–1067
Solanaceae Source website http://www.solanaceaesource.org
Schmiediche PE, Hawkes JG, Ochoa CM (1980) Breeding of the cultivated potato species Solanum × juzepczukii Buk. and S. × curtilobum Juz. et Buk. I. A study of the natural variation of S. × juzepczukii, S. × curtilobum and their wild progenitor S. acaule Bitt. Euphytica 29:685–704
Schmiediche PE, Hawkes JG, Ochoa CM (1982) The breeding of the cultivated potato species Solanum × juzepczukii and S. × curtilobum. II. The resynthesis of S. × juzepczukii and S. × curtilobum. Euphytica 31:395–707
Sotomayor D, Zorrilla C (2019) Identifying high priority areas for in situ conservation of wild relatives of potato. https://doi.org/10.6084/m9.figshare.7549976.v1
SPDA, CCTA, INIA (2015) Los cultivos de la sierra y el cambio climático, siete casos de la sierra centro y sur del Perú. Sociedad Peruana de Derecho Ambiental (SPDA)
Spooner DM, Sytsma KJ, Conti E (1991) Chloroplast DNA evidence for genome differentiation in wild potatoes (Solanum sect. Petota: Solanaceae). Amer J Bot 78:1354–1366
Spooner DM, Sytsma KJ (1992) Reexamination of series relationships of Mexican and Central American wild potatoes (Solanum sect. Petota): evidence from chloroplast DNA restriction site variation. Syst Bot 17:432–448
Spooner DM, Van den Berg RG (1992) An analysis of recent taxonomic concepts in wild potatoes (Solanum sect. Petota). Gen. Res. Crop Evol 39:23–37
Spooner DM, J. Anderson GJ, Jansen RJ, (1993) Chloroplast DNA evidence for the interrelationships of tomatoes, potatoes, and pepinos (Solanaceae). Am J Bot 80(6):676–688
Spooner DM, Castillo R (1997) Reexamination of series relationships of South American wild potatoes (Solanaceae: Solanum sect. Petota): evidence from chloroplast DNA restriction site variation. Am J Bot 84:671–685
Spooner DM, Hijmans RJ (2001) Potato systematics and germplasm collecting, 1989–2000. Am J Potato Res 78(237–268):395
Spooner DM, Van den Berg RG, Rodríguez A, Bamberg J, Hijmans RJ, Lara-Cabrera SI (2004) Wild potatoes (Solanum section Petota) of North and Central America. Syst Bot Monogr 68:1–209 + 9 plates
Spooner DM, Hetterscheid WLA (2005) Origins, evolution, and group classification of cultivated potatoes. In: Motley TJ, Zerega N, Cross H (eds) Darwin’s harvest: new approaches to the origins, evolution, and conservation of crops. Colombia University Press, New York, pp 285–307
Spooner DM, Nuñez J, Rodríguez F, Naik PS, Ghislain M (2005) Nuclear and chloroplast DNA reassessment of the origin of Indian potato varieties and its implications for the origin of the early European potato. Theor Appl Genet 110:1020–1026
Spooner DM, Salas A (2006) Structure, biosystematics, and genetic resources. In: Gopal J, Khurana SMP (eds) Handbook of potato production, improvement, and post-harvest management. Haworth's Press, Inc., Binghampton, New York
Spooner DM, Clausen AM, Peralta IE (2009). Taxonomic treatment of Solanum section Petota (wild potatoes). In: Zuloaga FO, Morrone O, Belgrano MJ (eds) Catálogo de plantas vasculares del Cono Sur (Argentina, Chile, Paraguay, Uruguay, y sur del Brasil). Monogr Syst Bot, Mo Bot Gard 107:3011–3053
Spooner DM, Van den Berg RG, Rodríguez A, Bamberg J, Hijmans RJ, Lara-Cabrera SI. (2004) Wild potatoes (Solanum section Petota) of North and Central America. Syst Bot Monogr 68, 209p + 9 plates
Spooner DM, McLean K, Ramsay G, Waugh R, Bryan GJ (2005) A single domestication for potato based on multilocus amplified fragment length polymorphism genotyping. Proc Natl Acad Sci USA 102:14694–14699
Spooner DM, Núñez J, Trujillo G, del Rosario Herrera M, Guzmán F, Ghislain M (2007) Extensive simple sequence repeat genotyping of potato landraces supports a major reevaluation of their gene pool structure and classification. Proc Natl Acad Sci USA 104:19398–19403
Spooner DM, Rodríguez F, Polgár Z, Ballard HE Jr, Jansky SH (2008) Genomic origins of potato polyploids: GBSSI gene sequencing data. The Plant Genome, a suppl. To Crop Sci 48:S27–S36
Spooner DM, Gavrilenko T, Jansky SH, Ovchinnikova A, Krylova E, Knapp S, Simon R (2010) Ecogeography of ploidy variation in cultivated potato (Solanum sect. Petota). Am J Bot 97:2049–2060
Spooner DM, Ghislain M, Simon R, Jansky SH, Gavrilenko T (2014) Systematics, diversity, genetics, and evolution of wild and cultivated potatoes. Bot Rev 80:283–383
Spooner DM, Alvarez N, Peralta IE, Clausen AM (2016) Taxonomy of wild potatoes and their relatives in southern South America (Solanum sects. Petota and Etuberosum). Syst Bot Monogr 100:1–240 + 10 plates
Spooner DM, Ruess H, Arbizu C, Rodríguez F, Solis-Lemus C (2018) Greatly reduced phylogenetic structure in the cultivated potato clade of Solanum section Petota. Am J Bot 105:60–70
Spooner DM, Shelley J, Rodríguez F, Simon R, Ames M, Fajardo D, Castillo R (2019) Taxonomy of Wild Potatoes in Northern South America (Solanum section Petota). Syst Bot Monogr 108:305p + 5 plates
Stout AB, Chandler C (1941) Change from self-incompatibility to self-compatibility accompanying change from diploidy to tetraploidy. Science 94:118
Stelly D, Peloquin SJ (1986) Formation of 2n megagametophytes in diploid tuber-bearing Solanums. Am J Bot 73:1351–1363
Stelly D, Peloquin SJ, Palmer R, Crane C (1984) Mayer’s hemalum-methy salicylate: a stain-clearing technique for observations within whole ovules. Stain Technol 59:155–161
Tepe EJ, Anderson GJ, Spooner DM, Bohs L (2016) Relationships among wild relatives of tomato, potato, and pepino. Taxon 65:262–276
Tucci M, Carputo D, Bile G, Frusciante L (1996) Male fertility and freezing tolerance of hybrids involving Solanum tuberosum haploids and diploid Solanum species. Potato Res 39:345–353
Ugent D (1968) The potato in Mexico: geography and primitive culture. Econ Bot 22:109–123
Ugent D (1970) The potato: what is the origin of this important crop plant, and how did it first become domesticated? Science 170:1161–1166
Ugent D, Pozorski S, Pozorski T (1982) Archaeological potato tuber remains from the Casma Valley of Peru. Econ Bot 36:182–192
Ugent D, Dillehay T, Ramirez C (1987) Potato remains from a late Pleistocene settlement in southcentral Chile. Econ Bot 41:17–27
UNCED (1992) Convention on Biological Diversity, United Nations Conference on Enviromental and Development, UNCED, Genova
Van den Berg RG, Miller JT, Ugarte ML, Kardolus JP, Villand J, Nienhuis J, Spooner DM (1998) Collapse of morphological species in the wild potato Solanum brevicaule complex (sect. Petota). Am J Bot 85:92–109
Vavilov NI (1922) The law of homologous series in variation. J Genet 12:47–90
Vavilov NI (1940) The new systematics of cultivated plants. In: Huxley J (ed) The new systematics. Clarendon Press, Oxford, pp 549–566
Vega SE, Bamberg JB (1995) Screening the US potato collection for frost hardiness. Amer. Potato J. 72:13–21
Veilleux R (1985) Diploid and polyploid gametes in crop plants: mechanisms of formation and utilization in plant breeding. Plant Breed Rev 3:253–288
Vollmer R, Villagaray R, Egusquiza V, Espirilla J, Garcia M, Torres A, Ellis D (2016) The potato cryobank at the International Potato Center (CIP): a model for long term conservation of clonal plant genetic resources collections of the future. Cryo Letters 37(5):318–329
Vollmer R, Villagaray R, Cárdenas J, Castro M, Chávez O, Anglin N L, Ellis D (2017). A large-scale viability assessment of the potato cryobank at the International Potato Center (CIP). In vitro Cell Dev Biol-Plant 53(4):309-317
Vollmer R, Villagaray R, Castro M, Anglin NL, Ellis D (2019) Cryopreserved potato shoot tips showed genotype-specific response to sucrose concentration in rewarming solution (RS). Plant Cell Tis Org Cult (PCTOC), pp 1–11
Wang MR, Lambardi M, Engelmann F, Pathirana R, Panis B, Volk GM, Wang QC (2020) Advances in cryopreservation of in vitro-derived propagules: technologies and explant sources. Plant Cell Tiss Org Cult, pp 1–14. https://doi.org/10.1007/s11240-020-01770-0
Watanabe K (2015) Potato genetics, genomics, and application. Breed Sci 65:53–68. https://doi.org/10.1270/jsbbs.65.53
Werner J, Peloquin SJ (1991) Occurrence and mechanisms of 2n egg formation in 2x potato. Genome 4:975–982
Whalen MD (1984) Conspectus of species groups in Solanum subgenus Leptostemonum. Gentes Herb 12:179–282
Zhang L, Rodriguez F (2015) The potato. In: Beaudri MC (ed) Metheny KB. An Encyclopedia. Rowman & Little field Publishers, The Archaeology of Food, pp 415–418
Zorrilla C, Sotomayor D, Gómez R, Salas A, Vergara P, Ellis D (2019a) Ensuring the long-term conservation of wild relatives of potato in Peru. https://doi.org/10.6084/m9.figshare.7549922.v1
Zorrilla C, Salas A, Roca W, Tay D (2019b) Changes in the genetic structure of seed populations in six South American wild potato species as a consequence of seed multiplication at CIP Genebank. https://doi.org/10.6084/m9.figshare.7844714.v1
Acknowledgements
This work is dedicated to David Spooner, botanist, indefatigable explorer and passionate taxonomist who led research using new methods and analytical approaches to elucidate the phylogeny and diversity of crop species and wild relatives, fundamentally potatoes, and inspire people and students with his insights.
The authors acknowledge Dr. Claudio Galmarini, for his critical revision of the present chapter. Iris Edith Peralta research supported by CONICET and SIIP UNCuyo projects.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Peralta, I.E., Clausen, A.M., Zorrilla, C., Ames, M., Digilio, A., Rodriguez, F. (2021). Wild and Cultivated Potato Species Diversity, Taxonomy, and Conservation. In: Carputo, D., Aversano, R., Ercolano, M.R. (eds) The Wild Solanums Genomes. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-030-30343-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-30343-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30342-6
Online ISBN: 978-3-030-30343-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)