Abstract
The discoveries of numerous theropod dinosaurs with filamentous integumentary structures in various stages of morphological complexity from the Upper Jurassic and Lower Cretaceous of China provided striking evidence that birds represent modern predatory dinosaurs and that feathers were originally filamentous. In the shadow of these impressive discoveries, two early juvenile theropod dinosaurs from the Upper Jurassic limestones of Bavaria (Germany), Juravenator starki and Sciurumimus albersdoerferi, were described. Both are preserved with phosphatized soft tissues, including skin and feathers. In the current study, the integumentary structures of both theropods are investigated and revised with the help of autofluorescence methods, using two different excitation wavelengths (UVA and cyan). Both theropods possessed monofilamentous feathers and scaleless skin. In J. starki, short feathers could only be traced in the tail region. The tubercle-like structures, originally described as scales, found in the anterior tail region of J. starki, show no autofluorescence signal and were reinterpreted as remains of adipocere, maybe indicating the presence of a fat body. S. albersdoerferi was probably entirely plumaged, possessing a filamentous crest on the dorsal edge in the anterior tail section. This current example emphasizes the importance of taphonomic reviews for the interpretation of integumentary structures. Furthermore, the new data give new insights into the early evolution of feathers. However, the placement of J. starki in multiple phylogenetic positions and differences in the morphological interpretation of filamentous feathers found in basal coelurosaurs produce contrasting reconstructions of character evolution that will need to be resolved in due course if greater clarity is to be obtained in this area.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
The discoveries of numerous theropod dinosaurs from the Upper Jurassic and Lower Cretaceous of China preserved with filamentous integumentary structures in various stages of morphological complexity provided new insights into the early evolution of feathers. These findings indicated that ancestral feathers were filamentous and did not originate in relation to flight, but for other purposes (Prum and Brush 2002; Norell and Xu 2005; Xu and Guo 2009; Foth et al. 2014; Xu et al. 2014). The feathers from these localities are usually preserved as black, carbonized traces related to melanosome preservation (Li et al. 2010, 2012, 2014; Zhang et al. 2010), which for the first time allowed conclusions about ancestral colour patterns in extinct animals (see Vinther et al. 2008).
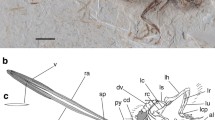
In the last decade, two early juvenile theropod dinosaurs from the Upper Jurassic limestones of Bavaria (Germany) were described, Juravenator starki (Göhlich and Chiappe 2006) and Sciurumimus albersdoerferi (Rauhut et al. 2012), both of which were preserved with large traces of soft tissue remains, including skin and feathers (Fig. 6.1). In contrast to the feathered theropods from China, the integumentary structures of both theropods were preserved in the form of apatite (see Frey and Martill 1998; Viohl and Zapp 2006; see below), resulting in a strong autofluorescence signal under UV light with respect to the surrounding matrix. In the present study, the integumentary soft tissues of J. starki and S. albersdoerferi are investigated with the help of autofluorescence techniques using two different wavelengths (UVA and cyan) and different light filter adjustments. This approach allows corroborating morphological observation on soft tissues due to different reactions on light colours and filter adjustments. These results in turn are used for taphonomic revision of the preservation of the integumentary structures. Finally, the morphology of the integuments of J. starki and S. albersdoerferi is compared with that of other feathered theropods and, after a short discussion on the phylogenetic position of J. starki, integrated into a phylogenetic-based overview of early feather evolution in theropods.
6.2 General Comments on Soft Tissue Preservation in Plattenkalks of Painten and Schamhaupten
The Upper Jurassic well bedded to laminated lithographic limestones of the southern Franconian Alb (the often so-called Solnhofener Plattenkalks or plattenkalks of the Solnhofen Archipelago) have a great potential for soft tissue (e.g. integuments, inner organs and muscles) preservation, including impressions (Wellnhofer 2009; Foth et al. 2014), melanosome preservation (Tischlinger 1998; Carney et al. 2012) and apatite preservation (Frey and Martill 1998; Briggs 2003; Viohl and Zapp 2006), which differ in their chemical compounds, respectively. The feather impressions of various Archaeopteryx lithographica specimens, for instance, are covered by a thin layer rich in phosphorus and sulphur, which might be organic in origin (Bergmann et al. 2010). The isolated A. lithographica feather, which is preserved in the form of melanosomes, is rich in copper, nickel and sulphur (Carney et al. 2012; Manning et al. 2013), whereas apatite preservation is, unsurprisingly, rich in calcium and phosphorus (Briggs and Kear 1994; Briggs and Wilby 1996; Briggs 2003). These different chemical compounds lead to different fluorescent characteristics, inasmuch as apatite shows a strong luminous signal under certain wavelengths such as UV light or cyan light (autofluorescence), while the melanosomes and organic cover of the impressions appear to be darker than the surrounding matrix (Frey and Martill 1998; Tischlinger and Frey 2002; Tischlinger and Unwin 2004; Tischlinger 2005, 2009).
Based on the strong luminescence under UV light (see Göhlich and Chiappe 2006; Göhlich et al. 2006; Chiappe and Göhlich 2010; Rauhut et al. 2012), the soft tissues of both Sciurumimus albersdoerferi and Juravenator starki are mainly preserved in the form of apatite, although Göhlich and Chiappe additionally reported tubercle scales and ventral fibres in the proximal tail region of J. starki, which are preserved as visible marks (see below), and in S. albersdoerferi, parts of the filaments can be traced as impressions under low-angle light (Rauhut et al. 2012: Fig. S1). According to Briggs and Kear (1994), apatite mineralization occurs mainly under anoxic conditions when the pH drops between 6 and 7, inducing the precipitation of calcium phosphate in the system. This, however, requires a high concentration of phosphate from the very first, which can, for example, originate from the organism itself due to decay of organic matter and dissolution of biogenic apatite or from external sources like microbial mats, in which bacteria can induce apatite precipitation (Hirschler et al. 1990; Briggs et al. 1993; Wilby et al. 1996). These experimental results are consistent with the supposed deposition process for the bindstones of the Painten and Schamhaupten localities, which were both formed under hypersaline and anoxic conditions by microbial mats (predominantly by cyanobacteria) (Link and Fürsich 2001; Viohl and Zapp 2006, 2007). Besides, the Schamhaupten bindstones are strongly silicified due to a higher abundance of representatives of Radiolaria and Silicispongia (Viohl and Zapp 2006, 2007). The almost complete and articulated preservation of J. starki and S. albersdoerferi indicates that both specimens sank to the sea bottom rapidly after death and did not float for a longer period in open water (see Reisdorf and Wuttke 2012). Thus, the anoxic conditions at the bottom and the overgrowth by microbial mats prevented further decay and finally induced soft tissue mineralization (Hirschler et al. 1990; Briggs and Kears 1993; Wilby et al. 1996). However, in contrast to bone, the main compounds of integumentary structures (i.e. keratin, collagen and colour pigments) are not very rich in phosphorus (Kühn 1974; Murayama et al. 1986; Schweitzer et al. 1999a; McGraw 2003), while various examples of fossilized integumentary structures show a strong phosphate signal (e.g. Schweitzer et al. 1999a; Tischlinger and Unwin 2004; Benton et al. 2008; Bergmann et al. 2010; Tischlinger and Rauhut 2015). This fact may favour a predominant external source of phosphate for apatite formation in the soft tissues of J. starki and S. albersdoerferi. On the other hand, integumentary structures often work as toxin storage organs (e.g. Reichholf 1996; Metcheva et al. 2006; Dumbacher et al. 2009), accumulating various biogenic and toxic elements, including phosphorus (Marlow and Caldwell 1934; Kelsall and Calaprice 1972; Liu et al. 2006; Metcheva et al. 2006), so that apatite formation still can partly source from the specimen itself.
6.3 Material and Methods
6.3.1 Taxon Sampling
The holotypes of Sciurumimus albersdoerferi (BMMS BK 11) and Juravenator starki (JME Sch 200) represent both early juvenile theropod dinosaurs from the Torleite Formation (upper Kimmeridgian) of the Jurassic limestones of Bavaria (see Niebuhr and Pürner 2014), in which J. starki was found in strongly silicified, laminated bindstones (a type of autochthonous limestone; see Embry and Klovan 1971) located in the Schamhaupten area (Göhlich et al. 2006; Viohl and Zapp 2006, 2007; Chiappe and Göhlich 2010; Viohl 2015a, b), while S. albersdoerferi comes from the thin-bedded micritic bindstones of the Painten area (Rauhut et al. 2012; Albersdörfer and Häckel 2015; Viohl 2015a).
Although both specimens resemble each other in general features and body proportions, it could be demonstrated that J. starki and S. albersdoerferi are taxonomically distinct from each other at the species level (Rauhut et al. 2012). In a phylogenetic assessment, S. albersdoerferi was found close to the base of tetanurans, most likely representing a juvenile megalosauroid (Rauhut et al. 2012). In contrast, J. starki was originally described as a basal coelurosaur (Göhlich and Chiappe 2006; Chiappe and Göhlich 2010), which was supported by several phylogenetic analyses (e.g. Butler and Upchurch 2007; Rauhut et al. 2012; Loewen et al. 2013; Brusatte et al. 2014; Choiniere et al. 2014). However, Rauhut et al. (2012) already argued that this phylogenetic position may result from the early juvenile stage of J. starki (see discussion).
6.3.2 Autofluorescence Methods
During the past 15 years, frequent investigations under ultraviolet (UV) and cyan radiation have shown that fossils from the Upper Jurassic limestones of southern Germany are often autofluorescent (e.g. Tischlinger 2002, 2005; Tischlinger and Unwin 2004; Haug et al. 2009, 2011a, b; Haug and Haug 2011; Frey and Tischlinger 2012; Tischlinger and Arratia 2013), i.e. the objects emit light in a longer certain wavelength after they were exposed to more energetic light. Due to an increased contrast between structure and matrix, this characteristic allows a more precise investigation of morphological details of both hard and mineralized soft tissues, including delicate structures that are poorly or not discernible in visible light. Thus, this technique can be used to establish compressed morphological structures more clearly and to differentiate them from each other, but also from cracks, preparation artefacts and the underlying matrix.
The UV investigations of Juravenator starki and Sciurumimus albersdoerferi were done with the help of powerful UVA lamps with intensities between 4000 and more than 50,000 μW per 10 mm2 and a wavelength of 365–366 nm. Because sometimes essential details of morphological structures are poorly or even not visible to the naked eye or under a microscope, they can still be documented with the help of UV light photography, using different filter techniques for the camera, which allow a selective visualization of peculiar fine structures. In most cases, a variety of different colour-correction filters are necessary, because each type of limestone, but also fossilized hard and soft tissues, reacts differently to different wavelengths. Thus, the right combination of filters and exposure times (1 second to several minutes) is needed to highlight the area of interest, depending on the nature of the fossil material and the magnification, intensity and incident angle of the UV lamps. In consequence, the optimum filtering and exposure time must be tested in a series of experiments (Tischlinger 2002; Frey and Tischlinger 2012). The UV photos were taken with analogue cameras (Pentax ME and MX) on slide film (Kodak Professional Elite Chrome ISO 100/21° and Kodak Elite Chrome Extra Colour 100) and with diverse digital cameras (Panasonic Lumix) partly in combination with a Raynox M-250 macro lens.
As stated above, we used a second autofluorescence method (cyan-red) for corroboration of the morphological observations on the integumentary structures. The investigation with cyan-red fluorescence was performed with a Canon EOS Rebel T3i camera and a Canon Macro Photo Lens MP-E 65 mm, in which the camera lens was equipped with a red filter foil taken from cyan-red stereo spectacles. The complementary cyan filter foil was attached to the light sources (three LED torches). After the objects of interest were excited by the cyan light, only the emitted light was able to pass through the complementary red filter, while light with other wavelengths was mostly blocked (Haug et al. 2011a; Haug and Haug 2011).
After documentation, the digital photos were processed in Adobe Photoshop CS3, including brightness adjustment and contrast enhancement. For the cyan-red light investigation, the photos were modified by deleting the blue and green colour channels and transformed afterwards into grey scale to increase the contrast between fluorescent and nonfluorescent structures.
6.4 Results
6.4.1 The Integumentary Structures of Juravenator starki
The soft tissues of Juravenator starki were first described by Göhlich and Chiappe (2006), Göhlich et al. (2006) and Chiappe and Göhlich (2010). Due to the fact that the bindstones from Schamhaupten are strongly silicified (Viohl and Zapp 2006, 2007), the preparation of the soft tissues was much more difficult in J. starki, and the quality of preservation is not as good as in Sciurumimus albersdoerferi. The majority of soft tissues in J. starki are preserved as apatite, illuminating bright yellow to bright green under filtered UV light (depending on the filter and camera system), and are also visible under cyan light (Figs. 6.2 and 6.3). The remains are restricted to the posterodorsal pelvic region, both tibiae and the tail, in which skin remains can be found in all areas (Fig. 6.2), while feather filaments are restricted to the mid-tail section (Fig. 6.3). Chiappe and Göhlich (2010) further mentioned soft tissue preservation dorsal to the rostrum of the skull, but the identification of this alleged soft tissue is currently impossible because it is covered with glue, illuminating cyan under filtered UV light.
Skin of Juravenator starki. (a–d) Tubercle and fibre structures of the proximal tail region under (a) normal light, (b) cyan light, (c) filtered UV light taken with an analogue camera and (d) filtered UV light taken with a digital camera, which are here interpreted as adipocere. (e) Skin remains on the ventral side of the tail. (f, g) Skin remains of the hindlimb under (f) filtered UV light and (g) cyan light. ca caudal vertebrae, f fibres, is ischum, s skin, t tubercles, ti tibia
Feather filaments (arrows) of Juravenator starki. (a, b) Filaments on the dorsal side of the tail under (a) filtered UV light taken with an analogue camera, (b) cyan light and (c) filtered UV light taken with a digital camera. (d) Filaments on the ventral side of the tail under filtered UV light taken with a digital camera
The most conspicuous structures are a large patch of tubercle-like structures along the laterodorsal side of the tail between the 9th and 18th caudal and a series of longitudinal orientated fibre-like structures along the ventral side of the tail between the 12th and 15th caudal (Fig. 6.2a), which are even visible under normal light conditions (see Chiappe and Göhlich 2010). The former structure was originally described as uniformly sized, nonoverlapping, smooth tubercle-like scales, as known from various dinosaur taxa (Osborn 1912; Czerkas 1997; Mayr et al. 2002; Coria and Chiappe 2007; Christiansen and Tschopp 2010; Bell 2012, 2014), while the latter was interpreted as possible remains of tendinal elements of the m. ilioischiocaudalis, remains of a reinforced layer of the vertical septum ventral to the chevrons or bundles of subcutaneous collagen fibres (see Göhlich and Chiappe 2006; Göhlich et al. 2006; Chiappe and Göhlich 2010). On the ventral side of the caudals, between the chevrons, further tubercle-like structures are present, but they are not as well preserved as those on the laterodorsal side. On the basis of the current investigation, performed with a digital camera, both structures illuminate green to brown under filtered UV light similar to the bone and surrounding matrix (Fig. 6.2d). Using a different filter arrangement and an analogue camera, both structures fluoresce in bright blue, while the matrix possesses a darker blue signal, and the bone appears green to brown (Fig. 6.2c). In both cases, the signal is very different from the phosphatized soft tissue remains (see above). Under cyan light, both structures show no signs of autofluorescence, like the surrounding matrix, but in contrast to the bone and phosphatized soft tissues (Fig. 6.2b, e). These findings indicate that the tubercle- and fibre-like structures may chemically be differently composed than both bone and phosphatized soft tissue remains, but also the surrounding matrix. In some areas, both tubercles and fibres are partly covered with a thin layer of phosphatized soft tissues. Consequently, the supposed tubercles are not superficial, but seem to be internal, like the fibre-like structures. Another aspect that should be taken into account is that the tubercle-like structures are not as uniform as originally described. In fact, dorsal to the 12th and 13th caudal, some tubercles converge to short, thick, parallel band-like structures, which are orientated perpendicular with respect to the tail axis. These bands in turn look very similar to the ventral, horizontally orientated fibres, only differing in orientation. In consequence, it is possible that the original interpretation of the structures may not be correct (see discussion).
In contrast, the skin remains from the tail and hindlimb region, which are preserved as apatite, appear smooth and show no signs of scales (Fig. 6.2e–g). Feather filaments are visible on the dorsal and ventral side of the tail. The dorsal feathers are found in a small area around the 18th to 20th caudal (Fig. 6.3a–c), while the ventral filaments are preserved between the 9th and 13th caudal vertebrae (Fig. 6.3d). The filaments are parallel oriented and angled 20–45° backwards with respect to the tail axis. The filaments are short, measuring about 4 mm as preserved, and show no sign of branching. However, it should be noted that these filaments could have been longer originally, but parts of these very delicate structures might have been removed during preparation. Due to the quality of preservation, no further observation can be made.
6.4.2 The Integumentary Structures of Sciurumimus albersdoerferi
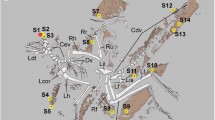
The holotype of Sciurumimus albersdoerferi shows both skin and feather preservation in various parts of the body (Figs. 6.4 and 6.5). Under filtered UV light, the skin remains fluoresce in a bright yellow, which can be well distinguished from the reddish bindstone. The skin remains further show a red autofluorescent signal when illuminated with cyan light, while they are not visible under normal light. The skin remains are preserved in the region of the scapula (dorsal to the acromion process of the left scapula), forelimb (along the right humerus, ulna and radius) and hindlimb (left femur and tibia). In the original description, the soft tissues along the posterior edge of the tibia of Sciurumimus were interpreted as possible fossilized muscle tissue (Rauhut et al. 2012). However, because the surface of this area is rather smooth and does not show any indication for internal structuring (i.e. fascicle and fibres; see Kellner 1996; Chin et al. 2003; Dal Sasso and Maganuco 2011) under UV and cyan-red light, we reinterpret this patch also as skin remains. Further small patches of skin are preserved in the anterior portion of the back, on the lateral and anteroventral side of the torso as well as ventral to the last sacral vertebra. The best-preserved relicts of skin can be found in the anterior and middle tail section (up to the 25th caudal vertebra), along both the dorsal and lateral side (Fig. 6.4c, d). The skin appears generally smooth and shows no signs of external tubercle-like scales. Within the tail region, the skin is even directly preserved in association with feather filaments (see below).
Skin of Sciurumimus albersdoerferi. (a, b) Skin remains of the forelimb under (a) filtered UV light taken with digital camera and (b) cyan light. (c) Overview of skin remains in the tail region. (d) Detail of large patch of skin on the ventral side of the tail. (e) Skin remains in the thorax region. White arrows mark skin remains and yellow arrows mark feather filaments. ca caudal vertebrae, dr dorsal ribs, hu humerus, ra radius
Feather filaments of Sciurumimus albersdoerferi. (a, b) Skin and filaments on the dorsal side of the thorax under filtered UV light taken with digital camera. (a) Overview of the integument structures. (b) Details of the filaments from the dorsal region. (c–f) Integument structure of the dorsal side in the proximal tail region. Long filaments under (c) cyan light and (d) under filtered UV light taken with digital camera. Orange arrows mark artificial overlaps of the monofilaments. Blue arrows mark the proximal ends of the feather filaments. (e) Skin remains (white arrows), short (yellow arrows) and long filaments with yellow and greenish fluorescence (orange arrows). (f) Detail of the association between skin (white arrows) and long filaments (orange filaments) indicating possible follicles. (g) Details of short filaments (yellow arrows) on the ventral side under filtered UV light taken with digital camera
The best feather remains are present along the back, as well as along the dorsal and lateroventral side of the tail (Fig. 6.5). The tail feathers are traceable until the midportion of the tail (27th caudal). Small patches of feathers are further preserved on the lateral side of the torso as well as in the belly region right in front of the knee joint. In the torso and the anterior tail region, the feather remains fluoresce under yellow-filtered UV light and are visible as red structures when illuminated with cyan light. More posteriorly the tail feathers shine greenish under filtered UV light and are barely visible under cyan light anymore, while the most distal tail feather remains appear yellow again (Fig. 6.5c–e).
The reasons for the different fluorescence signal of the feather filaments in the tail region probably result from different chemical compounds, which could be related to a preserved colour pattern. However, with the current method, this is not testable. Although it is principally possible to trace different chemical compounds with the help of unfiltered UV light due to specific characteristic fluorescence colour signals (Kane and Sell 2001), this does not work with the current approach, which works with multiple UV filters for increasing the contrast between matrix, bones and soft tissues (see above). As the identification via unfiltered UV light is further limited to only those chemical compounds being autofluorescent, the differences in the colour signals of the feathers have to be investigated with more sensitive methods using scanning electron microscopy and different spectroscopy techniques in a future approach (see Field et al. 2013; Egerton et al. 2015).
The general morphology of the feathers of S. albersdoerferi is described on the basis of the anterior dorsal tail feathers, which are best exposed. The feathers of this region are long, gently backward curved, stiff filaments, which measure 30–40 mm in length, 0.2 mm in width, and are almost perpendicularly orientated with respect to the axis of the tail (Fig. 6.5c–e). More posteriorly, the filaments become shorter, measuring c. 3 mm in the mid-tail portion. The filaments in the anterodorsal region show no signs of branching distally, although some filaments seem to stick closely together (Fig. 6.5d). Because those filaments are not fused both proximally and distally to the contact area, these structures certainly do not represent branching structures, but artificial attachments due to an overlapping in combination with taphonomic compaction of the single filaments (see Foth 2012). Proximally, the interpretation of the feather morphology is more difficult. Again, in some areas single filaments seem to join each other, creating the impression that they are fused into a calamus-like structure. However, the problem of overlapping and compaction of single filaments in this portion is probably even more evident due to denser packing of filaments at the base. Furthermore, most feathers are not joined proximally, but still solitary before reaching the border of the skin (Fig. 6.5d, f). Thus, we conclude that the branched morphology at the base is in fact artificial and that the actual morphology of the feathers is monofilamentous over their entire length. More proximal to the feathers described, further parallel-orientated filaments with greenish fluorescence (filtered UV) are preserved, which seem to be shorter and run horizontally with respect to the axis of the tail (Fig. 6.5d, f). However, due to their parallel arrangement, the stiff appearance and the colour, we consider these filaments as short feathers, which originate from the dorsolateral side of the tail.
Further feathers are preserved on the ventral side of the tail, which show their best preservation around the large, ventral patch of skin (Fig. 6.5g). In this region, the filaments are almost horizontally orientated with respect to the axis of the tail and are parallel to each other. The filaments are fine and straight and show no sign of branching. In the way they are preserved, the feathers are short, measuring about 2–3 mm. In the border region of the large ventral skin patch, some feathers extend over the ventral border of the skin.
The dorsal trunk feathers are preserved along the mid and posterior portion of the back (Fig. 6.5a, b). As the original matrix in this area was only several millimetres thin, it was not possible to prepare the complete plumage without risking the destruction of the specimen (Raimund Albersdörfer, pers. comm., 2011). Thus, only the proximal part of the feathers is exposed, and nothing can be said about their actual length. The arrangement of these feathers is less regular than in the tail region, showing a high degree of overlapping and artificial contacts. Like in the tail region, some filaments are closely attached to each other, but no real fusion can be detected. Thus, the dorsal trunk feathers seem to be monofilamentous, too. Further small areas with filaments are preserved on the lateral and posteroventral side of the torso (Fig. 6.4e). The preserved filaments are short (c. 4–5 mm) and very thin and show no signs of branching. Because first bone remains of the specimen were originally found in the field from this area, and the torso had to be carefully reconstructed (Raimund Albersdörfer, pers. comm., 2011), large areas of the torso region are infused with glue in order to repair fissures within the matrix. Thus, it is not clear if those filaments are entirely preserved or only small fractions are visible.
Along the dorsal edge of the tail, the association between skin and feathers can be studied best (Fig. 6.5e). Like in other body regions, the skin illuminates yellow under filtered UV light and is preserved as a thin stripe, horizontally orientated with respect to the tail axis. In contrast, the filaments of this region show a greenish fluorescence under the same light conditions. As preserved, the skin seems to be vertically structured in lateral view, while the proximal ends of the vertically orientated feather filaments pierce in between, probably indicating that the feathers were deeply anchored within the skin (Fig. 6.5f), i.e. that the feathers actually grew out of follicles (see discussions).
Ventral to the skin remains of the dorsal edge of the tail, a different kind of filament-like soft tissue is preserved. In contrast to the feather filaments from this region, these structures are short, thick (notably thicker than the individual feather filaments) and wrinkled and are obliquely orientated with respect to the axis of the tail, with a parallel arrangement. Furthermore, these filaments show a yellow signal under UV light, like the skin tissue (Fig. 6.5g). Therefore, we do not interpret these structures as feather filaments. Instead, it is possible that these structures are relicts of subcutaneous muscle tissue or thick dermal collagen bundles. Although both tissue types can potentially fossilize (Kellner 1996; Chin et al. 2003; Lingham-Soliar and Wesley-Smith 2008; Dal Sasso and Maganuco 2011), it is not possible to identify the true nature of these structures on the basis of the current methods and their preservation.
6.5 Discussion
6.5.1 Identification of Tubercles in the Tail Region of Juravenator starki
The present examination of the soft tissue in the tail region of Juravenator starki reveals that the original interpretation of the tubercles as scales is questionable on the basis of several observations, including the overall kind of preservation, the morphology, the absence of autofluorescence signal and the fact that the tubercles are partly covered with phosphatized soft tissue. In consequence, the tubercles are unlikely to represent a superficial integumentary structure. Furthermore, it is not clear why these very distinct and robust tubercles are only preserved in a single, restricted area, while phosphatized integumental tissue remains can be found along the entire anterior half of the tail. As an alternative explanation, we suggest that the tubercles of J. starki could be the result of adipocere formation. Adipocere (corpse or grave wax) is a decomposition product of subepidermal adipose body tissue containing predominantly saturated fatty acids, which are produced due to the hydrolysis and hydrogenation of neutral fats, especially during subaquatic decay (Forbes et al. 2005). Its formation occurs predominantly under moist, warm, anaerobic conditions, a pH range of approximately 5–9 and the presence of putrefactive bacteria (Forbes et al. 2005; O’Brien and Kuehner 2007). Thus, the same environmental conditions leading to apatite preservation of soft tissues (see above) can also form adipocere. Finally, adipocere precipitates into concretions of calcium carbonate CaCO3 (Berner 1968). In this context, the formation of adipocere has previously been hypothesized to play an important role in fossilization of vertebrates from the limestones of the Solnhofen Archipelago (de Buisonjé 1985; Reisdorf and Wuttke 2012). Similar tubercle-like structures have, for instance, also been preserved in the head region of an undescribed ichthyosaur from the Solnhofen limestones housed in the Bürgermeister-Müller-Museum in Solnhofen (Achim Reisdorf, pers. comm. 2015; see Maisch 2015). Because adipocere is generally soft and crumbly, its morphology is very variable, which includes tubercle-like, segmental, bundle-like or even amorphous granular appearances (see Wiman 1942; Reisdorf and Wuttke 2012). Thus, the fibre-like structures found on the ventral side of the tail of J. starki also fall in the morphological spectrum of adipocere.
Following this interpretation, the regional restriction of the tubercles can be explained by the presence of a large fat body in the anterior tail section, as known in extant crocodilians (Frey 1988; Huchzermeyer 2003) and turtles (Walter Joyce, pers. comm. 2015). Thus, the current case demonstrates that the discovery of tubercle-like structures in sediments surrounding dinosaur fossils or even on the bones themselves should not be identified by default as skin remains, but evaluated carefully for taphonomic alternatives. In this context, the conspicuous tubercles found in the mid-section of the tail of the French Compsognathus corallestris (Peyer 2006) might also be interpreted as remains of adipocere. To test the hypothesized adiposal origin using geochemical methods, the tubercles of Juravenator and Compsognathus shall contain a high concentration of CaCO3 with a very low 13C/12C ratio in the carbonate, as adipocere and lipid carbon are highly depleted in 13C relative to normal marine calcium carbonate (Sondheimer et al. 1966; Berner 1968).
6.5.2 The Skin Morphology of Juravenator starki and Sciurumimus albersdoerferi
If the tubercle- and fibre-like structures in Juravenator starki represent adipocere, as proposed, all integumentary structures are exclusively preserved in the form of apatite, in which the phosphatized skin remains found in the limb and tail region of J. starki and Sciurumimus albersdoerferi show no evidence for scales whatsoever. Scaly skin remains from the body region are rarely described in theropod dinosaurs (e.g. Bonaparte et al. 1990; Czerkas 1997; Ortega et al. 2010) as many theropod taxa possessed extensive feather plumage, which covered the whole body (Xu and Guo 2009; Xu et al. 2014). Only in the distal foot region scale preservation is more frequent (Gatesy 2001; Ji et al. 2001; Currie et al. 2003; Kundrát 2004; Cuesta et al. 2015), as this region was probably barely covered with feathers, like in the majority of modern birds (Lucas and Stettenheim 1972). Unfortunately, no integumental structures are preserved in the foot region of J. starki and S. albersdoerferi, which would allow a comparison.
The absence of scales in J. starki and S. albersdoerferi could be potentially taphonomic, if (a) this kind of soft tissue mineralization does not lead to scale preservation in general or (b) if the scales of these early juvenile theropods were very small or even not fully developed, prohibiting a proper preservation. However, various embryological studies of modern non-avian reptiles and birds (i.e. foot region) indicate that scales are already morphologically well developed and keratinized during hatching (Hamburger and Hamilton 1951; Dhouailly et al. 1980; Alibardi and Thompson 2000, 2001, 2002; Werneburg et al. 2009), a situation also found in fossil sauropod embryos (Coria and Chiappe 2007). Furthermore, several fossils of Rhynchocephalia and squamates from the limestones of the Solnhofen archipelago with phosphatized skin remains (e.g. Pleurosaurus goldfussi) demonstrate that scale preservation was not generally impossible in these deposits, although in smaller species (Sapheosaurus thiollierei/Piocormus laticeps) skin remains also sometimes appear smooth (Tischlinger and Rauhut 2015). Furthermore, preservation of other keratinous structures is not rare in vertebrates from the Solnhofen Archipelago, and at least parts of the keratinous sheaths of the manual and pedal unguals are also preserved in both Juravenator and Sciurumimus and are usually well visible under UV light (e.g. Chiappe and Göhlich 2010: Fig. 20; Rauhut et al. 2012: Fig. 1c). If we consider the absence of scales in the phosphatized skin remains of J. starki and S. albersdoerferi to not be taphonomic, the skin would resemble that of modern birds, where body parts covered with feathers (except of the foot region) are scaleless (Lucas and Stettenheim 1972; McNamara et al. 2018). As mentioned above, the skin remains of both theropods are usually found in association with feather filaments, except for the limb regions. However, based on the current data, it is unclear if scales were originally present and if skin morphology may have changed through ontogeny in these theropods.
6.5.3 The Feather Morphology of Juravenator starki and Sciurumimus albersdoerferi
The new examination reveals that in Juravenator starki both the dorsal and ventral sides of the tail were feathered, indicating full plumage coverage of the tail as in other coelurosaurs. Unfortunately, other body parts show no signs of feathers. Because the limestones from the Schamhaupten locality are strongly silicified and extremely hard to prepare, it cannot be ruled out that other areas with soft tissue preservation were accidentally removed during preparation. In contrast, the extension of the plumage can be far better reconstructed in Sciurumimus albersdoerferi, indicating that the whole thorax and tail were covered with feathers. The examination further reveals that the anterior tail feathers along the dorsal edge were extremely elongated, while the dorsolateral, lateral and ventral feather filaments were significantly shorter. Thus, the tail of S. albersdoerferi was not uniformly bushy, but formed an elongated, dorsal filamentous crest. Similar filamentous crests have been described in the ornithischian dinosaurs Psittacosaurus sp. (Mayr et al. 2002, 2016) and Tianyulong confuciusi (Zheng et al. 2009). Ornithomimus sp. also shows elongated filaments along the dorsal edge of the proximal tail portion (van der Reest et al. 2016).
Skin remains without feather preservation could indicate that tibial regions of both theropods were probably naked, as in Ornithomimus sp. (van der Reest et al. 2016), but in contrast to the compsognathid Sinocalliopteryx gigas (Ji et al. 2007) and various eumaniraptorians (Zheng et al. 2013; Foth et al. 2014). However, it cannot be ruled out that the absence of feathers in this region may simply be a preservational artefact given that scales are generally expected in unfeathered theropod hindlimbs (see Cuesta et al. 2015).
The feather morphology of J. starki and S. albersdoerferi was found to be monofilamentous over their entire length. Possible branching patterns in S. albersdoerferi were interpreted as taphonomic artefacts caused by the overlap of feathers and the two-dimensional preservation of the fossils itself (see Foth 2012). Thus, the feathers of both theropods represent feathers of stage I according to Prum (1999), or morphotype I and II according to Xu et al. (2010), which were hypothesized to represent the ancestral feather morphologies in both evolutionary scenarios (Fig. 3.6). Similar monofilamentous feathers have been described for the alvarezsaurid Shuvuuia deserti (Schweitzer et al. 1999b), the therizinosauroids Beipiaosaurus inexpectus (Xu et al. 2009) and Jianchangosaurus yixianensis (Pu et al. 2013), two undescribed large-bodied basal coelurosaurs (Xu et al. 2010), and in the manual region of a juvenile Ornithomimus sp. (Zelenitsky et al. 2012). Furthermore, monofilamentous filaments (or bristles) were also present in some ornithischian dinosaurs (Mayr et al. 2002, 2016; Zheng et al. 2009; Godefroit et al. 2014), and even pterosaurs (e.g. Kellner et al. 2010), although it is not entirely clear at the moment if these filaments are homologous with theropod feathers (see Barrett et al. 2015). However, in many basal coelurosaurs (see Currie and Chen 2001; Xu et al. 2004, 2012; Ji et al. 2007), the kind of plumage preservation makes the conclusion, if the filaments were still monofilamentous or already branched, representing a more derived feather type, very difficult (see Currie and Chen 2001; Xu and Guo 2009; Xu et al. 2010; Foth 2012; Saitta et al. 2018). Usually, the dense body plumage consists of filaments, which are parallelly oriented to each other over the entire length. Often, single filaments group closely together, especially close to the body. These groupings could be evidence that these filaments were proximally merged, forming a branched feather tuft of multiple barbs emerging from the calamus [radially symmetric branched feather without rhachis—stage II after Prum (1999) and morphotype 3 after Xu et al. (2010)] or joined into a short rhachis [bilaterally symmetric branched feathers with short rhachis—stage IIIa after Prum (1999) and morphotype 5 after Xu et al. (2010)]. However, no certain signs of fusion are identifiable (Fig. 3.5), and the described morphology could alternatively result from taphonomic compaction of overlapping filaments (see Foth 2012). Only for Pennaraptora, branched feather types could be identified without doubt so far (e.g. Ji et al. 1998; Xu et al. 2001, 2015; Foth et al. 2014), although van der Reest et al. (2016) report the presence of branched body feathers containing a rhachis in a newly discovered Ornithomimus sp. specimen. If this identification is correct, the origin of bilaterally symmetric feathers can be pushed back to the base of Maniraptoriformes. However, as stated above, this branching structure might also be a preservation artefact. Therefore, more discoveries and closer investigations of plumage morphologies of basal coelurosaurs are necessary to test ideas about feather branching evolution, and autofluorescence techniques could be a key to establish the data needed for this discussion. Besides the UV and cyan-red autofluorescence techniques, a further illumination method has been recently established, using laser-stimulated fluorescence (LSF), which will help in studying soft tissues in fossils in more detail (Kaye et al. 2015; Mayr et al. 2016; Wang et al. 2017).
In the dorsal tail region of S. albersdoerferi, skin and feathers were found in direct association with each other, in which the skin remains appear perpendicular to the long axis of the body, creating the impression that the feathers were anchored deep within the skin. If this interpretation is correct, this morphology indicates that the feathers grow out of follicles like modern feathers or mammal hairs (Lucas and Stettenheim 1972; Sengel 1976). However, due to the compaction of the specimen and possible preparation artefacts in this region, the described morphology is still arguable. On the other hand, the outer edge of the skin is the only region in a two-dimensional compressed fossil, where such association could potentially be preserved. Furthermore, the different fluorescence of skin and feathers in this region supports the view that both structures are distinct units and that the filaments pierce into the skin and are not just simple superficial outgrowths (Fig. 6.6e, f). Thus, S. albersdoerferi might present evidence for the hypothesis that follicles were associated with the origin of feathers, as suggested by Prum (1999) and Prum and Brush (2002). Furthermore, a very similar situation has been also found in the ornithischian dinosaur Psittacosaurus sp. (Mayr et al. 2002), where several bristles penetrate the skin. If these observations are not artificial (sensu Mayr et al. 2016), the presence of follicles could be a possible support for the homology of ornithischian filaments with theropod feathers (see Brusatte et al. 2010; Zheng et al. 2009; Rauhut et al. 2012; Godefroit et al. 2014; contra Barrett et al. 2015; Mayr et al. 2016).
Origin of monofilamentous and branched feather types within theropods with two alternative phylogenetic positions of Juravenator starki. (a) Traditional interpretation with J. starki as compsognathid. Filaments of Dilong paradoxus, Sinosauropteryx prima, Ornithomimus sp. and Beipiaosaurus inexpectus are interpreted as branched filaments. In this view, branched feathers are an apomorphic character of Coelurosauria, while several taxa show a secondary simplification of their filaments. (b) Alternative interpretation with J. starki as a basal tetanuran. The branching patterns of filaments in non-maniraptoriformes are interpreted as overlapping artefacts, so that the feathers in these theropods are actually monofilamentous. In Ornithomimus sp. and Beipiaosaurus inexpectus simple branched feathers might be present, indicating a potential origin of this feather type in Maniraptoriformes. However, if the assumed branched feathers of these two theropods turn out to be artificial (see above), monofilaments evolved quickly into pennaceous feathers within Maniraptora. 1 Tetanurae, 2 Coelurosauria, 3 Tyrannosauroidea, 4 Compsognathidae, 5 Maniraptoriformes, 6 Maniraptora
Based on the early ontogenetic stage of both J. starki and S. albersdoerferi, the feather remains found represent a juvenile plumage. The presence of filamentous feathers indicates that juvenile theropods probably used their feathers for body insulation. However, the presence of a conspicuous dorsal filamentous crest in S. albersdoerferi might further indicate a signal or camouflage function. Because of the phylogenetic position of S. albersdoerferi close to the base of tetanurans and also the size of this early post-hatchling individual in comparison with other basal tetanuran hatchlings and embryos (e.g. Rauhut and Fechner 2005; Araújo et al. 2013), it has to be assumed that adult representatives of this species were medium- to large-bodied theropods (e.g. Britt 1991; Zhao and Currie 1993; Rauhut 2005; Sadleir et al. 2008). As the increase of body size impacts the need of body insulation for thermoregulation, it is possible that adult individuals lost large parts of their body plumage and were secondarily partly or fully naked (see van der Reest et al. 2016), as it is also the case in many large mammals, such as rhinos or elephants. However, the presence of probably fully feathered, medium- to large-bodied theropods from the Lower Cretaceous of China (Xu et al. 2010, 2012) indicates that plumage reduction in large-bodied theropods during ontogeny is not obligatory.
6.5.4 The Phylogenetic Position of Juravenator starki
Before the new observations on the integumentary structures of Juravenator starki and Sciurumimus albersdoerferi can be included into an evolutionary framework, the phylogenetic position of both theropods has to be discussed. As mentioned above, Rauhut et al. (2012) demonstrated that S. albersdoerferi is a basal tetanuran and most probably a juvenile megalosauroid, although the exact phylogenetic position is difficult to establish, due to our still very poor knowledge of ontogenetic changes in basal tetanurans. In contrast, J. starki was originally described as basal coelurosaur (Göhlich and Chiappe 2006; Chiappe and Göhlich 2010). Indeed, J. starki possesses a number of characters present in basal coelurosaurs, e.g. an elongate anterior process of the maxillary body, which is longer than high; a maxillary fenestra that is situated posterior to rostral border of the antorbital fossa; an enlarged, round orbit, maxillary teeth without anterior serrations; axial epipophyses that do not extend beyond the posterior rim of the postzygapophyses; and long and hair-like cervical ribs (Göhlich and Chiappe 2006; Chiappe and Göhlich 2010).
However, in light of its early juvenile stage, Rauhut et al. (2012) already argued that the phylogenetic position of J. starki may be an artefact (see also Tsuihiji et al. 2011). For instance, enlarged, round orbits and maxillary teeth without anterior serrations were found to be juvenile characters of basal tetanurans, which probably evolved in basal coelurosaurs by paedomorphosis (Rauhut et al. 2012; Foth et al. 2016). An elongated anterior process of the maxillary body is present in some compsognathids (Currie and Chen 2001; Hwang et al. 2004) but is also known for megalosauroids (Britt 1991; Sereno et al. 1998; Allain 2002; Rauhut et al. 2012) and some other basal tetanurans (e.g. Zhao and Currie 1993). The slender morphology of the cervical ribs in J. starki may be an allometric feature due to its young age. Long cervical ribs are also present in more basal theropods, e.g. in the coelophysid Coelophysis bauri (Colbert 1989) or the carcharodontosaurid Concavenator corcovatus (Ortega et al. 2010). Due to their gracility, cervical ribs are often incompletely preserved, making a phylogenetic evaluation of this character difficult. Other characters presented by Göhlich and Chiappe (2006) and Chiappe and Göhlich (2010), like the missing mandibular fenestra or the short and low neural spines of the cervical vertebrae, are probably artefacts of deformation. In contrast, J. starki possesses several characters that are more typically seen in more basal theropods, like coelophysoids, ceratosaurs or basal tetanurans (Rauhut and Foth 2014, in prep.), such as a medially closed maxillary fenestra (see Megalosauroidea), a short maxillary antorbital fossa anterior to the antorbital fenestra or a supra-acetabular crest that is confluent with the lateral brevis shelf. Thus, an alternative phylogenetic position close to the base of Tetanurae is also plausible. Unfortunately, most of the analyses in which J. starki was included (see above) focused on the interrelationships of coelurosaurs and had only a poor sampling of basal theropods. Thus, the actual phylogenetic position of J. starki has to be tested in a future approach with a more comprehensive sampling, but this is beyond the scope of the current study.
6.5.5 Insights into Early Feather Evolution in Theropods
The evolutionary implications that can be drawn from the current data are impacted by two major aspects: (1) the phylogenetic position of Juravenator starki and (2) the actual morphology of the filamentous feathers in basal coelurosaurs, which has been discussed as monofilamentous or proximally branched (see above). In previous models, branched feathers were believed to be present in basal coelurosaurs (e.g. Prum and Brush 2002; Xu and Guo 2009; Xu et al. 2010, 2014), and J. starki was considered as a compsognathid (see Xu and Guo 2009; Barrett et al. 2015). Based on these assumptions (Fig. 6.6a), Sciurumimus albersdoerferi would be the phylogenetically most basal feathered theropod, while its monofilamentous feathers would represent the ancestral feather type. The possible anchorage of the filaments within the skin would further imply that follicles were primordial structures for monofilamentous feathers, too. Thus, the reconstructed ancestral stage is in agreement with the hypothesized ancestral feather type proposed by Prum (1999). In basal coelurosaurs, feathers became more complex, evolving proximal branching patterns due to the formation of multiple barbs emerging from the calamus (radially symmetric feathers—stage II after Prum 1999 and morphotype 3 after Xu et al. 2010) or even a short rhachis formation caused by barb fusion at the anterior side of the calamus (bilaterally symmetric feathers—stage IIIa after Prum 1999 and morphotype 5 after Xu et al. 2010). At this stage, the plumage was still filamentous, but contained both monofilamentous and simple-branched feathers (as hypothesized for Beipiaosaurus inexpectus). Assuming that the feather types found in the tail region are representative for the entire plumage, J. starki would only possess the ancestral feather type, as no branching could be documented. Pennaceous feathers covering the entire body (bilateral symmetric feather with long rhachis and barbs with barbules—stage IV after Prum 1999 and morphotype 8 after Xu et al. 2010) evolved in the clade called Pennaraptora, which includes Oviraptorosauria, Dromaeosauridae, Troodontidae and Avialae (Foth et al. 2014). This whole scenario is very much congruent with the evolutionary model proposed by Prum (1999), which is based on the morphological and developmental hierarchy of feathers (see Lucas and Stettenheim 1972; Prum and Dyck 2003).
However, the reconstructed character evolution might be different if J. starki is considered as a basal tetanuran and with the assumption that the filamentous plumages of basal coelurosaurs consisted only of monofilamentous feathers (Fig. 6.6b). In this case, J. starki and S. albersdoerferi would represent the phylogenetically most basal records for feathered theropods. As in the previous case, monofilamentous feathers growing from a follicle would represent the ancestral feather type, but in contrast to the previous scenario, feather morphology did not change until branched feather types (both radially and bilaterally symmetric feathers, including pennaceous feathers) evolved in the clade Pennaraptora (Foth et al. 2014) or perhaps Maniraptoriformes (van der Reest et al. 2016). This scenario, however, conflicts with the model of Prum (1999), as the proposed transformation of monofilamentous feathers to bilaterally symmetric pennaceous feathers would have skipped the proposed intermediate stage of radially symmetric feathers.
Although morphologically less complex, it has been argued that at least in modern birds radially symmetric feather is secondarily simplified due to the reduction of developmental time (Schaub 1912; Becker 1959; Foth 2011). On the other hand, developmental data of bilaterally symmetric feathers of recent birds show that the rhachis is the fusion product of barbs, merging successively towards each other on the anterior side of the feather anlage (Lucas and Stettenheim 1972; Harris et al. 2002). In consequence, barb formation has to precede rhachis formation in both ontogeny and evolution (Prum 1999; Prum and Brush 2002; Harris et al. 2002). Thus, the only way to solve this conflict without violating the concept of hierarchical organization of feathers would be to assume that both formation processes evolved quasi-contemporaneously. However, two newly discovered anurognathids from the Middle–Late Jurassic Yanliao Group in China (Yang et al. 2018) indicate that the filamentous integuments of ornithodirans could evolve a variety of feather-like branching patterns without a clear rhachis morphology so that more complex branching scenarios need to be considered for the early evolution of feathers.
6.6 Conclusions
The current study revised the integumentary structures of the early juvenile theropod dinosaurs Juravenator starki and Sciurumimus albersdoerferi from the Upper Jurassic limestones of Bavaria with the help of autofluorescence methods using two different excitation wavelengths (UVA and cyan). The preserved integumentary structures were primarily preserved in the form of phosphate-rich apatite, showing a strong signal of the autofluorescence signal. By this it was possible to describe the integumentary structures of both theropods in much more detail and review possible taphonomic artefacts related to soft tissue preservation. Both theropods possessed monofilamentous feathers and probably smooth skin without scales or with only tiny scales that cannot be distinguished from each other as preserved. In J. starki, short feathers could be only traced in the tail region, but their preservation is rather poor compared to S. albersdoerferi, which is probably caused by the higher silicification of the limestone matrix from the Schamhaupten area. S. albersdoerferi, however, was probably entirely covered with unbranched feathers growing out of follicles. On the dorsal edge of the anterior tail, it had a filamentous crest, which might extend onto the thorax. The tubercle-like structures found in the tail region of J. starki, previously described as scales, show no autofluorescence signal and were reinterpreted as remains of adipocere that formed shortly after death and inhibited further decay of specimen. The occurrence of adipocere in a small, restricted area may indicate the former presence of a fat body in the anterior tail region of J. starki, as present in recent crocodilians and turtles. This emphasizes the importance of taphonomic considerations in the interpretation of integumentary structures and soft tissues (see Vinther et al. 2008; Foth 2012) and the reconstruction of soft tissue evolution on the basis of fossil organism (Witmer 1995; Sansom et al. 2010; Foth 2014). Furthermore, the current study gives new insights into the early evolution of feathers, although patterns of reconstructed character evolution are sensitive to the phylogenetic position of J. starki and the absence or presence of simple branched feather types in basal coelurosaurs. However, current data reveal that J. starki and S. albersdoerferi might represent the phylogenetically most basal record of feathered theropods. Furthermore, if radially symmetric feathers described for some basal coelurosaurs turn out to be taphonomic artefacts, the transformation of monofilamentous feathers to bilaterally symmetric branched feathers (including pennaceous feathers) might have happened relatively fast and skipped the proposed intermediate proximally branched, radially symmetric stage (see Prum 1999). Due to hierarchical organization of feathers in terms of morphology and development, such transformation would be only possible if barb ridge and rhachis formation evolved quasi contemporaneously. However, given the recent discovery of complex feather-like branching patterns in pterosaur filaments (Yang et al. 2018), it is also possible that the Prum (1999) model may yet be true and that an outstanding gap in the fossil record remains.
References
Albersdörfer R, Häckel W (2015) Die Kieselplattenkalke von Painten. In: Arratia G, Schultze H-P, Tischlinger H, Viohl G (eds) Solnhofen – Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München, pp 126–133
Alibardi L, Thompson MB (2000) Scale morphogenesis and ultrastructure of dermis during embryonic development in the alligator (Alligator mississippiensis, Crocodilia, Reptilia). Acta Zool (Stockholm) 81:325–338
Alibardi L, Thompson MB (2001) Fine structure of the developing epidermis in the embryo of the American alligator (Alligator mississippiensis, Crocodilia, Reptilia). J Anat 198:265–282
Alibardi L, Thompson MB (2002) Keratinization and ultrastructure of the epidermis of late embryonic stages in the alligator (Alligator mississippiensis). J Anat 201:71–84
Allain R (2002) Discovery of megalosaur (Dinosauria, Theropoda) in the middle Bathonian of Normandy (France) and its implications for the phylogeny of basal Tetanurae. J Vertebr Paleontol 22:548–563
Araújo R, Castanhinha R, Martins R, Mateus O, Hendrickx C, Beckmann F, Schell N, Alves L (2013) Filling the gaps of dinosaur eggshell phylogeny: Late Jurassic Theropod clutch with embryos from Portugal. Sci Rep 3:1924
Barrett PM, Evans DC, Campione NE (2015) Evolution of dinosaur epidermal structures. Biol Lett 11:20150229
Becker R (1959) Die Strukturanalyse der Gefiederfolge von Megapodius feyc. reinw. und ihre Beziehungen zu der Nestlingsdune der Hühnervögel. Rev Suisse Zool 66:411–527
Bell PR (2012) Standardized terminology and potential taxonomic utility for hadrosaurid skin impressions: a case study for Saurolophus from Canada and Mongolia. PLoS One 7:e31295
Bell PR (2014) A review of hadrosaurid skin impressions. In: Eberth DA, Evans DC (eds) Hadrosaurs. Indiana University Press, Bloomington, pp 572–590
Benton MJ, Zhou Z, Orr PJ, Zhang F, Kearns SL (2008) The remarkable fossils from the Early Cretaceous Jehol Biota of China and how they have changed our knowledge of Mesozoic life. Proc Geol Assoc 119:209–228
Bergmann U, Morton RW, Manning PL, Sellers WI, Farrar S, Huntley KG, Wogelius RA, Larson PL (2010) Archaeopteryx feathers and bone chemistry fully revealed via synchrotron imaging. Proc Natl Acad Sci USA 107:9060–9065
Berner RA (1968) Calcium carbonate concretions formed by the decomposition of organic matter. Science 159:195–197
Bonaparte JF, Novas FE, Coria RA (1990) Carnotaurus sastrei Bonaparte, the horned, lightly built carnosaur from the Middle Cretaceous of Patagonia. Contrib Sci 416:1–42
Briggs DEG (2003) The role of decay and mineralization in the preservation of soft-bodied fossils. Annu Rev Earth Planet Sci 31:275–301
Briggs DEG, Kear AJ (1993) Fossilization of soft tissue in the laboratory. Science 259:1439–1442
Briggs DEG, Kear AJ (1994) Decay and mineralization of shrimps. PALAIOS 9:431–456
Briggs DEG, Wilby PR (1996) The role of the calcium carbonate-calcium phosphate switch in the mineralization of soft-bodied fossils. J Geol Soc Lond 153:665–668
Briggs DEG, Kear AJ, Martill DM, Wilby PR (1993) Phosphatization of soft-tissue in experiments and fossils. J Geol Soc Lond 150:1035–1038
Britt B (1991) Theropods of Dry Mesa Quarry (Morrison Formation, Late Jurassic), Colorado, with emphasis on the osteology of Torvosaurus tanneri. BYU Geol Stud 37:1–72
Brusatte SL, Nesbitt SJ, Irmis RB, Butler RJ, Benton MJ, Norell MA (2010) The origin and early radiation of dinosaurs. Earth Sci Rev 101:68–100
Brusatte SL, Lloyd GT, Wang SC, Norell MA (2014) Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur-bird transition. Curr Biol 24:2386–2392
Butler RJ, Upchurch P (2007) Highly incomplete taxa and the phylogenetic relationships of the theropod dinosaur Juravenator starki. J Vertebr Paleontol 27:253–256
Carney RM, Vinther J, Shawkey MD, D’Alba L, Ackermann J (2012) New evidence on the colour and nature of the isolated Archaeopteryx feather. Nat Commun 3:637
Chiappe LM, Göhlich UB (2010) Anatomy of Juravenator starki (Theropoda: Coelurosauria) from the Late Jurassic of Germany. Neues Jahrb Geol Palaontol Abh 258:257–296
Chin K, Eberth DA, Schweitzer MH, Rando TA, Sloboda WJ, Horner JR (2003) Remarkable preservation of undigested muscle tissue within a Late Cretaceous tyrannosaurid coprolite from Alberta, Canada. PALAIOS 18:286–294
Choiniere JN, Clark JM, Forster CA, Norell MA, Eberth DA, Erickson GM, Chu H, Xu X (2014) A juvenile specimen of a new coelurosaur (Dinosauria: Theropoda) from the Middle–Late Jurassic Shishugou Formation of Xinjiang, People’s Republic of China. J Syst Palaeontol 12:177–215
Christiansen NA, Tschopp E (2010) Exceptional stegosaur integument impressions from the Upper Jurassic Morrison Formation of Wyoming. Swiss J Geosci 103:163–171
Colbert EH (1989) The Triassic dinosaur Coelophysis. Mus North Ariz Bull 57:1–160
Coria RA, Chiappe LM (2007) Embryonic skin from Late Cretaceous sauropods (Dinosauria) of Auca Mahuevo, Patagonia, Argentina. J Paleontol 81:1528–1532
Cuesta E, Díaz-Martínez I, Ortega F, Sanz JL (2015) Did all theropods have chicken-like feet? First evidence of a non-avian dinosaur podotheca. Cretac Res 56:53–59
Currie PJ, Chen P (2001) Anatomy of Sinosauropteryx prima from Liaoning, northeastern China. Can J Earth Sci 38:1705–1727
Currie PJ, Badamgarav D, Koppelhus EB (2003) The first Late Cretaceous footprints from the Nemegt locality in the Gobi of Mongolia. Ichnos 10:1–13
Czerkas SA (1997) Skin. In: Currie PJ, Padian K (eds) Encyclopedia of dinosaurs. Academic, San Diego, CA, pp 669–675
Dal Sasso C, Maganuco S (2011) Scipionyx samniticus (Theropoda: Compsognathidae) from the Lower Cretaceous of Italy. Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 37:1–281
De Buisonjé PH (1985) Climatological conditions during deposition of the Solnhofen limestones. In: Hecht MK, Ostrom JH, Viohl G, Wellnhofer P (eds) The beginnings of birds. Freunde des Jura-Museums, Eichstätt, pp 45–65
Dhouailly D, Hardy MH, Sengel P (1980) Formation of feathers on chick foot scales: a stage-dependent morphogenetic response to retinoic acid. J Embryol Exp Morphol 58:63–78
Dumbacher JP, Menon GK, Daly JW (2009) Skin as a toxin storage organ in the endemic New Guinean genus Pitohui. Auk 126:520–530
Egerton VM, Wogelius RA, Norell MA, Edwards NP, Sellers WI, Bergmann U, Sokaras D et al (2015) The mapping and differentiation of biological and environmental elemental signatures in the fossil remains of a 50 million year old bird. J Anal At Spectrom 30:627–634
Embry AF, Klovan E (1971) A late Devonian reef tract on northeastern Banks Island, N.W.T. Bull Can Petrol Geol 19:730–781
Field DJ, D’Alba L, Vinther J, Webb SM, Gearty W, Shawkey MD (2013) Melanin concentration gradients in modern and fossil feathers. PLoS One 8:e59451
Forbes SL, Stuart BH, Dent B (2005) The effect of the burial environment on adipocere formation. Forensic Sci Int 154:24–34
Foth C (2011) The morphology of neoptile feathers: ancestral state reconstruction and its phylogenetic implications. J Morphol 272:387–403
Foth C (2012) On the identification of feather structures in stem-line representatives of birds: evidence from fossils and actuopalaeontology. Paläontol Z 86:91–102
Foth C (2014) Comment on the absence of ossified sternal elements in basal paravian dinosaurs. Proc Natl Acad Sci USA 111:E5334
Foth C, Tischlinger H, Rauhut OWM (2014) New specimen of Archaeopteryx provides insights into the evolution of pennaceous feathers. Nature 511:79–82
Foth C, Hedrick BP, Ezcurra MD (2016) Cranial ontogenetic variation in early saurischians and the role of heterochrony in the diversification of predatory dinosaurs. PeerJ 4:e1589
Frey E (1988) Anatomie des Körperstammes von Alligator mississippiensis Daudin. Stuttgarter Beiträge zur Naturkunde, A 424:1–106
Frey E, Martill DM (1998) Soft tissue preservation in a specimen of Pterodactylus kochi (Wagner) from the Upper Jurassic of Germany. Neues Jahrb Geol Palaontol Abh 210:421–441
Frey E, Tischlinger H (2012) The Late Jurassic pterosaur Rhamphorhynchus, a frequent victim of the ganoid fish Aspidorhynchus? PLoS One 7:e31945
Gatesy SM (2001) Skin impressions of Triassic theropods as records of foot movement. Bull Mus Comp Zool 156:137–149
Godefroit P, Sinitsa SM, Dhouailly D, Bolotsky YL, Sizov AV, McNamara ME, Benton MJ, Spagna P (2014) A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science 345:451–455
Göhlich UB, Chiappe LM (2006) A new carnivorous dinosaur from the Late Jurassic Solnhofen archipelago. Nature 440:329–332
Göhlich UB, Tischlinger H, Chiappe LM (2006) Juravenator starki (Reptilia, Theropoda), ein neuer Raubdinosaurier aus dem Oberjura der Südlichen Frankenalb (Süddeutschland): Skelettanatomie und Weichteilbefunde. Archaeopteryx 24:1–26
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Harris MP, Fallon JF, Prum RO (2002) Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool B Mol Dev Evol 294:160–176
Haug JT, Haug C (2011) Fossilien unter langwelligem Licht: Grün-Orange-Fluoreszenz an makroskopischen Objekten. Archaeopteryx 29:20–23
Haug C, Haug JT, Waloszek D, Maas A, Frattigiani R, Liebau S (2009) New method to document fossils from lithographic limestones of southern Germany and Lebanon. Palaeontol Electron 12:3.6T
Haug JT, Haug C, Kutschera V, Mayer G, Maas A, Liebau S, Castellani C, Wolfram U, Clarkson ENK, Waloszek D (2011a) Autofluorescence imaging, an excellent tool for comparative morphology. J Microsc 244:259–272
Haug JT, Haug C, Waloszek D, Schweigert G (2011b) The importance of lithographic limestones for revealing ontogenies in fossil crustaceans. Swiss J Geosci 104:S85–S98
Hirschler A, Lucas J, Hubert J-C (1990) Bacterial involvement in apatite genesis. FEMS Microbiol Ecol 73:211–220
Huchzermeyer FW (2003) Crocodiles. CABI, Wallingford
Hwang SH, Norell MA, Ji Q, Gao K (2004) A large compsognathid from the Early Cretaceous Yixian Formation of China. J Syst Palaeontol 2:13–30
Ji Q, Currie PJ, Norell MA, Ji S (1998) Two feathered dinosaurs from northeastern China. Nature 393:753–761
Ji Q, Norell MA, Gao K, Ji S, Ren D (2001) The distribution of integumentary structures in a feathered dinosaur. Nature 410:1084–1088
Ji S, Ji Q, Lü J, Yuan C (2007) A new giant compsognathid dinosaur with long filamentous integuments from Lower Cretaceous of northeastern China. Acta Geol Sin 81:8–15
Kane R, Sell H (2001) Revolution in lamps: a chronicle of 50 years of progress. The Fairmont Press, Lilburn
Kaye TG, Falk AR, Pittman M, Sereno PC, Martin LD, Burnham DA et al (2015) Laser-stimulated fluorescence in paleontology. PLoS One 10:e0125923
Kellner AWA (1996) Fossilized theropod soft tissue. Nature 379:32
Kellner AWA, Wang X, Tischlinger H, Campos D, Hone D, Meng X (2010) The soft tissue of Jeholopterus (Pterosauria, Anurognathidae, Batrachognathinae) and the structure of the pterosaur wing membrane. Proc R Soc B Biol Sci 277:321–329
Kelsall JP, Calaprice JR (1972) Chemical content of waterfowl plumage as a potential diagnostic tool. J Wildl Manag 36:1088–1097
Kühn K (1974) Struktur und Biochemie des Kollagens. Chem unserer Zeit 8:97–103
Kundrát M (2004) When did theropods become feathered? Evidence for pre-Archaeopteryx feathery appendages. J Exp Zool B Mol Dev Evol 302B:355–364
Li Q, Gao K, Vinther J, Shawkey MD, Clarke JA, D’Alba L, Meng Q, Briggs DEG, Prum RO (2010) Plumage color patterns of an extinct dinosaur. Science 327:1369–1372
Li Q, Gao K, Meng Q, Clarke JA, Shawkey MD, D’Alba L, Pei R, Ellison M, Norell MA, Vinther J (2012) Reconstruction of Microraptor and the evolution of iridescent plumage. Science 335:1215–1219
Li Q, Clarke JA, Gao K, Zhou Z, Meng Q, Li D, D’Alba L, Shawkey MD (2014) Melanosome evolution indicates a key physiological shift within feathered dinosaurs. Nature 507:350–353
Lingham-Soliar T, Wesley-Smith J (2008) First investigation of the collagen D-band ultrastructure in fossilized vertebrate integument. Proc R Soc Lond B 275:2207–2212
Link E, Fürsich FT (2001) Hochauflösende Feinstratigraphie und Mikrofaziesanalyse der Oberjura Plattenkalke von Painten, Südliche Frankenalb. Archaeopteryx 19:71–88
Liu X, Zhao S, Sun L, Yin X, Xie Z, Luo H, Wang Y (2006) P and trace metal contents in biomaterials, soils, sediments and plants in colony of red-footed booby (Sula sula) in the Dongdao Island of South China Sea. Chemosphere 65:707–715
Loewen MA, Irmis RB, Sertich JJW, Currie PJ, Sampson SD (2013) Tyrant dinosaur evolution tracks the rise and fall of Late Cretaceous oceans. PLoS One 8:e79420. https://doi.org/10.1371/journal.pone.0079420
Lucas AM, Stettenheim PR (1972) Avian anatomy: integument, part I & II. U.S. Government Printing Office, Washington, DC
Maisch MW (2015) Fischechsen (Ichthyosauria). In: Arratia G, Schultze HP, Tischlinger H, Viohl G (eds) Solnhofen – Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München, pp 422–430
Manning PL, Edwards NP, Wogelius RA, Bergmann U, Barden HE, Larson PL, Schwarz-Wings D et al (2013) Synchrotron-based chemical imaging reveals plumage patterns in a 150 million year old early bird. J Anal At Spectrom 28:1024–1030
Marlow HW, Caldwell MJ (1934) A chemical and X-ray study of “flightless” feathers. J Hered 25:265–268
Mayr G, Peters DS, Plodowski G, Vogel O (2002) Bristle-like integumentary structures at the tail of the horned dinosaur Psittacosaurus. Naturwissenschaften 89:361–365
Mayr G, Pittman M, Saitta E, Kaye TG, Vinther J (2016) Structure and homology of Psittacosaurus tail bristles. Palaeontology 59:793–802
McGraw KJ (2003) Melanins, metals, and mate quality. Oikos 102:402–406
McNamara ME, Zhang F, Kearns SL, Orr PJ, Toulouse A, Foley T et al (2018) Fossilized skin reveals coevolution with feathers and metabolism in feathered dinosaurs and early birds. Nat Commun 9:2072
Metcheva R, Yurukova L, Teodorova S, Nikolova E (2006) The penguin feathers as bioindicator of Antarctica environmental state. Sci Total Environ 362AD:259–265
Murayama K, Takahashi R, Yokote Y, Akahane K (1986) The primary structure of feather keratins from duck (Anas platyrhynchos) and pigeon (Columba livia). Biochim Biophs Acta 873:6–12
Niebuhr B, Pürner T (2014) Lithostratigraphie der Weißjura-Gruppe der Frankenalb (außeralpiner Oberjura) und der mittel- bis oberjurassischen Reliktvorkommen zwischen Straubing und Passau (Bayern). Schriftenr Dtsch Ges Geowissenschaften 83:5–72
Norell MA, Xu X (2005) Feathered dinosaurs. Annu Rev Earth Planet Sci 33:277–299
O’Brien TG, Kuehner AC (2007) Waxing grave about adipocere: soft tissue change in an aquatic context. J Forensic Sci 52:294–301
Ortega F, Escaso F, Sanz JL (2010) A bizarre, humped Carcharodontosauria (Theropoda) from the Lower Cretaceous of Spain. Nature 467:203–206
Osborn HF (1912) Integument of the iguanodontid dinosaur Trachodon. Mem Am Mus Nat Hist 1:33–54
Peyer K (2006) A reconsideration of Compsognathus from the Upper Tithonian of Canjuers, southeastern France. J Vertebr Paleontol 26:879–896
Prum RO (1999) Development and evolutionary origin of feathers. J Exp Zool B Mol Dev Evol 285:291–306
Prum RO, Brush AH (2002) The evolutionary origin and diversification of feathers. Q Rev Biol 77:261–295
Prum RO, Dyck J (2003) A hierarchical model of plumage: morphology, development, and evolution. J Exp Zool B Mol Dev Evol 298B:73–90
Pu H, Kobayashi Y, Lü J, Xu L, Wu Y, Chang H, Zhang J, Jia S (2013) An unusual basal therizinosaur dinosaur with an ornithischian dental arrangement from northeastern China. PLoS One 8:e63423
Rauhut OWM (2005) Osteology and relationships of a new theropod dinosaur from the Middle Jurassic of Patagonia. Palaeontology 48:87–110
Rauhut OWM, Fechner R (2005) Early development of the facial region in a non-avian theropod dinosaur. Proc R Soc Lond B 272:1179–1183
Rauhut OWM, Foth C (2014) New information on the theropod dinosaurs from the Late Jurassic lithographic limestones of southern Germany. J Vertebr Paleontol, Program and Abstracts: 212
Rauhut OWM, Foth C, Tischlinger H, Norell MA (2012) Exceptionally preserved juvenile megalosauroid theropod dinosaur with filamentous integument from the Late Jurassic of Germany. Proc Natl Acad Sci USA 109:11746–11751
Reichholf JH (1996) Die Feder, die Mauser und der Ursprung der Vögel. Archaeopteryx 14:27–38
Reisdorf AG, Wuttke M (2012) Re-evaluating Moodie’s opisthotonic-posture hypothesis in fossil vertebrates part I: reptiles – the taphonomy of the bipedal dinosaurs Compsognathus longipes and Juravenator starki from the Solnhofen Archipelago (Jurassic, Germany). Palaeobiodivers Palaeoenviron 92:119–168
Sadleir RW, Barrett PM, Powell HP (2008) The anatomy and systematics of Eustreptospondylus oxoniensis, a theropod dinosaur from the Middle Jurassic of Oxfordshire, England. Monogr Palaeontol Soc 627:1–82
Saitta E, Gelernter R, Vinther J (2018) Additional information on the primitive contour and wing feathering of paravian dinosaurs. Palaeontology 61:273–288
Sansom RS, Gabbott SE, Purnell MA (2010) Non-random decay of chordate characters causes bias in fossil interpretation. Nature 463:797–800
Schaub S (1912) Die Nestdunen der Vögel und ihre Bedeutung für die Phylogenie der Feder. Verh Naturforsch Ges Basel 23:131–182
Schweitzer MH, Watt JA, Avci R, Forster CA, Krause DW, Knapp LW, Rogers R, Beech I, Marshall M (1999a) Keratin immunoreactivity in the Late Cretaceous bird Rahonavis ostromi. J Vertebr Paleontol 19:712–722
Schweitzer MH, Watt JA, Avci R, Knapp LW, Chiappe LM, Norell MA, Marshall M (1999b) Beta-keratin specific immunological reactivity in feather-like structures of the Cretaceous alvarezsaurid, Shuvuuia deserti. J Exp Zool B Mol Dev Evol 285:146–157
Sengel P (1976) Morphogenesis of skin. Cambridge University Press, Cambridge
Sereno PC, Beck AL, Dutheil DB, Gado B, Larsson HCE, Lyon GH, Marcot JD et al (1998) A long-snouted predatory dinosaur from Africa and the evolution of Spinosaurids. Science 282:1298–1302
Sondheimer E, Dence WA, Mattick LR, Silverman SR (1966) Composition of combustible concretions of the alewife, Alosa pseudoharengus. Science 152:221–223
Tischlinger H (1998) Erstnachweis von Pigmentfarben bei Plattenkalk-Teleosteern. Archaeopteryx 16:1–18
Tischlinger H (2002) Der Eichstätter Archaeopteryx im langwelligen UV-Licht. Archaeopteryx 20:21–38
Tischlinger H (2005) Neue Informationen zum Berliner Exemplar von Archaeopteryx lithographica H. v. Meyer 1861. Archaeopteryx 23:33–50
Tischlinger H (2009) Der achte Archaeopteryx – das Daitinger Exemplar. Archaeopteryx 27:1–20
Tischlinger H, Arratia G (2013) Ultraviolet light as a tool for investigating Mesozoic fishes, with a focus on the ichthyofauna of the Solnhofen archipelago. In: Arratia G, Schultze HP, Wilson MVH (eds) Mesozoic fishes, vol 5: Global diversity and evolution. Verlag Dr. Friedrich Pfeil, München, pp 549–560
Tischlinger H, Frey E (2002) Ein Rhamphorhynchus (Pterosauria, Reptilia) mit ungewöhnlicher Flughauterhaltung aus dem Solnhofener Plattenkalk. Archaeopteryx 20:1–20
Tischlinger H, Rauhut OWM (2015) Schuppenechsen (Lepidosauria). In: Arratia G, Schultze HP, Tischlinger H, Viohl G (eds) Solnhofen – Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München, pp 431–447
Tischlinger H, Unwin DM (2004) UV-Untersuchungen des Berliner Exemplars von Archaeopteryx lithographica H. v. Meyer 1861 und der isolierten Archaeopteryx-Feder. Archaeopteryx 22:17–50
Tsuihiji T, Watabe M, Tsogtbaatar K, Tsubamoto T, Barsbold R, Suzuki S et al (2011) Cranial osteology of a juvenile specimens of Tarbosaurus bataar (Theropoda, Tyrannosauridae) from the Nemegt Formation (Upper Cretaceous) of Bugin-Tsav, Mongolia. J Vertebr Paleontol 31:497–517
Van der Reest AJ, Wolfe AP, Currie PJ (2016) A densely feathered ornithomimid (Dinosauria: Theropoda) from the Upper Cretaceous Dinosaur Park Formation, Alberta, Canada. Cretac Res 58:108–117
Vinther J, Briggs DEG, Prum RO, Saranathan V (2008) The colour of fossil feathers. Biol Lett 4:522–525
Viohl G (2015a) Die Plattenkalk-Typen der Südlichen Frankenalb. In: Arratia G, Schultze H-P, Tischlinger H, Viohl G (eds) Solnhofen – Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München, pp 72–77
Viohl G (2015b) Die Grabung in Schamhaupten. In: Arratia G, Schultze H-P, Tischlinger H, Viohl G (eds) Solnhofen – Ein Fenster in die Jurazeit. Verlag Dr. Friedrich Pfeil, München, pp 119–125
Viohl G, Zapp M (2006) Die Fossil-Lagestätte Schamhaupten (oberstes Kimmeridgium, Südliche Frankenalb, Bayern). Archaeopteryx 24:27–78
Viohl G, Zapp M (2007) Schamhaupten, an outstanding Fossil-Lagerstätte in a silicified Plattenkalk around the Kimmeridgian-Tithonian boundary (Southern Franconian Alb, Bavaria). Neues Jahrb Geol Palaontol Abh 245:127–142
Wang X, Pittman M, Zheng X, Kaye TG, Falk AR, Hartman S, Xu X (2017) Basal paravian functional anatomy illuminated by high-detail body outline. Nat Commun 8:14576. https://doi.org/10.1038/ncomms14576
Wellnhofer P (2009) Archaeopteryx: the icon of evolution. Verlag Dr. Friedrich Pfeil, München
Werneburg I, Hugi J, Müller J, Sánchez-Villagra MR (2009) Embryogenesis and ossification of Emydura subglobosa (Testudines, Pleurodira, Chelidae) and patterns of turtle development. Dev Dyn 238:2770–2786
Wilby PR, Briggs DEG, Bernier P, Gaillard C (1996) Role of microbial mats in the fossilization of soft tissues. Geology 24:787–790
Wiman C (1942) Über ältere und neuere Funde von Leichenwachs. Senckenbergiana 25:1–19
Witmer LM (1995) The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In: Thomason JJ (ed) Functional morphology in vertebrate paleontology. Cambridge University Press, Cambridge, pp 19–33
Xu X, Guo Y (2009) The origin and early evolution of feathers: insights from recent paleontological and neontological data. Vertebrata PalAsiatica 47:311–329
Xu X, Zhou Z, Prum RO (2001) Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature 410:200–204
Xu X, Norell MA, Kuang X, Wang X, Zhao Q, Jia C (2004) Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids. Nature 431:680–684
Xu X, Zheng X, You H (2009) A new feather type in a nonavian theropod and the early evolution of feathers. Proc Natl Acad Sci USA 106:832–834
Xu X, Zheng X, You H (2010) Exceptional dinosaur fossils show ontogenetic development of early feathers. Nature 464:1338–1341
Xu X, Wang K, Zhang K, Ma Q, Xing L, Sullivan C, Hu D, Cheng S, Wang S (2012) A gigantic feathered dinosaur from the Lower Cretaceous of China. Nature 484:92–95
Xu X, Zhou Z, Dudley R, Mackem S, Chuong C, Erickson GM, Varricchio DJ (2014) An integrative approach to understanding bird origins. Science 346:12532931–12532910
Xu X, Zheng X, Sullivan C, Wang W, Xing L, Wang Y, Zhang X, O’Connor JK, Zhang F, Pan Y (2015) A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings. Nature 251:70–73
Yang Z, Jiang B, McNamara ME, Kearns SL, Pittman M, Kaye TG, Orr PJ, Xu X, Benton MJ (2018) Pterosaur integumentary structures with complex feather-like branching. Nat Ecol Evol 3:24–30
Zelenitsky DK, Therrien F, Erickson GM, DeBuhr CL, Kobayashi Y, Eberth DA, Hadfield F (2012) Feathered non-avian dinosaurs from North America provide insight into wing origins. Science 338:510–514
Zhang F, Kearns SL, Orr PJ, Benton MJ, Zhou Z, Johnson D, Xu X, Wang X (2010) Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 463:1075–1078
Zhao X, Currie PJ (1993) A large crested theropod from the Jurassic of Xinjiang, People’s Republic of China. Can J Earth Sci 30:2027–2036
Zheng X, You H, Xu X, Dong Z (2009) An Early Cretaceous heterodontosaurid dinosaur with filamentous integumentary structures. Nature 458:333–336
Zheng X, Zhou Z, Wang X, Zhang F, Zhang X, Wang Y, Wei G, Wang S, Xu X (2013) Hind wings in basal birds and the evolution of leg feathers. Science 339:1309–1312
Acknowledgements
We thank Raimund Albersdörfer and Martina Köbl-Ebert for access to the two specimens and Michael Pittman for critical discussion and reviewing the manuscript. This work was supported by the Volkswagen Foundation under grant I/84 640 (to OR), the Deutsche Forschungsgemeinschaft under grant (FO 1005/2-1) and the Swiss National Science Foundation under grant PZ00P2_174040 (both to CF).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Foth, C., Haug, C., Haug, J.T., Tischlinger, H., Rauhut, O.W.M. (2020). Two of a Feather: A Comparison of the Preserved Integument in the Juvenile Theropod Dinosaurs Sciurumimus and Juravenator from the Kimmeridgian Torleite Formation of Southern Germany. In: Foth, C., Rauhut, O. (eds) The Evolution of Feathers. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-27223-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-27223-4_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27222-7
Online ISBN: 978-3-030-27223-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)