Abstract
Advances in cancer prevention, screening and early detection, diagnosis, treatment, and care delivery have yielded significant improvements in survival for cancer patients and decreases in cancer-related treatment toxicity. However, these advances have been accompanied by an increasing financial burden to patient and their caregivers. Financial toxicity, which draws a parallel between the side effects of cancer treatment and the side effects of undue financial distress, is increasingly recognized as being common and a source of significant morbidity and decreased health-related quality of life for head and neck cancer (HNC) patients and their caregivers. This chapter will (1) define financial toxicity following cancer treatment, (2) estimate its incidence in HNC patients, (3) highlight key risk factors in HNC patients, (4) describe various tools for its measurement, (5) discuss the impact of financial toxicity on HNC patients, and (6) review resources on financial toxicity for patients and caregivers. Knowledge gaps and research opportunities to improve the delivery of patient-centered cancer care that is attentive to the complex issue of financial toxicity will be explored at the conclusion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Financial toxicity
- Financial distress
- Treatment cost
- Caregiver burden
- Patient-reported outcomes
- Head and neck cancer
Since President Nixon declared war on cancer in 1971, the United States has seen a tremendous investment into cancer prevention, screening and early detection, diagnosis, treatment, and care delivery with a subsequent increase in survival and decrease in cancer-related morbidity [1]. As a result, there are now more than 15.5 million cancer survivors in the United States [2]. However, concomitant with these scientific advances in cancer treatment and improvements in cancer care delivery has been an exponential increase in the cost of cancer care [3]. Not only is the overall cost of cancer care to the healthcare system increasing, but the proportion of that cost that falls on cancer patients and their families continues to grow [4, 5]. Unfortunately, this trend is expected to worsen over time with the continued developed of targeted and immune-modulating anticancer therapies [6]. In addition, patients continue to increase enrollment in high-deductible plans on the insurance exchanges through the Patient Protection and Affordable Care Act, further increasing the burden of rising cancer care borne by patients [7].
As a result of the convergence between improved oncologic outcomes, increased cost of cancer care, and continued shifting of the burden of the cost of cancer care to the patient, there has been a growing awareness of a phenomenon known as financial toxicity. Financial toxicity is a multidimensional construct comprised of three conceptual domains: (1) material hardship that results from increased out-of-pocket [OOP] costs and lower income, (2) psychological distress resulting from the material hardship, and (3) compensatory coping strategies that families develop in response to the financial cost of cancer and its treatment [8, 9]. The downstream impact of financial toxicity is significant and includes altered cancer treatment preferences [10], decreased adherence to cancer treatment [11], increased symptom burden [12], decreased health-related quality of life (HRQOL) [13], and decreased survival [14]. In addition, because financial toxicity disproportionately burdens socioeconomically disadvantaged patients, it is expected to exacerbate disparities in cancer care treatment and outcomes. Understanding the impact of the financial cost of cancer care and its effect on patients and their caregivers is thus of critical importance to patients, clinicians, researchers, and policy makers [15].
This chapter will define financial toxicity following cancer treatment, estimate its incidence in head and neck cancer (HNC) patients, highlight key risk factors, describe various tools for its measurement, and discuss practical considerations for providers vis-à-vis financial toxicity. Knowledge gaps and research opportunities to improve the delivery of patient-centered cancer care that is attentive to the complex issue of financial toxicity will be explored at the conclusion.
What Is Financial Toxicity
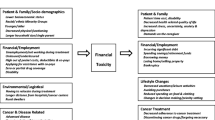
Financial toxicity is a multidimensional construct comprised of three conceptual domains: (1) material hardship that results from increased OOP costs and lower income, (2) psychological distress resulting from the material hardship, and (3) compensatory coping strategies that families develop in response to the financial cost of cancer and its treatment (Fig. 10.1) [8, 9]. The term financial toxicity draws a parallel to the well-known physical toxicity experienced by cancer patients due to treatment side effects [16] although other terms that are sometimes used interchangeably with cancer-related financial toxicity include financial distress, financial hardship, financial burden, and financial impact [9].
For cancer patients, financial distress is a result of the following three costs: (1) the direct (nonreimbursed) OOP medical costs of cancer care, (2) the direct nonmedical costs borne by the patient, and (3) the indirect and opportunity costs associated with cancer treatment [17, 18]. Components of direct OOP cancer costs include insurance premiums and deductibles as well as direct medical costs related to prescription and nonprescription medications, medical professional visits, hospital bills, nutritional services, physical/speech/occupational therapy and other rehabilitative services, home care, and devices and equipment. The direct nonmedical costs that contribute to the cost of cancer care include transportation and lodging for treatment, childcare/elder care, and other supportive services. Indirect and opportunity costs of cancer care include lost income during treatment due to missed workdays from illness and injury as well as future lost earned income from a lack of employment. These indirect costs of cancer care are a critically important factor for patients with HNC as they have the highest rate of disability or quitting work relative to any other cancer [19].
Direct OOP costs, missed workdays, and lost employment due to cancer all contribute to financial toxicity. Finkelstein et al. used the Medical Expenditure Panel Survey to perform a population-based study of the working age population (25–64 years) in the United States from 2000 to 2005 to quantify the impact of a cancer diagnosis on direct OOP costs, missed workdays, and lost employment [18]. They found that the mean annual OOP expenditure was $1170 greater for participants actively treated for cancer relative to those who are not treated for cancer [18]. Compared to those without cancer, cancer patients had a 4.5% relative decrease (and 3.3% absolute decrease) in the odds of employment and missed 22.3 more days of work annually [18]. Between the direct OOP costs and missed workdays, an average household could expect to have their annual medical bill increase from 5% of their annual income to 8% of their annual income [18].
While those being actively treated for cancer face significant financial toxicity from the direct OOP costs of medical care and the lost workdays due to illness, financial toxicity continues for long-term cancer survivors as well. A systematic review by Altice et al. demonstrated that cancer survivors have an annual mean loss in productivity ranging from $380 to $8236 [9]. Of cancer survivors in this review, 12–62% reported that cancer treatment caused them to go into debt and 49% experienced financial distress [9].
Incidence of Financial Toxicity
The incidence of financial distress among patients with cancer in the United States is quite high [20] with prevalence estimates ranging up to 50% [9]. Cancer-related financial toxicity is especially common in patients with HNC in the United States with cumulative incidence estimates ranging from 40% to 69% [4, 21, 22]. The higher rate of financial toxicity in patients with HNC relative to other types of cancer is likely due to the frequent use of trimodal treatment paradigms in HNC (surgery, radiation, and chemotherapy) combined with a patient population that tends to have a lower socioeconomic status. Interestingly, a study examining financial toxicity in 67 patients with HNC treated with (chemo)radiation in Norway described very low levels of financial difficulties arising from HNC and its treatment [23]. Similarly, only 18% of HNC survivors treated in Canada reported unmet needs about financial support [24]. These data suggest that findings about financial toxicity in HNC patients are probably not generalizable across vastly different healthcare delivery systems (e.g., the United States compared to Norway).
A single institution prospective cohort study of 33 patients with HNC treated with a primary surgical approach with/without adjuvant therapy published in 1989 assessed changes in financial burden over time [21]. Using a nonvalidated patient self-report of financial problems, the authors reported that the incidence of perceived financial difficulties was 9% preoperatively, peaked at 40% at 3 months after treatment, and decreased slightly at 1 year after treatment [21].
In a prospective cohort study of 73 insured patients with locally advanced HNC treated at a single tertiary care academic medical center between May 2013 and November 2014, 69% of patients needed to use at least one financial coping strategy (e.g., borrowing money or using credit, selling possessions or property) within 6 months of treatment [22]. The median OOP cost for this group of patients was $805.93 per month (range $6 to $10,156) and the median indirect cost was $135,271.10 (range $0 to $1,317,882) [22]. The authors suggested that the high OOP costs identified for HNC patients are likely a key contributor to the high incidence of financial distress in patients with HNC [22].
Risk Factors for Financial Toxicity
Identification of patients at risk for developing financial toxicity using pre-treatment, baseline characteristics would theoretically allow for improved multidisciplinary evaluation and prevention through appropriate referrals to financial advisors, patient navigators, and cancer psychologists. Although the incidence of financial toxicity is high for patients with HNC [22], there is a subset of patients who are at exceptionally high risk for financial toxicity. In a prospective cohort study of 73 patients with locally advanced HNC, patients with Medicaid, decreased wealth, higher perceived social isolation, and higher total out-of-pocket costs were independently associated with financial toxicity on multivariable logistic regression analysis [22].
These aforementioned risk factors lead not only to an increased financial stress, but in fact create more barriers in receiving optimal care with an association with medication nonadherence and more missed appointments [22]. This creates an undue cycle of financial toxicity with the initial financial burden of cancer treatment leading to an increased use of cost-coping mechanisms that generates further financial stress and poorer quality of life for patients and their caregivers.
Tools for Measuring Financial Toxicity
Following the conceptual model of financial toxicity outlined by Altice et al. (Fig. 10.1), measures of financial toxicity can be grouped into three categories: (1) measures of material hardship, (2) measures of psychological distress in response to financial hardship, and (3) measures of compensatory coping behaviors (typically medication nonadherence) [9].
There are a variety of quantitative measures of material hardship resulting from cancer treatment including direct OOP costs, financial burden, productivity loss, medical debt/depletion of assets, and bankruptcy. Financial burden is defined as the ratio of OOP health-related spending to household income [25]. A financial burden of 10–20% or more is considered significant [9, 26]. Quantitative assessment of cancer-related material hardship also includes measures of indirect/opportunity costs. The Work Productivity and Activity Impairment (WPAI) Questionnaire is a validated and reliable tool that measures time missed from work and impairment of work and regular activities due to overall health and symptoms that has been used in oncology patients [27]. Because the indirect costs of cancer care are particularly important for patients with HNC [19, 28], a robust assessment of financial toxicity should include measures of these opportunity costs.
In addition to monetary metrics of financial hardship, comprehensive assessments of financial toxicity should include patient-reported outcome measures (PROMs). Optimizing strategies to deliver patient-centered oncology care is a key priority for major funding, policy making, and regulatory entities [29, 30]. Harnessing patient-reported outcomes (PROs) to deliver patient-centered oncology care results in numerous improved outcomes including better symptom management, enhanced HRQOL, and increased survival [31,32,33]. Unfortunately, although PROMs have been developed to cover a range of physical and psychosocial aspects of cancer and its treatment, a PRO measure for cancer-related financial toxicity has been lacking until recently [15].
The COmprehensive Score for financial Toxicity (COST) questionnaire is a reliable, validated, single-domain 11-item PRO measure of financial toxicity in patients with cancer that addresses the material and psychological hardship of financial cancer care (Fig. 10.2) [15]. In the original validation study of 233 patients with AJCC Stage IV solid tumors receiving chemotherapy, the COST measure demonstrated appropriate psychometric performance with high internal consistency and test-retest reliability [34]. The COST PRO measure was found to correlate with income and psychosocial distress [34]. In addition, higher levels of financial toxicity (as measured by the COST) were associated with decreased HRQOL, suggesting that the new COST questionnaire is capturing clinically relevant patient-centered outcomes [34]. Although the COST measure is an exciting addition to the PRO armamentarium, it has not been specifically validated in HNC patients. The authors hope that this PRO measure of cancer-related financial toxicity will facilitate continued patient-centered research on the topic to minimize the adverse effects of financial toxicity and its potential to exacerbate existing disparities in cancer care and outcomes [34].
The InCharge Financial Distress/Financial Well-Being Scale (IFDFW) is another reliable and validated measure of financial toxicity [35]. Although not healthcare specific, it covers material and psychological hardship domains and has been used in numerous prior studies to assess cancer-related financial distress [36,37,38].
In addition to tools dedicated solely to measuring financial toxicity in cancer patients, questions about financial toxicity of cancer care also frequently embedded in multidomain HRQOL tools. The EORTC/QLQ-C30 is a 30-item reliable and validated multidomain HRQOL measure for oncology patients that addresses financial toxicity through the following single question: “Has your physical condition or medical treatment caused you financial difficulties?“ [39] The Cancer Survivors’ Unmet Needs Measure (CaSUN) is another tool that has been used to measure financial toxicity in cancer survivors [24]. Among the 35-items in the CaSUN questionnaire is one that assesses the cancer survivor’s unmet need regarding financial support [40]. In a study of 158 patients at a quaternary cancer care center using the CaSUN questionnaire, patients who endorsed needing help with financial support as an unmet need did so with moderate to strong strength [24].
The Impact of Financial Toxicity on Head and Neck Cancer Patients
The downstream impact of financial toxicity on cancer patients is significant as increased levels of financial toxicity are associated with decreased adherence to cancer treatment [11], increased symptom burden [12], decreased HRQOL [13], unmet needs for HNC survivors [41], and decreased survival [14]. To mitigate the adverse effects of financial toxicity, patients employ a variety of cost-coping strategies that mediate the relationship between financial toxicity and worse oncologic and HRQOL outcomes. A flowchart of the economic consequences of cancer treatment and how coping mechanisms lead to worse HRQOL and health outcomes is shown in Fig. 10.3 [6]. Cost-coping strategies have been conceptualized as care altering and lifestyle altering [42]. Examples of care-altering strategies to minimize the burden of cancer-related financial toxicity include not filling a prescription, taking less medication than prescribed, and missing tests, procedures, or appointments [42]. Lifestyle-altering cost-coping strategies include spending personal savings, selling possessions, borrowing money, having other family members work more, spending less on necessities (e.g., food, clothing), and decreasing spending on leisure activities [42].
Flowchart of economic consequences of cancer treatment on the patient and patient coping. (From Carrera et al. [6], with permission)
One of the key downstream impacts of care-altering strategies to mitigate against cancer-related financial toxicity is thus decreased treatment adherence. In a study of insured patients with diverse types of cancer, 20% of patients took less than the prescribed amount of cancer medications, 19% partially filled prescriptions, and 24% did not fill prescriptions at all in an attempt to defray out-of-pocket expenses [43]. Others have documented that higher prescription copayments for aromatase inhibitors are associated with higher rates of nonadherence and nonpersistence in patients with breast cancer [11].
Lifestyle-altering strategies to manage financial toxicity stemming from cancer treatment also significantly contribute to decreased HRQOL. In a sample of patients with locally advanced HNC, 69% of patients reported employing at least one cost-coping strategy within 6 months of treatment initiation [22]. These strategies included using all or a portion of their savings (62% of patients), borrowing money or using credit (42% of patients), selling possessions or property (26% of patients), and having family members work additional hours (23% of patients) [22]. In a different study examining lifestyle-altering behaviors as a consequence of cancer-related financial toxicity, 68% of patients reduced leisure activities, 46% reduced spending on food and clothing, 46% used savings, and 18% sold possessions as a consequence of high OOP cancer care expenses [43]. Other studies have shown that patients suffering from cancer-related financial toxicity are more likely to file for bankruptcy [14]. Furthermore, cross-sectional studies of HNC survivors have demonstrated that financial strain due to cancer is independently associated with unmet needs [41].
In addition to the negative impact that financial toxicity has at the individual patient level, cancer-related financial toxicity also has significant negative implications from a societal perspective. Specifically, as the impact of financial toxicity for cancer patients continues to grow, racial and socioeconomic disparities in cancer care and oncologic outcomes are expected to be exacerbated [34]. In a study of 400 insured cancer patients evaluating stylized cancer treatment scenarios, patients with higher income were more likely to select a treatment due to its perceived impact on survival while lower income patients were more likely to select a treatment due to its perceived impact on cost [10].
The Impact of Financial Toxicity on Head and Neck Cancer Caregivers
In addition to creating a devastating financial and psychosocial impact on the HNC patient, financial toxicity also impacts family members and caregivers [44]. Data collected from a study of informal cancer caregivers demonstrated that the average time spent caring for cancer patients was 8.3 hours per day for 13.7 months [45]. The estimated cost of caregiving time spent for cancer survivors over a 2-year period after the diagnosis was estimated to be $47,710 [45]. This value was even higher in patients with higher staging and distant or metastatic disease at diagnoses [45]. Informal caregivers spend valuable time with cancer patients and are at risk for making extended employment changes in order to provide the desired level of care [46].
A cross-sectional study of 180 HNC survivor-caregiver dyads revealed that caregivers with reported financial distress had survivors who reported significantly higher fear of cancer recurrence and cancer worry [47]. Financial distress among caregivers causes undue psychosocial concern as well, which may compromise optimal completion of caregiving tasks. A different cross-sectional study of 44 partners of HNC survivors quantified unmet survivorship care needs using the Cancer Survivors’ Partners Unmet Needs Survey (CaSPUN). In this study, the authors found that 21% of partners’ endorsed needing help finding out about financial support and/or government benefits [48].
In a qualitative study of 31 HNC caregivers, finance-related psychosocial distress was highly prevalent4. Caregivers frequently described direct nonmedical costs of HNC treatment (travel for appointments, overnight accommodations) and indirect costs (giving up or significantly reducing paid work to care for family/friends) during the treatment phase [4]. Flexible working arrangements for caregivers, practical community support (e.g., fundraising), private insurance, and access to medical and/or social welfare benefits were all found to mitigate the negative effects of financial toxicity for HNC caregivers [4].
The long-term financial impact of HNC care was particularly distressing to caregivers, but they often attempted to hide the financial situation from the HNC survivor in an attempt to protect him/her from an additional source of worry [4]. As a result of the paucity of attention to the impact of financial toxicity on HNC caregivers, it is not surprising that caregivers of HNC survivors rank “finding out about financial support” as a critical unmet need [49].
Resources on Financial Toxicity for Patients and Caregivers
Although HNC patients and their caregivers are interested in receiving additional resources about financial toxicity and strategies to manage financial issues, it nevertheless represents an unmet need for many. In a study of 158 HNC survivors treated at a single academic medical center in Toronto, 23% of patients reported being interested-very interested in receiving more resources about managing financial issues after treatment of HNC [24].
There are publicly available resources that can be used to help understand and manage financial toxicity. The National Cancer Institute provides up-to-date information regarding financial toxicity in the form of the Physician Data Query that is beneficial for both the patient [50] and provider [51]. This information summary defines terminology regarding financial distress that is comprehendible at the patient level and discusses risk factors, its effects, and ways to minimize financial toxicity [50].
Financial assistance resources that can be provided to HNC patients and their caregivers include the Head and Neck Cancer Alliance program, The Oral Cancer Foundation, Support for People with Head and Neck Cancer, CancerCare, and The Assistance Fund. These resources allow patients to better understand their financial burdens and also provide an opportunity to communicate with other patients with similar diagnoses and financial stressors. However, the provider should be involved as much as possible in helping patients and their caregivers with this process alongside providing these resources.
Practical Considerations About Financial Toxicity of Cancer Treatment for Head and Neck Cancer Providers
Although in many cases oncology providers do not discuss the financial toxicity of cancer treatment with patients and caregivers, there is clearly a compelling need to inquire about financial toxicity and engage and support patients and caregivers on this topic [4]. A survey conducted by Jagsi et al. demonstrated that over two-thirds of breast cancer patients that were worried about their finances noted that physicians and their accompanying staff did not help address these issues [52]. Moreover, it was noted that only one-half of medical oncologists and only 15% of surgeons believe that someone in their practices spoke with patients about financial toxicity [52].
Sociodemographic factors also play a role in the doctor-patient relationship and the discussion of financial burden. A study with video-recorded clinical interactions between African American cancer patients and non-African American oncologists revealed that cost discussions occurred in less than half of the encounters (45%) with patients mostly initiating discussion about cost (63%) [53]. Oncologists initiated cost discussions only 36% of times [53], demonstrating that clinicians should become more proactive in cost discussions and understanding of the financial toxicity that affects racial/ethnic minorities.
The management of HNC is a multidisciplinary collaborative effort, and clinicians should be actively engaged and understanding of patient’s justified fears of financial distress and its complex interplaying elements (Fig. 10.4). Providers should allow each patient diagnosed with HNC the opportunity to work with patient navigators, psychologists, financial advisors, and other physicians as to best manage medical care with regards to patient autonomy and team collaboration. Discussions of cost with HNC patients and their caregivers need to be further studied and evaluated with multimodal perspectives.
Conceptual framework relating severe illness, treatment choice, and health and financial outcomes. Cancer and financial strain and distress have a complexity of interplaying elements. (From Board PATE [51], with permission)
As in other aspects of multidisciplinary HNC care, no single HNC provider can completely manage financial toxicity. A critically important role for HNC surgeons, radiation oncologists, and/or medical oncologists is to help increase awareness that financial toxicity is common following treatment for HNC and that resources exist for its management. Other relevant players to whom referrals should be made to assess for and manage financial toxicity include social workers and patient navigators, who may further facilitate engagement with social welfare systems [4].
Knowledge Gaps and Research Opportunities
With the ever-growing awareness of patient-centered financial toxicity, more literature is becoming available to help clinicians and providers over time. However, further evidence-based research should be conducted to address significant knowledge gaps that preclude the screening, prevention, and treatment of financial toxicity in patients with HNC. One of the major gaps in the area of cancer-related financial toxicity research is inconsistent use of definitions, terms, and measures [9]. The lack of agreed-upon nomenclature and measures precludes identification of targets and development of interventions to prevent and manage financial toxicity in cancer survivors.
Additionally, there should be more transparency and solidarity in the quantification of financial burden to allow investigators to more accurately understand causality. Providers should have all financial resources available at hand based upon a systematic approach to understand and interpret the current types of charitable aid offered to cancer patients.
Research of both patient-level and caregiver-level financial distress is a fundamental pillar in understanding the overall meaning of financial toxicity itself. Adaptation of validated tools to quantify patient-level financial toxicity should be further implemented. Investigators should also take the opportunity to create and develop a validated instrument to quantify the financial burden for caregivers, including informal caregivers.
An overall understanding of the healthcare delivery system as it relates to financial toxicity to patients is also crucial. Providers, patients, and policy makers should value the importance of access to optimal and high-quality cancer treatment in the most efficient manner possible. Questions should be asked regarding which systemic issues are currently present in the healthcare model in order to generate solutions to further advance survival and quality of life and reduce financial toxicity.
There should be implementation of a universal screening tool to determine which patients are at a high risk for financial toxicity, and every patient should have the opportunity to be aware of the possibility of financial burden and the psychosocial impact of cancer at the earliest possible time in the treatment process. In addition, future research should seek to clarify the longitudinal course of financial distress in HNC patients and their caregivers. Only then can the HRQOL of HNC patients further improve as clinicians and patients learn more and more about the patient-centered financial toxicity.
There is much to learn regarding financial toxicity and the root cause of this issue that plagues HNC patients. Providers have a duty to provide the best possible financial resources and further educate themselves for HNC patients who not only suffer from the medical toxicity of cancer but also undue financial toxicity.
Conclusion
Financial toxicity is particularly common in patients with HNC and represents an unmet need for many patients and their caregivers. The armamentarium of tools to measure and identify financial toxicity, particularly in a patient-centered fashion, continues to grow. There is nevertheless significant future work to be done to develop and implement targeted prevention and treatment strategies for those at highest risk of developing financial toxicity.
References
Drake N. Forty years on from Nixon’s war, cancer research ‘evolves’. Nat Med. 2011;17:757.
Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89.
Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–28.
Balfe M, Butow P, O’Sullivan E, et al. The financial impact of head and neck cancer caregiving: a qualitative study. Psychooncology. 2016;25:1441–7.
Chang S, Long SR, Kutikova L, et al. Estimating the cost of cancer: results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J Clin Oncol. 2004;22:3524–30.
Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68:153–65.
Foundation KF. Kaiser family foundation. Available at: http://kaiserfamilyfoundation.files.wordpress.com/2013/01/8177.pdf. Accessed 25 Oct 2018.
Zafar SY, Abernethy AP. Financial toxicity, Part I: a new name for a growing problem. Oncology (Williston Park). 2013;27:80–1.. 149
Altice CK, Banegas MP, Tucker-Seeley RD, et al. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2017;109:djw205–djw05.
Wong YN, Egleston BL, Sachdeva K, et al. Cancer patients’ trade-offs among efficacy, toxicity, and out-of-pocket cost in the curative and noncurative setting. Med Care. 2013;51:838–45.
Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534–42.
Lathan CS, Cronin A, Tucker-Seeley R, et al. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal vancer. J Clin Oncol. 2016;34:1732–40.
Zafar SY, McNeil RB, Thomas CM, et al. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11:145–50.
Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34:980–6.
de Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer: the COST measure. Cancer. 2014;120:3245–53.
Zafar SY, Abernethy AP. Financial toxicity, Part I: a new name for a growing problem. Oncology (Williston Park, NY). 2013;27:80–149.
Drummond M, MJ S, GW T, et al. Methods for the economic evaluation of health care programmes. 3rd ed. New York: Oxford University Press; 2005.
Finkelstein EA, Tangka FK, Trogdon JG, et al. The personal financial burden of cancer for the working-aged population. Am J Manag Care. 2009;15:801–6.
Short PF, Vasey JJ, Tunceli K. Employment pathways in a large cohort of adult cancer survivors. Cancer. 2005;103:1292–301.
Stump TK, Eghan N, Egleston BL, et al. Cost concerns of patients with cancer. J Oncol Pract. 2013;9:251–7.
Krouse JH, Krouse HJ, Fabian RL. Adaptation to surgery for head and neck cancer. Laryngoscope. 1989;99:789–94.
de Souza JA, Kung S, O’Connor J, et al. Determinants of patient-centered financial stress in patients with locally advanced head and neck cancer. J Oncol Pract. 2017;13:e310–e18.
Egestad H, Nieder C. Undesirable financial effects of head and neck cancer radiotherapy during the initial treatment period. Int J Circumpolar Health. 2015;74:26686.
Giuliani M, McQuestion M, Jones J, et al. Prevalence and nature of survivorship needs in patients with head and neck cancer. Head Neck. 2016;38:1097–103.
Banthin JS, Cunningham P, Bernard DM. Financial burden of health care, 2001–2004. Health Aff (Millwood). 2008;27:188–95.
Banthin JS, Bernard DM. Changes in financial burdens for health care: national estimates for the population younger than 65 years, 1996 to 2003. JAMA. 2006;296:2712–9.
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353–65.
Pearce A, Timmons A, O’Sullivan E, et al. Long-term workforce participation patterns following head and neck cancer. J Cancer Surviv. 2015;9:30–9.
Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–55.
Lipscomb J, Gotay CC, Snyder CF. Patient-reported outcomes in cancer: a review of recent research and policy initiatives. CA Cancer J Clin. 2007;57:278–300.
Basch E. Patient-reported outcomes – harnessing Patients’ voices to improve clinical care. N Engl J Med. 2017;376:105–8.
Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557–65.
Rotenstein LS, Huckman RS, Wagle NW. Making patients and doctors happier – the potential of patient-reported outcomes. N Engl J Med. 2017;377:1309–12.
de Souza JA, Yap BJ, Wroblewski K, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: the validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123:476–84.
Prawitz A, Garman ET, Sorhaindo B, et al. InCharge financial distress/financial well-being scale: development, administration, and score Interpretation. J Financ Couns Plan. 2006;17(1):17.
Bestvina CM, Zullig LL, Rushing C, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10:162–7.
Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19:1135–40.
Meisenberg BR, Varner A, Ellis E, et al. Patient attitudes regarding the cost of illness in cancer care. Oncologist. 2015;20:1199–204.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Hodgkinson K, Butow P, Hunt GE, et al. The development and evaluation of a measure to assess cancer survivors’ unmet supportive care needs: the CaSUN (Cancer Survivors’ Unmet Needs measure). Psychooncology. 2007;16:796–804.
O’Brien KM, Timmons A, Butow P, et al. Associations between neighbourhood support and financial burden with unmet needs of head and neck cancer survivors. Oral Oncol. 2017;65:57–64.
Nipp RD, Zullig LL, Samsa G, et al. Identifying cancer patients who alter care or lifestyle due to treatment-related financial distress. Psychooncology. 2016;25:719–25.
Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18:381–90.
National Alliance for Caregiving. Available at: www.caregiving.org/wp-content/uploads/2016/06/CancerCaregivingReport_FINAL_June-17-2016.pdf. Accessed 1 June 2017.
Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115:4362–73.
de Moor JS, Dowling EC, Ekwueme DU, et al. Employment implications of informal cancer caregiving. J Cancer Surviv. 2017;11:48–57.
Maguire R, Hanly P, Balfe M, et al. Worry in head and neck cancer caregivers: the role of survivor factors, care-related stressors, and loneliness in predicting fear of recurrence. Nurs Res. 2017;66:295–303.
Giuliani M, Milne R, McQuestion M, et al. Partner’s survivorship care needs: an analysis in head and neck cancer patients. Oral Oncol. 2017;71:113–21.
Chen SC, Lai YH, Liao CT, et al. Unmet supportive care needs and characteristics of family caregivers of patients with oral cancer after surgery. Psychooncology. 2014;23:569–77.
Board PATE. Financial toxicity and cancer treatment (PDQ®): patient version. In: PDQ cancer information summaries [internet] 2018 Nov. 1st ed. Bethesda: National Cancer Institute (US); 2002.
Board PATE. Financial toxicity and cancer treatment (PDQ®): health professional version. In: PDQ cancer information summaries [internet] 2018 Oct. 24th ed. Bethesda: National Cancer Institute (US); 2002.
Jagsi R, Ward KC, Abrahamse PH, et al. Unmet need for clinician engagement regarding financial toxicity after diagnosis of breast cancer. Cancer. 2018;124(18):3668–76.
Hamel LM, Penner LA, Eggly S, et al. Do patients and oncologists discuss the cost of cancer treatment? An observational study of clinical interactions between African American patients and their oncologists. J Oncol Pract. 2017;13:e249–e58.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Graboyes, E.M., Gupta, A., Sterba, K.R. (2020). Financial Impact of Cancer Treatment. In: Fundakowski, C. (eds) Head and Neck Cancer. Springer, Cham. https://doi.org/10.1007/978-3-030-27881-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-27881-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27880-9
Online ISBN: 978-3-030-27881-6
eBook Packages: MedicineMedicine (R0)