Abstract
Gut microbiota composition and functionality can influence the pathophysiology of age-related cognitive impairment and dementia, according to a large number of animal studies. The translation of this concept to humans is still uncertain, due to the relatively low number of clinical studies focused on fecal microbiota and large number of environmental factors that influence the microbiota composition. However, the fecal microbiota composition of older patients with dementia is deeply different from that of healthy active controls, conditioning a different metabolic profile. The possible use of fecal microbiota-related parameters and microbiota-derived metabolites as biomarkers of cognitive performance and dementia is critically reviewed in this paper, focusing on the most promising areas of research for the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Alzheimer’s disease
- Mild cognitive impairment
- Biodiversity
- Firmicutes/Bacteroidetes ratio
- Microbial metabolites
1 Gut Microbiota and Cognitive Aging
1.1 The “Gut-Brain Axis” in Dementia
In the last decade, several studies have highlighted that the intestinal microbiome, i.e. the ensemble of bacteria symbiotically living with the host in the gut lumen, may influence the physiopathology ofa large number of human diseases, not involving only the gastrointestinal system [1,2,3]. For example, alterations of the composition of gut microbiota may promote the progression of chronic liver disease [4], chronic renal failure [5] and even the formation of kidney stones [6]. By modulating insulin sensitivity, anabolism and systemic inflammation, the gut microbiota could also have relevance in the onset of age-related bone diseases and sarcopenia [7, 8].
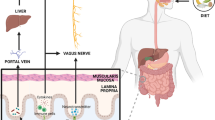
In this context, the existence of a possible “gut-brain axis” influencing cognition in aging has been hypothesized [9, 10]. A large number of animal studies, recently reviewed elsewhere [10], have demonstrated that alterations of the gut microbiota composition, the so-called dysbiosis, can be associated with reduction of performances at cognitive tasks and, conversely, the administration of probiotics or functional foods can prevent cognitive decline in transgenic models of Alzheimer’s disease. The hypothesized mechanisms involved in these phenomena include bacterial production of amyloid proteins able to promote β-amyloid deposition in the brain tissue, modulation of vagal nerve activity, production of bacterial metabolites that may act as endocrine modulators or neurotransmitters, modulation of neuroinflammation and microbiota-mediated transformation of nutrients into substances exerting toxic or protective activity on brain cells [10, 11].
Therefore, the current scientific literature suggests that the intestinal microbiome can influence the onset of dementia and its progression in multiple ways, making the “gut-brain axis” a promising area of research for the future.
1.2 Clinical Relevance of the “Gut-Brain Axis” in Humans with Dementia
Despite the results of pre-clinical studies, the translation of the “gut-brain axis” concept into human health is still uncertain. In fact, only a few investigators have comprehensively compared the intestinal microbiota composition of older patients with cognitive complaints (ranging from mild cognitive impairment to overt dementia) with that of healthy active older subjects (Table 8.1). The results of these studies suggest the presence of reduced gut microbiota biodiversity (i.e., lower numbers of species) and specific compositional signatures, such as over-representation of Bacteroides and Enterobacteriaceae and under-representation of Dialister, in subjects with cognitive symptoms [12,13,14,15]. In the largest of these studies, comparing the fecal microbiota composition of 34 older patients with dementia and 94 age-matched controls, dementia was associated with increased biodiversity, reduced representation of Bacteroides, and an increased Firmicutes/Bacteroidetes ratio [16]. These associations were independent of other known biomarkers of dementia, including ApoE ε4 polymorphism, presence of lacunar infarcts at brain computed tomography (CT) or amyloid deposition detected by positron-emission tomography (PET) [16].
These findings, although promising, should be interpreted with caution. Most of the existing studies were observational, with a cross-sectional design preventing to draw solid conclusions on causal inference. Low sample sizes of these studies also represent an issue (Table 8.1) and generalizability to the general population with cognitive impairment or dementia is not automatic.
These limitations could be overcome by rigorous and well-designed intervention studies, implying the manipulation of the intestinal microbiome by administration of probiotics or functional foods. However, despite the large number of human trials with pre- or probiotics, studies specifically focused on dementia are still lacking [17]. The few studies published to date included only subjects without cognitive complaints [17] or with advanced dementia [18,19,20], and thus had a low chance of detecting significant variations in cognitive performance. Moreover, the investigators in these studies concentrated on microbiological and inflammation-related, rather than on clinical outcomes [18,19,20]. In the only study which assessed cognition as the primary outcome, the intervention consisted in a 12-week resistance training program associated with the administration of a Bifidobacterium spp blend, so that the improvement of executive functions detected in the intervention arm could not be attributed with complete certainty to the benefits of gut microbiome manipulation [21].
In this scenario, the possibility of obtaining a clinically relevant modulation of cognitive symptoms with microbiome-targeted interventions in older patients still remains speculative.
1.3 Gut Microbiota at the Cross-Road Between Environment and Human Pathophysiology
An increasing body of evidence suggests that the human microbiome composition is strongly influenced by environmental factors [1]. These factors include dietary patterns and habits [2], exercise [22], drugs [23, 24], the presence of diseases [23, 24], climate and pollution [25]. Moreover, the gut microbiota composition is shaped in each human being according to his or her genetic background, and is greatly influenced by immune system functionality [1, 20]. Aging itself represents a process able to influence the gut microbiota composition in an independent way, implying a progressive reduction of biodiversity and resilience to stressors [26]. Finally, growing evidence indicates that the output of metagenomics analyses of fecal microbiota is dependent on the bacterial load, which is ultimately associated with stool moisture [27, 28]. Intestinal motility, number of bowel movements and hydration status are thus important variables influencing the microbiome composition.
In the largest population-based study to date, investigating the human gut microbiota composition and its determinants, the authors were able to identify a long list of environmental factors associated with a distinct microbiological signature [29]. These results put into question the role of the intestinal microbiome as an independent mediator of human health and disease. They also suggest the existence of complex relationships between the environment and the host physiopathology, with gut microbiota possibly positioned at the cross-road [8, 30]. In fact, it is very difficult to consider all of the possible environmental factors influencing the microbiome composition, acting as potential confounders in pathophysiological associations [1, 30].
From a clinical perspective, this means that the disease-associated microbiome alterations most likely represent only one component of a large network of pathophysiological factors [30]. It also helps to understand why microbiome-targeted interventions, including administration of probiotics and functional foods, rarely provide clinically significant results outside the field of gastroenterology. If the microbiome is a cross-road mediator between the environment and host pathophysiology, then environmental interventions have a greater potential of influencing disease onset and course than microbiome manipulations targeted to a single or a limited number of bacterial species.
Despite these possible limitations, the intestinal microbiome remains a promising target for anti-aging interventions [31], particularly in the field of cognitive disorders, in which studies conducted in mouse models have provided encouraging results [10]. However, much research must still be done before we can recommend therapeutic strategies centered on the microbiome for patients with cognitive impairment, mainly because of objective difficulties in identification and preparation of probiotic blends or functional foods able to induce significant modification in the overall structure of gut communities [32].
Possibly, the nearest goal in this field of medicine is the use of gut microbiota composition and gut microbiota-related metabolites as biomarkers of disease. The existing studies exploring the relationship between microbiome and dementia highlight that a certain number of abnormalities in microbiome composition and functionality may be of potential interest as biomarkers. To be useful as biomarker of cognitive function, a physiological parameter should be measurable, correlated with cognitive performance or other indicators of cognitive impairment, able to predict the onset and course of cognitive impairment as well as the therapeutic response to treatments [33, 34]. Correlation with other measures of disease and prediction of outcomes are thus necessary before a biomarker can enter into clinical practice [33, 34]. Unfortunately, most of the research on the gut-brain axis has been focused on correlations rather than predictions [1]. However, there are many microbiome-related parameters deserving careful evaluation as possible biomarkers of cognitive aging in future studies.
2 Gut Microbiota Composition as Biomarker of Cognitive Function
2.1 Microbiome Biodiversity
The latest next-generation sequencing techniques (16S rRNA microbial profiling, shotgun metagenomics) allow detection of virtually all different taxa harbored in biological samples [35, 36]. After bioinformatics processing, the output of these analyses retrieves several indexes of biodiversity, such as the Shannon Index, the Simpson Index or the Chao1 Index [37]. In microbial ecology, biodiversity (also called alpha diversity) is defined as species richness and relative species abundance in space and time [38]. Thus, the more elevated are biodiversity indices, the higher is the number of species harbored in the fecal sample [37].
A high fecal microbiome biodiversity is generally considered to be a marker of good health status, implying a virtuous symbiotic equilibrium between microbial communities and host [3]. Conversely, reduced fecal microbiome biodiversity is generally associated with poor health status, and represents the main feature of gut microbiota dysbiosis [3]. For example, studies performed in patients with critical illnesses admitted to intensive care units globally show a high level of fecal microbiota dysbiosis, with reduced species richness [39]. Moreover, older patients with prolonged hospital stay who develop Clostridium difficile infection, one of the most frequent healthcare-associated infections, exhibit a reduced level of fecal microbiota biodiversity, compared with those who have milder health problems and shorter hospital stays [40].
However, an elevated fecal microbiota biodiversity is not necessarily associated with a healthy microbiota. When patients are treated with systemic antibiotic therapy, a transient increase in gut microbiota biodiversity may be observed due to overexpansion of microbial populations, mainly pathobionts that are not sensitive to the administered treatment [41, 42]. Stressful events such as exercise to exhaustion, may also promote increased gut microbiota biodiversity due to increased colonization and expansion of opportunistic pathogens at the expense of symbionts [22]. Thus, a finding of increased biodiversity in fecal microbiota should be carefully interpreted, especially in the light of which taxa are present and their relative abundance.
In mouse models of senescence or dementia, the association between gut microbiota biodiversity and cognitive performance has provided inconsistent results. For example, in a group of senescence-accelerated mouse prone 8 (SAMP8) mice exhibiting symptoms of severe cognitive dysfunction, the gut microbiota biodiversity measured by Chao1 and Shannon indices was significantly reduced in comparison with controls [43]. However, another study showed that older mice with mild symptoms of cognitive dysfunction exhibited an increased, rather than decreased, gut microbiota biodiversity in comparison with younger mice [44]. Antibiotic-induced reduction of gut microbiota biodiversity resulted in worsened cognitive performance of mice in one study [45] but was associated with reduced Aβ amyloid deposition in transgenic mouse models of Alzheimer’s disease [46, 47]. In germ-free mice, harboring no intestinal microbiota, a massive reduction of Aβ amyloid brain deposition was also documented in comparison with controls [48].
In humans, the association between gut microbiota biodiversity and cognitive functions has been assessed in different settings. In patients with cirrhosis undergoing liver transplantation, a high gut microbiota diversity was associated with reduced risk of encephalopathy and thus cognitive symptoms [49]. However, species richness in gut microbiota was unable to predict long-term cognitive outcomes in a group of unselected patients with cirrhosis [50]. In obese subjects, the Shannon index of fecal microbiota was correlated with anatomical and functional parameters of brain magnetic resonance imaging (R2∗ and fractional anisotropy of the hypothalamus, caudate nucleus and hippocampus) and cognitive performance related to speed, attention and flexibility [51].
In a group of 85 HIV-infected subjects naïve to antiretroviral therapy, fecal microbiota biodiversity was significantly lower in the presence of HIV-associated neurological disease with cognitive symptoms [52]. However, this difference was not independent of educational level and HIV-infection-related clinical data, such as CD4 T-cell count, suggesting that gut microbiota dysbiosis is not pathophysiologically involved in the cognitive dysfunction [52].
In the largest human study to date, Verdi et al. [53] analyzed the fecal microbiota of 1551 individuals over the age of 40 and correlated microbial variables with the cognitive performance assessed by several tests (verbal fluency, Mini-Mental State Examination, Deary-Liewald Reaction Time test, Paired Associated Learning from the Cambridge Neuropsychological Test Automated Battery). Among the results of these tests, only the Deary-Liewald Reaction Time test score was inversely associated with alpha diversity independently of covariates, suggesting that the possible link between gut microbiota biodiversity and cognition may be mediated by several other environmental and host-related factors [53].
Fecal microbiota biodiversity was analyzed in patients with dementia in only three studies [13, 16, 54]. Araos et al. analyzed the fecal microbiota of 85 long-term-care facility residents with advanced dementia, demonstrating a very high level of dysbiosis with reduced species richness in all subjects [54]. Their results suggest that advanced dementia may be associated with low alpha diversity indices but the absence of a control group in the study design prevents solid conclusions. In another study performed in 25 patients with dementia and 25 controls, the Shannon index was significantly reduced in cases (Table 8.1) [13], supporting the possible association between reduced alpha diversity and cognitive symptoms. However, in the most recent and largest study comparing alpha diversity between 34 demented patients and 94 controls, Saji and colleagues documented that the Shannon index was significantly lower in controls, rather than in demented patients (Table 8.1) [16].
Overall, the current literature state-of-art does not support the use of fecal microbiota biodiversity indices as biomarkers of cognitive aging. The studies performed on this topic are too scarce, with reduced sample sizes, and have therefore provided inconclusive results with interpretations that may be challenging. However, they do suggest that an association between alpha diversity and cognitive symptoms may exist, although this is not independent of covariates. Future studies should better investigate this association, considering larger sample sizes, thorough selection of covariates and the use of longitudinal designs to assess whether variations in gut microbiome composition can predict variations in cognitive function.
2.2 Firmicutes to Bacteroidetes Ratio
Bioinformatics processing of next-generation sequencing metagenomics output allows identification of the number of bacterial taxa harbored in fecal samples as well as assignment of each of these to the corresponding taxonomic level and calculation of their relative abundance [35, 36]. These data are generally used to determine the relative abundance of the two most represented phyla in fecal microbiota, i.e., Bacteroidetes and Firmicutes, and their ratio. The Firmicutes/Bacteroidetes ratio is an index representing the overall qualitative composition of the fecal microbiota. The intestinal microbiota can in fact physiologically assume different states, called enterotypes, with a predominance of either Bacteroidetes or Firmicutes, according to individual factors and dietary habits [55]. The Firmicutes/Bacteroidetes ratio increases from infancy to adult life and then declines again with senescence [56]. The predominance of Firmicutes in fecal microbiota composition has also been associated with the presence of metabolic imbalances, especially obesity, insulin resistance and metabolic syndrome [57, 58]. In fact, it generally increases with increasing body mass indices [59]. The Firmicutes/Bacteroidetes ratio has thus become an important parameter in the evaluation of the relationship between gut microbiota, obesity and obesity-related disorders [57].
An altered Firmicutes/Bacteroidetes ratio can however also represent an index of dysbiosis in other diseases, including dementia. In mice genetically prone to dementia (3xtg breed), gut microbiota analyses revealed a marked elevation of the Firmicutes/Bacteroidetes ratio compared to controls [60]. In mice with diet-induced obesity, over-expression of Firmicutes and reduced expression of Bacteroidetes was associated with impaired cognitive performance, especially in recognition memory and spatial memory tasks [61]. Finally, in older mice, an increase in the Firmicutes/Bacteroidetes ratio was associated with compromised cognition and increased anxiety behaviors, and this was not observed in younger mice [44].
The Firmicutes/Bacteroidetes ratio was calculated in human beings with dementia in only two studies, where it appeared significantly higher than in healthy controls [15, 16]. However, in a population of cognitively healthy older adults, Manderino et al. showed that greater proportions of Firmicutes and smaller proportions of Bacteroidetes in fecal microbiota were associated with better performances at cognitive tests [14].
Thus, the current literature state-of-art seems to support the concept that the presence of cognitive impairment may be associated with variations in the Firmicutes/Bacteroidetes ratio in fecal microbiota, making this parameter a promising potential biomarker of cognitive aging, deserving investigation in future studies. The capacity of this ratio to predict the onset and worsening of cognitive symptoms in human beings, and its association with obesity and metabolic imbalance should be particularly assessed.
2.3 Abundance of Specific Taxa in Fecal Microbiota
Apart from the overall composition of the fecal microbiota, variations in the representation of specific taxa may show a significant association with the presence of cognitive symptoms. The outputs of metagenomics analyses and their bioinformatics elaborations generally retrieve, for each detected bacterial taxon, a ratio of relative abundance, representing the proportion of bacteria belonging to that taxon on the total bacterial load of the sample [28]. Thus, these relative abundances do not represent the absolute quantities of bacteria harbored in each sample, and this concept should be considered for interpreting any result [28]. However, relative abundances could represent promising biomarkers of health status, especially if their association with diseases is reproducible across different studies.
The main bacterial taxa found over-represented or under-represented in fecal samples of either patients or animal models with cognitive symptoms, cognitive impairment or dementia, according to the current literature state-of-art, are summarized in Table 8.2. The findings of studies on mouse models [43, 60, 62,63,64] showed some degree of inconsistency. For example, in mice with cognitive impairment, Christensenellaceae and Ruminococcaceae were found to be depleted in one study [43] and over-represented in another [60]. However, depletion of Bifidobacteria and over-representation of Anaeroplasmatales were both associated with dementia in two studies [60, 63]. The largest study which compared senescence-accelerated mouse prone 8 with senescence-accelerated mouse resistant 1 breeds, showed that the presence of cognitive symptoms was associated with depletion of 26 taxa and over-representation of only one taxon [43].
Human studies which investigated the relative abundances of specific microbial taxa in fecal microbiota composition in relation to cognitive performance were performed in healthy subjects [19, 53, 65, 66], patients with cirrhosis [67,68,69], Parkinsonism [70, 71], or dementia/cognitive impairment [12,13,14,15,16]. An overview of taxa that resulted in significant differences between subjects with and without signs of cognitive dysfunction is presented in Table 8.2. In a large population of twins aged 40 or older, Verdi and colleagues showed that under-representation of taxa belonging to the Burkholderiales order was associated with worse performance at cognitive tests independently of covariates such as frailty and treatment with proton pump inhibitors [53]. These findings suggest that microbiota depletion of Burkholderiales may represent a promising biomarker of cognitive aging, although the cross-sectional design of the study prevents drawing any conclusions on prediction of cognitive outcomes. In their studies on patients with cirrhosis, Bajaj and colleagues found other putative microbial biomarkers of cognitive impairment [67,68,69], namely Alcaligenaceae, Porphyromonadaceae and Enterobacteriaceae with positive associations, and Lactobacillales and Lachnospiraceae having negative associations with the condition (Table 8.2). These studies provide interesting data on the possible usefulness of gut microbiota-related parameters for predicting cognitive outcomes. However, they were conducted using a population of patients exhibiting a high burden of gut microbiota dysbiosis, due to cirrhosis. Therefore, their results may not be immediately transferred to the elderly population with cognitive impairment or dementia.
Decreased relative abundance of Lachnospiraceae, Butyricicoccus and Clostridium XIVb, and increased relative abundance of Lactobacillaceae and Christensenellaceae were associated with the presence of cognitive impairment in two distinct studies performed on patients with Parkinsonism [70, 71]. Interestingly, a decreased relative abundance of Lachnospiraceae associated with cognitive symptoms was also observed in patients with cirrhosis and, most importantly, in one of the few studies specifically conducted in the field of human dementia [15, 69]. Similarly, a decreased relative abundance of Clostridium XIVb was also detected in the study by Vogt and colleagues [13], in which the fecal microbiota of 25 patients with Alzheimer’s disease was compared with that of 25 healthy controls (Table 8.1). Thus, the abundance of Lachnospiraceae and Clostridium XIV b may deserve greater attention in future studies as possible biomarkers of cognitive aging.
The study by Vogt and colleagues [13] was also the most accurate one to date in the identification of microbial taxa with altered relative abundance in Alzheimer’s disease, and with significant correlation of these with other biomarkers, including p-tau and β-amyloid deposition. Specifically, the relative abundance of Blautia, Bacteroides, Phascolarctobacterium, Alistipes, Bilophila and Gemella was increased in Alzheimer’s disease, while the relative abundance of Clostridum, Bifidobacterium, Dialister, Adlerkreutzia and Turicibacter was decreased [13]. Future, larger studies should better clarify whether these bacteria may assume the role of microbial biomarkers of dementia or cognitive impairment.
It is also noteworthy that a significant association between the representation of some taxa in the fecal microbiome and imaging biomarkers of cognitive dysfunction [66] or genetic risk factors for Alzheimer’s disease (APOE polymorphisms) [65] has been detected in two distinct studies. Other investigations recently reviewed by Franceschi et al. [72] also suggest that the presence of Helicobacter pylori in the intestinal microbiome could be involved in the development of Alzheimer’s disease, by promoting systemic chronic inflammation or molecular mimicry mechanisms. These aspects will need further confirmation in future studies but may represent the bases for the discovery of other microbial biomarkers of dementia and cognitive impairment.
In summary, the current literature state-of-art suggests a list of possible gut microbiota-related biomarkers of cognitive aging, shown in Table 8.2. However, the evidence is too scarce to recommend the use of fecal microbiota analyses as a method to identify the risk of cognitive impairment or dementia. In addition, these studies were conducted in heterogeneous settings, included small numbers of participants and had a cross-sectional design, preventing study of the association of microbiome composition with clinical outcomes. Finally, some inconsistencies are present among the results of different studies, reinforcing the need to develop larger and sounder investigations in this field.
3 Microbiota-Related Metabolites as Biomarkers of Cognitive Function
3.1 The Role of Microbial Metabolites as Biomarkers in the Gut-Brain Axis
Many substances synthesized by components of the gut microbiota, either as metabolic byproducts or constituents of bacterial structures, may have a role in influencing the gut-brain axis functionality in cognitive function [10]. These substances, their characteristics and possible pathophysiological relevance have been recently reviewed by many authors [73,74,75,76,77]. These include neurotransmitters [73, 74], metabolites derived by amino acid catabolism [75], short-chain fatty acids (SCFAs) and other lipids [76], components of the outer membrane of bacteria [76] and even bacterial amyloid proteins [77]. Despite their possible involvement in the development and course of dementia, only a limited number of them could have potential usefulness as biomarkers of cognitive aging. In fact, neurotransmitters, such as acetylcholine, norepinephrine, histamine and γ-aminobutyric acid, are also synthetized by the host, and their microbial origin cannot be easily detected with standard laboratory methods [73]. Bacterial amyloid proteins may be involved in the pathophysiology of Alzheimer’s disease by promoting the formation of β-amyloid protein aggregates in the brain by molecular mimicry but they cannot easily be detected in biological samples [77]. Metabolites derived by amino acid catabolism and SCFAs have recently received considerable attention by researchers and show, in some cases, correlations with cognitive function [75, 76]. However, some technical issues associated with laboratory methods of detection are currently limiting their study and implementation in clinical practice.
Along with substances synthesized by the intestinal microbiota, there are also a number of substances derived from diet and transformed by certain metabotypes of gut microbiota that may have a relevant pathophysiological involvement in the gut-brain axis [8, 10]. However, since the intake of the precursors of these substances is variable and not easy to standardize in the diet, their use as biomarkers is in doubt. The most known of these substances is trimethylamine N-oxide (TMAO) , which is produced by the metaorganismal metabolism of dietary choline and has been implicated in the pathogenesis of several human diseases, including cardiovascular, cerebrovascular and metabolic diseases [78]. Other microbial products of nutrients introduced with diet include polyphenol metabolites and urolithins [8]. Finally, recent studies link cognitive performance to vitamin K status, which is partly produced by the intestinal microbiota [79], so that vitamin K metabolism may represent another promising area of research in the field of cognitive aging.
The putative biomarkers of cognitive aging derived by microbiota metabolism can be detected in several biological samples, ranging from blood to cerebrospinal fluid to feces. In fact, most of the substances produced by gut microbiota are absorbed into the systemic circulation and can be detected in blood. This phenomenon is particularly enhanced in the presence of a “leaky gut”. This means that increased intestinal mucosa permeability iscaused by inflammatory conditions of the gastrointestinal tract or by the presence of an extreme gut microbiota dysbiosis [80].
From the systemic circulation, some microbiota-derived substances may reach the central nervous system and may be best detected in cerebrospinal fluid samples. Some investigators have also studied metabolic byproducts of intestinal bacteria in stool samples, since their concentration may be directly related to the microbiota composition and functionality. SCFAs are among the most studied microbiota-derived biomarkers of disease in stool samples [81]. However, these molecules show low stability in fecal samples leading to huge intra-individual variability, depending on the timing of analyses after sample collection [81].
3.2 Structural and Functional Components of Bacteria as Biomarkers of Cognitive Aging
The structural and functional components of gut microbiota that have been detected in biological samples of the host and related with the onset of cognitive dysfunction or dementia, include lipopolysaccharide (LPS) and rhamnolipids (Table 8.3). LPS can be detected in human blood samples at low concentrations even in the absence of bacteremia or sepsis. This phenomenon is an expression of gut microbiota dysbiosis or altered intestinal permeability, and causes chronic subclinical activation of inflammation that is involved in the pathophysiology of several non-communicable diseases, including atherosclerosis, diabetes and obesity [82]. Since inflammation is also an important mechanism triggering age-related cognitive impairment and the onset of dementia, some researchers have investigated the possible association between the presence of LPS in biological samples of the host and dementia [10].
In mice with diet-induced obesity, an association between reduced performance at recognition and spatial memory tasks and increased serum levels of LPS was detected [61]. These findings were confirmed by an intervention study where genistein was administered in association with a high-fat diet to mice [83], suggesting that LPS levels may be inversely correlated with cognitive performance. However, the intracerebral injection of LPS in mouse models of Alzheimer’s disease did not induce significant alterations in cognitive function [84].
Human serum levels of LPS have not been associated with cognitive performance by any study to date. However, investigations on brain biopsies have demonstrated that lysates from the hippocampus and superior temporal lobe neocortex of patients with Alzheimer’s disease had significantly higher concentrations of LPS than brain lysates from non-demented controls [85,86,87]. Therefore, the relationship between LPS and cognitive performance in humans deserves more investigations and LPS could represent a promising biomarker of cognitive health.
Andreadou et al. have also demonstrated that rhamnolipids, other bacterial virulence factors possibly derived from the gut microbiota, exhibit higher serum and cerebrospinal fluid levels in patients suffering from Alzheimer’s disease than in non-demented controls [88]. These findings have not been replicated in other studies but the role of rhamnolipids as a possible biomarker of cognitive aging and pathophysiology of dementia should be investigated.
3.3 Microbiota, Amino Acid Metabolism and Cognitive Aging
Studies performed in mice have demonstrated that the decline of cognitive performance is associated with a distinct metabolic profile in serum and in the brain [44, 60]. In mouse genetically prone to dementia, an overabundance of ketone bodies, lactate and amino acids and depletion of unsaturated fatty acids and choline, was demonstrated in serum and feces, suggesting a role of the intestinal microbiota metabolism in driving these changes [60, 65]. Brain metabolomics of aged mice showed significantly lower concentrations of amino acids (including methionine, phenylalanine, cysteine and creatine) and lipidic cofactors (including hydroxycholesterol, prostagrandins, phosphocholine and docosapentaenoate) than control young mice [44]. Part of these brain metabolic signatures may be directly influenced by the gut microbiota. In fact, older mice with signs of cognitive impairment had significantly higher brain levels of two metabolites related to microbial metabolism of the amino acid tryptophan, i.e., 3-indoxyl-sulfate and phenol sulfate, compared to young mice [44]. Interestingly, these metabolites have been associated with neurological toxicity and inflammation [89]. In patients who underwent bone marrow transplantation, the levels these molecules were predictive of clinical outcomes, reflecting the presence of gut microbiota dysbiosis [90].
The metabolism of tryptophan has also been associated with brain function in humans. Aging is associated with reduced serum levels of tryptophan and increased activation of the main human metabolic pathway of tryptophan degradation, i.e. the kynurenine pathway [91]. The serum levels of some kynurenine pathway intermediates, such as kynurenic acid and 3-hydroxykynurenine, have also shown a positive association with the level of cognitive impairment [91]. In a recent large population-based study, the kynurenine-tryptophan ratio was inversely associated with the performance at the Kendrick Object Learning Test and Controlled Oral Word Association Test [92]. These findings can be explained by a possible neurotoxic effect of kynurenine pathway intermediates.
Gut microbiota can contribute to tryptophan degradation with its own metabolic pathways. The most studied intermediate is indole-3 acetic acid (IAA) , which is known as one of the main uremic toxins in patients suffering from chronic kidney failure [93]. Serum IAA levels were found to be negatively associated with Mini-Mental State Examination test score in patients undergoing chronic hemodialysis [94] and with depressive symptoms in subjects with severe chronic kidney disease not undergoing hemodialysis [95]. Another intermediate of microbial tryptophan degradation is indoxyl sulfate, which showed serum concentrations that were associated with poor executive functions in a group of 199 patients with early-stage chronic kidney disease [96]. Interestingly, indoxyl sulfate is able to induce apoptosis in human astrocytes, suggesting a possible involvement of this microbiota-derived metabolite in the pathophysiology of dementia [97].
Moreover, tryptophan is autonomously produced by bacteria through the shikimate pathway, a seven-step metabolic route allowing the synthesis of aromatic amino acids from two intermediates in carbohydrate metabolism, i.e., phosphoenolpyruvic acid and erythrose 4-phosphate. In a study performed on 20 patients with Alzheimer’s disease and 5 healthy controls, Paley et al. were able to identify gut bacterial gene sequences unique to patients with Alzheimer’s disease, belonging to the enzyme NADH:Ubiquinone reductase that is related to the optimal functioning of the shikimate pathway [98]. These findings suggest that the gut microbiota of patients with Alzheimer’s disease may have an enhanced production of tryptophan through the shikimate pathway and that this phenomenon may have relevance in the physiopathology of dementia.
In summary, the current scientific literature does not provide sufficient data to recommend the adoption of bacterial products of amino acid metabolism as biomarkers of cognitive aging. However, the complex interplay between the gut microbiota and the host in tryptophan metabolism may have relevance in the field of cognitive impairment and should be further investigated in the future. Kynurenine, kynurenic acid, 3-hydroxykynurenine, IAA and indoxyl sulfate could represent good candidates as biomarkers of cognitive aging.
3.4 Microbiota-Derived SCFAs and Cognitive Aging
SCFAs, namely acetate, butyrate and propionate, are among the most studied metabolic products of the gut microbiota. They are derived from dietary carbohydrates and absorbed into the systemic circulation, where they can exert a wide range of physiologic functions, including control of body weight, regulation of insulin sensitivity, regulation of inflammation and immune system activation [99, 100]. These mechanisms may have particular relevance for central nervous system diseases, in which inflammation is also involved in the pathogenesis of Alzheimer’s disease [101]. Butyrate has been implicated as a central mediator of the possible gut-brain axis, and the effects of the administration of butyrate or butyrate-producing bacteria on brain functions have been investigated through several animal studies, recently reviewed by Stilling and colleagues [102]. However, few of these studies were focused on dementia or cognitive impairment and, therefore, their clinical relevance remains uncertain.
In a study performed in mice, Fröhlich and colleagues demonstrated that the administration of antibiotics to induce a deep gut microbiota dysbiosis, was associated with cognitive dysfunction and lower colonic concentrations of SCFAs compared to administration of placebo [45]. Conversely, the administration of a symbiotic (Enterococcus faecium plus inulin) in a mouse model of cognitive aging was associated with an increase in the fecal concentration of butyrate and better cognitive performances than the placebo [103]. The administration of a butyrate-producing species, Clostridium butyricum, as a probiotic to mouse models of vascular dementia and cerebral ischemia/reperfusion injury resulted in improved cognitive function or reduced neurologic deficits [104, 105]. In another study, the administration of Bifidobacterium breve strain A1, a metabolically active strain able to produce acetate, to an Alzheimer’s disease mouse model resulted in improvements in cognitive function or in a less pronounced cognitive decline [106].
These data globally indicate that SCFAs, and particularly butyrate, could play an important physiopathological role in cognitive impairment, and thus may represent good biomarkers of cognitive aging. Fecal samples of patients with Alzheimer’s disease are generally abundant in butyrate-producing bacteria [107] but no comparison with healthy controls is currently available on this specific point. The role of SCFAs as biomarkers of cognition in older subjects should thus be more investigated in future studies. Unfortunately, the volatile nature of these compounds makes them difficult to detect in human samples, and analyses generally show a low degree of reproducibility [81].
3.5 Other Microbiota-Derived Compounds and Cognitive Aging
3.5.1 Bile Acids
Bile acids are not a primary product of intestinal microbiota but their metabolism is influenced by the microbiome composition and functionality. In fact, dysbiosis can be associated with important alterations of bile acid metabolism, contributing to the pathogenesis of several diseases [108]. Tauroursodeoxycholic acid, a bile acid found in bears and subject to gut microbiota metabolism, showed neuroprotective properties especially in animal models of Huntington’s disease [109]. These properties have also been recently demonstrated in mouse models of dementia, in which the administration of tauroursodeoxycolic acid was able to slow down amyloid precursor protein processing and amyloid-β deposition [110, 111].
These data have raised speculations that other bile acids produced by humans and subject to gut microbiota metabolism, may have a role in the pathophysiology of dementia and could serve as biomarkers of cognitive aging. Olzarán and colleagues were able to identify a metabolic fingerprint of Alzheimer’s disease by analyzing plasma samples of a large group of patients with mild cognitive impairment or dementia [112]. Interestingly, they found that the concentrations of one bile acid (deoxycolic acid) were independently associated with the presence of cognitive symptoms [112]. Another recent study showed that specific bile acid profiles in serum were associated with other well-established biomarkers of Alzheimer’s disease normally found in the cerebrospinal fluid (amyloid β1-42 and p-tau181) and with imaging markers of brain atrophy [113], reinforcing a possible role of bile acids as microbiota-derived biomarkers of cognitive aging.
3.5.2 TMAO
TMAO is a metabolic product derived from microbial metabolism of dietary choline and serum levels of this molecule have been associated with an increased risk of atherosclerosis in animal models and human beings [78]. Thus, it has been considered as a marker of risk of vascular dementia [78]. However, recent studies have highlighted that it may also have relevance in the pathogenesis of Alzheimer’s disease. First, the administration of exogenous TMAO to mice genetically prone to dementia resulted in an increased number of senescent cells in hippocampus, reduced synaptic plasticity-related protein expression and reduced cognitive performance [114]. Moreover, in a large sample of older patients, either with Alzheimer-type dementia, mild cognitive impairment or no cognitive symptoms, the levels of TMAO detected in cerebrospinal fluid were significantly different according to cognitive status and related with other biomarkers of Alzheimer’s disease (i.e., phosphorylated tau, total tau and neurofilament light chain protein) [115]. Thus, the elevation of TMAO in cerebrospinal fluid may represent a promising biomarker of cognitive dysfunction.
3.5.3 Polyphenols
Dietary polyphenols have been associated with reduced risk of a large number of cardiovascular disorders, based mainly on studies in vitro [116]. These compounds are generally metabolized by the gut microbiota into a large number of substances that may exert a protective function against neurodegeneration, and there is interest in these molecules as potential biomarkers of cognitive aging. For example, 3-hydroxybezoic acid and 3-(3′-hydroxyphenyl)propionic acid, two metabolites derived from microbiota degradation of anthocyanidins contained in grape seeds, accumulate in brain tissues of rats and exhibit the capacity of interfering with the assembly of β-amyloid into neurotoxic aggregates [117]. Polyphenol metabolites can also modulate cognitive resilience in mice, but this capacity is dependent on the gut microbiota composition and functionality, the so-called metabotype [118]. Therefore, the administration of polyphenols as food supplements or nutraceuticals may have different effects in different individuals, depending on the gut microbiota.
An example of this inter-individual variability of polyphenol metabolism, depending on gut microbiota composition, is the ellagitannins, a group of polyphenol compounds found mainly in pomegranates and walnuts [119, 120]. In human beings, two different gut microbiota metabotypes (A and B) have been identified, and each of these is able to produce different metabolites from ellagitannins, called urolithin-A and urolithin-B, respectively [119, 120]. Urolithin-A has been associated with reduced cardiometabolic risk factors and with beneficial health effects, unlike urolithin-B [119]. Thus, the gut microbiota urolithin-related metabotype may contribute to the observed variability of health benefits of pomegranate extracts found in humans [120].
A recent study performed in APP/PS1 mouse models of Alzheimer’s disease has demonstrated that the administration of urolithin A ameliorated cognitive performance and positively influenced several pathophysiological mechanisms of dementia, including amyloid-β deposition, neuronal apoptosis and neuroinflammation [121]. Urolithin A also seems to be able to enhance mitophagy, which is a mechanism protecting against cognitive impairment [122]. In a small human randomized trial, the administration of 8 ounces of pomegranate juice for 4 weeks to older patients reporting cognitive complaints resulted in a significant improvement in verbal memory tasks, which was paralleled by a significant increase in plasma concentrations of urolithin A-glucuronide [123]. These data underline the point that urolithin A and urolithin A-metabotype of gut microbiota may represent interesting biomarkers of cognitive aging viaidentification of a subset of patients that are susceptible to nutritional intervention.
3.5.4 Vitamin K
Vitamin K metabolism and the administration of vitamin K antagonists as oral anticoagulants have been recently studied as possible risk factors for cognitive decline in older individuals [124, 125]. Some studies support a positive association between vitamin K levels and cognitive performance [79, 124], while the administration of vitamin K antagonists to rats resulted in altered cognition [126]. However, the effect of vitamin K antagonists on cognitive function may depend on the pathophysiological mechanism of dementia, as it may be protective in cerebrovascular forms of dementia [125] and probably detrimental in neurodegenerative forms [79, 124]. Patients with cognitive impairment who need the administration of vitamin K antagonists are more likely to have multimorbidity and more severe forms of cognitive dysfunction at baseline, so that the putative association between vitamin K antagonists and progression to dementia may be spurious [127]. In this scenario, the intestinal microbiome could play an important role, since vitamin K is physiologically produced by intestinal bacteria [128]. However, no study has investigated the correlation between production of vitamin K by the gut microbiota and cognitive outcomes to date. Vitamin K could however represent another promising biomarker of cognitive aging meriting future investigations.
4 Conclusions
Gut microbiota composition and microbiota-derived metabolites or substances represent a promising area of research for the identification of novel biomarkers of cognitive aging. However, the current literature state-of-the-art does not support the implementation of microbiome-related biomarkers of cognitive aging for use in clinical practice. The study of the relationship between gut microbiota composition and functionality in mild cognitive impairment and different types of dementia should be translated from animal models to patients. In addition, these studies should be focused on alpha diversity, Firmicutes/Bacteroidetes ratio, abundance of specific key taxa and microbial metabolism of amino acids, SCFAs, bile acids, vitamin K and nutrients such as polyphenols and choline.
The use of new microbiome-related biomarkers of cognitive aging could bring to several advantages. If these biomarkers show alterations in the early phases of dementia, they could assist physicians in the diagnostic process, or even lead to early diagnosis. They could also help identifying those patients with a quicker evolution towards dementia, deserving more aggressive treatments and strict follow-up. From the patient’s perspective, it could bring improved management of cognitive diseases and give important information on neurological diseases, facilitating a comprehensive classification of health status. Finally, it could also have economic relevance, since the microbiome analyses are generally less expensive than brain imaging examinations. For all these reasons, more research is urgently needed in this field.
References
Schmidt TSB, Raes J, Bork P (2018) The human gut microbiome: from association to modulation. Cell 172(6):1198–1215
Gentile CL, Weir TL (2018) The gut microbiota at the intersection of diet and human health. Science 362:776–780
Ticinesi A, Nouvenne A, Tana C, Prati B, Cerundolo N, Miraglia C et al (2018) The impact of intestinal microbiota on bio-medical research: definitions, techniques and physiology of a “new frontier”. Acta Biomed 89(9S):52–59
Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A et al (2019) Gut-liver axis, gut microbiota, and its modulation in the management of liver disease: a review of the literature. Int J Mol Sci 20(2):395. pii: E395. https://doi.org/10.3390/ijms20020395
Cosola C, Rocchetti MT, Sabatino A, Fiaccadori E, Di Iorio BR, Gesualdo L (2019) Microbiota issue in CKD: how promising are gut-targeted approaches? J Nephrol 32(1):27–37
Ticinesi A, Milani C, Guerra A, Allegri F, Lauretani F, Nouvenne A et al (2018) Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut 67(12):2097–2106
Quach D, Britton RA (2017) Gut microbiota and bone health. Adv Exp Med Biol 1033:47–58
Ticinesi A, Lauretani F, Milani C, Nouvenne A, Tana C, Del Rio D et al (2017) Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: is there a gut-muscle axis? Nutrients 9(12):E1303. pii: E1303. https://doi.org/10.3390/nu9121303
Junges VM, Closs VE, Nogueira GM, Valle Gottlieb MG (2018) Crosstalk between gut microbiota and the central nervous system: a focus for Alzheimer’s disease. Curr Alzheimer Res 15:1–12.43
Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T (2018) Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging 13:1497–1511
Kowalski K, Mulak A (2019) Brain-gut-microbiota axis in Alzheimer’s disease. J Neurogastroenterol Motil 25(1):48–60
Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C et al (2017) Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49:60–68
Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC et al (2017) Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7(1):13537. https://doi.org/10.1038/s41598-017-13601-y
Manderino L, Carroll I, Azcarate-Peril MA, Rochette A, Heinberg L, Peat C et al (2017) Preliminary evidence for an association between the composition of gut microbiome and cognitive function in neurologically-healthy older adults. J Int Neuropsychol Soc 23(8):700–705
Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L et al (2018) Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis 63(4):1337–1346
Saji N, Niida S, Murotani K, Hisada T, Tsuduki T, Sugimoto T et al (2019) Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci Rep 9:1008. https://doi.org/10.1038/s41598-018-38218-7
Desmedt O, Broers VJV, Zamariola G, Pachikian B, Delzenne N, Luminet O (2019) Effects of prebiotics on affect and cognition in human intervention studies. Nutr Rev 77(2):81–95
Leblhuber F, Steiner K, Schuetz B, Fuchs D, Gostner JM (2018) Probiotic supplementation in patients with Alzheimer’s dementia – An explorative intervention study. Curr Alzheimer Res 15:1106–1113
Tran TTT, Cousin FJ, Lynch DB, Menon R, Brulc J, Brown JRM et al (2019) Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome 7:39. https://doi.org/10.1186/s40168-019-0654-1
Agahi A, Hamidi GA, Daneshvar R, Hamdieh M, Soheili M, Alinaghipour A et al (2018) Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front Neurol 9:662. https://doi.org/10.3389/fneur.2018.00662
Inoue T, Kobayashi Y, Mori N, Sakagawa M, Xiao JZ, Moritani T et al (2018) Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef Microbes 9(6):843–853
Ticinesi A, Lauretani F, Tana C, Nouvenne A, Ridolo E, Meschi T (2019) Exercise and immune system as modulators of intestinal microbiome: implications for the gut-muscle axis hypothesis. Exerc Immunol Rev 25:84–95
Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA et al (2017) Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep 7(1):11102. https://doi.org/10.1038/s41598-017-10734-y
Jackson MA, Verdi S, Maxan ME, Shin CM, Zierer J, Bowyer RCE et al (2018) Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun 9(1):2655. https://doi.org/10.1038/s41467-018-05184-7
Davenport ER, Mizrahi-Mian O, Michelini K, Barreiro LB, Ober C, Gilad Y (2014) Seasonal variation in human gut microbiome composition. PLoS One 9(3):e90731. https://doi.org/10.1371/journal.pone.0090731
O’Toole PW, Jeffery IB (2015) Gut microbiome and aging. Science 350(6265):1214–1215
Vandeputte D, Falony G, Vieiera-Silva S, Tito RY, Joossens M, Raes J (2016) Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65(1):57–62
Vandeputte D, Kathagen G, D’hoe K, Vieira-Silva S, Valles-Colomer M, Sabino J et al (2017) Quantitative microbiome profiling links gut community variation to microbial load. Nature 551(7681):507–511
Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T et al (2016) Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352(6285):565–569
Falony G, Vandeputte D, Caenepeel C, Vieira-Silva S, Daryoush T, Vermeire S et al (2019) The human microbiome in health and disease: hype or hope. Acta Clin Belg 74(2):53–64
Ticinesi A, Tana C, Nouvenne A (2019) The intestinal microbiome and its relevance for functionality in older persons. Curr Opin Clin Nutr Metab Care 22(1):4–12
Mancuso C, Santangelo R (2018) Alzheimer’s disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharm Res 129:329–336
Lozupone M, La Montagna M, D’Urso F, Daniele A, Greco A, Seripa D et al (2019) The role of biomarkers in psychiatry. Adv Exp Med Biol 1118:135–162
Ruan Q, D’Onofrio G, Sancarlo D, Greco A, Lozupone M, Seripa D et al (2017) Emerging biomarkers and screening for cognitive frailty. Aging Clin Exp Res 29:1075–1086
Ventura M, Turroni F, Canchaya C, Vaughan EE, O’Toole PW, Van Sinderen D (2009) Microbial diversity in the human intestine and novel insights from metagenomics. Front Biosci 14:3214–3221
Milani C, Hevia A, Foroni E, Duranti S, Turroni F, Lugli GA et al (2013) Assessing the fecal microbiota: an optimized ion torrent 16S rRNA gene-based analysis protocol. PLoS One 8(7):e68739. https://doi.org/10.1371/journal.pone.0068739
Kim BR, Shin J, Guevarra RB, Lee JH, Kim DW, Seol KH et al (2017) Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol 27(12):2089–2093
Schloss PD, Handelsman J (2006) Introducing SONS, a tool for operational taxonomic unit-based comparisons of microbial community memberships and structures. Appl Environ Microbiol 75:7537–7541
Ticinesi A, Milani C, Lauretani F, Nouvenne A, Tana C, Ventura M et al (2019) Gut microbiome in the elderly hospitalized patient: a marker of disease and prognosis? In: Faintuch J, Faintuch S (eds) Microbiome and metabolome in diagnosis, therapy, and other strategic applications. Associated Press, London, pp 287–296. isbn:978-0-12-815249-2
Milani C, Ticinesi A, Gerritsen J, Nouvenne A, Lugli GA, Mancabelli L et al (2016) Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci Rep 6:25945. https://doi.org/10.1038/srep2594
Ianiro G, Tilg H, Gasbarrini A (2016) Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 65(11):1906–1911
Perez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K et al (2013) Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 62(11):1591–1601
Zhan G, Yang N, Li S, Huang N, Fang X, Zhang J et al (2018) Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging 10(6):1257–1267
Hoffman JD, Parikh I, Green SJ, Chlipala G, Mohney RP, Keaton M et al (2017) Age drives distortion of brain metabolic, vascular and cognitive functions, and the gut microbiome. Front Aging Neurosci 9:298. https://doi.org/10.3389/fnagi.2017.00298
Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B et al (2016) Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav Immun 56:140–155
Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castillo P et al (2016) Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep 6:30028. https://doi.org/10.1038/srep30028
Minter MR, Hinterleitner R, Meisel M, Zhang C, Leone V, Zhang X et al (2017) Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PSΔE9 murine model of Alzheimer’s disease. Sci Rep 7:10411. https://doi.org/10.1038/s41598-017-11047-w
Harach T, Marungruang N, Duthilleul N, Cheatham V, McCoy KD, Frisoni G et al (2017) Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep 7:41802. https://doi.org/10.1038/srep41802
Bajaj JS, Fagan A, Sikaroodi M, White MB, Sterling RK, Gilles H et al (2017) Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver Transpl 23:907–914
Bajaj JS, Vargas HE, Reddy KR, Lai JC, O’Leary JG, Tandon P et al (2019) Association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis. Clin Gastroenterol Hepatol 17:756–765
Fernandez-Real JM, Serino M, Blasco G, Puig J, Daunis-i-Estadella J, Ricart W et al (2015) Gut microbiota interacts with brain microstructure and function. J Clin Endocrinol Metab 100(12):4505–4513
Zhang F, Yang J, Ji Y, Sun M, Shen J, Sun J et al (2019) Gut microbiota dysbiosis is not independently associated with neurocognitive impairment in people living with HIV. Front Microbiol 9:3352. https://doi.org/10.3389/fmicb.2018.03352
Verdi S, Jackson MA, Beaumont M, Bowyer RCE, Bell JT, Spector TD et al (2018) An investigation into physical frailty as a link between the gut microbiome and cognitive health. Front Aging Neurosci 10:398. https://doi.org/10.3389/fnagi.2018.00398
Araos R, Andreatos N, Ugalde J, Mitchell S, Mylonakis E, D’Agata EMC (2018) Fecal microbiome among nursing home residents with advanced dementia and Clostridium difficile. Dig Dis Sci 63(6):1525–1531
Costea PI, Hildebrand F, Arumugam M, Bäckhed F, Blaser MJ, Bushman FD et al (2018) Enterotypes in the landscape of gut microbial community composition. Nat Microbiol 3(1):8–16
Mariat D, Firmesse O, Levenez F, Guimarȃes V, Sokol H, Doré J et al (2009) The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 9:123. https://doi.org/10.1186/1471-2180-9-123
Sze MA, Schloss PD (2016) Looking for a signal in the noise: revisiting obesity and the microbiome. mBio 7(4):e01018–e01016. https://doi.org/10.1128/mBio.01018-16
Indiani CMDSP, Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, Parisotto TM (2018) Childhood obesity and Firmicutes/Bacteroidetes ratio in the gut microbiota: a systematic review. Child Obes 14(8):501–509
Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V et al (2017) Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukranian population. BMC Microbiol 17(1):120. https://doi.org/10.1186/s12866-017-1027-1
Sanguinetti E, Collado MC, Marrachelli VG, Monleon D, Selma-Royo M, Pardo-Tendero MM et al (2018) Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet. Sci Rep 8:4907. https://doi.org/10.1038/s41598-018-23261-1
Zhang P, Yu Y, Qin Y, Zhou Y, Tang R, Wang Q et al (2019) Alterations to the microbiota-colon-brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice. J Nutr Biochem 65:54–65
Bäuerl C, Collado MC, Diaz Cuevas A, Viña J, Pérez Martìnez G (2018) Shifts in gut microbiota composition in APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett Appl Microbiol 66(6):464–471
Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C et al (2017) Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep 7:2426. https://doi.org/10.1038/s41598-017-02587-2
Shen L, Liu L, Ji HF (2017) Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J Alzheimers Dis 56(1):385–390
Tran TTT, Corsini S, Kellingray L, Hegarty C, Le Gall G, Narbad A et al (2019) APOE genotype influences the gut microbiome structure and function in humans and mice: relevance for Alzheimer’s disease pathophysiology. FASEB J 33:8221–8231
Blasco G, Moreno-Navarrete JM, Rivero M, Pérez-Brocal V, Garre-Olmo J, Puig J et al (2017) The gut metagenome changes in parallel to waist circumference, brain iron deposition, and cognitive function. J Clin Endocrinol Metab 102:2962–2973
Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S et al (2012) Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 302:G168–G175
Bajaj JS, Ahluwalia V, Steinberg JL, Hobgood S, Boling PA, Godschalk M et al (2016) Elderly patients have an altered gut-brain axis regardless of the presence of cirrhosis. Sci Rep 6:38481. https://doi.org/10.1038/srep38481
Bajaj JS, Fagan A, White MB, Wade JB, Hylemon PB, Heuman DM et al (2019) Specific gut and salivary microbiota patterns are linked with different cognitive testing strategies in minimal hepatic encephalopathy. Am J Gastroenterol 114:1080–1090
Qian Y, Yang Y, Xu S, Wu C, Song Y, Qin N et al (2018) Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 70:194–202
Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S et al (2019) Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov Disord 34(3):396–405
Franceschi F, Ojetti V, Candelli M, Covino M, Cardone S, Potenza A et al (2019) Microbes and Alzheimer’s disease: lessons from H. pylori and GUT microbiota. Eur Rev Med Pharmacol Sci 23:426–430
Alkasir R, Li J, Li X, Jin M, Zhu B (2017) Human gut microbiota: the links with dementia development. Protein Cell 8(2):90–102
Giau VV, Wu SY, Jamerlan A, An SSA, Kim SY, Hulme J (2018) Gut microbiota and their neuroinflammatory implications in Alzheimer’s disease. Nutrients 10:1765. https://doi.org/10.3390/nu10111765
Sanz Y, Romanì-Perez M, Benìtez-Pàez A, Portune KJ, Brigidi P, Rampelli S et al (2018) Towards microbiome-informed dietary recommendations for promoting metabolic and mental health: opinion papers of the MyNewGut project. Clin Nutr 37:2191–2197
Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S (2017) Microbiome, probiotics and neurodegenerative diseases: deciphering the gut-brain axis. Cell Mol Life Sci 74:3769–3787
Friedland RP, Chapman MR (2017) The role of microbial amyloid in neurodegeneration. PLoS Pathog 13(12):e1006654. https://doi.org/10.1371/journal.ppat.1006654
Li S, Shao Y, Li K, HuangFu C, Wang W, Liu Z et al (2018) Vascular cognitive impairment and the gut microbiota. J Alzheimers Dis 63(4):1209–1222
Kiely A, Ferland G, Ouliass B, O’Toole PW, Purtill H, O’Connor EM (2018) Vitamin K status and inflammation are associated with cognition in older Irish adults. Nutr Neurosci 1–9
Quigley EMM (2016) Leaky gut – concept or clinical entity? Curr Opin Gastroenterol 32(2):74–79
Liebisch G, Ecker J, Roth S, Schweizer S, Öttl V, Schött HF et al (2019) Quantification of fecal short-chain fatty acids by liquid chromatography tandem mass spectrometry-investigation of pre-analytic stability. Biomol Ther 9(4):E121. https://doi.org/10.3390/biom9040121
Belizário JE, Faintuch J, Garay-Malpartida M (2018) Gut microbiome dysbiosis and immunometabolism: new frontiers for treatment of metabolic diseases. Mediat Inflamm 2018:2037838–2037812. https://doi.org/10.1155/2018/2037838
López P, Sánchez M, Perez-Cruz C, Velázquez-Villegas LA, Syeda T, Aguilar-López M et al (2018) Long-term genistein consumption modifies gut microbiota, improving glucose metabolism, metabolic endotoxinemia, and cognitive function in mice fed a high-fat diet. Mol Nutr Food Res 62:1800313
Hayashi K, Hasegawa Y, Takemoto Y, Cao C, Takeya H, Komohara Y et al (2019) Continuous intracerebroventricular injection of Porphyromonas gingivalis lipopolysaccharide induces systemic organ dysfunction in a mouse model of Alzheimer’s disease. Exp Gerontol 120:1–5
Zhao Y, Cong L, Jaber V, Lukiw WJ (2017) Microbiome-derived lipopolysaccharide enriched in the perinuclear region of Alzheimer’s disease brain. Front Immunol 8:1064. https://doi.org/10.3389/fimmu.2017.01064
Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, Sharp FR (2016) Gram-negative bacterial molecules associate with Alzheimer’s disease pathology. Neurology 87(22):2324–2332
Zhao Y, Jaber V, Lukiw WJ (2017) Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol 7:318. https://doi.org/10.3389/fcimb.2017.00318
Andreadou E, Pantazaki AA, Daniilidou M, Tsolaki M (2017) Rhamnolipids, microbial virulence factors, in Alzheimer’s disease. J Alzheimers Dis 59(1):209–222
Zgoda-Pols JR, Chowdhury S, Wirth M, Milburn MV, Alexander DC, Alton KB (2011) Metabolomics analysis reveals elevation of 3-indoxyl sulfate in plasma and brain during chemically-induced acute kidney injury in mice: investigation of nicotinic acid receptor agonists. Toxicol Appl Pharmacol 255:48–56
Weber D, Oefner PJ, Hiergeist A, Koestler J, Gessner A, Weber M et al (2015) Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood 126:1723–1728
Ramos-Chávez LA, Roldán-Roldán G, Garcia-Juárez B, González-Esquivel D, Pérez de la Cruz G, Pineda B et al (2018) Low serum tryptophan levels as an indicator of global cognitive performance in nondemented women over 50 years old. Oxidative Med Cell Longev 2018:8604718. https://doi.org/10.1155/2018/8604718
Hafstad-Solvang SE, Nordrehaug JE, Tell GS, Nygård O, McCann A, Ueland PM et al (2019) The kynurenine pathway and cognitive performance in community-dwelling older adults. The Hordaland Health Study. Brain Behav Immun 75:155–162
Castillo-Rodriguez E, Fernandez-Prado R, Esteras R, Perez-Gomez MV, Gracia-Iguacel C, Fernandez-Fernandez B et al (2018) Impact of altered intestinal microbiota on chronic kidney disease progression. Toxins (Basel) 10(7):E300. https://doi.org/10.3390/toxins10070300
Lin YT, Wu PH, Lee HH, Mubanga M, Chen CS, Kuo MC et al (2019) Indole-3 acetic acid increased risk of impaired cognitive function in patients receiving hemodialysis. Neurotoxicology 73:85–91
Karu N, McKercher C, Nichols DS, Davies N, Shellie RA, Hilder EF et al (2016) Tryptophan metabolism, its relation to inflammation and stress markers and association with psychological and cognitive functioning: Tasmanian Chronic Kidney Disease pilot study. BMC Nephrol 17:171. https://doi.org/10.1186/s12882-016-0387-3
Yeh YC, Huang MF, Liang SS, Hwang SJ, Tsai JC, Liu TL et al (2016) Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. Neurotoxicology 53:148–152
Lin YT, Wu PH, Tsai YC, Hsu YL, Wang HY, Kuo MC et al (2019) Indoxyl sulfate induces apoptosis through oxidative stress and mitogen-activated protein kinase signalinig pathway inhibition in human astrocytes. J Clin Med 8(2):E191. https://doi.org/10.3390/jcm8020191
Paley EL, Merkulova-Rainon T, Faynboym A, Shestopalov VI, Aksenoff I (2018) Geographical distribution and diversity of gut microbial NADH-Ubiquinone oxidoreductase sequence associated with Alzheimer’s disease. J Alzheimers Dis 61:1531–1540
Canfora EE, Jocken JW, Blaak EE (2015) Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11(10):577–591
Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E (2017) Dysbiosis and the immune system. Nat Rev Immunol 17(4):219–232
Russo R, Cristiano C, Avagliano C, De Caro C, La Rana G, Mattace Raso G et al (2017) Gut-brain axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr Med Chem 24:1–22
Stilling RM, van de Vouw M, Clarke G, Stanton C, Dinan TG, Cryan JF (2016) The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int 99:110–132
Romo-Araiza A, Gutiérrez-Salmeán G, Galván EJ, Hernández-Frausto M, Herrera-López G, Romo-Parra H et al (2018) Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front Aging Neurosci 10:416. https://doi.org/10.3389/fnagi.2018.00416
Liu J, Sun J, Wang F, Yu X, Ling Z, Li H et al (2015) Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int 2015:412946. https://doi.org/10.1155/2015/412946
Sun J, Wang F, Ling Z, Yu X, Chen W, Li H et al (2016) Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res 1642:180–188
Kobayashi Y, Sugahara H, Shimada K, Mitsuyama E, Kuhara T, Yasuoka A et al (2017) Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s diseases. Sci Rep 7:13510. https://doi.org/10.1038/s41598-017-13368-2
Nguyen TTT, Fujimura Y, Mimura I, Fujii Y, Nguyen NL, Arakawa N et al (2018) Cultivable butyrate-producing bacteria of elderly Japanese diagnosed with Alzheimer’s disease. J Microbiol 56(10):760–771
Staley C, Weingarden AR, Khoruts A, Sadowsky MJ (2017) Interaction of gut microbiota with bile acid metabolism and its influence on diseases states. Appl Microbiol Biotechnol 101(7):47–64
Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC (2002) Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc Natl Acad Sci U S A 99(16):10671–10676
Nunes AF, Amaral JD, Lo AC, Fonseca MB, Viana RJ, Callaerts-Vegh Z et al (2012) TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-β deposition in APP/PS1 mice. Mol Neurobiol 45(3):440–454
Dionisio PA, Amaral JD, Ribeiro MF, Lo AC, D’Hooge R, Rodrigues CM (2015) Amyloid-β pathology is attenuated by tauroursodeoxycholic acid treatment in APP/PS1 mice after disease onset. Neurobiol Aging 36(1):228–240
Olzarán J, Gil-de-Gómez L, Rodríguez-Martín A, Valentí-Soler M, Frades-Payo B, Marín-Muñoz J et al (2015) A blood-based, 7-metabolite signature for early diagnosis of Alzheimer’s disease. J Alzheimers Dis 45(4):1157–1173
Nho K, Kueider-Paisley A, MahmoudianDehkordi S, Arnold M, Risacher SL, Louie G et al (2019) Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: relationship to neuroimaging and CSF biomarkers. Alzheimers Dement 15:232–244
Li D, Ke Y, Zhan R, Liu C, Zhao M, Zeng A et al (2018) Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 10:e12768. https://doi.org/10.1111/acel.12768. [Epub ahead of print]
Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CM et al (2018) The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res Ther 10:124. https://doi.org/10.1186/s13195-018-0451-2
Potì F, Santi D, Spaggiari G, Zimetti F, Zanotti I (2019) Polyphenol health effects on cardiovascular and neurodegenerative disorders: a review and meta-analysis. Int J Mol Sci 20:351. https://doi.org/10.3390/ijms20020351
Wang D, Ho L, Faith J, Ono K, Janle EM, Lachcik PJ et al (2015) Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol Nutr Food Res 59:1025–1040
Frolinger T, Sims S, Smith C, Wang J, Cheng H, Faith J et al (2019) The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci Rep 9:3546. https://doi.org/10.1038/s41598-019-39994-6
Selma MV, González-Sarrías A, Salais-Salvadó J, Andrés-Lacueva C, Alasalvar C, Örem A et al (2018) The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: comparison between normoweight, overweight-obesity and metabolic syndrome. Clin Nutr 37(3):897–905
González-Sarrías A, García-Villalba R, Romo-Vaquero M, Alasalvar C, Örem A, Zafrilla P et al (2017) Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: a randomized clinical trial. Mol Nutr Food Res 61(5):1600830. https://doi.org/10.1002/mnfr.201600830
Gong Z, Huang J, Xu B, Ou Z, Zhang L, Lin X et al (2019) Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J Neuroinflammation 16(1):62. https://doi.org/10.1186/s12974-019-1450-3
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B et al (2019) Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22(3):401–412
Bookheimer SY, Renner BA, Ekstrom A, Li Z, Henning SM, Brown JA et al (2013) Pomegranate juice augments memory and FMRI activity in middle-aged and older adults with mild memory complaints. Evid Based Complement Alternat Med 2013:946298. https://doi.org/10.1155/2013/946298
Alisi L, Cao R, De Angelis C, Cafolla A, Caramia F, Cartocci G et al (2019) The relationship between vitamin K and cognition: a review of current evidence. Front Neurosci 10:239. https://doi.org/10.3389/fneur.2019.00239
Mongkhon P, Naser AY, Fanning L, Tse G, Lau WCY, Wong ICK et al (2019) Oral anticoagulants and risk of dementia: a systematic review and meta-analysis of observational studies and randomized controlled trials. Neurosci Biobehav Rev 96:1–9
Tamadon-Nejad S, Ouliass B, Rochford J, Ferland G (2018) Vitamin K deficiency induced by warfarin is associated with cognitive and behavioral perturbations, and alterations in brain sphingolipids in rats. Front Aging Neurosci 10:213. https://doi.org/10.3389/fnagi.2018.00213
Brangier A, Ferland G, Rolland Y, Gautier J, Féart C, Annweiler C (2018) Vitamin K antagonists and cognitive decline in older adults: a 24-month follow-up. Nutrients 10:666. https://doi.org/10.3390/nu10060666
LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M (2013) Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol 24(2):160–168
Acknowledgements
All authors have no conflict of interest to declare. No funding is reported for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ticinesi, A., Nouvenne, A., Tana, C., Prati, B., Meschi, T. (2019). Gut Microbiota and Microbiota-Related Metabolites as Possible Biomarkers of Cognitive Aging. In: Guest, P. (eds) Reviews on Biomarker Studies in Aging and Anti-Aging Research. Advances in Experimental Medicine and Biology(), vol 1178. Springer, Cham. https://doi.org/10.1007/978-3-030-25650-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-25650-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25649-4
Online ISBN: 978-3-030-25650-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)