Abstract
Several stem birds, such as Confuciusornithidae and Enantiornithes, were characterized by the possession of one or two pairs of conspicuous, elongated tail feathers with a unique morphology, so-called rhachis-dominated racket plumes. In the past, several studies reported contradictory interpretations regarding the morphology of these feathers, which sometimes failed to match with any morphology known from modern feathers. In this chapter, these interpretations are reviewed and compared with various modern feather types. The comparison confirms recent interpretations that the rhachis-dominated racket plumes are highly modified pennaceous feathers with ornamental function, originating at least two times independently from each other during evolution. While the gross organization (i.e., a short distal vane and a long, naked rhachis) of these feathers resembles that of filoplumes, they resemble pennaceous body feathers of penguins in terms of rhachis morphology and pigmentation pattern. As the rhachis-dominated racket plumes combine different morphologies that are apparent among modern feather types, this extinct morphotype does in fact not show any aberrant morphological novelties, but rather fall into the morphological and developmental spectrum of modern feathers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
10.1 Introduction
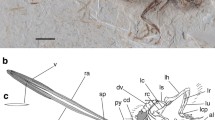
The tail plumage of Mesozoic Pennaraptora is characterized by a huge shape diversity (Foth et al. 2014; Wang et al. 2014), which is influenced by the length and morphology of the caudal series (e.g., Felice 2014; Rashid et al. 2014), the distribution of contour feathers along the tail (e.g., O’Connor et al. 2013; Foth et al. 2014; Wang et al. 2014), and the morphology of the tail feathers (=rectrices) (e.g., O’Connor et al. 2012; Wang et al. 2014). The tail plumage of Confuciusornithidae and many species of Enantiornithes is of special interest, as it frequently contains one or two pairs of conspicuously elongated, distally vaned tail feathers, herein called rhachis-dominated racket plumes (Fig. 10.1a, c), which are attached to the distal end of the pygostyle (e.g., Chiappe et al. 1999; Zheng et al. 2007; Zhang et al. 2008a, b; O’Connor et al. 2012; Carvalho et al. 2015a). Similar tail feathers were also described for the enigmatic scansoriopterygid Epidexipteryx hui (Zhang et al. 2008a, b) and an early juvenile individual of the oviraptorosaur Similicaudipteryx yixianensis (Xu et al. 2010b), but their likeness to rhachis-dominated racket plumes is not fully accepted. Apart from the unclear phylogenetic position of Scansoriopterygidae within Maniraptora (see Xu et al. 2010a, 2015; Agnolín and Novas 2013; O’Connor and Sullivan 2014), the distal portions of the tail feathers of Epidexipteryx are not preserved. Thus, it is not clear at the moment if they represent rhachis-dominated racket plumes or an own distinct feather type. The presence of rhachis dominated racket plumes in Similicaudipteryx as was questioned by various authors (Prum 2010; Foth 2012; O’Connor et al. 2012), and the structure can alternatively be interpreted as pin feathers, that are developing pennaceous feathers, which are still covered by the feather sheath. Thus, both species will not be included into the actual comparison.
Examples of elongated rhachis-dominated feathers in stem birds. (a) Confuciusornis sanctus (IVPP V13156) with rhachis-dominated racket plumes indicated by the arrow. (b) Eopengornis martini (STM24-1) with rhachis-dominated rectrices showing the common pennaceous morphology. (c) Enantiornithes indet. (GSGM-07-CM-001) with rhachis-dominated racket plumes. (d) Details of the distal end of the rhachis-dominated racket plumes of Confuciusornis sanctus. (e) Details of the distal end of the rhachis-dominated racket plumes of GSGM-07-CM-001. dv distal vane, ls lateral stripe, ms median stripe, r rhachis, v vane. Scale bars in (b)–(e) is 2 cm
In analogy to the elongated rectrices of modern birds (Andersson 1982; Bleiweiss 1987; Peters and Peters 2009), the elongated tail feathers of Confuciusornithidae and Enantiornithes probably had an ornamental function (Peters and Peters 2009; O’Connor et al. 2012), which in some cases may have been related to sexual dimorphism (Zheng et al. 2017). The actual morphology of rhachis-dominated racket plumes, however, seems to be quite different from those of modern examples. As a result, there is no true consensus regarding their morphology, and various interpretations have been published in the past (e.g., Xu and Guo 2009; Prum 2010; Foth 2012; O’Connor et al. 2012; Carvalho et al. 2015b), which often relied on differences in the quality of preservation. In the current chapter, these different morphological interpretations are reviewed and compared with each other. After extracting the main organization, the single individual morphological components of these enigmatic tail feathers are compared with different modern feather types. By extending this comparison beyond the morphological spectrum of tail feathers, it is possible to track down analog structures, helping to understand the actual morphology of this extinct feather type.
10.2 Institutional Abbreviations
GSGM Gansu Geological Museum, Lanzhou, China; IVPP Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China; NHMF Natural History Museum Fribourg, Switzerland; STM Shandong Tianyu Museum of Natural History, Linyi, China
10.3 Previous Morphological Interpretations
In the past, the enigmatic, elongated rectrices of Confuciusornithidae and Enantiornithes were addressed with varying terms, including elongate ribbonlike tail feathers (ETFs) (Zhang et al. 2008a, b), proximally ribbonlike pennaceous feathers (PRPFs) (Xu et al. 2010b), rhachis-dominated tail feathers (O’Connor et al. 2012), or rhachis-dominated racket plumes (Wang et al. 2014). Despite these different terms, the rectrices of the taxa in question possess a characteristic morphology, consisting of a broad, elongated central element with a dark, median stripe. The proximal portion of the central element is naked, exhibits dark lateral margins, and shows no sign of branching, while the distal quarter is vaned, being pennaceous (Fig. 10.1c–e). As is typical for fossilized plumages from the Jehol beds, the elongated tail feathers are usually preserved as carbonized traces, which has been shown to result from the preservation of melanosomes, showing the original pigmentation of the fossilized feathers, in several taxa (e.g., Vinther et al. 2008; Li et al. 2010; Zhang et al. 2010).
Originally, this feather type was described as scalelike (Zhang and Zhou 2000), which was classified as an ancestral unbranched feather type. However, this interpretation was based on an incomplete specimen, where the distal portions of the feathers were not preserved (O’Connor et al. 2012). On the basis of complete feathers, three different morphological interpretations were published so far as follows:
-
1.
Originally, the dark, median stripe of the central element was interpreted as a thin rhachis with two undivided, sheetlike vanes or laminae emerging on either side. In the distal portion, the pennaceous barbs were thought to extend outwards from the sheetlike vane and to not be directly connected with the rhachis (Zhang et al. 2006, 2008a, b; Xu and Guo 2009; Xu et al. 2010b; Fig. 10.2a).
-
2.
Later, Xu et al. (2010c) and O’Connor et al. (2012) argued that the whole central element represents an extremely long, broad rhachis, which ends in a distal, pennaceous portion (see also Prum 2010). The calamus, which is not preserved due to the probable lack of melanosomes in this region (see Benton et al. 2008; Vinther et al. 2008), is restricted to the most proximal portion of the feather, while the dark stripe in the middle of the rhachis was interpreted to be a preservational artifact resulting from a ventral furrow (Fig. 10.2b). Additionally, O’Connor et al. (2012) interpreted the dark lateral margins of the proximal ribbonlike portion of the central elements (Fig. 10.1c) to be possible remains of narrow, undifferentiated vanes (see above).
-
3.
Based on the branching pattern of the rectrices, Foth (2012) argued that the rhachis is only a broad and short element, restricted to the pennaceous portion, while the proximal ribbonlike structure in fact represents a prolonged, broadened calamus (Fig. 10.2c). The dark median line of the central element was interpreted as a pigmentation of the internal pith inside the rhachis and calamus or as remains of a ventral furrow (see O’Connor et al. 2012).
Different interpretations of the morphology of rhachis-dominated racket plumes in stem birds. (a) Morphology after Zhang et al. (2006, 2008a, b), Xu and Guo (2009), and Xu et al. (2010b). (b) Morphology after Xu et al. (2010c) and O’Connor et al. (2012). (c) Morphology after Foth (2012). (d) Current interpretation based on the comparison with various modern feather types, including penguin body feathers. b barbs of the distal vane, ca calamus, ipmr internal pigmented median ridge, iplr internal pigmented lateral ridge, mc medullary cavity, r rhachis, sb short barbs, sv sheetlike vanes, vf ventral furrow. Illustration of elongated tail feathers modified after Xu et al. (2010a)
All three interpretations are problematic for various reasons: The presence of sheetlike vanes, running along the whole central element, as proposed by Zhang et al. (2006, 2008a, b), Xu and Guo (2009), and Xu et al. (2010b), or being restricted to the lateral margin of the proximal ribbonlike portion (see O’Connor et al. 2012), is incorrect from a semantic point of view, as feather vanes consist of a series of parallel arranged barbs (Lucas and Stettenheim 1972) and thus, by definition, cannot be undifferentiated. Along those lines, the Enantiornithes Cratoavis cearensis from the Early Cretaceous Crato Formation of Brazil (see below), whose elongated rectrices are preserved as impression and not carbonized traces (Carvalho et al. 2015a, b), indicates that, at least in this bird, the whole central element is strap-like (see Prum 2010; Xu et al. 2010c; Foth 2012; O’Connor et al. 2012), showing no morphological signs of undifferentiated vanes in the proximal portion.
Based on the hierarchical organization of feathers in terms of morphology and development (Lucas and Stettenheim 1972; Prum and Dyck 2003), the whole central structure in the pennaceous portion has to be classified as the rhachis (see Prum 2010; Foth 2012; O’Connor et al. 2012). However, contrary to Foth (2012), the central element of the rectrices shows no sign of interruption in the form of a superior umbilicus between the distal pennaceous and proximal ribbonlike portion, which would mark the transition from rhachis to calamus. In fact, the median stripe runs without interruption along the entire central element, and the dark lateral margins are continuous with the barbs of the pennaceous portion (O’Connor et al. 2012). Thus, the whole central element can, in fact, be interpreted as one single strap-like structure, that is, an elongated, dorsoventrally flattened rhachis, as previously interpreted by Prum (2010), Xu et al. (2010c), and O’Connor et al. (2012).
The median stripe itself is usually preserved as a narrow carbonized trace, but not as an impression (O’Connor et al. 2012). According to the interpretation of Xu et al. (2010a) and O’Connor et al. (2012), the stripe would be exposed ventrally. As demonstrated by several studies, carbonized traces are often the result of melanosome preservation, which show the original pigmentation pattern of the fossilized feather (e.g., Vinther et al. 2008; Li et al. 2010; Zhang et al. 2010). Because the integument is usually preserved as a film, this type of preservation provides no direct evidence as to which side of the feather is exposed. By contrast, as the lateral parts of the strap-like rhachides, which surround the median stripe, are not pigmented (see O’Connor et al. 2012), the dark median stripe could be a potential “eye-catcher” for other members of the species. Thus, assuming an ornamental function, it is more plausible that the median stripe was located dorsally or part of the internal pith (see below) and is actually not homologous with the ventral furrow of the rhachis.
In this context, Carvalho et al. (2015b) described the presence of a thin midline furrow along the broadened rhachis of the racket plumes of Cratoavis, which was interpreted as dorsal groove, a structure unknown for recent bird feathers. The authors apparently presumed that the rhachis-dominated racket plumes of Cratoavis are preserved in dorsal view without giving any explanation other than that the proximal caudal vertebrae and pygostyle are preserved in that view as well. On the basis of the Berlin specimen of Archaeopteryx lithographica, this equation of skeletal and integumental orientation is taphonomically not always valid, as in this particular specimen the skeleton is visible in dorsolateral view (Wellnhofer 2009), while the wings clearly show the ventral aspect (Wellnhofer 2009; Longrich et al. 2012). Such preservational artifacts result when the fossil is unevenly split between the two plates. Thus, without providing further evidence that the rectrices of Cratoavis are actually preserved in dorsal view, it is alternatively possible that the longitudinal furrow actually represents the ventral furrow of the rhachis (see Lucas and Stettenheim 1972).
10.4 Morphological Comparison with Modern Feather Types
Hereinafter, the hierarchical organization and the morphology of single structures common in rhachis-dominated racket plumes are compared with similar-looking structures in modern feather types. As rhachis-dominated racket plumes are extinct as morphotype, this comparison is restricted to single feather structures, while it is simultaneously extended to feather types from other body regions, which often fulfill a very different biological role. In consequence, functional aspects cannot be transmitted to morphological structure one to one.
10.5 Modern Feather Types with a Distally Branching Portion
Distally restricted vanes are known in racket plumes (Bleiweiss 1987; Fig. 10.3a–d) and filoplumes (Lucas and Stettenheim 1972; Fig. 10.3e). Modern racket plumes represent a type of display feathers, which occurs in the head and tail regions of various recent birds (e.g., Prioniturus discurus, Ocreatus underwoodii, Loddigesia mirabilis, Tanysiptera carolinae, Parotia carolae). Their distal portion consists of a thin rhachis with distinct pennaceous vanes, which merge proximally into a thin, “naked” wire section. This wire section, however, is not truly naked, but consists of narrow vanes of densely packed, rudimentary barbs, running along both sides of the rhachis. The most proximal portion of the racket plumes, however, can be fully vaned again, showing the typical pennaceous morphology (Bleiweiss 1987). Despite overall similarities, the rhachis-dominated racket plumes of the stem birds discussed above seem to show no indication for the presence of short barbs in the proximal portion of the rhachis (see Cratoavis), at least under normal light (see below; Fig. 10.3a–d). This situation is also evident in various rhachis-dominated feathers found in the Upper Cretaceous Burmese amber (Xing et al. 2018). Instead, the feathers often possess a dark lateral stripe on each side of the rhachis (O’Connor et al. 2012, see below).
Examples of distal-vaned feather in extant birds. (a) Racket plumes of a female strange-tailed tyrant (Alectrurus risora, Tyrannidae). (b) Racket plumes of booted racket-tail (Ocreatus underwoodii, Trochilidae). (c) Racket plumes of king bird-of-paradise (Cicinnurus regius, Paradisaeidae). (d) Racket plumes of the Amazonian motmot (Momotus momota, Coraciiformes). (e) Drawing of a filoplume with details of the calamus morphology. ca calamus, dv distal vane, pv proximal vane, r rhachis, re rectrices. (a)–(d) Photos by Hans-Rüdiger Siegel (NHMF-2016). (e) Modified after Lucas and Stettenheim (1972)
By contrast, fully grown filoplumes, which fulfill a biological role as sensory organs, possess a small number of distal barbs, which are fused into a thin rhachis. The rhachis itself is elongated, showing a long naked portion, before it anastomoses ventrally into a short calamus (Lucas and Stettenheim 1972; Fig. 10.3e). Consequently, the gross organization of filoplumes (i.e., the portion of vanes and rhachis) resembles to some degree the morphology of the rhachis-dominated racket plumes as interpreted by Prum (2010), Xu et al. (2010c) and O’Connor et al. (2012). However, filoplumes are much smaller in size, possess a very short open (not pennaceous) vane, a thin rhachis, and, in contrast to most other feather types (except of bristles and semibristles), are associated with the nervous system located within the follicle (Lucas and Stettenheim 1972).
10.6 Modern Feather Types with Broadened Rhachis
In most feathers, the rhachis is a four-sided element and not conspicuously broadened and flattened (Lucas and Stettenheim 1972). However, display feathers of several bird species show a distal expansion. In the scale-feathered malkoha (Phaenicophaeus cumingi) and curl-crested aracari (Pteroglossus beauharnaesii), the distal expansions are caused by the lateral fusion of several barbs (Brush 1965, 1967). In contrast, in the rail species Rallus aquaticus, Rallus elegans, and Rallus longirostris and the cedar waxwing (Bombycilla cedrorum), a similar morphology results from the broadening of the terminal barb, which forms the tip of the rhachis (Brush 1967). The display feathers of the African openbill (Anastomus lamelligerus) show a mixture of both morphologies, as the most terminal barb is elongated and broadened, while additional, distally located barbs are fused to the terminal barb proximally (Vigneron et al. 2006). However, in all of these examples, the lateral expansion of the rhachis is restricted to the distal tip of the feather. More proximally, the rhachis thins to the common pennaceous condition.
The only example of modern feathers possessing a broadened, flattened rhachis over their entire length is known from penguins (Wohlauer 1901; Chandler 1916; Rutschke 1965). The rhachis of the body feathers, for instance, emerges from a short, cylindrical calamus. Proximally the rhachis is oval in cross section, but continuously expands laterally, while flattening dorsoventrally, before tapering at the feather tip (Chandler 1916; Rutschke 1965; Fig. 10.4). Feathers from the belly region possess a thin and shallow ventral furrow in the proximal half of the rhachis (Fig. 10.4b), while such a structure is absent in the back feathers. In contrast, prominent ventral furrows giving the rhachis an open C-shaped cross section are present in the remiges and rectrices of penguins (Rutschke 1965). Thus, although fully vaned and shorter in relative length, the rhachis morphology of penguin body feathers resembles the observations of rhachis-dominated racket plumes and the fully pennaceous rhachis-dominated rectrices of Eopengornis martini and Parapengornis eurycaudatus (see discussion). Recent discoveries of rhachis-dominated feathers from Upper Cretaceous Burmese amber seem to contradict this comparison, by showing a central (rhachidal) ridge surrounded by two undifferentiated laminae, which lack an internal pith, but having a ventrally opened C-shaped cross section (Xing et al. 2018). However, as the central element of these feathers measures less than 1 mm in a diameter, this particular morphology could result from miniaturization, showing a broadened rhachis without a pith. In fact, many modern feather types with delicate barbs (e.g., small down feathers, many neoptile down feathers) or rhachides (e.g., filoplumes, small bristle feathers) also lack an internal pith (Lucas and Stettenheim 1972; Foth 2011). Thus, due to significant size differences, the rhachis morphology of the Upper Cretaceous Burmese amber does not necessarily correspond to the larger tail streamers found in the birds from the Jehol group. However, as stated above, the rhachides of remiges and rectrices in penguins also have a C-shaped in cross section (Rutschke 1965), resembling to a certain degree the condition found in the Burmese feathers.
Morphology of a pennaceous body feather of the emperor penguin (Aptenodytes forsteri). (a) Dorsal view. (b) Ventral view. (c–e) Drawings of the cross section of rhachis of a pennaceous body feather of Aptenodytes forsteri from different portions. do dorsal, ve ventral, b pennaceous barbs, ca calamus, ipmr internal pigmented median ridge, r rhachis, vf ventral furrow. Scale bars in (a)–(b) is 1 cm. (c)–(e) modified after Rutschke (1965)
10.7 Rhachis Pigmentation in Modern Feathers
In analogy to the general preservation of feathers as dark carbonized traces, the dark median stripe found in rhachis-dominated racket plumes is most likely based on preservation of melanosomes (see Vinther et al. 2008; Li et al. 2010; Zhang et al. 2010) and thus indicates a color pattern along the rhachis. In modern feathers, very complex color patterns can be present, but are usually exposed on the dorsal surface of the vanes of pennaceous feathers (Prum and Williamson 2002). The rhachis itself is often monochromatic, sometimes shaded, but not complexly pigmented. Here, pigments can be concentrated in the pith or in the cortex of the rhachis (Rutschke 1965; Brush 1967). For instance, in the scale-feathered malkoha, curl-crested aracari, different rail species, and the cedar waxwing (see above), high concentrations of melanin are present in the rhachidal pith (Brush and Allen 1963; Brush 1967). This kind of pigmentation results in a plane, dark, monochromatic appearance of the rhachis, which is, however, different from the situation found in the fossil examples. In contrast, the whitish, broadened rhachis of penguin feathers possess a thin, dark median stripe, which is usually expressed on the dorsal side of the feather (Wohlauer 1901; Rutschke 1965; Fig. 10.4a). This structure results from the presence of a high concentration of melanin pigments, which are located within a longitudinal, internal ridge that runs along the dorsal side of the cortex, while the rest of the cortex is unpigmented (Rutschke 1965; Fig. 10.4a, c–e). In some penguin feathers, a similar, median stripe is additionally present on the ventral side, which fuses with the dorsal ridge in the distal portion of the rhachis (Rutschke 1965; Fig. 10.4b, e). Also slightly different in morphology, the rhachis-dominated feathers from the Upper Cretaceous Burmese amber show a median ridge along the rhachis that is strongly pigmented (Xing et al. 2018).
In this context, the dark, lateral margins, originally described as undifferentiated vanes (O’Connor et al. 2012), could be the result from similar, highly pigmented, internal cortical ridges, running along the lateral side of the rhachis. Alternatively, the dark, lateral stripes could be also a preservational artifact caused by the conservation of highly pigmented, very short, but densely packed barbs, which cannot be detected with the help of normal light microscopy techniques. In this case, the proximal portion of the elongated rectrices would not be truly naked, but similar to the wire structures found in racket plumes (Bleiweiss 1987, see above). Here, the usage of laser-stimulated fluorescence (LSF) may be able to help to clarify the morphology of these structures in the future, as this autofluorescence method was successfully employed to visualize the remains of tiny barbules in fossil feathers, which were hardly detectable under white and polarized light conditions (Kaye et al. 2015). However, the rhachis-dominated feathers from Cratoavis and the Upper Cretaceous Burmese amber (Carvalho et al. 2015a; Xing et al. 2018) indicate that the proximal portion of the rhachis was actually naked.
10.8 Discussion
Within Pygostylia, rhachis-dominated racket plumes evolved at least two times independently within the stem line of birds, in Confuciusornithidae, and Enantiornithes (Foth et al. 2014; Wang et al. 2014). The presence of a pair of elongated, fully pennaceous, but rhachis-dominated, rectrices in the two Enantiornithes Eopengornis and Parapengornis (Fig. 10.1b) and the discovery of an enantiornithine bird with a rectricial fan have led to the conclusion that rhachis-dominated racket plumes were highly modified pennaceous feathers (O’Connor et al. 2012, 2016; Wang et al. 2014; Hu et al. 2015). This is further supported by the occurrence of delicate median stripes in the rhachis of wing feathers (=remiges) of some stem birds like Confuciusornis spp. (Confuciusornithidae) and Eopengornis (Enantiornithes) (Wang et al. 2014, 2015). With this review, I attempt to clarify a number of problematic aspects regarding the morphology of these feathers that have been published in the last years. In particular, the gross organization of these feathers resembles either that of filoplumes, containing a distally branched portion fused into a long, naked rhachis. The comparison with the wire section of modern racket plumes might be inadequate, as it possesses a series of short, densely arranged barbs running along the elongated “naked” portion (Fig. 10.3). Nevertheless, the distal portion itself was fully pennaceous as in modern racket plumes. The long, central element probably represents a single, elongated, strap-like rhachis, which most likely merges proximally into a short, cylindrically shaped calamus, thereby resembling the condition of the fully pennaceous rhachis-dominated rectrices of Eopengornis martini and Parapengornis and potentially that of modern penguin body feathers (Fig. 10.4). However, this particular morphology could be modified to a more laminar shape (Xing et al. 2018) due to miniaturization, resulting in a reduction of the internal rhachidal pith (Lucas and Stettenheim 1972). In further analogy to penguin feathers, the dark median stripe running along the broadened rhachis might represent a strongly pigmented internal cortical ridge (Fig. 10.4). [The situation for the median stripe in the wing feathers of some stem birds (see above) is not evaluated here due to the unexplored situation in terms of the presence of this particular character in modern bird wing remiges.] If one assumes an ornamental function, this pigmented ridge would probably have been located on the dorsal side of the rhachis, although a (additional) ventral expression, as in some penguin feathers, cannot be ruled out. Taking the variety of pigmentation patterns of modern feathers into account and the fact that these ornamental feathers originated at least two times independently, the occurrence of the pigmented ridge on the dorsal or ventral side could be variable and differ between taxa. In the strong miniaturized feathers from the Upper Cretaceous Burmese amber, the median ridge is even externally recognizable from both dorsal and ventral side (Xing et al. 2018), which could be caused by the reduction of the internal pith, leading to the extreme lamination of the rest of the rhachis. In analogy to modern pennaceous feathers, the longitudinal groove found in the rectrices in Cratoavis most likely represents the ventral furrow of the rhachis and not a dorsal groove as originally interpreted. The dark lateral margins in the proximal ribbonlike portion (Fig. 10.1c) could result from either pigmented internal lateral cortical ridges or very short, densely packed pigmented barbs running along the rhachis. As the rhachis-dominated feathers of Cratoavis and the Upper Cretaceous Burmese amber seem to have smooth lateral margins, the second alternative seems to be less likely, at least for Enantiornithes. And, once again, given that this feather type evolved two times independently (see above), it cannot be ruled out that the dark lateral margins evolved differently among Confuciusornithidae and Enantiornithes. To test this, the morphology of the lateral margins has to be investigated in more detail in the future using autofluorescence methods (see Kaye et al. 2015).
Despite these uncertainties, all proposed structures can be verified with an analog example found in modern feather types. This in turn implies that this very specialized fossil feather type falls into the morphological, and therefore developmental (including the genetic control), spectrum of modern feathers. Previously, O’Connor et al. (2012) proposed a hypothetical molecular developmental model, where rhachis enlargement is caused by changes in the BMP (bone morphogenetic protein), Noggin, and Shh (sonic hedgehog) activity (see also Yu et al. 2002). Due to the great similarities with the rhachis morphology of penguin feathers, this model can now be tested directly by studying feather morphogenesis in this group of birds.
While the broad rhachides of penguin feathers represent one of the many morphological adaptions of the plumage to the semiaquatic lifestyle (Rutschke 1965), the enigmatic, rhachis-dominated racket plumes of Confuciusornithidae and Enantiornithes had probably an ornamental function (Peters and Peters 2009; O’Connor et al. 2012; Foth et al. 2014) similar to the distally expanded or elongated feather examples mentioned above (see Brush 1965, 1967; Bleiweiss 1987; Vigneron et al. 2006). In extant birds, the expanded portions are usually highlighted by color patterns created by pigments or nanostructural organization to the cortex and pith. For instance, the internal organization of parallel layers in the cortex of the body feathers of the Africa openbill (Vigneron et al. 2006) creates thin-film interferences due to refraction and reflection along the surfaces of each single layer, resulting in a gleaming color pattern. Thus, the presence of a broadened rhachis in the feathers discussed herein may be a strong indicator for a complex, gleaming color pattern with delicate dark highlights resulting from the median and lateral stripes. In addition to these ornamental functions, it was also hypothesized that the long rectrices had an aerodynamic function (Zhang et al. 2006). Vane asymmetry in the fully pennaceous rectrices of Eopengornis indicates that aerodynamics was an important biological role in the precursor of rhachis-dominated racket plumes (Wang et al. 2014). However, as the short pennaceous tip of the latter possesses a symmetric shape and cannot produce much lift, an evolutionary shift toward a stronger ornamental function was hypothesized (Wang et al. 2014).
10.9 Conclusions
The enigmatic, elongated tail feathers of Confuciusornithidae and Enantiornithes are here interpreted as highly modified pennaceous feathers that originated independently from each other during evolution. A review of previous morphological interpretations and taphonomic preservation of this feather type and a careful comparison with modern feather morphologies shows that these feathers are very similar to the body feathers of penguins in terms of rhachis morphology and pigmentation pattern, while the gross organization resembles that of filoplumes. Assuming a similar cortical structure to the rhachis of the African openbill, the rectrices of these stem birds can be inferred to probably have been iridescent, supporting a possible ornamental function. As all morphological structures can be verified with an analog example, found in modern feather types, this fossil feather type falls into the morphological spectrum of modern feathers. This in turn indicates that both the morphogenesis (including the genetic control) could be potentially studied with the help of the modern analogues presented herein.
References
Agnolín FL, Novas FE (2013) Avian ancestors. A review of the phylogenetic relationships of the theropods Unenlagiidae, Microraptoria, Anchiornis and Scansoriopterygidae. Springer, Dordrecht
Andersson M (1982) Female choice selects for extreme tail length in a widowbird. Nature 299:818–820
Benton MJ, Zhou Z, Orr PJ, Zhang F, Kearns SL (2008) The remarkable fossils from the early cretaceous Jehol biota of China and how they have changed our knowledge of Mesozoic life. Proc Geol Assoc 119:209–228
Bleiweiss R (1987) Development and evolution of avian racket plumes: fine structure and serial homology of the wire. J Morphol 194:23–39
Brush AH (1965) The structure and pigmentation of the feather tips of the scaled cuckoo (Lepidogrammus cumigi). Auk 82:155–160
Brush AH (1967) Additional observations on the structure of unusual feather tips. Wilson Bull 79:322–327
Brush AH, Allen K (1963) Astaxanthin in the Cedar Waxwing. Science 142:47–48
Carvalho IS, Novas FE, Agnolín FL, Isasi MP, Freitas FI, Andrade JA (2015a) A new genus and species of enantiornithine bird from the early cretaceous of Brazil. Brazilian J Geol 45:161–171
Carvalho IS, Novas FE, Agnolín FL, Isasi MP, Freitas FI, Andrade JA (2015b) A Mesozoic bird from Gondwana preserving feathers. Nat Commun 6:7141
Chandler AC (1916) A study of the structure of feathers, with reference to their taxonomic significance. Univ Calif Publ Zool 13:243–446
Chiappe LM, Ji S, Ji Q, Norell MA (1999) Anatomy and systematics of the Confuciusornithidae (Theropoda: Aves) from the late Mesozoic of northeastern China. Bull Am Mus Nat Hist 242:1–89.17
Felice RN (2014) Coevolution of caudal skeleton and tail feathers in birds. J Morphol 275:1431–1440
Foth C (2011) The morphology of neoptile feathers: ancestral state reconstruction and its phylogenetic implications. J Morphol 272:387–403
Foth C (2012) On the identification of feather structures in stem-line representatives of birds: evidence from fossils and actuopalaeontology. Paläontol Z 86:91–102
Foth C, Tischlinger H, Rauhut OWM (2014) New specimen of Archaeopteryx provides insights into the evolution of pennaceous feathers. Nature 511:79–82
Hu H, O’Connor JK, Zhou Z (2015) A new species of Pengornithidae (Aves: Enantiornithes) from the lower cretaceous of China suggests a specialized scansorial habitat previously unknown in early birds. PLoS One 10:e0126791
Kaye TG, Falk AR, Pittman M, Sereno PC, Martin LD, Burnham DA, Gong E, Xu X, Wang Y (2015) Laser-stimulated fluorescence in paleontology. PLoS One 10:e0125923
Li Q, Gao K, Vinther J, Shawkey MD, Clarke JA, D’Alba L, Meng Q, Briggs DEG, Prum RO (2010) Plumage color patterns of an extinct dinosaur. Science 327:1369–1372
Longrich NR, Vinther J, Meng Q, Li Q, Russell AP (2012) Primitive wing feather arrangement in Archaeopteryx lithographica and Anchiornis huxleyi. Curr Biol 22:1–6
Lucas AM, Stettenheim PR (1972) Avian anatomy. Integument, part I and II. U.S. Government Printing Office, Washington, DC
O’Connor JK, Sullivan C (2014) Reinterpretation of the early cretaceous maniraptoran (Dinosauria: Theropoda) Zhongornis haoae as a scansoriopterygid-like non-avian, and morphological resemblances between scansoriopterygids and basal oviraptorosaurs. Vertebrata PalAsiatica 52:3–30
O’Connor JK, Chiappe LM, Chuong C, Bottjer DJ, You H (2012) Homology and potential cellular and molecular mechanisms for the development of unique feather morphologies in early birds. Geosciences 2:157–177
O’Connor JK, Wang X, Sullivan C, Zheng X, Tubaro P, Zhang X, Zhou Z (2013) Unique caudal plumage of Jeholornis and complex tail evolution in early birds. Proc Natl Acad Sci 110:17404–17408
O’Connor JK, Wang X, Zheng X, H H, Zhang X, Zhou Z (2016) An enantiornithine with a fan-shaped tail, and the evolution of the rectricial complex in early birds. Curr Biol 26:114–119
Peters WS, Peters DS (2009) Life history, sexual dimorphism and “ornamental” feathers in the mesozoic bird Confuciusornis sanctus. Biol Lett 5:817–820
Prum RO (2010) Moulting tail feathers in a juvenile oviraptorosaur. Nature 468:E1
Prum RO, Dyck J (2003) A hierarchical model of plumage: morphology, development, and evolution. J Exp Zool (MOL DEV EVOL) 298B:73–90
Prum RO, Williamson S (2002) Reaction-diffusion models of within-feather pigmentation patterning. Proc R Soc Lond B 269:781–792
Rashid DJ, Chapman SC, Larsson HCE, Organ CL, A-G Bebin CS, Merzdorf RB, Horner JR (2014) From dinosaurs to birds: a tail of evolution. EvoDevo 5:1–20
Rutschke E (1965) Beiträge zur Morphologie der Pinguinfeder. Z Morphol Okol Tiere 55:835–858
Vigneron JP, Lousse V, Colomer J-F, Rassart M, Louette M (2006) Complex optical structure in the ribbon-like feathers of the African open-bill stork. Proc SPIE 6320:632014
Vinther J, Briggs DEG, Prum RO, Saranathan V (2008) The colour of fossil feathers. Biol Lett 4:522–525
Wang X, O’Connor JK, Zheng X, Wang M, Hu H, Zhou Z (2014) Insights into the evolution of rhachis dominated tail feathers from a new basal enantiornithine (Aves: Ornithothoraces). Biol J Linn Soc 113:805–819
Wang M, Zheng X, O’Connor JK, Lloyd GT, Wang X, Wang Y, Zhang X, Zhou Z (2015) The oldest record of Ornithuromorpha from the early cretaceous of China. Nat Commun 6:6987
Wellnhofer P (2009) Archaeopteryx: the icon of evolution. Verlag Dr. Friedrich Pfeil, München
Wohlauer E (1901) Die Entwicklung des Embryonalgefieders von Eudyptes chrysocome. Z Morphol Anthropol 4:149–178
Xing L, Cockx P, McKellar RC, O’Connor JK (2018) Ornamental feathers in cretaceous Burmese amber: resolving the enigma of rachis-dominated feather structure. J Palaeogeogr 7:13
Xu X, Guo Y (2009) The origin and early evolution of feathers: insights from recent paleontological and neontological data. Vertebrata PalAsiatica 47:311–329
Xu X, Ma Q, Hu D (2010a) Pre-Archaeopteryx coelurosaurian dinosaurs and their implications for understanding avian origins. Chin Sci Bull 55:3971–3977
Xu X, Zheng X, You H (2010b) Exceptional dinosaur fossils show ontogenetic development of early feathers. Nature 464:1338–1341
Xu X, Zheng X, You H (2010c) Reply: moulting tail feathers in a juvenile oviraptorosaur. Nature 468:E2
Xu X, Zheng X, Sullivan C, Wang W, Xing L, Wang Y, Zhang X, O’Connor JK, Zhang F, Pan Y (2015) A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings. Nature 521:70–73
Yu M, P W, Widelitz RB, Chuong C (2002) The morphogenesis of feathers. Nature 420:308–312
Zhang F, Zhou Z (2000) A primitive enantiornithine bird and the origin of feathers. Science 290:1955–1959
Zhang F, Zhou Z, Dyke GJ (2006) Feathers and “feather-like” integumentary structures in Liaoning birds and dinosaurs. Geol J 41:395–404
Zhang F, Zhou Z, Benton MJ (2008a) A primitive confuciusornithid bird from China and its implications for early avian flight. Sci China Ser D Earth Sci 51:625–639
Zhang F, Zhou Z, Xu X, Wang X, Sullivan C (2008b) A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 455:1105–1108
Zhang F, Kearns SL, Orr PJ, Benton MJ, Zhou Z, Johnson D, Xu X, Wang X (2010) Fossilized melanosomes and the colour of cretaceous dinosaurs and birds. Nature 463:1075–1078
Zheng X, Zhang Z, Hou L (2007) A new enantiornitine bird with four long rectrices from the early cretaceous of northern Hebei, China. Acta Geol Sin 81:703–708
Zheng X, O’Connor JK, Wang X, Pan Y, Wang Y, Wang M, Zhou Z (2017) Exceptional preservation of soft tissue in a new specimen of Eoconfuciusornis and its biological implications. Natl Sci Rev 4:441–452
Acknowledgements
I thank Jingmai O’Connor and Gao Wei (both Institute of Vertebrate Paleontology and Paleoanthropology) for photos of the elongated tail feathers of basal birds. I further thank Emanuel Gerber and Hans-Rüdiger Siegel (both Natural History Museum Fribourg, NHMF-2016) for photos of the elongated tail feathers of recent birds. Norma Schmitz and Ragnar Kinzelbach (both Zoological collection of the University of Rostock) are acknowledged for feather samples of Aptenodytes forsteri and Walter Joyce (University of Fribourg) for proofreading the manuscript. Jingmai O’Connor is further thanked for her critical comments on the previous version of the chapter. The study was supported by the Swiss National Science Foundation under grant PZ00P2_174040.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Foth, C. (2020). A Morphological Review of the Enigmatic Elongated Tail Feathers of Stem Birds. In: Foth, C., Rauhut, O. (eds) The Evolution of Feathers. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-27223-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-27223-4_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27222-7
Online ISBN: 978-3-030-27223-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)