Abstract
Two decades of paleontological discoveries of basal birds and non-avian theropods with preserved integumentary structures, especially in Late Jurassic to Early Cretaceous deposits from northeastern China, have greatly improved our understanding of the origin and early evolution of birds and their plumage. Here, we present a concise review of the plumage evolution within pennaraptora, the most inclusive clade containing Oviraptorosauria and Paraves. Feather or feather-like morphotypes were particularly diversified in non-avialan paravians, suggesting that they probably already fulfilled a wide array of biological roles, including thermoregulation and visual display. The feather-like structures in non-eumaniraptoran paravians were obviously not adapted for flight. However, Microraptor and maybe some of its relatives preserve large pennaceous feathers along the limbs and tail, similar in morphology and organization to those in modern birds, so that they could have functioned in active flight or passive gliding. Several aerodynamic innovations and flight-related morphological adaptations were (likely independently) experimented within the paravian clade close to the origin of birds. The origin and early evolution of complex feathers and flight abilities in paravian theropods were not linear processes, but more complex than previously thought.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
7.1 Introduction
Before the discovery of Sinosauropteryx, a small Early Cretaceous coelurosaurian theropod from Liaoning Province in China (Ji and Ji 1996; Chen et al. 1998), feathers were thought to be an exclusively avialian character. After two decades of discoveries, feathers and feather-related structures are known to be quite widespread among dinosaurs based on fossils with a great range of size, osteology, limb proportions, and integumentary coverings (Hopson 2001; Ji et al. 2001). Moreover, feathers and feather-related structures may have been present in the common ancestors of both saurischian and ornithischian dinosaurs (Witmer 2009; Godefroit et al. 2014). Here, we briefly describe the feathers and plumage of pennaraptoran theropods, a monophyletic clade containing Oviraptor philoceratops, Deinonychus anthirrhopus, and Passer domesticus) but not Therizinosauroidea (Therizinosaurus cheloniformis). This monophyletic group encompasses five groups of bipedal dinosaurs characterized by a trend in size reduction and the presence of pennaceous feathers (Foth et al. 2014; Lee et al. 2014): oviraptorosauria, scansoriopterygidae (a group of hypothetized bat-winged dinosaurs), Troodontidae, Dromaeosauridae, and Avialae.
The presence of feather-related structures is not limited to pennaraptoran dinosaurs. The first evidence of integumentary structures among theropods was reported in Pelecanimimus, a small ornithomimosaur from the Lower Cretaceous of Spain (Pérez-Moreno et al. 1994). The specimen shows preserved impressions of subparallel fibers arranged perpendicular to the bone surface below the neck and around the right humerus and elbow (Pérez-Moreno et al. 1994). Two years later, Sinosauropteryx, a small coelurosaurian dinosaur from the Lower Cretaceous of China (Chen et al. 2005), was reported by Ji and Ji (1996). Integumentary structures are preserved in this specimen along the dorsal surface of the neck, the back, the hip, and along the upper and lower margins of the tail (Chen et al. 1998). The nature of these appendages has been largely discussed (Currie and Chen 2001; Zhou and Zhang 2006; Lingham-Soliar 2012) and their affinities with feathers are still debated. Despite the debate about the relationship between these integumentary structures and feathers, integumentary structures have been reported in a medium-sized therizinosaurid theropod, Beipiaosaurus, which shows integumentary appendages close to those of Sinosauropteryx (Xu et al. 1999a). Zhou and Zhang (2006) suggested that the finely preserved filaments near the ulna of Beipiaosaurus were the precursors to secondary flight feathers. Protofeathers were also reported in Dilong and Yutyrannus, two basal tyrannosauroids from the Cretaceous of China (Xu et al. 2004, 2012). The filamentous protofeathers of Dilong were probably branched as in modern feathers and preserved around the posterior left mandible and around the tail (Xu et al. 2004). Filaments composing the feathers were joined at their bases along a central filament (Xu et al. 2004). In Yutyrannus, the filamentous integumentary structures were preserved close to the tail, the pelvis, the pes, the neck, and near a limb bone (identified as a humerus) (Xu et al. 2012). It seems that the feather covering in tyrannosauroids was used for thermoregulation and/or display structures rather than in flight (Xu et al. 2004, 2012).
7.2 Oviraptorosauria
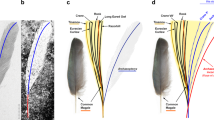
Some plumage characteristics present in birds and, in a wider context, in paravians were inherited from this clade of Cretaceous theropod dinosaurs. The presence of integumentary structures in oviraptorosauran theropods was recently reported in Caudipteryx, Protarchaeopteryx, and Similicaudipteryx (Ji et al. 1998; Xu et al. 2010) (Fig. 7.1). The presence of remiges (i.e., flight feathers of modern birds and paravians) were reported in Caudipteryx and Similicaudipteryx (Ji et al. 1998; Xu et al. 2010). Fourteen remiges were attached to the hand of Caudipteryx (i.e., primaries) (Ji et al. 1998), whereas 10 primary and 12 secondary remiges were preserved along each arm of Similicaudipteryx (Xu et al. 2010). No flight feathers were reported in Protarchaeopteryx (Ji et al. 1998). In Caudipteryx and Similicaudipteyrx, the remiges were symmetrical (i.e., both vanes had the same width along the rachis) and elongated (Ji et al. 1998; Xu et al. 2010). Unlike in modern birds, in which the first primaries are the longest ones, the middle primaries were reported to be the longest ones in Caudipteryx and Similicaudipteryx (Lucas and Stettenheim 1972; Ji et al. 1998; Xu et al. 2010). One of the main characteristics of Oviraptorosauran dinosaurs is the reduction of the tail, which ends with a stiffened portion (even a pygostyle in Nomingia) (Osmólska et al. 2004). The reduced tail of Caudipteryx, Protarchaeopteryx, and Similicaudipteryx bears several rectrices (i.e., tail feathers of modern birds) organized as a fan (Persons et al. 2014), which are restricted to last caudal vertebrae (Ji et al. 1998; Xu et al. 2010). These rectrices are symmetrical and their basal portion is plumulaceous as in modern birds (Ji et al. 1998). Barbules have been reported in the distal portion of the rectrices in Protarchaeopteryx (Ji et al. 1998). Plumulaceous feathers were found around the chest, the tail, and the femora of Protarchaeopteryx, whereas plumulaceous feathers were found around the tail of Caudipteryx (Ji et al. 1998). Long branching filamentous structures were found all around the skeleton of Similicaudipteryx (Xu et al. 2010).
Although the presence of a simple wing (i.e., formed by a single row of remiges) and a tail fan of feathers have been reported among some oviraptorosaurs, they are seen as flightless (Persons et al. 2014). Their feathering was not adapted to ensure an effective role in flight: the remiges were symmetrical, restricted to the forearm, and only slightly longer than the humerus; the distal remiges were shorter than the proximal ones; the forelimbs were proportionally shorter than that of modern birds; and the rectrices were more implied in sexual/visual display than to produce thrust for flight (Ji et al. 1998; Persons et al. 2014).
7.3 The Strange Case of Scansoriopterygidae
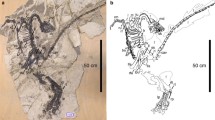
Scansoriopterygidae is a clade of basal paravians that was first characterized by the extreme elongation of the third digit. This is in contrast with birds that retain the plesiomorphic condition of neotheropods in which the second digit is the longest (we follow the “traditional” morphological digital identity homology pattern, in which the digit numbering of the manus of modern birds is I-II-III) (Zhang et al. 2002). The plumage of Epidendrosaurus, the first described scansoriopterygid, is limited to faint feather imprints more or less resembling those of Microraptor (Zhang et al. 2002). Two types of feather-like structures were subsequently described in Epidexipteryx: the dorsal part of the neck, the back, the pectoral region, and the distal part of the hip are covered by filamentous parallel barbs [called non-elongated ribbon-like tail feathers or non-ETF; Zhang et al. (2008a)] comparable to the down-like feathers in some feathered dinosaurs and primitive birds (e.g., Sinornithosaurus, Sinosauropteryx, Anchiornis, Xiaotingia) (Xu 2006; Zhang et al. 2008a) (Fig. 7.2). The ten caudal vertebrae of Epidexipteryx bear four elongated ribbon-like tail feathers with a broad central element, presumably the rachis, as encountered in Confuciusornithiformes and Enantiornithines (Zhang et al. 2008a, b). As in Confuciusornithiformes, those ornamental feathers may have played a role in visual display (Zhang et al. 2008a). Pennaceous feathers are absent along both the forelimbs and the hindlimbs, so scansoriopterygids were first regarded as secondary flightless paravians (Zhang et al. 2008a). However, the recently discovered Yi is characterized by the presence of rod-like structures (i.e., styliform elements) extending from each wrist and delimiting a membranous tissue preserved between the rod-like bones and the manual digits (Xu et al. 2015) (Fig. 7.2b). Those structures are thought to play the same role as the pteroid bone of pterosaurs or the styliform cartilage of flying squirrels and anomalures in supporting a membranous wing (Benett 2007; Xu et al. 2015). Thin stiff filamentous feathers are preserved around the skull, the neck, the forelimbs, and the hindlimbs up to the metatarsus (Xu et al. 2015). Some feathers in the skull and neck regions present a simple branching pattern with multiple radiating filaments, whereas feathers attached to the limbs are composed of an undifferentiated shaft-like structure with numerous parallel distal filaments (Xu et al. 2015). The presence of a membranous wing in scansoriopterygidae has been recently confirmed with the discovery of Ambopteryx (Wang et al. 2019) (Fig. 7.2c). A styliform element is preserved in articulation with the left wrist, and the matrix surrounding the left hand, right forelimb, and abdomen is coated in a brownish continuous layer bearing ripple-like striations (Wang et al. 2019). These elements confirm that the elongated third digit and the styliform element serve as supports for a membranous wing (Xu et al. 2015; Wang et al. 2019).
Selected specimens of Scansoriopterygidae. (a) STM 31-2, the holotype of Yi characterized by the presence of rod-like structures extending from each wrist (white arrows) (from Xu et al. 2015). (b) IVPP V24192, the holotype of Ambopteryx (white arrow points to the styliform element) (from Wang et al. 2019)
Initially, the long manus of Scansoriopterygidae was thought to be an adaptation for grasping or grabbing tree trunks rather than for flapping flight (Zhang et al. 2002). It has also been proposed that the elongated third digit of scansoriopterygids serves as a tool for extracting grubs during foraging (Wang et al. 2019). Now this trait is viewed as an adaptation to hold a membranous wing (Xu et al. 2015; Wang et al. 2019). The apparent lack on pennaceous feathers along the forelimbs in Scansoriopterygidae is therefore tentatively viewed as a secondary loss within this clade to allow the presence of a bat wing.
7.4 Anchiornithidae
This new informal group of small-bodied paravian theropods from the Upper Jurassic of China is characterized by extensive feathering. Depending on the phylogenetic analysis, anchiornithids are seen as basal paravians (Lefèvre et al. 2017), basal troodontids (Hu et al. 2009; Turner et al. 2012), or basal deinonychosaurians (Xu et al. 2011, 2015; Hu et al. 2018). The clade includes Anchiornis, Pedopenna, Eosinopteryx, Aurornis, Serikornis, Caihong, and Ostromia (Haarlem specimen) (Fig. 7.3). Only the last species comes from Germany, whereas the first five are from the Upper Jurassic of northeastern China (Inner Mongolia and Liaoning provinces). The presence of a feathered wing along the forelimb in Anchiornithidae is a pennaraptoran symplesiomorphy shared with Oviraptorosauria, although it may represent a symplesiomorphy shared with Ornithomimosauria because the presence of feathers is assumed in an adult specimen of Ornithomimus (Zelenitsky et al. 2012). Although the forewing of Oviraptorosauria seems to be formed by a single layer of long remiges, the wing of the basal paravians Anchiornis and Serikornis is formed by multiple layers of indistinct contour and flight feathers (Longrich et al. 2012; pers. obs.). The forewing of Anchiornis is formed by short symmetrical feathers (Hu et al. 2009; Pei et al. 2017), but some primary remiges show a slight curvature in the rachis (Saitta et al. 2017). The barbs of the remiges and major coverts of Anchiornis are not tight together, especially at their tip (Saitta et al. 2017). Saitta et al. (2017) suggested that the absence of differentiation between proximal and distal barbules results in feathers with open vanes, such as those reported in Anchiornis. In Serikornis, remiges are short, symmetrical, straplike, and undifferentiated (i.e., there are no significant length differences between remiges and wing coverts) with coverts contributing substantially to the airfoil (Lefèvre et al. 2017). The feathers are devoid of barbules along the forelimb and hindlimb (Lefèvre et al. 2017). The same configuration was observed in the secondary wing feathers of Eosinopteryx (Godefroit et al. 2013a). In Aurornis, forewing feathers are not preserved, with the exception of bundles of parallel filaments joined together proximally in the proximal third of the tail (Godefroit et al. 2013b). In Caihong, the feathers are too densely preserved to be adequately described (Hu et al. 2018). However, the forelimb is covered by vaned feathers with the proximal primaries and the distal secondaries being the longest remiges (Hu et al. 2018). These feathers are 2.4 times as long as the humerus with narrow rachises (Hu et al. 2018). One striking feature is the presence of alular feathers on the first digit of Caihong (considering the I-II-III pattern for digit numbering in birds and bird relatives) (Hu et al. 2018).
Selected specimens of Anchiornithids with the best preserved plumage. (a) IVPP V12721, the holotype of Pedopenna (from Xu and Zhang 2005). (b) YFGP-T5197, the holotype of Eosinopteryx (from Godefroit et al. 2013b). (c) YFGP-T5199, a specimen of Anchiornis (from Lindgren et al. 2015). (d) PMOL-AB00200, the holotype of Serikornis (from Lefèvre et al. 2017). (e) PMOL-B00175, the holotype of Caihong (from Hu et al. 2018)
Unlike in oviraptorosaurs, hindlimb feathers are well developed in Anchiornithidae. Trousers of elongated and symmetrical pennaceous tibial and metatarsal feathers are present in Pedopenna, Eosinopteryx, Serikornis, Caihong and Anchiornis (Xu and Zhang 2005; Hu et al. 2009, 2018; Godefroit et al. 2013a; Lefèvre et al. 2017; Pei et al. 2017; Saitta et al. 2017). The hindlimb feathers in Caihong are reported to be relatively more elongated than those of other paravians (Hu et al. 2018). The anterior side of the legs of Caihong is covered by numerous small feathers that extend on the pedal digits (Hu et al. 2018). This trait is also reported in Anchiornis and Serikornis, where the extensive feathering formed by a bundle of filaments extends up to the end of each toe (Hu et al. 2009; Lefèvre et al. 2017). This character is also seen in some modern birds, with an insulating or protective function (Weidensaul 1995; Hu et al. 2009).
Although elongated rectrices are present along the distal half of the tail in Oviraptorosauria (Ji et al. 1998; Xu et al. 2010) and Microraptor (Li et al. 2012), rectrices are absent in Serikornis, Aurornis, and Eosinopteryx; however, the absence of tail feathering may be a taphonomic artefact of melanosome preservation. In Caihong, large pennaceous feathers are attached to all caudal vertebrae together with covert feathers (Hu et al. 2018). The tail feathers in the most anterior part of the tail are less organized than in the middle and posterior regions but are supposed to be pennaceous (Hu et al. 2018). In Anchiornis, the proximal portion of the tail appears to be covered with plumulaceous features because no rachises were observed, whereas the middle and distal portions of the tail are associated with elongated pennaceous feathers that appear almost parallel to the caudal column (Hu et al. 2009; Pei et al. 2017). However, at this point, the tail of these basal paravians seems to have no aerodynamic function in increasing the total lift of the animal, nor any display function. The long bony tail was completely covered by elongated but symmetrical pennaceous rectrices in Anchiornis (Hu et al. 2009; Pei et al. 2017), resembling the rectrices in Archaeopteryx (in this species, the lateral rectrices are asymmetrical) (Foth et al. 2014).
7.5 Troodontidae
This phylogenetic position of this clade of small, lightly built maniraptorans from Cretaceous deposits of Asia and North America has been recently challenged. Pending on the phylogenetic analysis, it is positioned as the sistergroup of Dromaeosauridae (Turner et al. 2012; Lefèvre et al. 2017) in a larger clade named Deinonychosauria or as the sistergroup of Avialae (Godefroit et al. 2013b; Foth and Rauhut 2017). While dromaeosaurids from the Yixian and Jiufotang formations of Liaoning Province are well-documented concerning feather types and plumage, most troodontids appeared to lack integumentary structures; although numerous troodontid specimens have been discovered in the Yixian Formation of Liaoning Province (Sinovenator, Mei, Sinusonasus), most have been found in the Lujiatun Bed, which favorable for the three-dimensional preservation of specimens but not for the preservation of integumentary structures. Therefore, the apparent lack of feathers is surely due to an artifact of taphonomy or is caused by an erasing during preparation rather than a true absence. However, the discovery of Jianianhualong in the Lower Cretaceous Yixian Formation has greatly improved our understanding of troodontid feathering (Xu et al. 2017) (Fig. 7.4). The body of Jianianhualong is covered by long feathers, with the dorsal and sacral ones longer than the cervical feathers (Xu et al. 2017). Large pennaceous feathers are preserved dorsally and ventrally to nearly all caudal vertebrae as in Jinfengopteryx, another troodontid from China (Fig. 7.4a), forming a frond-like feathery tail (Xu et al. 2017). These feathers are curved with the distal edge convex and the proximal edge concave (Xu et al. 2017). In one well-exposed tail feather, the trailing vane is about twice as wide as the leading vane, with the blunt distal end wider than the proximal region (Xu et al. 2017). Feathers are also preserved near the humerus, ulna, and the middle portion of the tibia, but few details are preserved (Xu et al. 2017). Integumentary structures are also reported in Jinfengopteryx, another presumably basal troodontid theropod from the Early Cretaceous Huajiying Formation Heibei Province in China (Ji and Ji 2007). This specimen displays two patches of feathers on the right fingers, but no trace of flight feathers were preserved (Ji et al. 2005; Agnolin and Novas 2013). Only long and symmetrically-vaned tail feathers are well preserved and seem to attach to practically all caudal vertebrae (Ji et al. 2005). This configuration of the tail feathers is closer to that of Anchiornis, Archaeopteryx, and Jianianhualong, in which pennaceous feathers are attached to all caudal vertebrae, than that of some oviraptorosaurs and Serikornis, in which the tail feathers are gathered on the distal caudal vertebrae (Gatesy and Dial 1996; Ji et al. 2005; Xu et al. 2010).
7.6 Dromaeosauridae
Integumentary structures in dromaeosaurids have been well documented (Fig. 7.5) since the description of Sinornithosaurus, the first feathered dromaeosaurid discovered by Xu et al. (1999b). The body of this small dinosaur is covered by filamentous feathers that usually reach 40 mm in length around the postcranial elements and that are shorter around the skull (Xu et al. 1999b). No remiges or rectrices with prominent rachis were observed after a second preparation of the specimen (Xu et al. 2001). However, pennaceous feathers resembling the forewing feathers of Caudipteryx are attached along the ulnar region of NGMC 91, another specimen tentatively referred to Sinornithosaurus (Ji et al. 1998, 2001; Han et al. 2014; Lü and Brusatte 2015). Two additional types of integumentary structures can be observed in Sinornithosaurus: (1) filaments that are equal in length and remain parallel as they extend distally from their common point of insertion and (2) filaments that form the margin of a composed structure in a manner similar to a pennaceous feather vane (Xu et al. 2001). The first type resembles the downy feathers of natal down feathers of bird hatchlings (Foth 2011) and the second one resembles of the structure of a pennaceous feather formed by a central rachis from which multiple barbs arise (Lucas and Stettenheim 1972; Xu et al. 2001). Those structures cover the entire body of NGMC 91 except its distal hindlimbs (Ji et al. 2001).
Xu et al. (2000) described large patches of integumentary structures in the dromaeosaurid Microraptor, including contour impressions of narrow and remige-like feathers containing rachis-like structures near the femur (Fig. 7.5a). Integumentary structures are extraordinarily well preserved in Microraptor (Xu et al. 2003), including two types of feather-like structures (Xu et al. 2003): its body is covered by numerous 25- to 30-mm-long plumulaceous feathers, whereas the feathers around its skull roof are up to 40 mm long, with some of them displaying well-organized pennaceous vanes (Xu et al. 2003). Large pennaceous feathers are present on the distal end of the tail of Microraptor, but in a pattern far different from that in Archaeopteryx and Anchiornis, in which each caudal vertebra bears rectrices (i.e., large vaned pennaceous feathers) (Hu et al. 2009; Foth et al. 2014). In Microraptor, the first rectrices attach from the 15th or 18th 202 caudal vertebrae up to the distal end of the tail (Xu et al. 2003). However, they are inserted more proximally than in Caudipteryx and Protarchaeopteryx (Ji et al. 1998; Xu et al. 2003) and may have acted as stabilizers and additional lifts against the destabilizing effect of the hindwings during the flight of Microraptor (Chatterjee and Templin 2007; Alexander et al. 2010). The hindlimbs of Microraptor are covered by numerous pennaceous feathers. Tibial feathers are shorter than the 14 large pennaceous metatarsal feathers. Metatarsal feathers are roughly perpendicular to their corresponding bone, with short symmetrical vanes in the proximal portion of the metatarsus and long asymmetrical vanes in the distal region (Xu et al. 2003). This pattern is far from that in basal non-eumaniraptoran paravians, in which all tibial and metatarsal feathers are symmetrical and equal in length. The forelimb feathers of Microraptor have a modern bird pattern, with approximately 12 primaries and 18 longer secondaries that are more than twice as long as the humerus (Lucas and Stettenheim 1972); the distal primaries are more parallel to the manus than the proximal ones. The primaries and distal secondaries have asymmetrical vanes, whereas the proximal secondaries have symmetrical vanes (Xu et al. 2003). The presence of an alular feather on the first digit of Microraptor and small alular feathers in Caihong have been reported by Foth et al. (2014) and Hu et al. (2018). Coverts are present and are variable in size, although they are considerably shorter than the primaries and the secondaries—a pattern closer to modern birds than to other basal paravians (Xu et al. 2003; Longrich et al. 2012).
Changyuraptor, also from the Yixian Formation of Liaoning Province, is one meter-long dromaeosaurid with extremely long tail feathers (about one third the length of the skeleton), although forelimb feathers are poorly preserved (Han et al. 2014) (Fig. 7.5b). Some of the pennaceous primaries show a shaft that projects from the distal end of the left hand (Han et al. 2014). Changyuraptor preserve numerous pennaceous feathers that form an extensive hindwing as in Anchiornis, Xiaotingia, Pedopenna, Archaeopteryx, and Microraptor. The wing extends up to the distal end of the metatarsus but the feathers do not extend onto the digits, unlike in Anchiornis (Hu et al. 2009; Han et al. 2014). Although it is impossible to know whether they are asymmetrical or not, these hindlimb feathers overlap each other, apparently forming an airfoil all along the tibia and the femur (Han et al. 2014). The tail is almost completely covered by long and symmetrical rectrices that project backwards with an acute angle of 20° (Han et al. 2014). As in Microraptor, rectrices from the distal one fourth of the tail are more elongated and form a fan composed of at least eight or nine pairs of long feathers (Xu et al. 2003; Han et al. 2014). Those elongated tail feathers may have acted as a display structure, a pitch control structure during the flight, a way to compensate for the weight of the tail during the flight, or a way to reduce descent speed during landing thanks to the passive flexion of the long bony tail (Hone 2012; Han et al. 2014). Filamentous appendages similar to those of Sinornithosaurus are found along the ventral side of the neck and dorsal to the pelvis and base of the tail (Ji et al. 2001; Xu et al. 2001; Han et al. 2014).
Feathers are not limited to small-sized dromaeosaurids. Turner et al. (2007) showed that the posterior forearm of Velociraptor bears quill knobs for insertion of feathers, as in modern birds. They also suggested that the reduction or absence of quill knobs in larger and more derived dromaeosaurids may reflect the loss of aerodynamic capabilities (Turner et al. 2007). Lü and Brusatte (2015) described Zhenyuanlong, a larger-bodied (about 2 m long), short-armed dromaeosaurid from the Yixian Formation of Liaoning Province (Fig. 7.5c). Unlike in Tianyuraptor, another large-bodied dromaeosaurid from the Yixian Formation, integumentary structures are finely preserved around the skeleton of Zhenyuanlong. The body of Zhenyuanlong is covered by numerous small (<30 mm) filaments (Lü and Brusatte 2015). Their structure cannot be accurately determined because of their poor preservation. Large pennaceous feathers form broad wings along the forearms, with short coverts overlapping longer primaries and secondaries, resembling the condition in modern birds (Lucas and Stettenheim 1972; Lü and Brusatte 2015). Coverts from the ulnar region are perpendicularly inserted, whereas those from the metacarpal part are more obliquely inserted and more parallel to the long axis of the hand (Lü and Brusatte 2015). Even though other pennaceous feathers are not as well preserved as the coverts, approximately 10 primaries and 20 secondaries can be identified along the forelimb (Lü and Brusatte 2015). Secondaries are inserted perpendicularly to the long axis of the bones, like the secondary coverts, whereas primaries form an acute angle with the long axis of the manus (Lü and Brusatte 2015), as in Archaeopteryx, Microraptor, and modern birds (Lucas and Stettenheim 1972; Xu et al. 2003; Foth et al. 2014; Lü and Brusatte 2015). Although the vane arrangement of the forelimb feathers remains unclear due to poor preservation, those feathers were apparently asymmetrical (Lü and Brusatte 2015). The tail of Zhenyuanlong was likely covered by large pennaceous feathers, as suggested by the presence of thin, long, and straight rachides projecting roughly 45° posterodorsally from the longest axis of the tail. Small and simple feathers were apparently inserted on the proximal part of the tail, whereas much larger and more complex feathers were inserted distally, as also observed in Microraptor (Xu et al. 2003; Lü and Brusatte 2015). The presence or absence of a tail fan in Zhenyuanlong cannot be assessed because the distal part of the tail is not preserved. Hindlimb feathers are apparently absent in Zhenyuanlong, although it cannot be ruled out that it is an artifact of preservation (Lü and Brusatte 2015). Because of the small size of its forelimbs, Zhenyuanglong could not fly or glide. Therefore, the presence of a complex bird-like plumage along the forelimbs of this large dromaeosaurid can be viewed as a probable heritage from a flying or gliding ancestor, secondarily used for sexual or social display (Lü and Brusatte 2015).
7.7 General Pattern of Feather Evolution in Pennaraptoran Theropods
Feathers and feather-related structures are widely distributed among dinosaurs, from small ornithischians to large-bodied theropods such as Yutyrannus (Xu et al. 2012; Godefroit et al. 2014). The presence of pennaceous feathers along the forelimb, as observed in anchiornithids (e.g., Eosinopteryx, Anchiornis, and Serikornis) and more derived paravians, was likely inherited from a common ancestor shared with Oviraptorosauria or possibly Ornithomimosauria because quill-knobs have been described in one specimen of Ornithomimus (Zelenitsky et al. 2012). We hypothesize that those feathers were secondarily lost in scansoriopterygids to accommodate for the bat wing. Unlike in ornithomimosaurs and oviraptosaurs, the forelimb wing of anchiornitids is multi-layered, formed by several layers of undifferentiated coverts and flight feathers (Longrich et al. 2012).
The undifferentiated forelimb pennaceous feathers in anchiornithids were obviously not adapted for flight: their vanes were short and symmetrical, and their rachis was probably too slender and flexible (Godefroit et al. 2013a, b). The presence of open-vaned feathers in Anchiornis also underlines this lack of aerodynamic function (Saitta et al. 2017). Moreover, the forelimbs of anchiornithids were probably proportionally too short and their pectoral girdle lacks adaptations for flapping flight (Godefroit et al. 2013a). It might therefore be hypothesized that those basal paravians principally used their forelimb feathers as visual displays for social and/or sexual behaviors, although we do not rule out that they could use their primitive wings for gliding from tree to tree or prolonging leaps during cursorial locomotion.
Remiges became progressively better adapted for flight in more derived paravians, including some small dromaeosaurids and Avialae thought to be at least good gliders (Xu et al. 2003; Longrich 2006; Foth et al. 2014). Feather specialization in short coverts overlapping longer flight feathers is present both in Dromaeosauridae [e.g., Microraptor and Changyuraptor; Xu et al. (2003); Han et al. (2014)] and basal Avialae (e.g., Archaeopteryx and Enantiornithes; Foth et al. (2014); Xing et al. (2016). Forelimb plumage is unfortunately not preserved in troodontids, so it remains unclear whether feather specialization is a synapomorphy for Eumaniraptora (Dromaeosauridae + Troodontidae + Avialae) or a convergent evolution in Dromaeosauridae and Avialae.
Asymmetry of flight feathers also progressively appeared in dromaosaurids and avialans. As in modern birds, the forelimb feathers of Microraptor and Archaeopteryx are characterized by well-defined asymmetrical primaries that become less asymmetrical towards the last secondary feather covered by short coverts (Lucas and Stettenheim 1972; Foth et al. 2014; Feo et al. 2015). The development of feather asymmetry on the forearm is thought be an adaptation to the air pressure applied on the feathers during the flight for the reason that this asymmetrical configuration prevents feathers to twist due to pressure (Feo et al. 2015).
The alula (bastard wing) is formed by more or less symmetrical feathers attached to the alular (first) digit, allowing birds to reach a higher angle of attack during low-speed flight or landings (Lee et al. 2015). The alula is apparently not well documented in non-avialan Paraves and in basal avialans [although a patch of pennaceous feathers seems to be attached to the first digit in Microraptor and small alular feathers are reported in Caihong (Hu et al. 2018)], clearly appearing later within the Ornithothoraces clade (e.g., Protopteryx and Eoalulavis) (Sanz et al. 1996; Zhang and Zhou 2000; Xu et al. 2003; Lee et al. 2014).
Elongated hindlimb pennaceous feathers (the “tetrapterygian type”) appeared in anchiornithids (Pedopenna, Eosinopteryx, Serikornis, Anchiornis) and were retained in dromaeosaurids (e.g., Changyuraptor, Microraptor). It has been hypothesized that the development of hindlimb wings might be linked with improvement of the aerodynamic capacities of basal paravians (Longrich 2006). However, as already discussed, those basal paravians were likely not adapted for active flight or even for gliding. The development of hindlimb feathers is therefore tentatively regarded as a social and/or sexual display, as it is also the case for the forelimb feathers in those basal paravians (Foth et al. 2014; O’Connor and Chang 2015). In dromaeosaurids (e.g., Changyuraptor, Microraptor), it seems that the hindlimb wings could have helped to stabilize the animals during flight (Chatterjee and Templin 2007; Alexander et al. 2010), adding an aerodynamical function to the social and/or sexual display. Hindlimb feathers were also elongated in several basal Avialae. A distal-to-proximal reduction pattern for leg feathers can be subsequently observed in avialan evolution, and extensively scaled feet might have appeared secondarily at an early stage in ornithuromorph evolution (Zheng et al. 2013). However, Foth et al. (2014) suggest that only a tibial plumage is plesiomorphic and the metatarsal extension of feather trousers evolved independently in Microraptorine, Anchiornithidae, and Sapeornis.
Pennaceous tail feathers are present in Oviraptorosauria, in some anchiornithids, and in paravians. It might therefore be hypothesized that they were secondarily lost in the basal paravians Eosinopteryx and partially in Serikornis. The tail plumage has evidently a visual display function in oviraptorosaurs (Persons et al. 2014), as also in many modern birds. Although the long bony tail in non-avalian paravians and basal birds is reduced to a pygostyle in more advanced Avialae, it can be hypothesized that the elongated pennaceous feathers in non-avialan eumaniraptorans had a similar function to the rectrices in modern birds (Balmford et al. 1993; Thomas 1993; Fitzpatrick 1998). In the same way, the feathered tail of paravians could have helped to act as a stabilizer during flight (Han et al. 2014). Tail feathers may also be used as a pitch control structure or as a way to compensate for the weight of the long bony tail (Han et al. 2014). In Archaeopteryx, the lateral rectrices are asymmetrical, whereas the distal ones are long and symmetrical (Foth et al. 2014). The asymmetrical shape of the lateral tail feathers in Archaeopteryx tends to indicate that a secondary aerodynamic function took place with the display function in order to increase the total lift of the animal (Foth et al. 2014). In Caihong, the tail surface area formed by pennaceous feathers is proportionally larger than that of Archaeopteryx (Hu et al. 2018); it can be presumed that this airfoil acts as an additional aerodynamic display to increase the total lift of the animal. The troodontid Jianianhualong also shows asymmetrical tail feathers, suggesting that feather asymmetry in rectrices appeared in the ancestors of Troodontidae + Dromaeosauridae + Avialae (Xu et al. 2017). Xu et al. (2017) suggested that feather asymmetry first evolved in the troodontid tail and then appeared in other parts of the body to assist with locomotion on the ground or through the air.
7.8 Conclusion
Two decades of research and discoveries have shown that the feathers and plumage of modern birds have gone through various evolutionary stages. Feather or feather-like morphotypes were already particularly diversified in non-avialan paravians, including filamentous feathers, monofilaments (associated filaments extending distally from a common point of insertion), asymmetrical pennaceous feathers that are nearly identical or identical to those in modern birds, and more exotic feather-like structures that are unknown in modern birds (ribbon-like tail “feathers” in scansoriopterygids). The important diversity of integumentary structures in non-avialan paravians therefore suggests that they probably already fulfilled a wide array of biological roles, including thermoregulation and visual display, but also potentially maneuverability, brooding, and camouflage (Foth et al. 2014). Flight was obviously one of the last adaptations to appear in feather evolution (Xu and Guo 2009). The plumage structures in some basal paravians were obviously not adapted for flight. However, in Microraptor and maybe some of its relatives, the large pennaceous feathers along the forelimb, hindlimb, and tail were similar in morphology (large asymmetrical vane, curved rachis) and organization (specialization in coverts, primaries, and secondaries) to those in modern bird wings, supporting the hypothesis that they could have functioned in flight or gliding.
Several aerodynamic innovations and flight-related morphological adaptations were, likely and independently, experimented within the paravian clade close to the origin of birds, including bat-like membranous aerodynamic surfaces in scansoriopterygids (Xu et al. 2015), elongated hindlimb pennaceous feathers (the “tetrapterygian type”) in dromaeosaurids, and the important battery of modifications of the scapular girdle and forelimbs allowing flapping flight in Avialae (Senter 2006). The recent discoveries of exquisitely preserved specimens of feathered dinosaurs, particularly in Late Jurassic and Early Cretaceous deposits from Northeastern China, definitely demonstrate that the origin and early evolution of complex feathers and flight abilities in paravian theropods were not linear processes, but rather were more complex than previously thought.
Institutions
CAGS: Chinese Academy Of Geological Sciences, Beijing, China; DLXH: Dalian Xinghai Museum, Dalian, China; HG: Paleontological Center, Bohai University, Jinzhou City, China; IVPP: Institute Of Vertebrate Paeontology And Paleanthropology, Beijing, China; JPM: Jinzhou Paleontological Museum, Jinzhou, China; NGMC: National Geological Museum Of China, Beijing, China; PMOL: Paleontological Museum Of Liaoning, Shenyang, China; STM: Shandong Tianyu Museum Of Nature, Shandong, China; YFGP: Yizhou Fossil And Geology Park, Yizhou, China.
References
Agnolin FL, Novas FE (2013) Avian ancestors: a review of the phylogenetic relationships of the theropods Unenlagiidae, Microraptoria, Anchiornis and Scansoriopterygidae. Springer, Dordrecht
Alexander DE, Gong E-P, Martin LD, Burnham DA, Falk AR (2010) Model tests of gliding with different hindwing configurations in the four-winged dromaeosaurid Microraptor gui. Proc Natl Acad Sci USA 107:2972–2976
Balmford A, Jones IL, Thomas ALR (1993) On avian asymmetry: evidence of natural selection for symmetrical tails and wings in birds. Proc R Soc B 252:245–251
Benett SC (2007) Articulation and function of the pteroid bone of pterosaur. J Vertebr Paleontol 27:881–891
Chatterjee S, Templin JR (2007) Biplane wing planform and flight performance of the feathered dinosaur {Microraptor} gui. Proc Natl Acad Sci USA 104:1576–1580
Chen P-J, Dong Z-M, Zhen S-N (1998) An exceptionally well-preserved theropod dinosaur from the Yixian Formation of China. Nature 391:147–152
Chen P, Wang Q, Zhang H, Cao M, Li W, Wu S, Shen Y (2005) Jianshangou Bed of the Yixian Formation in West Liaoning, China. Sci China Ser D Earth Sci 48:298–312
Currie PJ, Chen P (2001) Anatomy of Sinosauropteryx prima from Liaoning, northeastern China. Can J Earth Sci 38:1705–1727
Feo TJ, Field DJ, Prum RO (2015) Barb geometry of asymmetrical feathers reveals a transitional morphology in the evolution of avian flight. Proc R Soc Lond B 282
Fitzpatrick S (1998) Birds’ tails as signaling devices: markings, shape, length, and feather quality. Am Nat 151:157–173
Foth C (2011) The morphology of neoptile feathers: ancestral state reconstruction and its phylogenetic implications. J Morphol 272:387–403
Foth C, Rauhut WM (2017) Re-evaluation of the Haarlem Archaeopteryx and the radiation of maniraptoran theropod dinosaurs. BMC Evol Biol 17:236
Foth C, Tischlinger H, Rauhut OWM (2014) New specimen of Archaeopteryx provides insights into the evolution of pennaceous feathers. Nature 511:79–82
Gatesy SM, Dial KP (1996) From frond to fan: Archaeopteryx and the evolution of short-tailed birds. Evolution 50:2037–2048
Godefroit P, Demuynck H, Dyke GJ, Hu D, Escuillié F, Claeys P (2013a) Reduced plumage and flight ability of a new Jurassic paravian theropod from China. Nat Commun 4:1394
Godefroit P, Cau A, Hu D, Escuillié F, Wenhao W, Dyke GJ (2013b) A Jurassic avialan dinosaur from China resolves the early phylogenetic history if birds. Nature 498:359–362
Godefroit P, Sinitsa SM, Dhouailly D, Bolotsky YL, Sizov AV, McNamara ME, Benton MJ, Spagna P (2014) A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science 345:451–455
Han G, Chiappe LM, Ji S-A, Habib M, Turner AH, Chinsamy AT, Liu X, Han L (2014) A new raptorial dinosaur with exceptionally long feathering provides insights into dromaeosaurid flight performance. Nat Commun 5:4382
Hone DWE (2012) Variation in the tail length of non-avian dinosaurs. J Vertebr Paleontol 32:1082–1089
Hopson JA (2001) New perspectives on the origin and early evolution of birds. Peabody Museum of Natural History, New Haven, CT
Hu D, Hou L, Zhang L, Xu X (2009) A pre-{Archaeopteryx} troodontid theropod from China with long feathers on the metatarsus. Nature 461:640–643
Hu D, Clarke JA, Eliason CM, Qiu R, Li Q, Shawkey MD, Zhao C, D’Alba L, Jiang J, Xu X (2018) A bony-crested Jurassic dinosaur with evidence of iridescent plumage highlights complexity in early paravian evolution. Nat Commun 9:217
Ji Q, Ji SA (1996) On discovery of the earliest bird fossil in China and the origin of birds. Chin Geol 233:30–33
Ji S, Ji Q (2007) {Jinfengopteryx} compared to {Archaeopteryx}, with comments on the mosaic evolution of long-tailed avialan birds. Acta Geol Sin 81:337–343
Ji Q, Currie PJ, Norell MA, Ji S-A (1998) Two feathered dinosaurs from the northeastern China. Nature 393:753–761
Ji Q, Norell MA, Gao K, Ji S (2001) The distribution of integumentary structures in a feathered dinosaur. Nature 410:1084–1088
Ji Q, Ji S, Lu J, You H, Chen W, Liu Y, Liu Y (2005) First avialan bird from China (Jinfengopteryx elegans gen. et sp. nov.). Geol Bull China 24:197–205
Lee MSY, Cau A, Naish D, Dyke GJ (2014) Sustained miniaturization and anatomical innovation in the dinosaurian ancestors of birds. Science 345:562–566
Lee S-I, Kim J, Park H, Jablonski PG, Choi H (2015) The function of the alula in avian flight. Sci Rep 5:9914
Lefèvre U, Cau A, Cincotta A, Hu D, Chinsamy A, Escuillié F, Godefroit P (2017) A new Jurassic theropod from China documents a transitional step in the macrostructure of feathers. Sci Nat 104:1–13
Li Q, Gao K-Q, Meng Q, Clarke JA, Shawkey MD, D’Alba L, Pei R, Ellison M, Norell MA, Vinther J (2012) Reconstruction of Microraptor and the evolution of iridescent plumage. Science 335:1215–1219
Lindgren J, Sjövall P, Carney RM, Cincotta A, Uvdal P, Hutcheson SW, Gustafsson O, Lefèvre U, Escuillié F, Heimdal J, Engdahl A, Gren JA, Kear BP, Wakamatsu K, Yans J, Godefroit P (2015) Molecular composition and ultrastructure of Jurassic paravian feathers. Sci Rep 5(1):13520
Lingham-Soliar T (2012) The evolution of the feather: Sinosauropteryx, life, death and preservation of an alleged feathered dinosaur. J Ornithol 153:699–711
Longrich N (2006) Structure and function of hindlimb feathers in Archaeopteryx lithographica. Paleobiology 32:417–431
Longrich NR, Vinther J, Meng Q, Li Q, Russell AP (2012) Primitive wing feather arrangement in Archaeopteryx lithographica and Anchiornis huxleyi. Curr Biol 22:2262–2267
Lü J, Brusatte S (2015) A large, short-armed, winged dromaeosaurid (Dinosauria: Theropoda) from the Early Cretaceous of China and its implication for feather evolution. Sci Rep 5:11775
Lucas AM, Stettenheim PR (1972) Avian anatomy: integument, parts I. U.S. Agricultural Research Service, Washington, DC
O’Connor JK, Chang H (2015) Hindlimb feathers in paravians: primarily “wings” or ornaments? Biol Bull 42:616–621
Osmólska H, Currie PJ, Barsbold R (2004) Oviraptorosauria. In: Weishampel DB, Dodson P, Osmólska H (eds) The Dinosauria. University of California Press, Berkeley, CA
Pei R, Li Q, Meng Q, Norell MA, Gao K-Q (2017) New specimens of Anchiornis huxleyi (Theropoda: Paraves) from the Late Jurassic of northeastern of China. Bull Am Mus Nat Hist 411:1–66
Pérez-Moreno BP, Sanz JL, Buscalioni AD, Moratalla JJ, Ortega F, Rasskin-Gutman D (1994) A unique multitoothed ornithomimosaur dinosaur from the Lower Cretaceous of Spain. Nature 370:363–367
Persons SW, Currie PJ, Norell MA (2014) Oviraptorosaur tail forms and functions. Acta Palaeontol Pol 59:553–567
Saitta ET, Gelernter R, Vinther J (2017) Additional information on the primitive contour and wing feathering of paravian dinosaurs. Palaeontology 61:273–288
Sanz JL, Chiappe LM, Perez-Moreno BP, Buscalioni AD, Moratalla JJ, Ortega F, Poyato-Ariza FJ (1996) An Early Cretaceous bird from Spain and its implications for the evolution of avian flight. Nature 382:442–445
Senter P (2006) Scapular orientation in theropods and basal birds, and the origin of flapping flight. Acta Palaeontol Pol 51:305–313
Thomas ALR (1993) On the aerodynamics of birds’ tail. Philos Trans R Soc B 340:361–380
Turner AH, Makovicky PJ, Norell MA (2007) Feather quill knobs in the dinosaur Velociraptor. Science 317:1721
Turner AH, Makovicky PJ, Norell MA (2012) A review of dromaeosaurid systematics and paravian phylogeny. Bull Am Mus Nat Hist 371:1–206
Wang M, O’Connor JK, Xu X, Zhou Z (2019) A new Jurassic scansoriopterygid and the loss of membranous wings in theropod dinosaurs. Nature 569:256–259
Weidensaul S (ed) (1995) Raptors: the birds of prey. Michigan University Press, New York, NY
Witmer LM (2009) Fuzzy origins for feathers. Nature 458:293–295
Xing L, McKellar RC, Wang M, Bai M, O’onnor JK, Benton MJ, Zhang J, Wang Y, Tseng K, Lockley MG, Li G, Zhang W, Xu X (2016) Mummified precocial bird wings in mid-Cretaceous Burmese amber. Nat Commun 7:12089
Xu X (2006) Scales, feathers and dinosaurs. Nature 440:287–288
Xu X, Guo Y (2009) The origin and early evolution of feathers: insights from recent paleontological and neontological data. Vertebrata PalAsiatica 47:311–329
Xu X, Zhang F (2005) A new maniraptoran dinosaur from China with long feathers on the metatarsus. Naturwissenschaften 92(4):173–177
Xu X, Tang Z, Wang X (1999a) A therizinosaurid dinosaur with integumentary structures from China. Nature 399
Xu X, Wang X, Wu X-C (1999b) A dromaeosaurid dinosaur with a filamentous integument from the Yixian Formation of China. Nature 401:262–266
Xu X, Zhou Z, Wang X (2000) The smallest known non-avian theropod dinosaur. Nature 408:705–708
Xu X, Zhou Z-H, Prum RO (2001) Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature 410:200–204
Xu X, Zhou Z, Wang X, Kuang X, Zhang F, Du X (2003) Four winged dinosaurs from China. Nature 421:335–340
Xu X, Norell MA, Kuang X, Wang X, Zhao Q, Jia C (2004) Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids. Nature 431:680–684
Xu X, Zheng X, You H (2010) Exceptional dinosaur fossils show ontogenetic development of early feathers. Nature 464:1338–1341
Xu X, You H, Du K, Han F (2011) An Archaeopteryx-like theropod from China and the origin of Avialae. Nature 475:465–470
Xu X, Wang K, Zhang K, Ma Q, Xing L, Sullivan C, Hu D, Cheng S, Wang S (2012) A gigantic feathered dinosaur from the Lower Cretaceous of China. Nature 484:92–95
Xu X, Zheng X, Sullivan C, Wang X, Xing L, Wang Y, Zhang X, O’Connor JK, Zhang F, Pan Y (2015) A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings. Nature 521:70–73
Xu X, Currie P, Pittman M, Xing L, Meng Q, Lü J, Hu D, Yu C (2017) Mosaic evolution in an asymmetrically feathered troodontid dinosaur with transitional features. Nat Commun 8:14972
Zelenitsky DK, Therrien F, Erickson GM, DeBuhr CL, Kobayashi Y, Eberth DA, Hadfield F (2012) Feathered non-avian dinosaurs from North America provide insight into wing origins. Science 338:510–514
Zhang F, Zhou Z (2000) A primitive enantiornithine bird and the origin of feathers. Science 290:1955–1959
Zhang F, Zhou Z, Xu X, Wang X (2002) A juvenile coelurosaurian theropod from China indicates arboreal habits. Naturwissenschaften 89:394–398
Zhang F, Zhou Z, Xu X, Wang X, Sullivan C (2008a) A bizarre Jurassic maniraptoran from China with elongated ribbon-like feathers. Nature 455:1105–1108
Zhang F, Zhou Z, Benton MJ (2008b) A primitive confuciusornithid bird from China and its implications for early avian flight. Sci China Ser Earth Sci 51:625–639
Zheng X, Zhou Z, Wang X, Zhang F, Wang Y, Wei G, Wang S, Xu X (2013) Hind wings in basal birds and the evolution of leg feathers. Science 339:1309–1312
Zhou Z, Zhang F-C (2006) Origin and early evolution of feathers: evidence from the early cretaceous of China. Acta Zool Sin 52:125–128
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lefèvre, U., Cau, A., Hu, D., Godefroit, P. (2020). Feather Evolution in Pennaraptora. In: Foth, C., Rauhut, O. (eds) The Evolution of Feathers. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-27223-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-27223-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27222-7
Online ISBN: 978-3-030-27223-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)