Abstract
Chemokine receptors, a family of G-protein-coupled receptors (GPCRs), bind in a specific manner to chemokines and elicit cellular responses. Their involvement in inflammatory diseases is predominant. Although the main function of chemokine receptors is enrolment of leukocytes at the site of inflammation, they are also widely explored as drug discovery targets. This is due to the fact that blockage of chemokine receptor may provide novel therapeutic interventions. This chapter discusses the various chemokine receptors, involvement of chemokine receptors in the pathogenesis of various diseases, and receptor-mediated strategies to tackle such afflictions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chemokines
- G protein-coupled receptor
- Receptor-mediated targeting
- Signaling
- Ligands
- Cytokines
- Transmembrane
1 Introduction
Chemokines are secretory and transmembrane proteins which are structurally related, and whose main functions are to recruit leukocyte populations employing specific receptors into the target. Based on their function chemokines can be classified into two major classes, inflammatory chemokines and immune chemokines. Inflammatory chemokines attract and activate monocytes and neutrophils, thereby playing a major role in inflammatory conditions that are acute [1]. Promiscuity of the receptor and significant redundancy of ligands, which is their major characteristic, facilitates recruitment of inflammatory cells in severe conditions. The immune chemokines play a significantly different role by attracting dendritic and lymphoid cells reflecting their involvement in immune reactions and inflammatory diseases which are chronic. This suggests their great promise and involvement in the therapy of immunological and inflammatory diseases [2]. The receptor has also been studied for therapeutic applications through targeted drug delivery strategies. This chapter discusses chemokine receptors, importance and targeted drug delivery strategies, and their potential clinical applications.
2 The Chemokine Receptors
Chemokines are small signaling peptide molecules with molecular weight 8–10 kDa secreted by the cells of the immune system in the presence of endogenous stimuli like IFN, IL-1, and tumor necrosis factor (TNF) and external stimulus like bacterial lipopolysaccharide (LPS) or viruses [3]. These chemokines cause migration of immune cells and help in recruiting them to the site of action through a variety of mechanisms. The process by which activated immune cells move toward the site of infection is called chemotaxis, that is, the movement of cells in response to chemical stimuli. Chemokines are therefore chemotactic cytokines comprising of >50 small secretory proteins that exhibit their effect on target tissues by interacting with seven heterotrimeric transmembrane (7TM) spanning GPCR family [4]. Chemokine receptors present on the surface of immune cells that interact with chemokine, a type of cytokine, are also known as cytokine receptors [5]. They belong to the large family of GPCR which includes receptors for inflammatory mediators, neurotransmitters, paracrine substances, odorant molecules, calcium ions, proteinases, hormones, and even photons [6].
Immune cells such as leukocytes primarily express chemokine receptors. Chemokines bind to GPCR giving rise to responses such as change in conformation in the receptor, triggering intracellular events that drive cell polarization, migration, and adhesion. This results in the induction of homing and leukocyte trafficking [7]. There are two major classes of chemokine receptors, namely, (CC) containing adjacent cysteines and (CXC) containing cysteines separated by amino acid. The CC receptors bind to CC chemokines and are named CCR1 to 9, and the CXC chemokines selectively bind to the CXC receptors termed CXCR1 to CXCR5. The Duffy antigen chemokine receptor (DARC) is known to bind both CXC and CC chemokine receptors as illustrated in Table 9.1 [3, 8,9,10]. Inflammatory mediators such as basophils, eosinophils, macrophage, and dendritic cells primarily express CC chemokine receptors, neutrophils mainly express CXC chemokine receptors, whereas lymphocytes express both CC and CXC types of cytokines receptors. Another study has shown that chemokine receptors that have been differently expressed cause nonimmunogenic antigens to become immunogenic. This “differently expressed” receptor is in fact the fusion of chemokine with antigen-presenting cells [11]. Chemokine targeting is now seen as a novel target for the treatment of atherosclerosis. This may be possible due to interference with disease progression at a particular stage of disease [12].
2.1 CXCR1 and CXCR2
CXCR1 receptor contains 350 amino acids, N-terminal domain-containing N-linked glycosylation site and all other features similar to that of a 7TM GPCR. IL-8, a CXC chemokine shows high affinity toward CXCR1 and CXCR2 [13]. CXCR1 binds to neutrophil-activating peptide 2 (NAP-2), granulocyte chemotactic protein 2 (GCP-2), and epithelial neutrophil-activating peptide 78 (ENA-78) with low affinity, while binding to IL-8 is highly selective. Growth-related oncogene family are the additional chemokines which bind to CXCR2 [14]. IL-8, a potent chemoattractant, displays its role in acute inflammation by activating CXCR2 and CXCR1 expressed on the surface of neutrophils [15]. Literature reports in vitro production of IL-8 by various cells like keratinocytes, fibroblasts, mast cells, neutrophils, monocytes, endothelial cells, and macrophages [16, 17]. CXCR1 and CXCR2 are expressed in all cell types including CD56+ NK cells, some CD8+ T cells, mast cells, monocytes, and granulocytes. Neutrophils express equal amount of CXCR1 and CXCR2, while expression of CXCR2 is predominant in monocytes and positive lymphocytes compared to CXCR1. TNF and LPS are known to downregulate expression of both CXCR1 and CXCR2 upon activation of tyrosine kinase-dependent signaling pathway. On the contrary, CXCR1 and CXCR2 expression is upregulated by bacterial-derived molecule fmlp and granulocyte colony-stimulating factor [18, 19].
2.2 CXCR3
CXCR3, a member of CXC chemokine receptor family whose primary role is in T-cell trafficking, is expressed majorly on effector T cells. It shares 41% similar amino acids to that of CXCR1 and CXCR2 receptors [20]. CXCR3 shows high expression of Th1-type CD4+ T cells, effector CD8+ T cells. Three interferon-inducible ligands activating CXCR3 are CXCL9 which is also referred as monokine induced by gamma-interferon or MIG, CXCL10 referred as interferon-induced protein of 10 kDa or IP-10 and I-TAC, that is, interferon-inducible T-cell alpha chemoattractant, or CXCL11. It is also worth noting that dendritic cells of plasma and subset of B cells show CXCR3 expression which could play a critical role in favoring their movement into inflamed lymph nodes [21].
2.3 CXCR4
The stromal-derived factor 1 (SDF-1) which is a CXC chemokine, attaches to CXCR4 and activates it leading to stimulation of cellular migration and polymerization of actin in a dose-dependent manner. It has been recently reported to be a vital HIV-1 coreceptor. SDF1α is a highly promising lymphocyte chemical attractant which also inhibits CD4+ permissive HIV-1 infection depending on CXCR4 expression pattern. A recent study suggests IL-4 causes overexpression of CXCR4 on resting T cells, while T-cell stimulation by CD28, CD3, and CD2 causes its downregulation [22, 23].
2.4 CXCR5
CXCR5 also known as Burkitt’s lymphoma receptor 1 (BLR1) is a 7TM domain G protein-coupled receptor which under normal conditions shows predominant expression by follicular helper T cells and mature B cells and controls their migration in the secondary lymphoid organs. CXC chemokine CXCL13 binds to CXCR5 and causes activation of multiple intracellular signaling pathways which regulates cellular functions like cell proliferation, survival, and migration [24].
2.5 XCR1
The receptor for lymphotactin is XCR1. This receptor is expressed strongly in placenta and weakly in spleen and thymus, which correlates with the expression of lymphotactin in these tissues.
2.6 CX3CR1
A CXC chemokine called as fractalkine is known to bind CX3CR1 chemokine receptor. Fractalkine, a glycoprotein attached to membrane with the chemokine, appears perched on an elongated mucin-like strand. CX3CR1 shows high-affinity binding with fractalkine [25]. In the bound form, fractalkine promotes adhesion of monocytes, T lymphocytes, natural killer cells to dendritic, endothelial, and epithelial cells [26].
2.7 CCR1
A cytomegalovirus protein, US28, binds to CCR1 receptor. CCR1 receptor is 33% homologous to the 7-transmembrane protein US28 [27]. Expression of CCR1 receptor causes increase in the F actin content, inhibition of cAMP formation, and basal migration of leukocytes. CCR1 receptor responds to binding of MIP-1a, MCP-2, RANTES (regulated on activation normal T-cell expressed and secreted), along with MCP-3 show specific binding toward HCC1 and CK8b. T-cell expression of chemokine receptor was induced by IL-2 and IL-15, whereas selective upregulation of CCR1 in human monocytes was facilitated by IL-10 [28].
2.8 CCR2A and CCR2B
Charo et al. produced two clones of CCR2 receptor, that is, CCR2A and CCR2B. Structurally CCR2A and CCR2B contain similar transmembrane and 58 untranslated regions but they differ in their C-terminus. A 36% similarity was observed between carboxy tail of CCR1 and CCR2B, whereas CCR2A bears no such similarity [29]. Lipopolysaccharide and IFN-γ have been reported to decrease expression of CCR2 receptors, whereas IL-2 causes increased expression. Expression of CCR2 in monocytes is enhanced by IL-10 [30].
2.9 CCR3
Eotaxin and eotaxin-2, two CC chemokines, represent the primary ligands for the 7TM GPCR CCR3 and bind them with greatest affinity hence, serve as predominant eosinophil activators which represent a variety of pathological conditions like hypereosinophilic syndrome asthma and urticaria [31, 32]. Thus, CCR3 plays a central role in controlling eosinophil migration. CCR3 has been reported in progressing M tropic HIV-1 infection of permissive cells in conjunction with CD4. CCR3 receptor shows 63% resemblance to CCR1 and 51% to CCR2B and is involved in binding of several CC chemokines. Chemokines which show specific binding toward CCR3 receptor includes MCP-4, RANTES, eotaxin, MCP-3, eotaxin-2, and MIP-5, which altogether are important in eosinophil recruitment and activation [33, 34].
2.10 CCR4
CCL17 or the thymus and activation-regulated chemokine and a macrophage-derived chemokine CCL22, both CC chemokine ligands bind specifically to CCR4. These two chemokine ligands are known to selectively activate CD4+ Th2 T lymphocyte. T-cell receptor and CD28 predominantly enhance expression of CCR4 on Th2 cells [35, 36].
2.11 CCR5
CCR5 shows affinity toward CC chemokines RANTES, MIP-1, MCP-2, MIP-1α, and MIP-1β [37]. CCR5 shows resemblance to CCR2B receptors with 71% identical amino acids. R5 strains of the human immunodeficiency virus (HIV-1 and -2) enter permissive cells aided by CCR5 as co-receptor in association with CD4. IL-10, an immunosuppressive and anti-inflammatory cytokine, causes upregulation of CCR5 in human monocytes by activation of MAP and STAT kinases [38, 39].
2.12 CCR6
CCR6 receptor shows selective affinity toward LARC (liver and activation-regulated chemokine) and macrophage inflammatory protein (MIP)-3α//Exolus-1/CCL20 [40]. B-lymphocytes, memory T cells, and dendritic cells show CCR6 expression, while peripheral blood leukocytes do not. IL-2 has been reported to cause upregulation of CCR6 mRNA [41]. However, contradictory results have been reported [42]; hence, the expression is not completely elucidated.
2.13 CCR7
CCR7 shows activation upon binding of a CC chemokine ligand CCL21. Dendritic cells and lymphocytes express CCR7 in lymph node, the site for medullary (MTC) and papillary thyroid carcinomas (PTC). T- and B-lymphocytes and dendritic cells show CCR7 expression. CCR7 is highly upregulated in herpesvirus-infected T cells and B cells infected with Epstein–Barr virus [43].
2.14 CCR8
I-309, a CC chemokine, is the only ligand which binds to CCR8. Monocytes and T lymphocytes are mainly activated by I-309 [44]. Type 2 T lymphocyte (Th2)-polarized cells show preferential expression of CCR8 receptor thus postulating that Th2 responses are mainly restricted to CCR8. CCR8 in association with CD4+ acts as a coreceptor for chemokine ligand I-309 of M tropic HIV-1 strains and is reported to be a binding and fusion inhibitor of HIV-1 [45].
2.15 CCR9
CCR9 is the recently identified chemokine receptor specific for the β chemokine thymus-expressed chemokine (TECK)/CCL25 [46]. Activation of dendritic cells and thymocytes by TECK indicates the role played by this CC chemokine in T-cell development [47]. Expression of CCR9 receptor is predominant in thymus, whereas lymph node and spleen show relatively less expression of this receptor [48].
2.16 D6
D6 receptor lacks intracellular signaling on binding of ligand and therefore it appears to be nonfunctional [49]. Monocyte chemoattractant protein (MCP-1 and -2) binds to D6 with high affinity, whereas some researchers reported the receptor to be promiscuous due to its ability to bind several chemokines with similar affinity [50].
2.17 Duffy Antigen Receptor for Chemokines (DARC)
DARC shows affinity toward both CC chemokine and CXC chemokine ligand MCP-1 and IL-8. Other ligands include MCP-3, MCP-4, GRO-α, RANTES, I-309, and eotaxin [51]. DARC is primarily involved in the pathogenesis of malaria. It shows predominant expression on erythrocytes but has also been detected on the capillary endothelium of kidney and spleen. CXCL8-binding proteins have properties similar to human erythrocyte blood group antigen known as duffy that facilitates entry of Plasmodium vivax to the malarial parasite. Duffy antigen displays promiscuity to CC and CXC chemokines. Based on these findings, duffy antigen DARC was renamed as atypical chemokine receptor 1 (ACKR1). Also, binding of chemokine to DARC caused inhibition of Plasmodium vivax infection [52].
3 Chemokine Receptor in Pathogenesis of Diseases
Chemokine receptors participate in the progression of various diseases either by causing overexpression of the receptors or by facilitating access of virus into the target tissues to develop infection. They take part in leucocyte trafficking, recirculation, and recruiting. The chemokine receptor redundancy allows specific receptor to bind several chemokines signaling through 7 TM GPCR. Infectious diseases like malaria and HIV have been shown to use chemokine receptors as entry receptors and coreceptors, respectively. Studies showing the genetics of these receptors and their significance in the pathogenesis of infectious diseases have been possible due to the genetic mutations in these receptor genes that encode the entry receptors for the two pathogens [53].
3.1 Chemokine Receptors as Virus Entry Mediators in HIV Infection
An interesting finding with respect to HIV infection is the fact that leukocyte recruitment and its regulation is central to the process [54].
HIV envelope proteins interact with CD4+ cell surface receptor to ensure efficient binding of virus. The conformational changes in the virus envelope are seen upon interaction with the CCR5 and CXCR4 coreceptors to produce fusion between the viral and cell membrane of the host [55]. Different coreceptors used by different HIV 1 isolates help understand their biological variability. Discovery of coreceptors has caused the change in the nomenclature of the HIV strain [56]. HIV virus strain using CXCR4 as coreceptor are named as X4, strains which involve CCR5 as coreceptor are named as R5, whereas strains which use both the receptors are named as X4R5 virus strains. In progressive stages of AIDS, large percentage of T cells express CXCR4 than CCR5 which allows more cells to be infected and destroyed [57].
Stromal cell-derived factor 1 (SDF-1) exhibits affinity for CXCR4, whereas RANTES, MIP-1 beta, and MIP-1 alpha which belong to β chemokine family are ligands for CCR5, MCP-5 promotes binding of (MCP-1) to CCR2. Eotaxin 1 and 2, MCP-4, and MCP-3 readily bind to CCR3. Thus, R5 strain of virus can be inhibited by ligands which bind CCR5. SDF-1 acts as a specific inhibitor of CXCR4. Hence, chemokine receptor expression in a specific cellular type can be coined as inducible or constitutive [58]. RANTES, MIP-1alpha, and MIP-1beta, the ligands of CCR5, are known to inhibit HIV-1 infection. In a particular study, a chemokine selective for eosinophil was isolated from allergen-challenged guinea pigs and was called “eotaxin.” This eotaxin has two crucial roles that it allows for recruitment of eosinophils at the local site and also enhances movement of eosinophils of the bone marrow [59].
Eotaxin’s receptor is CCR3. Eotaxin has been known to be a selective ligand for CCR3 but it can also interact with CCR2 and CCR5. Eotaxin is expressed on eosinophils, basophils, and Th2 lymphocytes. It has been understood from a study that eotaxin has antagonistic effect on CCR3. In addition, it has been shown eotaxin is a CCR2 antagonist [30]. A study indicated that eotaxin-3 has modulatory function and not inflammatory as suggested previously [60].
3.2 Malaria
Malaria, a severe parasitic infection of Plasmodium species, the most common being P. falciparum, is easily transmitted to humans hosts by the female anopheles mosquito. Species of the Plasmodium family that can cause malaria include P. falciparum, P. malariae, P. ovale, and P. vivax. Progression of the disease is mainly regulated by RANTES expression [61]. A study has shown that RANTES levels were upregulated at the peak of malarial infection in mouse. Hence, it was concluded that RANTES mediates inflammation that is a crucial part of malarial pathogenesis or in other words, the leukocyte recruitment helps in the pathogenesis of the disease. The leukocytes are recruited by chemokines and their receptors [62]. It was also shown that CCR1, CCR3, and CCR5 the receptors for RANTES which are expressed by macrophages, basophils, etc., also have a role in malaria upregulation.
In case of cerebral malaria (CM), the blood–brain barrier is affected causing the release of chemokines and variety of inflammatory cells inside the brain [63]. As mentioned before, CCR1, CCR3, and CCR5 have been implicated in the progression of malaria. According to a study, CCR2 does not play any role in the development of CM (caused by P. falciparum) [64].
Another way to look at the CM pathogenesis is to see the CD8+ T-cell proliferation and activation that causes cerebral damage. These CD8+ T cells are activated via chemokine receptors such as CCR5 and CXCR3. CCR5 that binds CCL3, CCL4, and CCL5 is revealed as a potent chemoattractant for CD8+ T cell. A study has shown that CCR5 deficiency reduces the likeliness of CM occurrence [65]. CXCR3 has also been given attention and in a study, mice lacking CXCR3 were seen less likely to be suffering from CM with fewer T cells implying that lesser chemokine production did not lead to recruitment of T cells. Interestingly, the same study concluded that mice with CM showed increased release of chemokines that bind to CXCR3 [66]. Furthermore, a study illustrates that in the mice with CM, NK cells aid in enhanced recruitment of CXCR3+ T cells [30]. CXCR3 knockouts were protected remarkably from CM, hence confirming the important role of CXCR3 in CM. Continuing on CXCR3, a study suggested that CXCR3 and its ligand CXCL9 recruit T cells [67].
An interesting finding by a study conducted in 1998 was interaction between HIV and malaria [68]. It was later shown that placentas of malaria-infected mothers often contain macrophages in a high concentration [69]. A study concluded that the coinfection state in pregnant Malawi women could be linked to CCR5 expression (in turn, the macrophages recruited by them) which led to higher malarial parasitemias as well as the fact that it was associated with HIV infection [70].
In yet another coinfection study of two Plasmodium species, it was found that the coinfection state led to reduced levels of chemokine receptor and its ligands leading to lesser accumulation of CD8+ T cells, and hence prevents CM in such mice. A study with children suffering from acute malaria showed that there is dysregulation of the CC chemokines that is MIP-1alpha and MIP-1beta levels were high, while RANTES level was low at the mRNA and protein level [71].
Looking at another major type of malaria, the placental malaria (PM), a study wherein the investigators tested the chemokine concentration in placental intervillous blood plasma of four different types, that is, {HIV+ PM+/PM−, HIV− PM+ PM−} found that MIP-1beta (belonging to chemokine CC subfamily) was higher in PM+ women than in HIV+ PM- and was not related to their HIV status. The MIP-1alpha levels were invariable though [72]. CCR5 being a coreceptor for macrophage-trophic HIV-1, it can be concluded that CCR5 upregulation can indeed be used in treating coinfection of HIV and PM. A study suggests that CCR3 and CCR5 both help/promote infection of HIV-1 in the central nervous system [73].
3.3 Atherosclerosis
There exists comprehensive literature on the role of chemokines in atherosclerosis (AS) and presently, several in vivo studies have suspected the involvement of several chemokine ligands and receptors in the process of AS [74]. AS is a disease that is known to cause high fatality worldwide. It was known that CCR5 had a role to play in AS but what effect it had was clearly assumed only after a study that stated that CCR5 expression is usually upregulated during AS plaques [75]. CCR1 and CCR5 have been known to bind to those cell types that are associated with AS. RANTES, CCL3, and CCL4 have been present in monocytes/macrophages/Th1 that have implications in the disease. Met-RANTES is an antagonist for RANTES which is a peptide receptor. This Met-RANTES has been studied in mice and has shown reduction in AS-related lesions. Earlier reports have been tentative, as reports have said that CCR5 deletion led to decline in the formation of atherosclerotic lesions. Another study stated that mice with CCR5 deletion enhanced the plaque quality but had no effect on its size. Thus, a study was performed to give a conclusion to the same and it was found that deficiency in CCR5 protected the cells against the formation of lesions and the accumulation of cells that was associated with the disease. It has been reported that genetic deletion of CX3CR1 and CCR2 decreases AS [76].
3.4 Miscellaneous
The chemokine receptor plays an important role in various other ailments. The same is summarized in Table 9.2 [28, 77,78,79,80,81,82,83].
4 Chemokine Receptor Structure
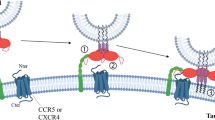
Chemokine receptors, a superfamily of GPCR, are known to bind chemokines in a specific manner and to elucidate a cellular response. They are membrane-bound molecules comprising of parallel strands of 7TM domain that couple with G proteins. Literature reports 18 human chemokine receptors till date. Due to their promiscuous nature, certain CXC chemokines show selective binding toward CXCR1 to CXCR5 receptors. In addition, nine receptors (CCR1 to CCR9) belong to CC chemokine receptor family. CX3CR1 and CXCR1-specific chemokine, Fractalkine has been identified. DARC binds indiscriminately to both CC and CXC chemokine [84]. A schematic representation of a chemokine receptor is illustrated in Fig. 9.1.
4.1 Structural Requirements for Chemokine Receptor Binding
4.1.1 Cysteine
The presence of cysteine residue on the extracellular loop is essential for proper alignment of the receptor on the cell membrane to ensure receptor signaling [85]. Out of the four conserved cysteine residues, one is present on the N-loop and other three on each of the three extracellular loops. Formation of disulfide bond on loop 1 and 2 is a prerequisite to elicit a cellular response. Formation of disulfide bond between N-loop and extracellular loops of CCR6 receptor is absent. However, in CCR5, all four cysteines are essential for functioning of chemokine receptor [86].
4.1.2 Sulfated Tyrosine
The HIV progression is initiated by the presence of sulfated tyrosine in the N-terminal loop. Posttranslational sulfation of tyrosine in Golgi apparatus affects ligand-binding affinity of chemokine receptors. CCR5 receptor exists in two forms, namely, sulfated and nonsulfated CCR5 based on the presence and absence of sulfated tyrosine. Interaction of N-loop of CCR5 containing sulfated tyrosine with the HIV envelope protein gp120 facilitates the progression of HIV infection, whereas entry of HIV virus is restricted in nonsulfated N-loop due to the absence of interaction with CD4 complexes/gp120 thus causing inhibition of binding and fusion [87].
4.2 Chemokine Receptor Activation and Signaling
Chemokine receptors are stimulated by several ligands, demonstrating that activation is not necessarily due to similar modes of ligand binding but due to similar molecular mechanisms. Though some chemokine receptors show monogamous binding to their ligand, majority of them show promiscuous binding which is however restricted to the same chemokine class [88].
Upon binding of chemokine ligand to the chemokine receptor conformational changes occur in the 7TM domain of the receptor, thereby triggering downstream processes by heterotrimeric (αβγ) G proteins bound to the intracellular loops which in turn leads to activation of intracellular signaling [89, 90]. In an inactive state, G alpha subunit is attached to guanosine diphosphate (GDP) which contains a GTPase domain which promotes hydrolysis and binding of guanosine triphosphate (GTP). Exchange of GDP for GTP takes place which ultimately causes cleavage of the G alpha subunit from the βƳ subunit of the heterotrimer upon activation of receptor by the ligand. The G alpha subunit then interacts with the Gβγ subunit in heterodimer. The dimer can act as an inhibitor for Gα because it facilitates interaction between Gα and GDP. Gα subunit then dissociates from Gα-GTP and Gβγ heterodimer complex of which the latter participates in the signaling cascade [2, 91,92,93]. G alpha subunits are of four types depending on their sequence and function. Phospholipase C (PLC) is stimulated by Gαq to facilitate intracellular Ca+ mobilization, membrane-associated enzyme phospholipase C2 (PLC2) is activated by the Gβγ heterodimer which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) resulting in the formation of two products intracellularly, namely, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). Calcium from intracellular stores is triggered by IP3, while DAG can also activate protein kinase C (PKC) isoforms among other targets [94]. PKC stimulation eventually leads to physiological response. There are extensive reports suggesting downstream signaling of low molecular weight Rho and Ras proteins, tyrosine kinase, phosphatidylinositol 3-kinase, phospholipase A2, and the MAP kinase pathway. The signaling event involves phosphorylation of amino acid residues of threonine and serine in the carboxy loop of the receptor by GPCR kinases sequestration of receptor by internalization [95,96,97]. The nature of the ligand dictates the conformation changes which are likely to occur on the exterior of the receptor. As the chemokine ligands are large molecules, the shift in 7TM domain which occurs due to binding of ligand is different from β2 adrenergic receptor, another GPCR which binds small agonists or rhodopsin. Small molecules like rhodopsin lack proline residues which constitute the 7TM domain of chemokine receptors. Proline produces kinks and bends in 7TM helix which affects its folding and orientation of intra- and extracellular loop in chemokine receptors. Researchers postulated that CCR2 and CCR5 binding is determined by the presence of the motif threonine-x-proline (TxP) in TM2 [98,99,100].
Binding of IL-8, the agonist for chemokine receptor CXCR1/CXCR2 to its receptor, results in stimulation of the receptor and causes GDP/GTP exchange which further hydrolyzes the G alpha protein subunit from the heterodimer (GβƳ). Phospholipase D is activated giving rise to the signaling cascade, namely, MAP kinase pathway and phosphorylation of various amino acid residues like serine/threonine on the carboxy-terminal of the receptor through secretion of various secondary messengers like DAG and IP3 which trigger the intracellular calcium pool. A series of events which follow intracellular calcium mobility include movement of chemotactic cytokines, release of inflammatory granules and free radicals, and finally modification of avidity of cell adhesion molecules like integrin [101, 102].
5 Ligand Binding
Several mutagenesis studies have demonstrated the ligand-binding region of chemokine receptor. The studies revealed that N terminals were important especially for certain receptors, for example, CCR2, CCR3, CCR5, and CXCR1 [84]. Further receptors bind to specific chemokines which could be considered as their ligand partners (CCR-CC, CXCR-CXC). In contrast, DARC exhibits high affinity for inflammatory chemokines of different subfamilies. Chemokine receptors involved in leukocyte migration exhibit high promiscuity. One such receptor is CCR3 which can bind to 10 different chemokines. Furthermore, such binding elicits varied cellular responses which can be very complex [85, 103]. For instance, internalization is triggered when CCL19 binds to CCR7, while the same is not observed when CCL21 binds to CCR7 [13]. In a similar manner, interaction of CCR4 with CCL22 induces internalization, while the same is not observed with CCL17. On the other hand, chemokine receptors which play a role in homeostatic function bind to single chemokines as illustrated by CCR9 which binds only to CCL25, while CXCR5 exhibits binding to CXCL13 [15]. Various ligands for chemokine receptors are depicted in Table 9.1.
Similarly, CXCR1 exhibits specific affinity for CXCL8, CXCR2 is less selective and can bind also to many CXC. More involved studies have demonstrated the role of specific regions of the receptor in binding and have associated the first extracellular loop in ligand binding by CXCR2, and have demonstrated related differences in the affinity of ligands to CXCR2 by eliciting their mechanisms. This has been substantiated by other studies. A multisite binding model has therefore been proposed for CCR1 and CCR3 [22]. Further, in case of CCR1, the role of second extracellular loop in ligand binding has been established using the cross-linkable macrophage inflammatory protein (MIP)-1α. Similarly for CCR2, the relevant site for ligand binding is the amino terminus [104].
Promiscuous chemokine ligand–receptor relationships are common. As a result, defining the chemokine receptor responsible for stimulus–response coupling in primary cells is often not straightforward due to overlapping specificities of receptors for ligands and leukocytes, and a paucity of receptor subtype-selective blocking agents. Although anti-receptor monoclonal antibodies and mice with targeted gene disruptions are now being used to resolve ligand-binding specificities in vivo, problems of interpretation persist due to the inequality of chemokine and chemokine receptor repertoires, tissue distribution, and biological usage among species [8].
6 Antagonists for Ligand Binding
A plethora of diseases have been identified which has postulated the role of chemokine receptors in their progression. Though chemokines have been evolved as regulators of immune response, their improper exploitation has contributed toward many afflictions. This has led to the discovery of a wide array of antagonists. Specific chemokine receptors like CXCR4 and CCR5 on cell surface of CD4+ T cells bind with HIV-1 envelope proteins, act as entry portals for HIV-1 virus which has raised significant interest by pharmaceutical companies to develop small molecules and antibodies against chemokine receptors [105, 106]. Numerous studies report molecules that potentially inhibit the interaction between gp120 proteins and chemokine receptors, thus preventing internalization. Amino oxypentane (AOP) RANTES an isoform of RANTES which act as a potential inhibitor for the eradication of HIV virus is been identified. In the same context, a series of CCR5 inhibitors have been developed for the treatment of HIV [107,108,109].
Though a number of biologics and GAG-based therapeutics which possess significant potential in inhibition of chemokine receptor function already exist, research efforts have also aimed toward the discovery of low molecular weight therapeutics with ability to act as chemokine receptor antagonists. CXCR4 and CCR5 have been identified as potential targets due to their roles in HIV entry [110].
A sequence of events follows the entry of human immunodeficiency virus (HIV-1) into the host.
Viral replication cycle starts with the formation of gp120/CD4+ complexes following the interaction between viral envelope protein and host cell membrane. The complex activates the chemokine receptors by undergoing conformational modifications, followed by a series of events which cause fusion between host and viral cell membranes [106]. T-tropic strains of HIV gain entry into the host predominantly through CXCR4, whereas CCR5 is essential for invasion of M-tropic HIV [68, 111, 112]. D6 receptor which lacks intracellular signaling is also reported to play a role [113]. This has led to the development of a number of CXCR4/CCR5 antagonists which have entered clinical trials and are depicted under the Sect. 8 on clinical studies later in this chapter.
7 Receptor-Mediated Targeting Strategies
Nanocarriers have been the major vehicles for receptor-mediated targeting due to the manifold advantages they offer. While they can enable passive targeting which is influenced by their physicochemical properties, for example, size, shape, surface charge, and hydrophobicity, attachment of ligands which can recognize cell membrane components can facilitate active cellular targeting. Using ligands for chemokine receptors can enable such active targeting via receptor-mediated endocytosis. However, despite major studies in the development of new drugs as chemokine agonists and antagonists, exploitation of chemokine receptor-based targeted drug delivery based on nanocarrier strategies is limited.
7.1 Liposomes
Immunoliposomes loaded with siRNA have been successfully employed for systemic targeting of LFA-1 (lymphocyte function-associated antigen-1) integrin present exclusively on leukocytes and other immune cells that act as mediators of HIV-1 infection [114].
An anti-HIV liposomal composition containing cardiolipin as phospholipid exhibited anti-HIV activity by inhibiting the binding and fusion of gp 120 with cell surface receptors CCR5 and CXCR4 of host cells [115]. Non-phospholipidic cationic liposomes containing free fatty acids, their monoesters and cholesterol, namely, Novasomes® 7474 loaded with a combination of 2 RANTES (a CCR5-specific inhibitor) and fusion inhibitor sifuvirtide enabled downregulation of CCR5 [116, 117].
7.2 Nanoparticles
Nanoparticles containing a specific siRNA sequence showed a marked reduction in the expression of CD4+ and CCR5 in explants of HIV-1 negative female, which was evident by decrease in the biomarker CD45. This proposed the ability of the nanoparticles to cause suppression of receptor-specific genes [118]. Intravenous infusion of nanoparticles encapsulated with CCR5-specific siRNA complexed with CD4+ T-cell-specific antibody downregulated expression of CCR5 receptors in preclinical mice model confirming their anti-HIV potential [119].
PLGA nanoparticles incorporating PSC-RANTES (amino terminus-modified synthetic analog of RANTES) showed greater mucosal penetration of the protein and improved activity as HIV-1 entry inhibitors in rhesus macaque model compared to their non-polymeric counterparts [120, 121]. PEG-stabilized gold nanoparticles are reported to be effective HIV-1 fusion inhibitors causing inhibition of binding of HIV envelope protein gp120 and CD4+ T cell. The gold nanoparticles showed efficient inhibition against X4, R5, and X4R5 virus strains of HIV-1 infection [122].
Gold nanoparticles coated with multiple sulfate-modified amphiphilic ligand showed great promise as HIV-1 inhibitor by competitive inhibition of gp 120 glycoproteins on virus and inhibit binding of the virus to dendritic cells and subsequent transfer to T lymphocytes (CCR8), thereby preventing viral replication [123]. Anchoring neutralizing antibodies (NABs) on silver nanoparticles induced the ability to neutralize HIV-1, not observed when NAB alone was used [124].
7.3 Miscellaneous
Atherosclerosis, an inflammatory process is strongly influenced by chemokine/chemokine receptor-like CCR2 which is majorly involved in leukocyte recruitment to the atherosclerotic plaque. Dextran nanoparticles encapsulating siRNA were employed to silence mRNA, thus downregulating CCR2 expression. Dextran nanoparticles labeled with Zr89 also served as a targeted theranostic. When injected in ApoE knockout mice, the nanoparticles showed reduced PET/MRI signal used to spot macrophage in the atherosclerosis-inflamed tissue, suggesting the role of dextran nanoparticles in targeting atherosclerotic plaque [125].
8 Clinical Studies
CXCR4 and CCR5 being the main hallmark for progression of HIV-1 infection have prompted several pharmaceutical players to rapidly develop potent inhibitors against these receptors which could block the selective pathway. Selective inhibition of receptors has given rise to drug-resistant strains of HIV due to the peculiarity of the virus to switch tropism. Hence, current research efforts are aimed to develop antagonists against dual tropic strains of virus. Among the specific inhibitors of HIV-1 infection, CCR5 antagonist has gained much of interest as HIV-1 inhibitor, though it plays a role in the progression of autoimmune diseases like type 1 diabetes and multiple sclerosis. Clinical studies using CCR5/CXCR4 antagonists are depicted in Table 9.3. The role of chemokines in other non-infectious diseases has led their evaluation for other applications. Clinical trials for such applications are recorded in Table 9.4.
9 Conclusion and Future Prospects
Chemokine receptors play a multifaceted role in the progression of infectious diseases like HIV, malaria, atherosclerosis, and multiple sclerosis. A number of antagonists are developed and are under clinical trials. Nevertheless, synergizing such developments with targeted drug delivery strategies using nanocarriers could play a major role in harnessing chemokine receptor-based targeting for improved therapeutic outcomes.
Abbreviations
- ACKR:
-
Atypical chemokine receptor
- AIDS:
-
Acquired immune deficiency syndrome
- AS:
-
Atherosclerosis
- BLR1:
-
Burkitt’s lymphoma receptor 1
- cAMP:
-
Cyclic adenosine monophosphate
- CD4:
-
Cluster of differentiation 4
- CHO:
-
Chinese hamster ovary
- CM:
-
Cerebral malaria
- CNS:
-
Central nervous system
- COPD:
-
Chronic obstructive pulmonary disease
- DAG:
-
Diacylglycerol
- DARC:
-
Duffy antigen receptor for chemokines
- EBV:
-
Epstein–Barr virus
- ELC:
-
EBI1 ligand chemokine
- GCP:
-
Granulocyte chemotactic Protein
- GDP:
-
Guanosine diphosphate
- GPCR:
-
G-protein-coupled receptors
- GTP:
-
Guanosine triphosphate
- GvHD:
-
Graft versus host disease
- HCMV:
-
Human cytomegalovirus
- HIV:
-
Human immunodeficiency virus
- HIVE:
-
HIV encephalitis
- HSV:
-
Herpes simplex virus
- IBD:
-
Inflammatory bowel disease
- IC 50:
-
inhibitory concentration 50
- IFN:
-
Interferon
- IL-1/IL-8:
-
Interleukin
- I-TAC:
-
interferon-inducible T-cell alpha chemoattractant
- LPS:
-
Lipopolysaccharide
- MCP:
-
Monocyte chemotactic/chemoattractant protein
- MIG:
-
monokine induced by gamma interferon
- MIP1α/1β:
-
Macrophage inflammatory protein
- mRNA:
-
Messenger ribonucleic acid
- MTC:
-
Medullary thyroid carcinomas
- NAP:
-
Neutrophil-activating peptide
- NK:
-
Natural killer
- PIP2:
-
Phosphatidylinositol 4, 5-biphosphate
- PLC:
-
Phospholipase C
- PLGA:
-
Polylactic-co-glycolic acid
- PM:
-
Placental malaria
- PTC:
-
Papillary thyroid carcinomas
- RA:
-
Rheumatoid arthritis
- RANTES:
-
Regulated on activation normal T-cell expressed and secreted
- RR-MS:
-
Relapsing–remitting multiple sclerosis
- SDF:
-
Stromal-derived Factor
- SDP:
-
Spirodiketopiperzine
- SLC:
-
Secondary lymphoid tissue chemokine
- TECK:
-
Thymus-expressed chemokine
- Th2:
-
T lymphocytes
- TxP:
-
Threonine x proline
- WNV:
-
West Nile virus
References
Solari R, Pease JE. Targeting chemokine receptors in disease – a case study of CCR4. Eur J Pharmacol. 2015;763:169–77.
Murphy PM. Chemokine receptors: structure, function and role in microbial pathogenesis. Cytokine Growth Factor Rev. 1996;7(1):47–64.
Mahalingam S, Karupiah G. Chemokines and chemokine receptors in infectious diseases. Immunol Cell Biol. 1999;77(6):469–75.
Gao J-L, Murphy PM. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J Biol Chem. 1994;269(46):28539–42.
Mukaida N. The roles of cytokine receptors in diseases. Rinsho Byori Jpn J Clin Pathol. 2000;48(5):409–15.
Moser B, Willimann K. Chemokines: role in inflammation and immune surveillance. Ann Rheum Dis. 2004;63:ii84–i9.
Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–16.
Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820.
Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1(2):95–104.
Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95(10):3032–43.
Biragyn A, Ruffini PA, Coscia M, Harvey LK, Neelapu SS, Baskar S, et al. Chemokine receptor-mediated delivery directs self-tumor antigen efficiently into the class II processing pathway in vitro and induces protective immunity in vivo. Blood. 2004;104(7):1961–9.
Koenen RR, Weber C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat Rev Drug Discov. 2010;9(2):141–53.
Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol. 1995;17(2):103–8.
Murphy PM, editor. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin Hematol. 1997;34(4):311–8.
Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) α, GROβ, GROγ, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271(34):20545–50.
Kaplanski G, Farnarier C, Kaplanski S, Porat R, Shapiro L, Bongrand P, et al. Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood. 1994;84(12):4242–8.
Hashimoto S, Yoda M, Yamada M, Yanai N, Kawashima T, Motoyoshi K. Macrophage colony-stimulating factor induces interleukin-8 production in human monocytes. Exp Hematol. 1996;24(2):123–8.
Khandaker MH, Mitchell G, Xu L, Andrews JD, Singh R, Leung H, et al. Metalloproteinases are involved in lipopolysaccharide–and tumor necrosis factor–mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood. 1999;93(7):2173–85.
Lloyd AR, Biragyn A, Johnston JA, Taub DD, Xu L, Michiel D, et al. Granulocyte-colony stimulating factor and lipopolysaccharide regulate the expression of interleukin 8 receptors on polymorphonuclear leukocytes. J Biol Chem. 1995;270(47):28188–92.
Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184(3):963–9.
Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–31.
Hesselgesser J, Liang M, Hoxie J, Greenberg M, Brass LF, Orsini MJ, et al. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J Immunol. 1998;160(2):877–83.
Jourdan P, Abbal C, Nora N, Hori T, Uchiyama T, Vendrell J-P, et al. Cutting edge: IL-4 induces functional cell-surface expression of CXCR4 on human T Cells1. J Immunol. 1998;160(9):4153–7.
Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–7.
Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Mol Interv. 2010;10(5):263–70.
Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91(4):521–30.
Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188(8):1413–9.
Chee MS, Satchwell SC, Preddie E, Weston KM, Barrell BG. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990;344(6268):774–7.
Bartoli C, Civatte M, Pellissier JF, Figarella-Branger D. CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol. 2001;102(4):385–92.
Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–21.
Elsner J, Petering H, Kluthe C, Kimmig D, Smolarski R, Ponath P, et al. Eotaxin-2 activates chemotaxis-related events and release of reactive oxygen species via pertussis toxin-sensitive G proteins in human eosinophils. Eur J Immunol. 1998;28(7):2152–8.
Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, et al. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85(7):1149–58.
Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183(5):2349–54.
Dairaghi DJ, Oldham ER, Bacon KB, Schall TJ. Chemokine receptor CCR3 function is highly dependent on local pH and ionic strength. J Biol Chem. 1997;272(45):28206–9.
Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272(23):15036–42.
D’Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, et al. Cutting edge: selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161(10):5111–5.
Ruffing N, Sullivan N, Sharmeen L, Sodroski J, Wu L. CCR5 has an expanded ligand-binding repertoire and is the primary receptor used by MCP-2 on activated T cells. Cell Immunol. 1998;189(2):160–8.
Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1β, and MIP-1α. J Biol Chem. 1996;271(29):17161–6.
Makuta Y, Sonoda Y, Yamamoto D, Funakoshi-Tago M, Aizu-Yokota E, Takebe Y, et al. Interleukin-10-induced CCR5 expression in macrophage like HL-60 cells: involvement of Erk1/2 and STAT-3. Biol Pharm Bull. 2003;26(8):1076–81.
Baba M, Imai T, Nishimura M, Kakizaki M, Takagi S, Hieshima K, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem. 1997;272(23):14893–8.
Greaves DR, Wang W, Dairaghi DJ, Dieu MC, Saint-Vis B, Franz-Bacon K, et al. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J Exp Med. 1997;186(6):837–44.
Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162(1):186–94.
Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, et al. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272(21):13803–9.
Endres MJ, Garlisi CG, Xiao H, Shan L, Hedrick JA. The Kaposi’s sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J Exp Med. 1999;189(12):1993–8.
Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, et al. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273(1):386–91.
Carramolino L, Zaballos A, Kremer L, Villares R, Martin P, Ardavin C, et al. Expression of CCR9 beta-chemokine receptor is modulated in thymocyte differentiation and is selectively maintained in CD8(+) T cells from secondary lymphoid organs. Blood. 2001;97(4):850–7.
Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, et al. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7(2):291–301.
Zaballos A, Gutierrez J, Varona R, Ardavin C, Marquez G. Cutting edge: identification of the orphan chemokine receptor GPR-9-6 as CCR9, the receptor for the chemokine TECK. J Immunol. 1999;162(10):5671–5.
Graham GJ. D6 and the atypical chemokine receptor family: novel regulators of immune and inflammatory processes. Eur J Immunol. 2009;39(2):342–51.
Nibbs RJ, Wylie SM, Yang J, Landau NR, Graham GJ. Cloning and characterization of a novel promiscuous human beta-chemokine receptor D6. J Biol Chem. 1997;272(51):32078–83.
Szabo MC, Soo KS, Zlotnik A, Schall TJ. Chemokine class differences in binding to the Duffy antigen-erythrocyte chemokine receptor. J Biol Chem. 1995;270(43):25348–51.
Horuk R. The Duffy antigen receptor for chemokines DARC/ACKR1. Front Immunol. 2015;6(279):1–3.
Suresh P, Wanchu A. Chemokines and chemokine receptors in HIV infection: role in pathogenesis and therapeutics. J Postgrad Med. 2006;52(3):210–7.
Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–5.
Stone MJ, Hayward JA, Huang C, EHuma Z, Sanchez J. Mechanisms of regulation of the chemokine-receptor network. Int J Mol Sci. 2017;18(2) https://doi.org/10.3390/ijms18020342.
Berger EA, Doms RW, Fenyo EM, Korber BT, Littman DR, Moore JP, et al. A new classification for HIV-1. Nature. 1998;391(6664):240.
Simmons G, Wilkinson D, Reeves JD, Dittmar MT, Beddows S, Weber J, et al. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70(12):8355–60.
Wu L, LaRosa G, Kassam N, Gordon CJ, Heath H, Ruffing N, et al. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186(8):1373–81.
Bartels J, Maune S, Meyer JE, Kulke R, Schluter C, Rowert J, et al. Increased eotaxin-mRNA expression in non-atopic and atopic nasal polyps: comparison to RANTES and MCP-3 expression. Rhinology. 1997;35(4):171–4.
von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103(13):1772–7.
Greenwood B. Malaria mortality and morbidity in Africa. Bull World Health Organ. 1999;77(8):617–8.
Dunst J, Kamena F, Matuschewski K. Cytokines and chemokines in cerebral malaria pathogenesis. Front Cell Infect Microbiol. 2017;7(324):1–16.
Belnoue E, Costa FT, Vigario AM, Voza T, Gonnet F, Landau I, et al. Chemokine receptor CCR2 is not essential for the development of experimental cerebral malaria. Infect Immun. 2003;71(6):3648–51.
Sarfo BY, Armah HB, Irune I, Adjei AA, Olver CS, Singh S, et al. Plasmodium yoelii 17XL infection up-regulates RANTES, CCR1, CCR3 and CCR5 expression, and induces ultrastructural changes in the cerebellum. Malar J. 2005;4(63):1–13.
Chaisavaneeyakorn S, Moore JM, Mirel L, Othoro C, Otieno J, Chaiyaroj SC, et al. Levels of macrophage inflammatory protein 1 alpha (MIP-1 alpha) and MIP-1 beta in intervillous blood plasma samples from women with placental malaria and human immunodeficiency virus infection. Clin Diagn Lab Immunol. 2003;10(4):631–6.
Chandramohan D, Greenwood BM. Is there an interaction between human immunodeficiency virus and Plasmodium falciparum? Int J Epidemiol. 1998;27(2):296–301.
Clark C, Phillips R. Cerebral malaria protection in mice by species-specific Plasmodium coinfection is associated with reduced CC chemokine levels in the brain. Parasite Immunol. 2011;33(11):637–41.
Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):872–7.
Hansen DS, Bernard NJ, Nie CQ, Schofield L. NK cells stimulate recruitment of CXCR3+ T cells to the brain during Plasmodium berghei-mediated cerebral malaria. J Immunol. 2007;178(9):5779–88.
Hochman S, Kim K. The impact of HIV coinfection on cerebral malaria pathogenesis. J Neuroparasitol. 2012;3:235547.
Ochiel DO, Awandare GA, Keller CC, Hittner JB, Kremsner PG, Weinberg JB, et al. Differential regulation of β-chemokines in children with Plasmodium falciparum malaria. Infect Immun. 2005;73(7):4190–7.
Pollina E, Chaluluka E, Carr R, Lucas S, Molyneaux M, Rogerson S. Monocytic infiltration of the placenta in malaria and its relation to HIV. Am J Trop Med Hyg. 1999;61:1–11.
Sarfo B, Singh S, Lillard J, Quarshie A, Gyasi R, Armah H, et al. The cerebral-malaria-associated expression of RANTES, CCR3 and CCR5 in post-mortem tissue samples. Ann Trop Med Parasitol. 2004;98(3):297–303.
Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94(2):253–61.
Boisvert WA, Curtiss LK, Terkeltaub RA. Interleukin-8 and its receptor CXCR2 in atherosclerosis. Immunol Res. 2000;21(2–3):129–37.
Reape TJ, Groot PH. Chemokines and atherosclerosis. Atherosclerosis. 1999;147(2):213–25.
Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357(9267):1513–8.
Sorensen LN, Paludan SR. Blocking CC chemokine receptor (CCR) 1 and CCR5 during herpes simplex virus type 2 infection in vivo impairs host defence and perturbs the cytokine response. Scand J Immunol. 2004;59(3):321–33.
Kopp SJ, Banisadr G, Glajch K, Maurer UE, Grunewald K, Miller RJ, et al. Infection of neurons and encephalitis after intracranial inoculation of herpes simplex virus requires the entry receptor nectin-1. Proc Natl Acad Sci U S A. 2009;106(42):17916–20.
Beisser PS, Laurent L, Virelizier J-L, Michelson S. Human Cytomegalovirus chemokine receptor gene US28 is transcribed in latently infected THP-1 monocytes. J Virol. 2001;75(13):5949–57.
Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12(9):1021–6.
Cheng W, Chen G. Chemokines and chemokine receptors in multiple sclerosis. Mediat Inflamm. 2014;2014:1–8.
Kaplan AP. Chemokines, chemokine receptors and allergy. Int Arch Allergy Immunol. 2001;124(4):423–31.
Kufareva I, Salanga CL, Handel TM. Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies. Immunol Cell Biol. 2015;93(4):372–83.
Blanpain C, Lee B, Vakili J, Doranz BJ, Govaerts C, Migeotte I, et al. Extracellular cysteines of CCR5 are required for chemokine binding, but dispensable for HIV-1 coreceptor activity. J Biol Chem. 1999;274(27):18902–8.
Lagane B, Ballet S, Planchenault T, Balabanian K, Le Poul E, Blanpain C, et al. Mutation of the DRY motif reveals different structural requirements for the CC chemokine receptor 5-mediated signaling and receptor endocytosis. Mol Pharmacol. 2005;67(6):1966–76.
Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, et al. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96(5):667–76.
Jensen PC, Rosenkilde MM. Activation mechanisms of chemokine receptors. Methods Enzymol. 2009;461:171–90.
Preininger AM, Hamm HE. G protein signaling: insights from new structures. Sci STKE. 2004;2004(218):re3.
Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, et al. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24(6):765–81.
Bokoch GM. Chemoattractant signaling and leukocyte activation. Blood. 1995;86(5):1649–60.
Wu D, LaRosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261(5117):101–3.
Kuang Y, Wu Y, Jiang H, Wu D. Selective G protein coupling by CC chemokine receptors. J Biol Chem. 1996;271(8):3975–8.
Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25(8):413–22.
Turner SJ, Domin J, Waterfield MD, Ward SG, Westwick J. The CC chemokine monocyte chemotactic peptide-1 activates both the class I p85/p110 phosphatidylinositol 3-kinase and the class II PI3K-C2α. J Biol Chem. 1998;273(40):25987–95.
Huang R, Lian JP, Robinson D, Badwey JA. Neutrophils stimulated with a variety of chemoattractants exhibit rapid activation of p21-activated kinases (Paks): separate signals are required for activation and inactivation of paks. Mol Cell Biol. 1998;18(12):7130–8.
Mellado M, Rodriguez-Frade J, Aragay A, Del Real G, Martin A, Vila-Coro A, et al. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol. 1998;161(2):805–13.
Gether U, Lin S, Ghanouni P, Ballesteros JA, Weinstein H, Kobilka BK. Agonists induce conformational changes in transmembrane domains III and VI of the β2 adrenoceptor. EMBO J. 1997;16(22):6737–47.
Arias DA, Navenot J-M, Zhang W-B, Broach J, Peiper SC. Constitutive activation of CCR5 and CCR2 induced by conformational changes in the conserved TXP motif in transmembrane helix 2. J Biol Chem. 2003;278(38):36513–21.
Govaerts C, Blanpain C, Deupi X, Ballet S, Ballesteros JA, Wodak SJ, et al. The TXP motif in the second transmembrane helix of CCR5. A structural determinant of chemokine-induced activation. J Biol Chem. 2001;276(16):13217–25.
Kamp M, Liu Y, Kortholt A. Function and regulation of heterotrimeric G proteins during chemotaxis. Int J Mol Sci. 2016;17(1):90.
Karnik SS, Gogonea C, Patil S, Saad Y, Takezako T. Activation of G-protein-coupled receptors: a common molecular mechanism. Trends Endocrinol Metab. 2003;14(9):431–7.
Baldwin JM. Structure and function of receptors coupled to G proteins. Curr Opin Cell Biol. 1994;6(2):180–90.
Pease JE, Wang J, Ponath PD, Murphy PM. The N-terminal extracellular segments of the chemokine receptors CCR1 and CCR3 are determinants for MIP-1α and eotaxin binding, respectively, but a second domain is essential for efficient receptor activation. J Biol Chem. 1998;273(32):19972–6.
Wells TN, Power CA, Shaw JP, Proudfoot AE. Chemokine blockers–therapeutics in the making? Trends Pharmacol Sci. 2006;27(1):41–7.
Olson WC, Rabut GE, Nagashima KA, Tran DN, Anselma DJ, Monard SP, et al. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J Virol. 1999;73(5):4145–55.
Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–5.
Simmons G, Clapham PR, Picard L, Offord RE, Rosenkilde MM, Schwartz TW, et al. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276(5310):276–9.
Mack M, Luckow B, Nelson PJ, Cihak J, Simmons G, Clapham PR, et al. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187(8):1215–24.
Doranz BJ, Grovit-Ferbas K, Sharron MP, Mao S-H, Goetz MB, Daar ES, et al. A small-molecule inhibitor directed against the chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. J Exp Med. 1997;186(8):1395–400.
Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, et al. CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272(5270):1955–8.
Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381(6584):661.
Neil SJ, Aasa-Chapman MM, Clapham PR, Nibbs RJ, McKnight A, Weiss RA. The promiscuous CC chemokine receptor D6 is a functional coreceptor for primary isolates of human immunodeficiency virus type 1 (HIV-1) and HIV-2 on astrocytes. J Virol. 2005;79(15):9618–24.
Kim S-S, Peer D, Kumar P, Subramanya S, Wu H, Asthana D, et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. Mol Ther. 2010;18(2):370–6.
Malavia NK, Zurakowski D, Schroeder A, Princiotto AM, Laury AR, Barash HE, et al. Liposomes for HIV prophylaxis. Biomaterials. 2011;32(33):8663–8.
Kish-Catalone T, Pal R, Parrish J, Rose N, Hocker L, Hudacik L, et al. Evaluation of-2 RANTES vaginal microbicide formulations in a nonhuman primate simian/human immunodeficiency virus (SHIV) challenge model. AIDS Res Hum Retrovir. 2007;23(1):33–42.
Franquelim HG, De-Sousa FF, Veiga AS, Santos NC, Castanho MA. Cationic liposomes are possible drug-delivery systems for HIV fusion inhibitor sifuvirtide. Soft Matter. 2011;7(23):11089–92.
Asin SN, Eszterhas SK, Rollenhagen C, Heimberg AM, Howell AL. HIV type 1 infection in women: increased transcription of HIV type 1 in ectocervical tissue explants. J Infect Dis. 2009;200(6):965–72.
Kumar P, Ban H-S, Kim S-S, Wu H, Pearson T, Greiner DL, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134(4):577–86.
Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm Res. 2009;26(3):502–11.
Fumakia M, Yang S, Gu J, Ho EA. Protein/peptide-based entry/fusion inhibitors as anti-HIV therapies: challenges and future direction. Rev Med Virol. 2016;26(1):4–20.
Vijayakumar S, Ganesan S. Gold nanoparticles as an HIV entry inhibitor. Curr HIV Res. 2012;10(8):643–6.
Di Gianvincenzo P, Marradi M, Martínez-Ávila OM, Bedoya LM, Alcamí J, Penadés S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorg Med Chem Lett. 2010;20(9):2718–21.
Lara HH, Ixtepan-Turrent L, Treviño ENG, Singh DK. Use of silver nanoparticles increased inhibition of cell-associated HIV-1 infection by neutralizing antibodies developed against HIV-1 envelope proteins. J Nanobiotechnol. 2011;9(1):38.
Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127(20):2038–46.
https://clinicaltrials.gov/ct2/show/NCT02128828?term=TAK+652+AND+Cenicriviroc
https://clinicaltrials.gov/ct2/show/NCT02483078?term=pro+140&rank=1
https://clinicaltrials.gov/ct2/show/NCT00102778?term=aplaviroc&rank=1
https://clinicaltrials.gov/ct2/show/NCT00393120?term=INCB9471&rank=1
https://clinicaltrials.gov/ct2/show/NCT00686829?term=VICRIVIROC&rank=1
https://clinicaltrials.gov/ct2/show/NCT00361101?term=AMD11070&rank=1
https://clinicaltrials.gov/ct2/results?cond=HIV&term=AMD3100&cntry=&state=&city=&dist=&Search=Search
Gladue RP, Brown MF, Zwillich SH. CCR1 antagonists: what have we learned from clinical trials. Curr Top Med Chem. 2010;10:1268.
https://clinicaltrials.gov/ct2/show/NCT00542022?term=mk0812&rank=2
https://clinicaltrials.gov/ct2/show/NCT00699790?term=BMS+741672&rank=1
https://clinicaltrials.gov/ct2/show/NCT01440257?term=CCX140&rank=3
https://clinicaltrials.gov/ct2/show/NCT00638755?term=CAT+213&rank=4
https://clinicaltrials.gov/ct2/show/NCT00990327?term=DPC+168&rank=1
https://clinicaltrials.gov/ct2/show/NCT00688467?term=SCH527123&rank=3
https://clinicaltrials.gov/ct2/show/NCT00504439?term=Sb656933&rank=1
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 American Association of Pharmaceutical Scientists
About this chapter
Cite this chapter
Maithania, H.V., D’Souza, A.A., Dandekar, P., Devarajan, P.V. (2019). Role of Chemokines and Chemokine Receptors in Infectious Diseases and Targeting Strategies. In: Devarajan, P., Dandekar, P., D'Souza, A. (eds) Targeted Intracellular Drug Delivery by Receptor Mediated Endocytosis. AAPS Advances in the Pharmaceutical Sciences Series, vol 39. Springer, Cham. https://doi.org/10.1007/978-3-030-29168-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-29168-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29167-9
Online ISBN: 978-3-030-29168-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)