Abstract
The Barí, an Amerindian society in the Sierra de Perijá between Venezuela and Colombia, have a mixed subsistence economy that integrates horticulture with fishing and hunting. Hunting produced just a quarter of the meat consumed in the 1970s, and although monkeys accounted for less than 6% of that amount, they still play an important cultural role. In the Barí territory, four species of monkey are known to exist: white-bellied spider (Ateles hybridus), red howler (Alouatta seniculus), white-fronted capuchin (Cebus leucocephalus), and gray-handed night monkeys (Aotus griseimembra), all of which are quite abundant. In an ethnobotanical study in Barí homeland, out of all trees censused (N = 3664) in 5.4 hectares of forest, 2476 individual trees (67.8%) provide food for monkeys. One hundred and two species of tress provided food for many animals, including monkeys. As humans “anthropogenically” create “cultural forests,” primates must be doing something very similar, by making their forest a “primatogenical” one. Therefore, most Neotropical forests are primatogenical since monkeys and humans disperse fruits of most trees, but monkeys still do most of it. Therefore, the preservation of these monkeys is key to the future of this forest, which could support larger fauna. Based on this research, primates are likely to be essential species in the reproduction of two-thirds of the Barí forest.
Resumen
Los Barí, una etnia indígena de la Sierra de Perijá entre Venezuela y Colombia, tienen una subsistencia mixta que integra horticultura, pesca y cacería. La cacería produce sólo un cuarto de la carne consumida en 1970s, si bien los monos representan menos del 6% de tal cantidad, éstos aun juegan un rol cultural. En el territorio Barí existen cuatro especies de primates: el mono araña norteño (Ateles hybridus), el aullador rojo o araguato (Alouatta seniculus), el capuchino cariblanco (Cebus leucocephalus) y el mono nocturno (Aotus griseimembra). Este pueblo indígena tiene un conocimiento muy detallado de la dieta de estos animales. De todos los árboles estudiados (N = 3.664) en 5.4 hectáreas de selva, existen 2.476 árboles (67.8%) que proporcionan alimentos a los primates. De los 227 taxones de árboles en estos sectores, 102 especies son alimenticias para muchos animales, incluyendo los primates. Como los humanos crean selvas “antropogénicas” que se suelen llamar “selvas culturales”, se podría decir que los monos hacen algo muy similar, al convertir la selva en “primatogénica” por jugar un rol significativo en el repoblamiento forestal. Por lo tanto, la preservación de los monos es clave para el futuro de estas selvas que, a su vez, pueden alimentar a un gran número de otras especies de animales. Esta investigación argumenta que los primates pueden ser unas especies primordiales en la reproducción de dos tercios de la selva Barí.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Palabras clave
1 Introduction
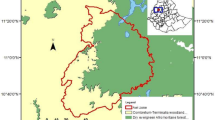
The Barí are an Amerindian group of approximately 4000 people living on the southwestern side of the Lake Maracaibo region of northwestern South America, on both sides of the Venezuela–Colombia border (Fig. 6.1). Their language belongs to the Chibcha linguistic family. This society practices subsistence, swidden horticulture complemented by fishing, hunting, and gathering of forest products. The Barí way of living is mostly associated with Amazonian cultures. They depend on manioc, supplemented with bananas and plantains as the main starches, and bocachico fish and monkeys as main sources of protein, in addition to pacas, peccaries, tapir, curassows, and river turtles as seasonal sources (Beckerman 1975, 1983; Beckerman and Lizarralde 2013).
Their environment is classified as a hyper-humid, tall tropical forest (Pittier 1948; Huber and Alarcón 1988; Aymard 2011). Based on its biodiversity of both fauna and flora, this region was denominated “Refugio del Catatumbo” by Steyermark (1982) and like the Southern Central American wet forests shares floristic similarities with the Amazonian and the Guayana Shield lowlands (Steyermark 1982; Gentry 1990; Aymard 2011).
However, Barí forest biodiversity is not as high as that found in the Amazon in places such as Yasuni Park in Ecuador (Finer et al. 2009) or Manu in Peru (Terborgh 1999). Ecological and cultural aspects of Barí ethnoprimatological work have been published in two book chapters (Lizarralde 2004, 2019). This book chapter focuses on combining ethnobotanical and ethnoprimatological information to detail the holistic understanding that the Barí have of their natural resources.
A word for primate does not exist in the Barí language. However, when asked in Spanish about all of their monkeys, they provide a list of six different animals. Among them are four species found in their forest which are considered the proper primates: (1) white-bellied or variegated spider monkey (Ateles hybridus, I. Geoffroy Saint-Hilaire, 1829, su’shà in Barí (the apostrophe (‘) in the Barí words is to represent a glottal occlusion sound similar to a combined “g” and “k” sound), average weight 9.5 kg; see Fig. 6.2), (2) red howler monkey (Alouatta seniculus, Linnaeus, 1766, borò or kámashkò’da in Barí (the two names are dialectical variations in the Barí language), average weight 6.0–8.5 kg; see Fig. 6.3a), (3) Sierra de Perijá white-fronted capuchin (Cebus leucocephalus, Gray, 1865, barashì in Barí, weight 1.0–3.5 kg), and (4) gray-handed night or owl monkey (Aotus griseimembra, Elliot, 1912, kogchi’bà or bá’bora in Barí, weight 0.8–1.3 kg; see Fig. 6.3b [Linares 1998]). The Barí also include under the Spanish gloss of “monos” (monkeys), or related to them, two other species of mammals: kinkajou (Potos flavus, Schreber, 1774, called bíshwì in Barí) and eastern lowland olingo (Bassaricyon alleni, Thomas, 1880, called bo’sábù in Barí), both associated with night monkeys because of their nocturnal arboreal habitat and/or prehensile tails (Lizarralde 2002).
Primates have always played a significant role in Barí culture. This importance can be observed in their mythology where monkeys have much more complex and rich mythological stories than any other animals, including fish. Even though the work of Stephen Beckerman (1983) in the 1970s and 1980s shows that fish were even the most important source of protein, monkeys likely surpassed them in precontact times (Lizarralde 2019). According to Beckerman (1975), by weight, monkeys accounted for 5.6% of the consumed proteins and 25% of hunted animals. This figure seems to be relatively low, especially in contrast to the great role monkeys play within the Barí culture. The consumption of monkeys must have been much higher in the past in order for them to figure so prominently in their mythological stories (Lizarralde 2019). Before the 1920s, Barí territory was much larger and supported a fraction of the population density that it has in recent times (Lizarralde 1991; Lizarralde and Lizarralde 2018). Their traditional hunting did not likely have a negative impact on communities of forest animals in the early 1900s as it has had, for instance, in Amazonian Peru due to the recent increase in population density of the Matsigenka (Ohl-Schacherer et al. 2007).
The Barí people have a detailed knowledge of their flora and fauna due to their intimate relationship with their environment and hunting activity (Lizarralde 1997). They recognize close to a thousand plant taxa and more than 350 animals. Also, they have developed a rich understanding of the behavior and diet of the animals they hunt, which enables them to predict the movement and location of these animals. While walking in the forest, the Barí are constantly examining fresh fruits found on the ground in order to determine which animals are eating them, for they can recognize the biting patterns of most taxa. To anticipate the movement of these animals, the Barí hunters check which foods are available, and their seasonalities can help to predict the movements of hunted forest animals.
Besides providing a food source, the other important role monkeys play in indigenous cultures is as pets, which is also common in many other indigenous cultures in the Amazon (Cormier 2003; Cormier and Urbani 2008; Lizarralde 2002; Shepard 2002; Stafford et al. 2016). Cormier (2003: 115) has observed that the Guajá people of Brazil have many pet monkeys, sometimes outnumbering the human inhabitants at some hearths. The Barí definitely enjoy having monkeys as pets, and children continually ask their parents to bring them home from hunting expeditions. The most common pet monkeys among the Barí are spider and night monkeys, while capuchin monkeys are rare. Howler monkeys have never been recorded as pets because they are harder to feed according to the Barí. Monkey pets play a complex role of socialization and enculturation of children to develop a hunter’s ability to discern vocalizations and scents of monkeys while in the forest (Cormier 2003).
The term ethnoprimatology was first coined by Leslie Sponsel (1997) as the subdiscipline that studies indigenous people’s relationship with their nonhuman primate populations. For the New World, there are relatively limited ethnoprimatological studies (e.g., Cormier 2003, 2004; Cormier and Urbani 2008; Lizarralde 2002, 2019; Parathian and Maldonado 2010; Shepard 2002; Stafford et al. 2016). However, with the exception of Cormier (2003, 2004), which provided brief information on the number of useful plants (N = 275) and the percentage (65.2%) of those feeding monkeys, there have been no studies in which ethnoprimatology and ethnobotany overlap in the present. This chapter combines these two subdisciplines for the case of the Barí, providing not only the number and percentage of trees that provide food for primates but also details about tree species demographics and the proportion of the forest providing food for monkeys. This is a new ethnobotanical perspective not applied to the present in ethnoprimatological studies with the exception of the work of L. Cormier (2004).
The theory behind this work is that Barí people have complex and detailed knowledge of their flora, like many indigenous people in the world (Lizarralde 1997, 2004). However, this knowledge is quite variable in a given population, and it is important to recognize and address this variability. Indigenous societies do have their experts who maintain complex and rich information about their flora and fauna and then share this information with community members. Ethnobotanical work completed by Brent Berlin (1992), Berlin et al. (1974), William Balée (2013), and Eglee and Stanford Zent (2002) has provided detailed examples of this extensive local knowledge by various indigenous people in the New World. The work of Shepard et al. (2001) has demonstrated that indigenous people such as the Matsigenka of Peru also have rich local ecological knowledge of their forest, which can provide excellent examples of animals’ relations to their food sources. Berlin’s proposal that saliency (e.g., abundance, size of tree, outstanding characteristics such as a big colorful flower or fruit) would make a tree better known and more utilized (Berlin 1992) has directly been observed in the case of the Barí knowledge of food-providing trees for monkeys.
Another important theoretical aspect of this chapter is historical ecology, since people and animals make changes in their environment and adapt to it (Balée 2013). Cormier (2003: 157) states, “historical ecological perspective takes into account the mutual influence of culture change and environmental change over time.” This perspective contrasts with the notion of an “ethnographic present” and the belief that cultures are unchanged and traditions are being maintained unchanged by millennia. In fact, culture and the environment are constantly changing, perhaps slower in indigenous societies where technology does not evolve as fast as it is in developed nations, but still, changes do occur. Understanding these changes can only be accomplished by taking into account historical events and framing them with people’s notions of adaptation and evolution of behaviors, similar to the way the work of Fikret Berkes has expanded our cognition of the Cree people in Canada (2012). According to Berkes, the Cree have been able to manage their fish and game and adapt to changes of their faunal population without detrimental effect. Drawing on these different theoretical approaches, I am elaborating below on the Barí case.
2 Primate Frugivory
The coevolution of angiosperms and frugivores started 80 million years ago, and the diversity of seed and fruit sizes and types peaked between 55 and 50 million years B.C.E. (Eriksson 2016). This evolutionary process developed as a mutualistic interaction, with fruit trees providing nutritive resources to frugivores (mostly birds and mammals) and frugivores offering seed dispersal services to plants (Eriksson 2016; Herrera 2002; Jordano 2000). The relationship between fruit trees and frugivores is often diffuse, with most frugivores taking advantage of various species of fruiting trees at any given time and vice versa (Lambert and Garber 1998). The larger the animal is, like a spider monkey (Fig. 6.4) or a tapir, the greater the number of seeds of different species of plants they can consume and disperse.
Trees with very large fruit (12–20 cm diameter) are rare in the Barí forest and are species that were once dispersed by megafauna that became extinct with the arrival of humans in the region at the end of the Pleistocene (Guimarães et al. 2008). These tree species have likely survived due to their dispersal by humans and/or agoutis and pacas. For example, in the Barí territory, we have at least three species of trees that were food for extinct megafauna (Gentry 1974, Janzen 1982): algarrobo or copal (bwai boj’bá in Barí, Hymenaea courbaril), the tree gourd (shiima in Barí and tapara in Venezuelan Spanish, Crescentia cujete), and the cannonball (kóba in Barí and coco-de-mono, taparo chuco, muco in Venezuelan Spanish, Couroupita guianensis). These trees produce thick 10–20 cm pods that were broken open by megafauna such as native horses, gomphotheres, and ground sloths (Janzen and Martin 1982; Janzen 1983). However, these trees are very rare in the Barí forests, with very few specimens recorded. For example, the cannonball tree has only been recorded once in all my forest plots; the copal has only been observed one time, a few kilometers northeast of Saimadoyi; and only a few tree gourds have been found at old Barí longhouse sites. Despite the literature suggesting that cannonball fruits can be dispersed by peccaries (Prance and Mori 1978) and copal by agoutis (Hallwachs 1986, Asquith et al. 1999), this does not seem to be the case in the Barí territory, as there appears to be little regeneration of these species. The rarity of these uncommon trees with their extralarge fruits could help to explain the abundance of other trees with medium and large fleshy fruits since the latter are dispersed by primates (Terborgh 1992).
Since the 1970s, many studies have provided detailed information on the topic of fruit trees and frugivores. For example, in the Peruvian Amazon, Terborgh (1986) recorded that 80% of mammalian and avian biomass is frugivorous. In the Eastern Amazon, Balée (1987) observed that 86% of 138 species of trees produced fruits eaten by hunted animals in Tembé territory. Cormier (2004) reported that in the Guajá territory, 90 species of plants were consumed by spider monkeys, 88 for capuchin monkeys, and 74 for black bearded saki monkey. In another study in Barro Colorado Island in Panama, out of the 291 species of trees, 78% produced fleshy fruits (Howe 1984). According to various other studies (Fleming et al. 1987; Jordano 2000, Smythe 1986), 70–94% of the Neotropical trees produce fleshy fruits in the low rainforest. The amount of fruits produced by central Amazonian forest trees is quite high and ranges from 0.85 to 1.3 metric tons per hectare annually (Fittkau and Klinge 1973). Therefore, multiple research studies confirm that lowland Neotropical forest trees provide an important source of food for birds and mammals and in significant amounts that facilitated their coevolution (Eriksson 2016; Fleming et al. 1987; Jordano 2000; Lambert and Garber 1998; Terborgh 1983).
The size of the fruit is an important consideration in their selection by monkeys according to Terborgh (1983: 75) and Eriksson (2016). Larger species of monkeys have preferences for larger fruits (>3 cm), while smaller monkeys focus on smaller fruits (<1 cm). Most of the fruits consumed by primates are medium- (1–3 cm) to large-size (>3 cm) seed drupes or berries. In the New World, primates consume mostly fruits that are “medium (44%) to large (45%) sized drupes (36.3%) and berries (22.2%) that contain seeds of between 0.5 and 2 cm (45.6%) in maximum dimension” (Lambert and Garber 1998: 16).
According to Lambert and Garber (1998), New World monkeys have the tendency of swallowing bigger seeds than Old World monkeys. Capuchin monkeys can swallow seeds as big as 1.8 cm and spider monkeys as big as 3 cm (Rowell and Mitchell 1991; van Roosmalen 1985, cited in Lambert and Garber 1998: 16–17). This is important because New World monkeys can disperse seeds from a greater number of species of trees, especially ones that are large seeded and are therefore not effectively dispersed by smaller-bodied frugivores.
Various studies in the New World detail the diversity of fruiting tree species consumed by primate communities. One of the most extensive studies of monkey diets in the New World is based in Manu Park, Peru (Terborgh 1983). This study recorded that 170 species of plants and 55 botanical families provided food for the primates studied, which represent about 20% of the total flora of Manu (1983: 62). Also in Manu, Lambert and Garber (1998: 16) indicated “153 plant species from 37 families in [their] sampled of the most common fruits eaten by the 14 platyrrhine taxa.” According to Link and Di Fiore (2006), spider monkeys in Ecuador consume 152 species of fruit and disperse 133 of these. All of these studies support primates having a quite diversified diet in different regions.
According to the Barí, monkeys feed on many species of trees, and their forest has plenty of food for them. This view is quite common in the literature about frugivory and primate diet (e.g., Eriksson 2016; Milton 1978, 1980, 1982; Milton et al. 2005; Stevenson et al. 2015; Terborgh 1983). For example, Lambert and Garber (1998: 21) offer a compelling observation: “Given the tens of millions of fruits eaten by primates each year and the hundreds of millions of seeds transported by primates and other animals, dispersers can have a collective ecological effect on the local history of the forest and the present day distribution of trees.”
In conclusion, primates are not passive agents in their forest but actively influencing the distribution and composition of tree species in their forest because fruit trees rely on seed dispersers to move their seeds away from the maternal tree, thereby increasing the seeds’ chances of survival. The Janzen–Connell effect shows the importance of frugivores for maintaining ecological health of forests (Terborgh et al. 2008). This movement of seeds also influences genetic mixing and gene flow within and between populations of trees (Wilson and Traveset 2000). Therefore, anthropogenic changes to forest, such as defaunation and fragmentation, can affect these processes and therefore the regeneration of forest trees (Neuschulz et al. 2016).
3 Methodology
Data for this chapter were collected over 34 months of fieldwork starting in 1988 and ending in 2002 as well as many more months of analyzing it and writing about the Barí ethnoecology. The focus of the research was ethnobotanical looking at not only knowledge variation among individual Barí people but also data on species of trees edible for different animals, and their relative densities were recorded. While collecting information about their flora, the Barí eagerly volunteered information about their fauna and their diet, especially in regard to those that they hunted. In multiple interviews, the Barí were able to consistently indicate the types of fruits or plants that were consumed by different kind of animals, including primates.
I marked off 36 small 30 × 50 m forest plots (including two smaller plots of 20 × 50 m which were added for logistical purposes), covering 5.4 hectares of forest, in order to learn about the variation of the Barí knowledge of trees and to capture a representative census of the forest composition of species (see Fig. 6.1 for the map with locations of places mentioned in the text). I chose this size for the plots because it fits well on an 8.5″ × 11″ piece of paper at the scale of 1:200, facilitating the ease of manipulating this map while presenting a questionnaire for interview informants. From these plots, a list of Barí tree taxa was compiled. The trees censused were equal or bigger than 10 cm dbh (diameter at breast height, 130 cm), which is a standard measurement botanists use for collecting tree species information in forest plots. For this study, 20 adult Barí (7 women and 13 men) were interviewed for each of those forest plots to document their consensus and variation regarding the ethnotaxa. Six Barí men who were good hunters then went over the list of 227 species of trees and confirmed which species provided food for primates and other animals.
For the identification of these species, 398 botanical vouchers were collected between 1988 and 1995, most of them fertile and containing either flower or fruit, if not both. Six sets of each of the vouchers were deposited in the Victor Manuel Ovalles Herbarium (MYF) located in the Pharmacy School at the Universidad Central de Venezuela in Caracas. Three hundred and two of the vouchers were identified by a number of taxonomists who visited the V. M. Ovalles Herbarium as well as by Dr. Stephen Tillet, the director at that time. A set of approximately 100 vouchers, out of the 302, were identified by Dr. Paul Berry, Gerardo Aymard, and other taxonomists in 1996 at the herbarium of the Missouri Botanical Garden. In the last 4 years, a Venezuelan taxonomist, Jose Ramón Grande Allende, also worked on 48 vouchers that were not identified or that were misidentified. Ninety-six vouchers have not been identified, as some of these vouchers may have been discarded in the V. M. Ovalles Herbarium collection due to poor preservation which resulted in the destruction of identifiable features. Scientific names were corrected and updated by G. Aymard in 2018. Originally more vouchers were planned to be collected with the goal of collecting from one and two thousand of them. Also, due to a strong case of hepatitis, the author had to leave the field earlier and was not able to collect more later since the two Colombian guerrilla groups (FARC and ELN) entered the area of research, making this research rather too risky to continue.
The current Barí territory covers 231,000 hectares that are still mostly forested. It is important to indicate that the 5.4 hectares of forest censused consisted of mostly primary forests with a few sections of secondary forests. Not only the forests in this study are focused mostly around the village of Saimadoyi (22 plots), but also a dozen plots extend from the Santa Rosa River on the north to Río de Oro on the south, and two plots on the mid-altitude section of the Sierra de Perijá were added to try to capture a greater number of tree species. Two additional plots (#25 and #26) are by the Ariquaisa River east and outside the Barí reservation (see Fig. 6.1). These last two plots are particularly interesting since they represent what was the largest type of forest originally found in the 1900s Barí territory which originally had a large population of monkeys but which today has been mostly deforested by cattle ranchers.
4 Number of Forest Trees That Produce Food for Primates
In tropical rainforests, we commonly find that species of trees have populations that have extreme numbers ranging from many (potentially hundreds in some cases) to very few individuals, with some being extremely rare with only one or few individuals known by the local population in the region (Terborgh 1992; Gentry 1996; Lizarralde 1997; Brent Berlin 1987: pers. comm.; Glenn Shepard Jr. 1996: pers. comm.; Zizka et al. 2018). The contribution of rare species to the tropical diversity has been recognized (Wills et al. 2006; Kenfack et al. 2007); however, their spatial distribution remains poorly understood (Zizka et al. 2018). The latter recent work on rarity in the Neotropics identified 26,315 species for Amazonia, of these 10,080 species as putatively rare within this region (Zizka et al. 2018). Inside Amazonia most collections of rare species were in the sub-Andean region and on the Guayana Shield and in few areas scattered across the study area. The authors also found that rare species are homogeneously distributed through most parts of the lowland Neotropics and Amazonia but more concentrated in highlands, with no clear disjunction patterns within lowland areas. These results suggest that a considerable proportion of rare plant species has surprisingly large distribution ranges (e.g., Peridiscus lucidus; see Aymard and Arellano 2018) and that collections of rare species across most of the lowland Neotropics, and in particular in Amazonia, show no clear directionality.
These sparse recordings were also observed in the Barí territory with almost half of the species represented only once or twice in the plots censused (Lizarralde 1997). Therefore, it is also relevant to include the number of individual trees since this information offers the demographics and densities of these species, and it indicates the relative amount of food available for the region. The important information missing here is the basal size of the trees that is key to relative abundance of fruits (which will be provided in the near future). Even though the Barí mentioned hog plum (baróo in Barí, Jobo in Spanish, Spondias mombin, Anacardiaceae) or breadnut tree (barúu in Barí; Ramón, Charo, or guaimaro in Spanish; and Mayan nut in English, Brosimum alicastrum, Moraceae) as important for their population of monkeys, having the number of individual trees representing different species might provide a more accurate perspective of which tree species are more important primate food in the Barí forests (instead of only listing species without their number of individual trees representing their species). Further in this chapter, the most important botanical families of trees and species are ranked.
Out of all the trees censused (N = 3664) in the 5.4 hectares of forest, 2476 individual trees (67.8%) provide food for monkeys. Among the 28 botanical families that provide food for monkeys, the top 10 represent 57.5% of all the trees that were censused in the forest plots in the Sierra de Perijá (see Table 6.1). They also represent 86.2% of all the trees that provide food for monkeys.
These leading botanical families are consistent with other regional studies (Russo et al. 2005). Terborgh (1983) also pointed out that the most important botanical families that provide food for the monkeys in Manu Park (Peru) are Moraceae, Annonaceae, Palmae, and Fabaceae. In another study, Stevenson (2004: 277) stated that the most important botanical families of fruit for wooly monkeys were Moraceae, Lecythidaceae, Anacardiaceae, Mimosaceae (in the Fabaceae), Urticaceae, Burseraceae, and Sapotaceae. Also in Manu park, Lambert and Garber (1998: 16) pointed out that 53% of these trees were represented by five botanical families: “Moraceae (23 species, including 12 Ficus species), Leguminosae (16 species), Sapotaceae (8 species), Palmae (8 species), and Annonaceae (6 species).” These findings indicate the Barí forest is not unique or exceptional in terms of the diversity of species feeding its primates.
5 Species of Trees that Produce Food for Primates
Looking at the number of species of trees based on this research, mostly around the village of Saimadoyi (but also around Bachichida, Kumanda, Bokshí, and Bagsarani), we note that 102 species of trees provide food for many animals, including monkeys (see Table 6.2 for the complete list and their details). Therefore, 44.9% of the 227 species of trees censused are food sources for primates.
The 18 most important species of trees noted below represent 46% of all the trees included in this study that provide foods for monkeys (see Table 6.3). This statistic, however, does not represent the preference of these fruits by monkeys but only the most abundant of all fruits known to feed them. Among the most important tree species in this list of trees, the Barí indicated that hog plum (Spondias mombin, Anacardiaceae) was among the top, especially in the mid to high altitudes, between 700 and 1000 meters above sea level on the Sierra de Perijá, where, according to the Barí, the population of spider and howler monkeys is much higher than in other parts of their territory. In fact, the Barí indicated that it was quite obvious that spider monkeys feasted on these fruits because the fat in their abdomen becomes yellowish to orange during the seasons of hog plums, from November to December and June to September. The author was able to confirm this observation, while the Barí were butchering a dead monkey in June of 1999 (see Fig. 6.4).
Another important source of food is breadnut (Brosimum alicastrum, Moraceae), which is also a favorite fruit tree for the monkeys of other Neotropical regions (Russo et al. 2005; Stevenson 2015; Terborgh 1983). It is not surprising that hog plum and breadnut are essential fruit trees for primates and many other animals in these forests. This observation is based on the fruit mass these two trees normally produce (Stevenson et al. 2015). Even though the density of breadnut is not very high (0.46%, with 17 individual trees registered) in the plots recorded, it is much more abundant at higher altitudes between 250 and 700 meters above sea level on the Sierra de Perijá (around plot 27–28) and Serrania de Abusanqui (around Bagsarani village), where the population of spider and howler monkeys is also very high according to the Barí, which was also observed several times between 1995 and 1999.
Similarly, in Yalbac (Belize), in an area surrounded by Mayan ruins and on a large man-made mound, it was observed that the breadnut tree attracts wildlife, including spider and howler monkeys, parrots, and toucans (personal observation in mid-March of 2008, 2011, and 2013). In Perijá, the Barí indicated that the fruits of the breadnut tree are abundant in April, in the beginning of the rainy season, when avocados are also ripe (M. Lizarralde 1995: pers. obs.). In these periods, the Barí also consume a substantial amount of breadnut tree fruits too. Cormier and Urbani (2008: 381) stated that breadnut tree density is very high near Mayan ruins and it is the preferred food for spider monkeys. Terborgh (1983) also mentioned several times that Brosimum fruit was very popular for monkeys in Manu as well as Kinzey and Norconk (1993) for monkeys in the Venezuelan Guayana region of Guri Lake, Bolivar state, mainly B. alicastrum subsp. bolivarense and B. guianense (Aymard et al. 1997).
Russo et al. (2005) stated that Brosimum was among the most preferred genus in four regions for spider monkeys: Tinigua (Colombia), Yasuni (Ecuador), Voltzberg (Surinam), and Barro Colorado Island (Panama). Stevenson et al. (2015) listed three species of Brosimum (B. guianensis, B. alicastrum, and B. lactescens) as very common for monkeys in Tinigua. In the Barí plotted forest, we recorded 47 Brosimum trees (17 B. alicastrum and 30 B. lactescens).
Most tree fruits favored by primates are fleshy and juicy like hog plum or have oily flesh like American oil palm (Attalea butyracea). Others such as breadnut or Mayan nut (Brosimum alicastrum) also provide starchy seeds that attract a large number of not only monkeys but also birds and other animals. A few trees such as Bacú (Cariniana pyriformis) and other medium-size palm fruits provide oily seeds too. Apparently, some fruits are alternative choices if the most preferred fruits are not available in particular times of the year.
Also, the large seeded fruits of different palms, bamboo palm (Oenocarpus mapora) and seje or patauá palm (Oenocarpus bataua var. bataua), are other highly preferred food sources found near the communal houses or where these longhouses once stood. Anthropogenic forests were created where people discarded fruits near their villages or homes, dispersing different tree species and ultimately shaping the forest community structure after villages were abandoned (Balée 2013; Rival 2016). Rival (2016) stated that Oenocarpus bataua was found frequently in old encampments sites. We have observed this pattern clearly with palm species like seje-patauá and American oil palm. The Barí confirmed this, which is also evident from aerial photographs of longhouses that show higher densities of these palms in their vicinity. In fact, further to confirm this observation, plot #36 was established on a former longhouse location where spider monkey hunting was the primary source of protein. This plot had a very high density of different species of palm. In this plot, there were 41 bamboo palms (Oenocarpus mapora, 19.9% density out of 206 individual trees 10 cm dbh were recorded), 12 prickly palms or cubarro (Bactris major var. major, 5.8% density), and 35 seje palm (Oenocarpus bataua var. bataua, 17.0% density). Therefore, these three palm species had a higher density with 42.7% of them recorded in this forest plot. This finding could be an indication that this entire region (the Lora River basin) also has a high density of these palms because they also appear to have been the most commonly gathered wild (anthropogenic) fruits. Also, this region has a very high abundance of monkeys, especially spider monkey with troops of up to 50 individuals (Lizarralde 2002, 2019), who are at the same time dispersing these seeds. Other studies suggest that the genus Oenocarpus is also eaten and dispersed by monkeys (Link and Di Fiore 2006; Russo et al. 2005; Stevenson 2015).
There are two other groups of important trees belonging to the various species of legumes/ice-cream-beans and figs that play an important role in the diet of monkeys as well. For example, one of the types of wild ice-cream-bean trees is called the “howler-monkey-ice-cream-bean” (kamashkorou nondyiruku, Inga cocleensis subsp. megantha). It is not surprising that the fleshy fruits preferred by the Barí people are the same ones that monkeys also consume due to their high caloric content in the form of sugars, which is needed to sustain our large brains. According to Lambert and Garber (1998: 24), “Legume pods … were among the top ten fruit species eaten by many New World monkeys.” In Voltzberg, Surinam, Inga was the second most consumed fruit among spider monkeys (Russo et al. 2005). Stevenson et al. (2015) also reported five species of Inga to be very important to primates. This situation is similar for the Barí since they have 13 species of legume trees with edible pods making up to 380 (15.5%) of individual trees in the plots.
The use of figs for food by monkeys has been consistently cited in the literature (e.g., Díaz-Martín et al. 2014; Terborgh 1983; Lambert and Garber 1998; Stevenson et al. 2015). However, Terborgh (1983, 1986) points out that some figs are the last resort when other fruits are not available, even though figs are important choices in other studies (Lambert and Garber 1998). Stevenson et al. (2015) reported three species of figs to be high in terms of proportions of fruits handled by primates. However, these trees can produce a significant amount of fruits throughout the year, attracting an array of animals (including various species of monkeys) and therefore enhancing their dispersal (Díaz-Martín et al. 2014). For this reason, the Barí like to use fig trees as a place to hunt monkeys by placing a blind with platforms on the crown of nearby trees (Lizarralde 2019). In their forest, we have recorded 23 individual fig trees, many of which tended to be large in general, reaching a diameter of the trunk at breast high over 2 meters and a height of 30–40 meters.
According to Terborgh (1983), the most important tree genera that feed monkeys are Ficus (Moraceae), Brosimum (Moraceae), Guatteria (Annonaceae), Casearia (Salicaceae), Inga (Fabaceae), Cecropia (Urticaceae), Celtis (Cannabaceae), and Cissus (Vitaceae). The main reason is that all of these genera “contain soft pulpy material surrounding the seeds” (Terborgh 1983: 74). Another study by Russo et al. (2005: 1033) notes that “consistent preferences by spider monkeys for Brosimum, Cecropia, Virola, and Ficus. Brosimum may be preferred because of the generally large quantities of fruit produced and long periods of fruit availability on individual trees.”
Primates also have certain preferences for fruits that have bright colors, and these preferences can vary from region to region according to Lambert and Garber (1998). The colors of choice are yellow and red, which account for 50% of fruits (and 80% including also orange and purple) in Manu (Lambert and Garber 1998). A similar pattern emerges among the Barí primates with yellow and red as their preferred fruit colors followed by orange, black, brown, and purple. Therefore, yellow, orange, or red fleshy or pulpy fruits, starchy or oily seeds, figs, and sweet pods are among the preferred fruits among primates in Perija.
Green edible fruits are the most popular color preferred by monkeys in Sierra de Perijá with 665 individual trees (26.9%) and 29 species (28.4%). The reason for it is that they are produced by two of the most important and populous families that commonly produce green fruits (Moraceae and Leguminosae). However, vibrant colors are also very popular if we combine all colors (except green and brown; see Table 6.4). These account for 62.5% (1548 individual trees) of fruits. If we include in all plots censused (N = 3664), this represents 42.2% of all trees. Unfortunately, we do not have information for 25 species and 219 (8.8%) trees that produced edible fruits for monkeys nor information for all other trees that do not produce fruit for monkeys. Like other studies (Lambert and Garber 1998), red fruits are represented with 407 (16.4%) individuals and 10 (9.8%) species, followed by yellow with 345 (13.9%) individuals and 23 (22.6%) species and orange with 251 (10.1%) species. Combining yellow, red, and orange fruits, there are 1062 (42.9%) individual trees and 40 (39.2%) species. Surely, bright color fruits attract monkeys and indicates their ripeness, but surprisingly, green fruits seem to be preferred in Perijá.
6 Discussion
As previously discussed, this high proportion of two-thirds of the forest trees feeding monkeys in the Sierra de Perijá is not unusual in other Amazonian forest regions. It is interesting to note that some of these fruit trees’ common names are associated with primates (e.g., Herrania albiflora as “monkey cacao,” Posoqueria latifolia as “monkey apple,” and “coco-de-mono” for Couroupita guianensis). We also see the “howler-monkey-ice-cream-bean” (a translation of the Barí name kamashkorou nondyiruku, Inga cocleensis). These names clearly indicate the trees’ functions of providing important primate foods.
We need to address the fact that primates are clearly dispersing seeds of the fruit trees that they feed on, especially those which have fleshy or oily mesocarps with smaller-size seeds not exceeding more than 2 or 3 cm. Apparently, spider monkeys can consume seeds as big as 3 cm (van Roosmalen 1985) and white-faced capuchins as big as 1.8 cm without not only destroying but also dispersing them (Rowell & Mitchell 1991, in Lambert and Garber 1998). This dissemination makes these trees more abundant since it is well known in the literature that primates play an important role in dispersing tropical rainforest seeds in the tropical rainforests (Eriksson 2016; Karubian et al. 2015; Stevenson et al. 2015; Terborgh 1986). It is known that white-faced capuchin monkeys disperse live seeds at an average distance of 235 m and as far as 700 m (Valenta and Fedigan 2009). Spider monkeys disperse seeds even farther (average of 443 m and as far away as 1280 m) since they cover a larger territory and move much faster than other monkeys (Link and di Fiore 2006).
Primates clearly exist in an intricate relationship with their forest. They not only are dependent on many fruit trees but also actively change the composition of their forest. As humans “anthropogenically” create “cultural forests,” primates must be doing something very similar, by making their forest a “primatogenical” one. Therefore, we could argue that most of the Neotropical forests are primatogenical since monkeys and humans disperse fruits of most trees, but monkeys still do most of it. For example, the work of Anzures-Dadda et al. (2011) indicated that with the absence of howler monkeys in southern Mexico, the sapling densities and recruitment of Brosimum alicastrum, Dialium guianense, Manilkara zapota, and Damburneya ambigens decrease. Chaves et al. (2011: 177) state that “spider monkeys are effective seed dispersers” with most seeds (>86%) undamaged from 71 species of trees from 23 plant families in the Mexican Lacandon rainforest. Russo et al. (2005: 1033) states that “[s]pider monkeys are one of the first animals to be hunted out of forests…, and their loss is likely to have important consequences for demography, community structure, and gene flow of trees and lianas in tropical forests.” These primates are the largest arboreal frugivorous species that can disperse a greater number of larger seeds, which are still viable.
In this chapter, I am not questioning the Balée (2013) concept of cultural or anthropogenic forest but wonder the role of monkeys in the reproduction of Neotropical forests. It is clear Amerindians have been responsible for the distribution of many species of trees (like Attalea butyracea, Bactris gasipaes, Brazil nuts, canon ball, copal, and tree gourd). Even monkeys help in the reproduction of these cultural forests. According to Balée (1994: 149), the Ka’apor people of Brazil reported that capuchin monkeys frequented old or abandoned gardens dispersing the seeds of Spondias mombin and wild cacao nearby, therefore “responsible for the high frequency and sensitivity of other species in fallows.” However, monkeys seem too play a major role in the reproduction of the forest in general. For example, “Link & De Luna (2004), for example, estimated that spider monkeys could ingest up to 1352 g (mean: 289 g, N = 90) of fruits … in a single feeding bout of the palm Oenocarpus bataua” (Link and Di Fiore 2006: 243). Therefore, larger monkeys like spider monkeys can consume and disperse wider range of fruits that other animals cannot consume and disperse (e.g., birds, bats, agouties, and pacas), being responsible to the reproduction of more species of trees in the rainforest. Link and di Fiore (2006: 243) stated “spider monkeys and other ateline primates are significant dispersers for many species of plants throughout the Neotropics.”
In the late Pleistocene, South America lost a large number of its megafauna that were seed dispersers (7 genera of large mammals that were more than a ton heavy and many more that weighted more over 44 kg, Guimarães et al. 2008). Today, the largest land mammal is the tapir that disperses the large quantities of largest seeds. But because “tapirs frequently defecate in salty lakes, a site unsuitable for successful seedling recruitment” (Donatti et al. 2007: 118), largest monkeys do play a major role dispersing many species of fruiting trees. In the late Miocene Amazon, there used to exist a 20 kg monkey related to spider monkey named Caipora bambuiorum found in NE Brazil. It became extinct only few thousand years ago likely due to human action of hunting (Defler 2019). This species was probably dispersing larger seeds of fruit trees, and these could explain the existence of some of the rare trees that lost their dispersers 10–12 thousands years ago.
7 Conclusion
Of the 227 species of trees found in the Barí territory, 102 species provide food for monkeys. The question is whether this 44.9% of trees that primates feed on is a low, normal, or high percentage. This percentage would have been higher if my plots were bigger and fewer in number since they would not include larger number of rare species that are mostly not food for monkeys. However, this is not abnormally low for other studies. There are only a few studies that provide some potential references. According to Stevenson et al. (2015: 2), primates in the Colombian forests were “responsible for 64% of the fruits manipulated across species.” Stevenson’s percentages are potentially higher than would be the case for all species of trees since they preselected a group of trees (73 species) that are “zoochorous ripe fruiting plants that had good crown visibility” (Stevenson et al. 2015: 3). In another project, Cormier (2003) also stated that 65.2% of the plant species known by the Guajá people of Brazil are also food for monkeys. It is clear that increased numbers of monkey species and therefore size variation will result in subsequent increases in the number of trees that feed monkeys. Perhaps also because the Barí forests have only four species of monkeys, this percentage is smaller (versus seven for the Guajá in the Brazilian Amazon). However, this could have been different if forest plots farther from the Barí village and in areas where monkey densities are higher would have been included in this research.
In the Barí territory, primates are reproducing a type of forest that can continue to provide them with food. The absence of primates could change the future and nature of this forest to one that promotes bird- or wind-dispersed seed trees. Therefore, the conservation of monkeys is key to the future of this forest, which could continue to support larger fauna. This research shows that primates are likely to be keystone species in the reproduction of two-thirds of the Barí forest. Another important point is that the field of ethnobotanical and ethnoecological ethnoprimatology work with indigenous people could provide new lights on the ecology and diet of primates in the tropical forests.
References
Anzures-Dadda A, Andresen E, Martínez ML, Manson RH (2011) Absence of howlers (Alouatta palliata) influences tree seedling densities in tropical rain forest fragments in southern Mexico. Int Primatol 32(3):634–651. https://doi.org/10.1007/s10764-011-9492-0
Asquith NM, Terborgh J, Arnold AE, Rivero CM (1999) The fruits the agouti ate: Hymenaea courbaril seed fate when its disperser is absent. J Trop Ecol 15:229–235
Aymard G (2011) Bosques húmedos macrotérmicos de Venezuela. Biollania (Spanish Edition) 10:33–46
Aymard G, Arellano PH (2018) First report of Peridiscaceae for the vascular flora of Colombia. Harv Pap Bot 23(1):109–121
Aymard G, Norconk M, Kinzey W (1997) Composición florística de comunidades vegetales en islas en el embalse de Guri. Río Caroní. Estado. Bolívar. Venezuela. Biollania 6 (Spanish Edition), pp 195–233
Balée W (1987) A etnobotánica quantitativa dos índios Tembé (Rio Gurupi, Pará). Bol Mus Para Emílio Goeldi, Botanica 3(1):29–50
Balée W (1994) Footprints of the forest: Ka’apor ethnobotany—the historical ecology of plant utilization by an Amazonian people. Columbia University Press, New York
Balée W (2013) Cultural Forest of the Amazon: a historical ecology of people and their landscapes. University Alabama Press, Birmingham
Beckerman S (1975) The cultural energetics of the Bari (Motilones Bravos) of Northern Colombia. Ph.D. thesis, Department of Anthropology, University of New Mexico
Beckerman S (1983) Carpe diem: an optimal foraging approach to Barí fishing and hunting. In: Hames RB, Vickers W (eds) Adaptive responses of native Amazonians. Academic Press, New York, pp 269–299
Beckerman S, Lizarralde R (2013) The ecology of the Barí: rainforest horticulturalists of South America. University of Texas Press, Austin
Berkes F (2012) Sacred ecology, 3rd edn. Editorial Routledge, New York
Berlin B (1992) Ethnobiological classification. Princeton University Press, Princeton
Berlin B, Breedlove DE, Raven PH (1974) Principles of Tzeltal plant classifications. Academic Press, New York/London
Chaves ÓM, Stoner KE, Arroyo-Rodríguez V, Estrada A (2011) Effectiveness of spider monkeys (Ateles geoffroyi vellerosus) as seed dispersers in continuous and fragmented rain forests in southern Mexico. Int J Primatol 32(1):177–192. https://doi.org/10.1007/s10764-010-9460-0
Cormier LA (2003) Kinship with Monkeys: the Guajá foragers of eastern Amazonia. Columbia University Press, New York
Cormier LA (2004) Monkey ethnobotany. In: Stepp JR, Wyndham FS, Zarger RK (eds) Ethnobiology and cultural diversity. Proceedings of the seventh international congress of ethnobiology. International Society of Ethnobiology, pp 313–325
Cormier L, Urbani B (2008) The ethnoprimatology of spider monkeys (Ateles spp.): from past to present. In: Campbell CJ (ed) Spider monkeys: behaviour, ecology and evolution of the Genus Ateles. Cambridge University Press, Cambridge, pp 377–403
Defler T (2019) History of terrestrial mammals in South America: how South American mammalian fauna changed from the Mesozoic to recent times. Springer, Dordrecht
Diaz-Martin Z, Swamy V, Terborgh J, Alvarez-Loayza P, Cornejo F (2014) Identifying keystone plant resources in an Amazonian forest using a long-term fruit-fall record. J Trop Ecol 30:291–301. https://doi.org/10.1017/S0266467414000248
Donatti CI, Galetti M, Pizo MA, PR Guimarães JR, Jordado P (2007) Living in the Land of Ghosts: Fruit Traits and the Importance of Large Mammals as Seed Dispersers in the Pantanal, Brazil. In AJ Dennis et al. Seed Dispersal: Theory and its Application in a Changing World. CAB International, Wallingford pp 104–123
Eriksson O (2016) Evolution of angiosperm seed disperser mutualisms: the timing of origins and their consequences for coevolutionary interactions between angiosperms and frugivores. Biol Rev 91:168–186
Finer M, Vijay V, Ponce F, Jenkins C, Kahn T (2009) Ecuador’s Yasuní Biosphere Reserve: a brief modern history and conservation challenges. Environ Res Lett 4(3):034005. https://iopscience.iop.org/article/10.1088/1748-9326/4/3/034005/fulltext/. Accessed 15 Dec 2016
Fittkau EJ, Klinge H (1973) On biomass and trophic structure of the central Amazonian rain forest ecosystem. Biotropica 5:2–14
Fleming TH, Breitwisch R, Whitesides GH (1987) Patterns of tropical vertebrate frugivore diversity. Annu Rev Ecol Evol Syst 18:91–109
Gentry A (1974) Coevolutionary patterns in Central America Bignoniaceae. Ann Mo Bot Gard 61:728–759
Gentry A (1990) Floristical similarities and differences between Southern Central America and Upper and Central Amazonia. In: Gentry AH (ed) Four neotropical rainforests. Yale University Press, New Haven, pp 141–160
Gentry A (1996) A field guide to the families and genera of woody plants of Northwest South America: (Colombia, Ecuador and Peru) with supplementary notes on herbaceous taxa. Chicago University Press, Chicago
Guimarães PR Jr, Galetti M, Jordano P (2008) Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS One 3(3):1–13., e1745. https://doi.org/10.1371/journal.pone.0001745
Hallwachs W (1986) Agoutis (Dasyprocta punctata): the inheritors of guapinol (Hymenaea courbaril: Leguminosae). In: Estrada A, Fleming TH (eds) Frugivores and seed dispersal. W. Junk Publishers, Dordrecht, pp 285–304
Herrera CM (2002) Seed dispersal by vertebrates. In: Herrera CM, Pellmyr O (eds) Approach plant-animal interactions: an evolutionary. Oxford Press, Blackwell, pp 185–208
Howe HF (1984) Implications of seed dispersal by animals for tropical reserve management. Biol Conserv 30:261–281
Huber O, Alarcón C (1988) Mapa de vegetación de Venezuela, con base en criterios fisiográfico-florísticos. 1:2.000.000. MARNR, The Nature Conservancy, Caracas
Janzen DH (1982) Fruit traits, and seed consumption by rodents, of Crescentia alata (Bignoniaceae) in Santa Rosa National Park, Costa Rica. Am J Bot 69(8):56–57
Janzen DH (1983) Hymenaea courbaril: (Guapinol, Stinking Toe). In: Janzen DH (ed) Costa Rican natural history. University of Chicago Press, Chicago, pp 253–256
Janzen DH, Martin PS (1982) Neotropical anachronisms: the fruits the gomphotheres ate. Science 215:19–27
Jordano P (2000) Fruits and frugivory. In: Fenner M (ed) The ecology of regeneration in plant communities, 2nd edn. CAB International, Wallingford, pp 125–166
Karubian J, Ottewell K, Link A, Di Fiore A (2015) Genetic consequences of seed dispersal to sleeping trees by white-bellied spider monkeys. Acta Oecol 68:50–58. https://doi.org/10.1016/j.actao.2015.07.005
Kenfack D, Thomas DW, Chuyong G, Condit R (2007) Rarity and abundance in a diverse African forest. Biodivers Conserv 16:2045–2074
Kinzey W, Norconk MA (1993) Physical and Chemical Properties of Fruits and Seeds Eaten by Pithecia and Chiropotes in Surinam and Venezuela. Int J Primatol 14(2):207–227
Lambert JE, Garber PA (1998) Evolutionary and ecological implications of primate seed dispersal. Am J Primatol 45:9–28
Linares O (1998) Mamíferos de Venezuela: Sociedad Conservacionista Audubon de Venezuela. Caracas, Venezuela
Link A, De Luna AG (2004) The importance of Oenocarpus bataua (arecaceae) in the diet of spider monkeys at Tinigua National Park, Colombia. Folia Primatol (abstract) 75:391
Link A, Di Fiore A (2006) Seed dispersal by spider monkeys and its importance in the maintenance of neotropical rain-forest diversity. J Trop Ecol 22(3):235–246
Lizarralde R (1991) Barí settlement patterns. Hum Ecol 19(4):428–452
Lizarralde M (1997) Perception, knowledge and use of the rainforest: ethnobotany of the Barí of Venezuela. Ph.D. thesis. Anthropology Department, California University at Berkeley
Lizarralde M (2002) Ethnoecology of monkeys among the Barí of Venezuela: perception, use and conservation. In: Fuentes A, Wolfe L (eds) Primates face to face: the conservation implications of human and nonhuman primate interactions. Cambridge University Press, Cambridge, pp 85–100
Lizarralde M (2004) Indigenous knowledge and conservation of the rainforest: ethnobotany of the Barí of Venezuela. In: Carlson TJ, Maffi L (eds) Ethnobotany and conservation of biocultural diversity, Advances in economic botany, vol 15. The New York Botanical Garden Press, Bronx, pp 112–129
Lizarralde M (2019) Etnoprimatología Barí en la Sierra de Perijá del estado Zulia. In: Urbani B, Ceballos-Mago N (eds) La Primatología en Venezuela. Tomo I Academia Nacional de Ciencias Exactas, Físicas y Naturales/Universidad Simón Bolívar (Colección Conjunta Acfiman/USB), Caracas
Lizarralde M, Lizarralde R (2018) Los Barí. In: Pereira MA, Rivas P (eds) Los Aborígenes de Venezuela, Volumen 5 (Monografía 52). Fundación La Salle de Ciencias Naturales, Caracas, pp 725–886
Milton K (1978) Behavioral adaptations to leaf-eating by the mantled howler monkey (Alouatta palliata). In: Montgomery GG (ed) The ecology of arboreal folivores. Smithsonian Institution Press, Washington, D.C., pp 535–549
Milton K (1980) The foraging strategy of howler monkeys: a study in primate economics. Columbia University Press, New York
Milton K (1982) Dietary quality and demographic regulation in a howler monkey population. In: Leigh EG, Rand AS, Windson DM (eds) The ecology of a tropical forest: seasonal rhythms and long-term changes. Smithsonian Institution Press, Washington, D.C., pp 273–289
Milton K, Giacalone J, Wright SJ, Stockmayer G (2005) Do frugivore population fluctuations reflect fruit productions? Evidence from Panama. In: Dew L, Boubli JP (eds) Tropical fruits and frugivores: the search for strong interactors. Springer, Dordrecht, pp 5–36
Neuschulz EL, Mueller T, Schleuning M, Böhning-Gaese K (2016) Pollination and seed dispersal are the most threatened processes of plant regeneration. Sci Rep 6:29839. www.nature.com/scientificreports/. https://doi.org/10.1038/srep29839
Ohl-Schacherer J, Shepard GH Jr, Kaplan H, Peres CA, Levi T, Yu DW (2007) The sustainability of subsistence hunting by Matsigenka native communities in Manu National Park, Peru. Conserv Biol 21:1174–1185
Parathian HE, Maldonado AM (2010) Human-nonhuman primate interactions amongst Tikuna people: perceptions and local initiatives for resource management in Amacayacu in the Columbian Amazon. Am J Primatol 71:1–11
Pittier H (1948) Trabajos Escogidos. Ministerio de Agricultura y Cria, Caracas
Prance GT, Mori SA (1978) Observations on the fruits and seeds of neotropical Lecythidaceae. Brittonia 30:21–33
Rival L (2016) Huaorani transformations in twenty-first century ecuador: treks into the future of time. The University of Arizona Press, Tucson
Rowell TE, Mitchell BJ (1991) Comparison of seed dispersal by guenons in Kenya and capuchins in Panama. J Trop Ecol 7:269–274
Russo SE, Campbell CJ, Dew JL, Stevenson PR, Suarez SA (2005) A multi-forest comparison of dietary preferences and seed dispersal by Ateles spp. Int J Primatol 26(5):1017–1037. https://doi.org/10.1007/s10764-005-6456-2
Shepard G Jr (2002) Primates in Matsigenka subsistence and world view. In: Fuentes A, Wolfe K (eds) Primates face to face: the conservation implications of human and nonhuman primate interactions. Cambridge University Press, Cambridge, pp 101–136
Shepard G Jr, Yu D, Lizarralde M, Italiano M (2001) Rainforest habitat classification among the Matsigenka of the Peruvian Amazon. J Ethnobiol 21(1):1–38
Smythe N (1986) Competition and resource partitioning in the guild of neotropical terrestrial frugivorous mammals. Annu Rev Ecol Syst 17:169–188
Sponsel LE (1997) The human niche in Amazonia: exploration in ethnoprimatology. In: Kinzey WG (ed) New World primates: ecology, evolution, and behavior. Aldine, Chicago, pp 143–165
Stafford CA, Alarcon-Valenzuela J, Patino J, Preziosi RF, Sellers WT (2016) Know your monkey: identifying primate conservation challenges in an Indigenous Kichwa community using an ethnoprimatological approach. Folia Primatol 87(1):31–47
Stevenson PF (2004) Fruit choice by woolly monkeys in Tinigua National Park, Colombia. Int J Primat 25(2):367–381
Stevenson PR, Link A, González-Caro S, Torres-Jiménez MF (2015) Frugivory in canopy plants in a Western Amazonian forest: dispersal systems, phylogenetic ensembles and keystone plants. PLoS One 10(10):1–22., e0140751. https://doi.org/10.1371/journal.pone.0140751
Steyermark JA (1982) Relationships of some Venezuelan forest refuges with lowland tropical floras. In: Prance GT (ed) Biological diversification in the Tropics. Columbia University Press, New York, pp 182–220
Terborgh J (1983) Five New World primates: a study in comparative ecology. Princeton University Press, Princeton
Terborgh J (1986) Community aspects of frugivory in tropical forests. In: Estrada A, Fleming TH (eds) Frugivores and seed dispersal. Dr. W. Junk Publishers, Dordrecht, pp 371–384
Terborgh J (1992) Diversity and tropical rain forest. Scientific American Library, New York
Terborgh J (1999) Requiem for nature. Island Press, Washington, D.C.
Terborgh J, Nuñez-Iturri G, Pitman NCA, Cornejo Valverde FH, Alvarez P, Swamy V, Pringle EG, Timothy Paine CE (2008) Tree recruitment in an empty forest. Ecology 89(6):1757–1768
Valenta K, Fedigan L (2009) Spatial patterns of seed dispersal by White-Faced Capuchins in Costa Rica: evaluating distant-dependent seed mortality. Biotropica 42(2):223–228
van Roosmalen MGM (1985) Habitat preferences, diet, feeding strategy and social organization of the black spider monkey (Ateles paniscus paniscus Linnaeus 1758) in Surinam. Acta Amazon 15:1–238
Wills C, Harms KE, Condit R, King D, Thompson J, He F, Muller-Landau HC, Ashton P, Losos E, Comita L, Hubbell S, Lafrankie J, Bunyavejchwin S, Dattaraja HS, Davies S, Esufali S, Foster R, Gunatilleke N, Gunatilleke S, Hall P, Itoh A, John R, Kiratiprayoon S, Kassim SR, Sukumar R, Suresh SH, Fang Sun I, Tan S, Yamakura T, Zimmerman J (2006) Nonrandon processes maintain diversity in tropical forests. Science 311:527–531
Wilson MF, Traveset A (2000) The ecology of seed dispersal. In: Fenner M (ed) The ecology of regeneration in plant communities, 2nd edn. CAB International, Wallingford, pp 85–110
Zent E, Zent S (2002) Los Jodï: sabios botánicos del Amazonas venezolano. Antropologica 97–98:29–70
Zizka A, ter Steege H, Do Céo M, Pessoa R, Antonelli A (2018) Finding needles in the haystack: where to look for rare species in the American tropics. Ecography 41:321–330
Acknowledgments
I am very thankful to all the Barí people for allowing the author to conduct research and reside in their cultural and natural environment and for providing prodigious amount of information and support for this research, especially from Akirihda, Iribi, Arukbá, Sarukbá, Abuyokba, Abokoré, Abohkín, Ataktabá, Mandabó, Ashkoró, Oroksá, Elizabeth Asigbera, and Emilio Aleobaddá. The help of Andres Achirabu, David Aleobaddá, and all their family members, in particular, made this work possible. Without their generosity and kind support, this chapter could not have been written. Funding for research was provided by Connecticut College (R. F. Johnson Faculty Development Fund). I want to thank my friend Jorge Cruz, anthropologist and writer, for reviewing and correcting my abstract in Spanish, Christopher Cobalth for editorial suggestions, my former student Zoe Diaz-Martin’14 and my mentor/padrino Dr. Stephen Beckerman for making many useful suggestions and corrections. I am also very grateful to Gerardo Aymard (PORT) because he corrected the botanical and taxonomical nomenclature of trees in this chapter and Paul Berry for the identification of my vouchers. Finally, I am extremely grateful to my wife, Anne-Marie, for her meticulous editorial assistance with this chapter. I am very grateful for all the helpful comments that Leslie Sponsel and William Balée provided in this chapter to improve it and the other editor of this volume, Bernardo Urbani, for all the work involved in the production of it.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lizarralde, M. (2020). Frugivorous Monkeys Feeding in a Tropical Rainforest: Barí Ethnobotanical Ethnoprimatology in Venezuela. In: Urbani, B., Lizarralde, M. (eds) Neotropical Ethnoprimatology. Ethnobiology. Springer, Cham. https://doi.org/10.1007/978-3-030-27504-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-27504-4_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27503-7
Online ISBN: 978-3-030-27504-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)