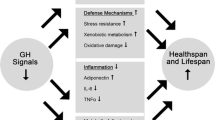
Abstract
Growth hormone (GH) is a metabolic hormone that has major functions in the liver, muscle, and adipose tissue (AT). In the past 20 years, numerous studies have demonstrated that decreased growth hormone (GH) action is clearly linked to alterations in longevity. Therefore, it is not surprising that mechanisms underlying the extended longevity of GH-mutant animals include alterations in AT function. This Review aims to describe the basics of AT biology, GH secretion and action, and the effects of altered GH signaling in mice and humans. Lastly, this Review discusses the intersection of GH and AT, and how the influence of GH on AT may play a critical role in determining lifespan and healthspan.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The growth hormone (GH) and insulin-like growth factor 1 (IGF-1) axis (collectively known as the somatotropic axis) was demonstrated to be a major determinant of mammalian longevity more than 20 years ago [1, 2]. Since then, numerous laboratories have attempted to elucidate mechanisms underlying the role of this axis in longevity, leading to an ever-growing list of possible mechanisms to disentangle [3, 4]. During the same timeframe, the growing obesity epidemic in the developed world has resulted in a dramatic increase in adipose tissue (AT) research. Since GH plays integral roles in the physiology of AT, there has been a surge in research attempting to understand how AT impacts longevity in GH-mutant mice. This review is aimed to give an overview of AT, GH, and how the interplay between the two influences longevity.
2 Adipose Tissue
Traditionally, AT was believed to be metabolically inactive, solely acting as a tissue to store excess calories. However, paradigm-shifting studies demonstrated that AT secretes adiponectin [5], leptin [5, 6] and resistin [7], which paved the way for future work on AT as an endocrine organ. Since then, our understanding of AT has been greatly expanded. We now know that there are at least three distinct types of adipose tissue: white adipose tissue (WAT); brown adipose tissue (BAT); and beige AT. As predicted, each type of AT depot has specific functions. Further differentiating these types of AT is the significant cellular heterogeneity within an AT depot itself [8]. Despite this heterogeneity, AT is largely made-up of postmitotic adipocytes and their replicative precursors, termed preadipocytes. The differentiation of preadipocytes into mature adipocytes is transcriptionally controlled through the coordination of CCAAT/enhancer-binding proteins (C/EBPs) and peroxisome proliferator-activated receptor gamma (PPARγ) [9]. This section is dedicated to defining the similarities and differences between WAT, BAT and beige AT, which are summarized in Table 11.1.
2.1 WAT
The defining characteristic of WAT in both humans and mice is the storage of excess energy. Morphologically, WAT is characterized by a large, unilocular lipid droplet, with few mitochondria. WAT has both similarities and differences in mice and humans. In mice, WAT is present in superficial subcutaneous depots, mainly in the scapular and inguinal regions. WAT is also present in the intra-abdominal region of mice in the form of perigonadal (epididymal and paraovarian in males and females, respectfully), mesenteric, and retroperitoneal AT. In humans, WAT is more widely distributed and is present subcutaneously in the gluteal, femoral, clavicular, and abdominal regions. WAT in humans is also present intra-abdominally in intraperitoneal, retroperitoneal, mesenteric, and omental AT depots. The major difference in WAT distribution between mice and humans is the large perigonadal depot in mice, and the large omental depot in humans. Most mouse studies in the context of metabolism and aging use inguinal WAT (iWAT) to represent subcutaneous AT, and perigonadal AT to represent the so-called visceral AT. Although this practice is widely used and accepted, it is worth mentioning that some investigators prefer a more stringent use of the term “visceral” to include only AT that directly drains into the portal vein, rather than any intra-abdominal AT depot. By this definition, only mesenteric AT in mice would be considered “visceral” [10].
In obese subjects, there are critical changes in WAT physiology. AT itself is surrounded by a thick extracellular matrix (ECM). During weight gain, the ECM in WAT must expand to accommodate hypertrophic adipocytes. However, this results in poor vascularization [11] and subsequent hypoxia. Hypoxia in the adipocyte is just one of several instances that cause an increased secretion of proinflammatory cytokines to be released from WAT during obesity [12]. Another unfavorable impact of obesity on WAT is ectopic lipid distribution. For example, intra-myocellular lipids [13,14,15,16,17] and intra-hepatic lipids [18,19,20,21] are associated with insulin resistance, while epicardial fat is associated with an increased risk of coronary artery disease [22,23,24].
2.2 Bat
The differences between BAT and WAT begin during development, as BAT comes from a mesoderm lineage that is myogenic factor 5 (MYF5) positive [25]. Unlike WAT, BAT is characterized by multilocular lipid droplets, many mitochondria, and is rich in both innervation and microvasculature. These anatomical traits are important for the main function of BAT, thermogenesis. Sympathetic nerves provide a source of norepinephrine (NE) to stimulate thermogenesis, blood vessels provide nutrients to the tissue as well as aid in heat dissipation, and multilocular lipid droplets have an increased surface area to facilitate an increased rate of lipolysis. The thermogenic circuit relies on several transcriptional inputs including peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) [26] and PR domain containing 16 (PRDM16) [27]. Moreover, cues from other transcriptional machinery such as thyroid hormone receptor [28] and retinoic acid receptor [29] play important roles in the thermogenic circuit. Readers interested in learning more about the transcriptional control of the thermogenic program are directed to the following reviews [30,31,32]. Central to the function of BAT is uncoupling protein 1 (UCP1), which dissociates the electron transport chain to release chemical energy in the form of heat.
Thermogenesis itself begins with the release of NE from the sympathetic nervous system, which acts on β3-adrenergic receptors, which are associated with G-protein coupled receptors (GPCRs) of the Gs subtype [33, 34]. Subsequently, a rise in cytosolic cAMP results in the activation of protein kinase A (PKA) [35], which has several functions including activating mitogen-activated protein kinase (MAPK) p38 [36] and increasing cytosolic free fatty acid (FFA) levels in the cell by phosphorylating perilipin [37]. This, in turn, causes the release of comparative gene identification-58 (CGI-58) to activate adipose triglyceride lipase (ATGL), the major triglyceride lipase in BAT [38,39,40]. The breakdown of triglycerides into FFAs in BAT is critical for two reasons. First, the resulting FFAs can be shuttled into the mitochondria where they can undergo β-oxidation to produce ATP and reduced electron carriers to maintain thermogenesis [30]. Second, the FFAs act as activators of UCP1 [41, 42]. Conversely, purine nucleotides act as inhibitors of UCP1 [41].
It was long believed that BAT in humans was non-existent past adolescence. However, a decade ago, seminal studies that “rediscovered” BAT in adult humans proved otherwise [43,44,45]. Because a few hundred milligrams of BAT can oxidize up to 60% of consumed glucose and lipids in a cold-acclimated mouse [46, 47], investigators became interested in using BAT therapeutically to combat the growing obesity epidemic. Although humans do possess BAT, it differs in several ways from BAT in mice. Mice have several distinct BAT depots including the large interscapular depot, as well as the axillary, cervical, paraaortic, cardiac, and perirenal depots. In humans, brown adipocytes appear interspersed in WAT, mainly in the supraclavicular region, but are also present in the para-aortic, cervical, axillary, perirenal, and paravertebral regions. It is worth noting that recently a supraclavicular BAT depot was discovered in mice [48]. There is an interscapular BAT depot in humans, although it disappears during adolescence. Currently, the gold standard for assessing the location and activity of BAT in humans is positron emission tomography coupled with computed tomography (PET/CT) during the infusion of radiolabeled 18-fluoro-deoxyglucose (18FDG) in a patient either wearing a “cold vest” or receiving a β3-agonist, such as mirabegron [49]. However, this method does not always accurately reflect BAT activity, and has led to vastly different estimates of the total volume of BAT present in humans, ranging over two orders of magnitude from only a few, to a few hundred milliliters [50]. The measurement of BAT in humans, along with a detailed description of its pitfalls has been reviewed elsewhere [49]. Regardless, we know that BAT is present in adult humans and has a significant impact on metabolism. A clear example of this is a study in which type 2 diabetics spent several hours a day over a 10-day period at 15 °C, which resulted in a significant increase in their glucose infusion rate (GIR) during a euglycemic clamp [51].
One of the biggest advances in our understanding of thermogenic AT in the past few years is the presence of UCP1-independent thermogenic mechanisms. For example, both brown and beige AT thermogenesis can occur through a creatine-based substrate cycle [52,53,54]. Moreover, beige AT thermogenesis can be controlled through ATP-dependent calcium cycling [55]. These findings can begin to explain why UCP1 null mice only become obese under thermoneutral temperatures [56, 57], while BAT-deficient mice are obese and insulin resistant at standard room temperature [58, 59]. To-date, however, these UCP1-independent forms of thermogenesis have not been examined in GH mutant mice.
2.3 Beige AT
Beige AT is distinct from both WAT and BAT. Beige adipocytes reside within WAT depots, contain mitochondria expressing UCP1, and are therefore thermogenic. Under basal conditions, thermogenic output of beige adipocytes is relatively low, however, stimulants such as cold exposure, exercise, or treatment with PPARγ agonists significantly increase the expansion and energy expenditure in these cells in a process termed beiging. Although beiging was described more than 30 years ago [60, 61], only recently have the specific lineages and molecular regulators that give rise to beige AT been worked out [62, 63]. It is worth noting that this is an area of ongoing investigation, with no clear consensus. For example, some studies have shown that beige AT derives from a lineage distinct from BAT that is positive for myosin heavy chain 11 (MYH11) and platelet derived growth factor receptor alpha (PDGFRα) in mice [64,65,66,67], while other beige adipocytes have been found to be positive for paired box 3 (PAX3) and MYF5 [68]. Adding further complexity are conflicting studies in which some investigators demonstrate that beige adipocytes are formed de novo in response to external cues [66, 69], while others argue they arise from the transdifferentiation of white adipocytes [70, 71]. Regardless of the developmental origin of beige adipose tissue, during cold-exposure, thermogenic beige adipocytes replace non-thermogenic white adipocytes, which is reversible when cold-acclimated mice are placed at thermoneutrality [72].
3 Properties of Adipose Tissue
3.1 Adipose Tissue Heterogeneity
Beyond the previously discussed inter-depot differences, AT is highly heterogenous within each depot. By volume, the majority of AT is composed of mature adipocytes. These adipocytes have a turnover rate of approximately 10% annually in humans, with a much faster turnover rate of 5% daily in mice [73, 74]. Because of this, preadipocytes, or committed adipocyte progenitors, are critical to the AT niche. Work has already been done to identify markers of white, brown, and beige adipocytes, however, studies identifying novel cell surface markers of preadipocytes that give rise to these adipocytes is mostly lacking, although some markers have been identified [75]. For example, sorting preadipocytes with high CD29 expression enriches for a population of preadipocytes that differentiate into cells with a high expression of UCP1 [75]. Preadipocytes are also a major source of tumor necrosis factor α (TNF-α), suggesting their role in AT extends beyond acting as a precursor cell [76].
Immune cells are another cell type with a large role in AT. It has been appreciated for years that macrophages are present in AT, and that their presence increases with obesity. However, considerable improvements have been made to our understanding of different subpopulations of macrophages in AT [77,78,79]. For example, there is good evidence that both M1 (referred to as classically activated) and M2 (referred to as alternatively activated) macrophages exist within AT [80, 81]. Along with macrophages, natural killer cells are recruited to AT during obesity, causing insulin resistance [82]. Changes in resident immune cells may also play a beneficial role in the physiology of AT. For example, cold-exposure causes an influx of M2 macrophages and eosinophils that aid in thermogenesis [83,84,85].
AT stores energy as triglycerides during caloric excess, and must liberate FFAs during periods of caloric demand through lipolysis. Since lipolysis is stimulated by the sympathetic release of NE, it makes sense that nerves are a core component of AT [86]. To transport nutrients to AT, or dissipate heat and FFAs (in BAT and WAT, respectfully), there must also be the presence of microvasculature, including endothelial cells and smooth muscle cells [8]. Certainly, AT heterogeneity has garnered attention in recent years. With advances in technologies such as single cell RNA-sequencing, the makeup and functional importance of the AT niche is sure to be further developed in the near future.
3.2 Adipose Tissue as a Secretory Organ
Studying WAT as an endocrine organ began 20 years ago with the discovery of leptin, resistin, and adiponectin being secreted from WAT [5,6,7]. WAT has also been known to secrete proinflammatory cytokines such as TNF-α and IL-6, which facilitate the development of insulin resistance during obesity. What has been much less studied, however, is the role of BAT as a secretory organ. Recently, this has changed as secreted factors from BAT (referred to as batokines) have been demonstrated to have both autocrine/paracrine and endocrine effects. For example, the lipokine 12,13-diHOME has been demonstrated to have an autocrine effect on BAT that results in increased lipid uptake [87]. Other factors that have paracrine/autocrine action in BAT are vascular endothelial growth factor A (VEGFa) and nitric oxide (NO), which increase angiogenesis [88, 89]. Moreover, fibroblast growth factor 2 (FGF2) and nerve growth factor (NGF) increase innervation and the recruitment of preadipocytes [90,91,92]. Endocrine factors that are secreted from BAT include insulin-like growth factor-binding protein 2 (IGFBP2) [93], WNT10b [93], and FGF21 [94]. BAT also secretes microRNAs. For example, both mice and humans show an inverse relationship between BAT activity and circulating levels of miR-92a [95]. Readers interested in learning more about the secretory function of BAT are encouraged to read a relevant review [96].
3.3 Alterations in AT during Aging
The main changes in WAT during aging is the gradual decline in tissue mass, the redistribution from subcutaneous to intra-abdominal depots, and the ectopic distribution of lipids in organs such as the liver and muscle [97,98,99,100]. Metabolically, aged WAT has a decline in its sensitivity to insulin and fatty acids [97, 101,102,103]. Moreover, aged WAT has an increased secretion of harmful proinflammatory cytokines such as TNF-α and IL-6 [104, 105]. There does appear to be an increase in macrophage infiltration in subcutaneous WAT, although this does not seem to apply to intra-abdominal WAT [106]. A final means through which WAT changes during aging is through the preadipocyte pool. Preadipocytes from aged tissue have lower levels of the transcription factors PPARγ C/EBPα, and their target genes [107, 108], which may explain the reduction in their capacity to differentiate into mature adipocytes. Moreover, senescent preadipocytes accumulate in aged WAT, which could contribute to metabolic impairment and increased systemic inflammation [97]. Readers interested in learning more about WAT remodeling during aging are directed to a relevant review [97].
Some of the changes mentioned above apply to BAT. For example, BAT mass decreases with age. Although senescence in BAT has been understudied, it is plausible to assume that brown preadipocytes also senesce, and lose the ability to differentiate into mature brown adipocytes with age. Many of the age-related changes in BAT relate to impaired thermogenic capacity. One mechanism for this is through the increased visceral AT expression of forkhead box protein A3 (FOXA3), which impairs BAT mass and function [109]. Interestingly, deletion of FOXA3 increases BAT late into life and extends longevity [109]. Another deleterious change in aged BAT is the presence of sympathetic neuron-associated macrophages, which chelate NE, resulting in decreased thermogenic output [110]. Finally, the well-documented age-dependent mitochondrial dysfunction impairs thermogenesis. The decline in the thermogenic function of BAT likely plays a critical role in the metabolic impairment and obesity observed during middle-age.
4 Growth Hormone
GH is a 22 kDa peptide hormone that is secreted from somatotrophs in the anterior pituitary. Its secretion is induced by the release of growth hormone releasing hormone (GHRH), and inhibited by the release of somatostatin (SST), both of which are released from the hypothalamus. GH has negative feedback on GH release from the pituitary, as well as on GHRH from the hypothalamus. Another level of feedback is through IGF-1 which acts on both the pituitary and hypothalamus [111]. Ghrelin, a “hunger” hormone is another factor that stimulates the release of GH [112]. AT can regulate GH production through FFAs and leptin which inhibit and stimulate GH production, respectively [113, 114].
In circulation, GH acts by binding to growth hormone receptor (GHR) on target tissues. Mainly, GH acts on the liver to stimulate the production of IGF-1, but GH can also act on other tissues such as muscle and AT [115]. Therefore, GH can elicit direct effects, or indirect effects through the action of IGF-1. Once bound to a homodimerized GHR, there is a conformational change in the receptor structure which brings together the associated janus kinase 2 (JAK2) domains together, allowing for transactivation [116] and subsequent phosphorylation of signal transducer and activator of transcription 5 (STAT5). Activated STAT5 can then enter the nucleus and act as a transcription factor [117]. GH has been demonstrated to signal through other non-canonical pathways including mammalian target of rapamycin (mTOR) and extracellular signaling-regulated kinase (ERK) [118].
5 Examples of Altered Growth Hormone Action
5.1 Humans
The two main ways that GH is altered in humans is through its overproduction in acromegaly and GH resistance, or through its under production in GH deficiency. Patients with acromegaly suffer from increased GH secretion, and subsequent increases in IGF-1 production. The increased secretion of GH is oftentimes the result of a pituitary adenoma. Acromegaly patients are more prone to cancer [119,120,121], diabetes [122], and are often short-lived compared to people with normal GH secretion [123, 124]. GH deficiency has multiple etiologies that influence the age of the onset of disease. In children, congenital GH deficiency is usually the result of mutations in genes encoding GH, GHRH, or other pituitary factors involved in the secretion of GH [125]. In adults, acquired GH deficiency is typically the result of hypopituitarism or irradiation of a pituitary adenoma [126]. Beyond deficiency, patients can be resistant to GH through mutations in the gene encoding GHR [127]. This, disease, termed Laron syndrome, causes patients to have low IGF-1, with elevated levels of GH [128]. Patients with both GH deficiency and resistance demonstrate decreased height, increased obesity, decreased bone mineral density, and altered lipid metabolism [3]. Interestingly, patients with Laron syndrome appear to be protected from cancer [129, 130] and diabetes [131], although Laron syndrome patients from cohorts in Israel and Turkey appear to still develop diabetes [132, 133], making the “protected” status from diabetes less clear.
5.2 Mice
To further understand the impact of GH signaling, several transgenic lines over-expressing GH have been created, the most commonly used being the bovine GH (bGH) transgenic line [134, 135]. These mice have a transgene that ectopically expresses GH under a strong promoter such as phosphoenolpyruvate carboxykinase (PEPCK). bGH mice are noticeably larger than their control littermates, and exhibit increased muscle mass. bGH mice demonstrate increased insulin resistance, and severe hyperplasia and hypertrophy of their hepatocytes [136]. Many of these mice die of hepatic cancer. Aging in these mice appears to be accelerated, and lifespan is reduced to around 1 year-of-age [135, 137].
Ames dwarf mice were first described in 1961, and suffer from a spontaneous mutation in the gene encoding prophet of pituitary factor 1 (Prop1) [138]. Snell dwarf mice were first described in the 1929, and suffer from a spontaneous mutation in the gene encoding pituitary factor 1 (Pit1) [139]. Since Prop1 is a transcription factor for the Pit1 gene, the phenotypes of Ames and Snell dwarfs are essentially identical, with both strains of dwarf mice lacking the production of GH, thyroid-stimulating hormone (TSH) and prolactin [140]. The downstream consequences of these mutations are a decreased production of IGF-1 and the thyroid hormones, T3 and T4. Both Ames and Snell dwarf mice are extremely long-lived, with an ~50% increase in longevity in Ames dwarf mice (1) and ~42% increase in Snell dwarf mice [141]. Along with increased longevity, these animals have a decreased incidence of cancer [142], increased insulin sensitivity [143] and increased adiposity, particularly in the subcutaneous depot [144].
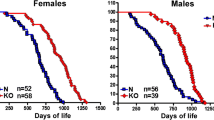
Growth hormone receptor knockout (GHRKO) mice were developed to replicate Laron syndrome in mice by disrupting the gene encoding GHR/GH binding protein [145]. As with Laron syndrome patients, GHRKO mice are small, have increased adiposity, and have increased circulating GH with low circulating IGF-1 [145]. GHRKO mice also live ~38% longer than control mice [146]. Studies of cognitive function [147, 148] and tissue histopathology [149] revealed that GHRKO mice have a delay in aging, similar to that of Ames and Snell dwarf mice. To study the effects of GH action in specific tissues, many tissue-specific lines of GHRKO mice have been created [150,151,152,153]. The first line attempting to knockout the GHR gene in AT was done using Cre driven by the adipocyte protein 2 (Ap2) promoter (termed FaGHRKO) [154]. Unlike the global GHRKO mice, these animals had an increase in IGF-1 and had no improvement in insulin sensitivity [154]. It has since been reported that the Ap2 promoter is “leaky” and is expressed in macrophages, endothelial cells, and in the brain [155, 156]. Therefore, an AT-specific GHRKO mouse (termed AdGHRKO) was generated using the more specific adiponectin promoter-driven Cre [157]. These animals show no difference in GH or IGF-1, have increased fat mass, decreased liver triglyceride content, and are insulin sensitive [157].
Other mouse lines such as the GHRH knockout (GHRHKO) mice live ~45% longer than their control littermates, and have decreased IGF-1 production and increased adiposity [158]. “Little” mice have a mutation of the GHRH receptor gene, live ~25% longer than their normal littermates, and develop increased adiposity [141]. Several current reviews discuss mechanisms of altered longevity in all these mouse lines in more detail [4, 159, 160].
6 Adipose Tissue in Mice and Humans with Altered Growth Hormone Action
What is clear is that a defining phenotype of GH-related mutations is alterations in adiposity. For example, patients with acromegaly and bGH mice have decreased adiposity [161,162,163,164], while patients and mice that are GH-deficient or GH-resistant have increased adiposity [144, 165,166,167]. Particularly noteworthy is that the iWAT depot in GHRKO mice is equal in mass to that of their control littermates, despite GHRKO mice weighing approximately 66% less [166, 168]. It has also been demonstrated that dwarf and GHRKO mice maintain a higher extra- to intra-peritoneal distribution of lipids [169], which is the opposite of acromegaly patients which have a higher ectopic distribution of lipids [170]. Beyond alterations in mass and distribution, there appears to be an alteration in the endocrine function of AT in GH-mutant animals. For example, leptin and adiponectin are decreased in bGH mice, and increased in dwarf and GHRKO mice [171, 172]. In fact, altered endocrine function may partially explain an unexpected phenotype, where surgical removal of the epidydimal WAT (eWAT) in dwarf and GHRKO mice results in decreased insulin sensitivity [173, 174]. This finding may be due to the fact that eWAT in long-lived GH-mutant mice secretes less pro-inflammatory cytokines such as TNF-α and IL-6, while secreting more adiponectin [173, 174]. The secretory function of BAT in these animals has not yet been assessed. Lowering senescent cell burden can extend longevity [175]. Therefore, it is important that 18-month-old dwarf and GHRKO mice have a reduced senescent cell burden, and that their preadipocytes demonstrate an increased differentiation capacity, suggesting that their AT has a “younger” phenotype [169]. Senescence in BAT of long-lived GH-mutant mice has not yet been investigated.
Although many studies have been conducted on WAT in GH-mutant mice, far less has been done relating to the BAT of these animals. To date, we know that BAT in GHRKO and Ames dwarf mice has an increased expression of Ucp1, and that BAT in bGH mice has a decreased expression of Ucp1 [176, 177]. This is accompanied by an increase or decrease in BAT mass in Ames dwarf/GHRKO and bGH mice, respectively [176, 177]. The highly active BAT observed in Ames dwarf and GHRKO mice may at least partially explain the increased rate of energy expenditure and reduced respiratory quotient observed in these animals [178]. Particularly curious in Ames dwarf mice is the increased BAT activity despite having a depleted thyroid hormone axis. This suggests that other circulating factors may be responsible for the increased BAT activity, although this hypothesis remains to be tested. It does appear that the increased BAT activity of these animals may play a role in several biomarkers of healthy aging. For example, placing Ames dwarf mice at thermoneutrality (30 °C) eliminates differences in oxygen consumption rate and respiratory quotient, and reduces their enhanced insulin sensitivity [179]. Further testing will need to be done to determine if thermoneutral housing also impacts longevity in these animals.
7 Final Thoughts
GH has a critical role in metabolism due to its profound effects highly metabolic tissues such as the liver, muscle and AT. Increased adiposity is associated with comorbidities ranging from diabetes to Alzheimer’s disease. Therefore, it is of major consequence that the AT in long-lived GH-mutant mice functions in a more metabolically beneficial way. These differences include AT distribution (extra- instead of intraperitoneal), endocrine function (shift from pro- to anti-inflammatory cytokines), and replication and senescence status (differentiates well and has a low senescent cell burden), as well as an increase in thermogenesis and BAT activity. In terms of whole-body physiology, these alterations cannot be understated. For example, both WAT and BAT in these animals most likely significantly contribute to improved glycemic control, which is believed to be a major factor in their improved healthspan and lifespan. There are still areas that should be examined in the context of AT in GH animals. For example, we now know that AT secretes many proteins, metabolites, lipids, miRNAs, and is a rich source of exosomes. Examining how these are alteredin GH-mutant mice is a currently unexplored area. Moreover, extrapolating these findings to human subjects would be of considerable interest.
References
Brown-Borg HM, Borg KE, Meliska CJ, Bartke A (1996) Dwarf mice and the ageing process. Nature 384(6604):33. https://doi.org/10.1038/384033a0
Steger RW, Bartke A, Cecim M (1993) Premature ageing in transgenic mice expressing different growth hormone genes. J Reprod Fertil Suppl 46:61–75
Aguiar-Oliveira MH, Bartke A (2018) Growth hormone deficiency: health and longevity. Endocr Rev. https://doi.org/10.1210/er.2018-00216. [Epub ahead of print]
Bartke A, Quainoo N (2018) Impact of growth hormone-related mutations on mammalian aging. Front Genet 9:586. https://doi.org/10.3389/fgene.2018.00586
Hu E, Liang P, Spiegelman BM (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271(18):10697–10703
Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ et al (1997) Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387(6636):903–908
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM et al (2001) The hormone resistin links obesity to diabetes. Nature 409(6818):307–312
Lynes MD, Tseng YH (2018) Deciphering adipose tissue heterogeneity. Ann N Y Acad Sci 1411(1):5–20
Farmer SR (2006) Transcriptional control of adipocyte formation. Cell Metab 4(4):263–273
Cinti S (2005) The adipose organ. Prostaglandins Leukot Essent Fatty Acids 73(1):9–15
Lemoine AY, Ledoux S, Queguiner I, Calderari S, Mechler C, Msika S et al (2012) Link between adipose tissue angiogenesis and fat accumulation in severely obese subjects. J Clin Endocrinol Metab 97(5):E775–E780
Rosen ED, Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156(1–2):20–44
Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL et al (1999) Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42(1):113–116
Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C et al (1999) Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48(8):1600–1606
Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C et al (1997) Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46(6):983–988
Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W et al (1999) Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 48(5):1113–1119
Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC et al (1996) Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism 45(8):947–950
Korenblat KM, Fabbrini E, Mohammed BS, Klein S (2008) Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 134(5):1369–1375
Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S et al (2005) Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 48(4):634–642
Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK et al (2001) Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120(5):1183–1192
Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A et al (2002) Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87(7):3023–3028
Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY et al (2008) Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart 94(3):e7. https://doi.org/10.1136/hrt.2007.118471
Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC et al (2009) Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol 29(5):781–786
Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS et al (2008) Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 117(5):605–613
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S et al (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454(7207):961–967
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92(6):829–839
Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M et al (2007) Transcriptional control of brown fat determination by PRDM16. Cell Metab 6(1):38–54
Cassard-Doulcier AM, Larose M, Matamala JC, Champigny O, Bouillaud F, Ricquier D (1994) In vitro interactions between nuclear proteins and uncoupling protein gene promoter reveal several putative transactivating factors including Ets1, retinoid X receptor, thyroid hormone receptor, and a CACCC box-binding protein. J Biol Chem 269(39):24335–24342
Alvarez R, de Andres J, Yubero P, Vinas O, Mampel T, Iglesias R et al (1995) A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J Biol Chem 270(10):5666–5673
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359
Lynes MD, Tseng YH (2015) The thermogenic circuit: regulators of thermogenic competency and differentiation. Genes Dis 2(2):164–172
Villarroya F, Peyrou M, Giralt M (2017) Transcriptional regulation of the uncoupling protein-1 gene. Biochimie 134:86–92
Granneman JG (1988) Norepinephrine infusions increase adenylate cyclase responsiveness in brown adipose tissue. J Pharmacol Exp Ther 245(3):1075–1080
Marette A, Bukowiecki LJ (1991) Noradrenaline stimulates glucose transport in rat brown adipocytes by activating thermogenesis. Evidence that fatty acid activation of mitochondrial respiration enhances glucose transport. Biochem J 277(Pt 1):119–124
Thonberg H, Lindgren EM, Nedergaard J, Cannon B (2001) As the proliferation promoter noradrenaline induces expression of ICER (induced cAMP early repressor) in proliferative brown adipocytes, ICER may not be a universal tumour suppressor. Biochem J 354(Pt 1):169–177
Cao W, Medvedev AV, Daniel KW, Collins S (2001) beta-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J Biol Chem 276(29):27077–27082
Chaudhry A, Granneman JG (1999) Differential regulation of functional responses by beta-adrenergic receptor subtypes in brown adipocytes. Am J Phys 277(1):R147–R153
Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z (2007) Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem 282(8):5726–5735
Granneman JG, Moore HP, Krishnamoorthy R, Rathod M (2009) Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J Biol Chem 284(50):34538–34544
Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M et al (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306(5700):1383–1386
Shabalina IG, Jacobsson A, Cannon B, Nedergaard J (2004) Native UCP1 displays simple competitive kinetics between the regulators purine nucleotides and fatty acids. J Biol Chem 279(37):38236–38248
Fedorenko A, Lishko PV, Kirichok Y (2012) Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151(2):400–413
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360(15):1509–1517
Nedergaard J, Bengtsson T, Cannon B (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293(2):E444–E452
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360(15):1500–1508
Cannon B, Nedergaard J (2011) Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214(Pt 2):242–253
Golozoubova V, Gullberg H, Matthias A, Cannon B, Vennstrom B, Nedergaard J (2004) Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol Endocrinol 18(2):384–401
Mo Q, Salley J, Roshan T, Baer LA, May FJ, Jaehnig EJ et al (2017) Identification and characterization of a supraclavicular brown adipose tissue in mice. JCI Insight 2(11). pii: 93166. https://doi.org/10.1172/jci.insight.93166
Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte EE (2018) Brown adipose tissue energy metabolism in humans. Front Endocrinol (Lausanne) 9:447. https://doi.org/10.3389/fendo.2018.00447
Lee P, Greenfield JR, Ho KK, Fulham MJ (2010) A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 299(4):E601–E606
Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ et al (2015) Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 21(8):863–865
Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P et al (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163(3):643–655
Kazak L, Chouchani ET, Lu GZ, Jedrychowski MP, Bare CJ, Mina AI et al (2017) Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab 26(4):660–671.e3
Bertholet AM, Kazak L, Chouchani ET, Bogaczynska MG, Paranjpe I, Wainwright GL et al (2017) Mitochondrial patch clamp of beige adipocytes reveals UCP1-positive and UCP1-negative cells both exhibiting futile creatine cycling. Cell Metab 25(4):811–822.e4
Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M et al (2017) UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23(12):1454–1465
Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME et al (1997) Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387(6628):90–94
Feldmann HM, Golozoubova V, Cannon B, Nedergaard J (2009) UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9(2):203–209
Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S (2013) EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 504(7478):163–167
Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ et al (2014) Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156(1–2):304–316
Young P, Arch JR, Ashwell M (1984) Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett 167(1):10–14
Loncar D, Afzelius BA, Cannon B (1988) Epididymal white adipose tissue after cold stress in rats. I. Nonmitochondrial changes. J Ultrastruct Mol Struct Res 101(2–3):109–122
Kajimura S, Spiegelman BM, Seale P (2015) Brown and beige fat: physiological roles beyond heat generation. Cell Metab 22(4):546–559
Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19(10):1252–1263
Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X et al (2014) A smooth muscle-like origin for beige adipocytes. Cell Metab 19(5):810–820
Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB et al (2016) Pdgfrbeta+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab 23(2):350–359
Berry DC, Jiang Y, Graff JM (2016) Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun 7:10184. https://doi.org/10.1038/ncomms10184
Lee YH, Petkova AP, Mottillo EP, Granneman JG (2012) In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 15(4):480–491
Sanchez-Gurmaches J, Guertin DA (2014) Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun 5:4099. https://doi.org/10.1038/ncomms5099
Wang QA, Tao C, Gupta RK, Scherer PE (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19(10):1338–1344
Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K et al (2010) The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298(6):E1244–E1253
Cinti S (2009) Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab 297(5):E977–E986
Loncar D (1991) Convertible adipose tissue in mice. Cell Tissue Res 266(1):149–161
Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O et al (2008) Dynamics of fat cell turnover in humans. Nature 453(7196):783–787
Rigamonti A, Brennand K, Lau F, Cowan CA (2011) Rapid cellular turnover in adipose tissue. PLoS One 6(3):e17637
Xue R, Lynes MD, Dreyfuss JM, Shamsi F, Schulz TJ, Zhang H et al (2015) Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat Med 21(7):760–768
Cartier A, Lemieux I, Almeras N, Tremblay A, Bergeron J, Despres JP (2008) Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab 93(5):1931–1938
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112(12):1821–1830
Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante AW Jr et al (2009) Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes 58(2):385–393
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117(1):175–184
Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR (2008) Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57(12):3239–3246
Lee BC, Kim MS, Pae M, Yamamoto Y, Eberle D, Shimada T et al (2016) Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity. Cell Metab 23(4):685–698
Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC et al (2015) Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160(1–2):74–87
Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I et al (2014) Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157(6):1279–1291
Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF et al (2015) Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519(7542):242–246
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR et al (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518(7538):197–206
Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL et al (2017) The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med 23(5):631–637
Asano A, Kimura K, Saito M (1999) Cold-induced mRNA expression of angiogenic factors in rat brown adipose tissue. J Vet Med Sci 61(4):403–409
Nisoli E, Tonello C, Briscini L, Carruba MO (1997) Inducible nitric oxide synthase in rat brown adipocytes: implications for blood flow to brown adipose tissue. Endocrinology 138(2):676–682
Nisoli E, Tonello C, Benarese M, Liberini P, Carruba MO (1996) Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology 137(2):495–503
Nechad M, Ruka E, Thibault J (1994) Production of nerve growth factor by brown fat in culture: relation with the in vivo developmental stage of the tissue. Comp Biochem Physiol Comp Physiol 107(2):381–388
Yamashita H, Sato Y, Kizaki T, Oh S, Nagasawa J, Ohno H (1994) Basic fibroblast growth factor (bFGF) contributes to the enlargement of brown adipose tissue during cold acclimation. Pflugers Arch 428(3–4):352–356
Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B (2013) Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology 154(8):2687–2701
Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T et al (2011) Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem 286(15):12983–12990
Chen Y, Buyel JJ, Hanssen MJ, Siegel F, Pan R, Naumann J et al (2016) Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Commun 7:11420. https://doi.org/10.1038/ncomms11420
Villarroya F, Cereijo R, Villarroya J, Giralt M (2017) Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 13(1):26–35
Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H et al (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9(5):667–684
Visser M, Pahor M, Tylavsky F, Kritchevsky SB, Cauley JA, Newman AB et al (2003) One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol (1985) 94(6):2368–2374
Raguso CA, Kyle U, Kossovsky MP, Roynette C, Paoloni-Giacobino A, Hans D et al (2006) A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin Nutr 25(4):573–580
Bertrand HA, Lynd FT, Masoro EJ, Yu BP (1980) Changes in adipose mass and cellularity through the adult life of rats fed ad libitum or a life-prolonging restricted diet. J Gerontol 35(6):827–835
Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT (1982) Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. J Gerontol 37(2):130–141
Kirkland JL, Dax EM (1984) Adipocyte hormone responsiveness and aging in the rat: problems in the interpretation of aging research. J Am Geriatr Soc 32(3):219–228
Yki-Jarvinen H, Kiviluoto T, Nikkila EA (1986) Insulin binding and action in adipocytes in vitro in relation to insulin action in vivo in young and middle-aged subjects. Acta Endocrinol 113(1):88–92
Morin CL, Pagliassotti MJ, Windmiller D, Eckel RH (1997) Adipose tissue-derived tumor necrosis factor-alpha activity is elevated in older rats. J Gerontol A Biol Sci Med Sci 52(4):B190–B195
Starr ME, Evers BM, Saito H (2009) Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci 64(7):723–730
Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr et al (1999) Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 106(5):506–512
Karagiannides I, Tchkonia T, Dobson DE, Steppan CM, Cummins P, Chan G et al (2001) Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol Regul Integr Comp Physiol 280(6):R1772–R1780
Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP (2008) Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg 60(5):538–544
Ma X, Xu L, Gavrilova O, Mueller E (2014) Role of forkhead box protein A3 in age-associated metabolic decline. Proc Natl Acad Sci U S A 111(39):14289–14294
Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sanchez NM, Mahu I et al (2017) Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med 23(11):1309–1318
Muller EE (1990) Clinical implications of growth hormone feedback mechanisms. Horm Res 33(Suppl 4):90–96
Muller AF, Lamberts SW, Janssen JA, Hofland LJ, Koetsveld PV, Bidlingmaier M et al (2002) Ghrelin drives GH secretion during fasting in man. Eur J Endocrinol 146(2):203–207
Pombo M, Pombo CM, Astorga R, Cordido F, Popovic V, Garcia-Mayor RV et al (1999) Regulation of growth hormone secretion by signals produced by the adipose tissue. J Endocrinol Investig 22(5 Suppl):22–26
Carro E, Senaris R, Considine RV, Casanueva FF, Dieguez C (1997) Regulation of in vivo growth hormone secretion by leptin. Endocrinology 138(5):2203–2206
Green H, Morikawa M, Nixon T (1985) A dual effector theory of growth-hormone action. Differentiation 29(3):195–198
Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K et al (2005) Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol 12(9):814–821
Wells JA (1996) Binding in the growth hormone receptor complex. Proc Natl Acad Sci U S A 93(1):1–6
Brooks AJ, Waters MJ (2010) The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol 6(9):515–525
Jenkins PJ (2006) Cancers associated with acromegaly. Neuroendocrinology 83(3–4):218–223
Rogozinski A, Furioso A, Glikman P, Junco M, Laudi R, Reyes A et al (2012) Thyroid nodules in acromegaly. Arq Bras Endocrinol Metabol 56(5):300–304
Rokkas T, Pistiolas D, Sechopoulos P, Margantinis G, Koukoulis G (2008) Risk of colorectal neoplasm in patients with acromegaly: a meta-analysis. World J Gastroenterol 14(22):3484–3489
Abreu A, Tovar AP, Castellanos R, Valenzuela A, Giraldo CM, Pinedo AC et al (2016) Challenges in the diagnosis and management of acromegaly: a focus on comorbidities. Pituitary 19(4):448–457
Holdaway IM, Bolland MJ, Gamble GD (2008) A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol 159(2):89–95
Melmed S (2009) Acromegaly pathogenesis and treatment. J Clin Invest 119(11):3189–3202
Mullis PE (2007) Genetics of growth hormone deficiency. Endocrinol Metab Clin N Am 36(1):17–36
Alatzoglou KS, Webb EA, Le Tissier P, Dattani MT (2014) Isolated growth hormone deficiency (GHD) in childhood and adolescence: recent advances. Endocr Rev 35(3):376–432
Laron Z, Blum W, Chatelain P, Ranke M, Rosenfeld R, Savage M et al (1993) Classification of growth hormone insensitivity syndrome. J Pediatr 122(2):241
Laron Z, Pertzelan A, Karp M (1962) Pituitary dwarfism with high serum levels of growth hormone. Isr J Med Sci 4(4):883–894
Shevah O, Laron Z (2007) Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Hormon IGF Res 17(1):54–57
Steuerman R, Shevah O, Laron Z (2011) Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol 164(4):485–489
Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW et al (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3(70):70ra13. https://doi.org/10.1126/scitranslmed.3001845
Laron Z, Kauli R (2016) Fifty seven years of follow-up of the Israeli cohort of Laron Syndrome patients-From discovery to treatment. Growth Hormon IGF Res 28:53–56
Agladioglu SY, Cetinkaya S, Savas Erdeve S, Onder A, Kendirci HN, Bas VN et al (2013) Diabetes mellitus with Laron syndrome: case report. J Pediatr Endocrinol Metab 26(9–10):955–958
Kopchick JJ, Bellush LL, Coschigano KT (1999) Transgenic models of growth hormone action. Annu Rev Nutr 19:437–461
Kopchick JJ, List EO, Kelder B, Gosney ES, Berryman DE (2014) Evaluation of growth hormone (GH) action in mice: discovery of GH receptor antagonists and clinical indications. Mol Cell Endocrinol 386(1–2):34–45
Miquet JG, Freund T, Martinez CS, Gonzalez L, Diaz ME, Micucci GP et al (2003) Hepatocellular alterations and dysregulation of oncogenic pathways in the liver of transgenic mice overexpressing growth hormone. Cell Cycle 12(7):1042–1057
Bartke A (2003) Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology 78(4):210–216
Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM et al (1996) Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384(6607):327–333
Snell GD (1929) Dwarf, a new mendelian recessive character of the house mouse. Proc Natl Acad Sci U S A 15(9):733–734
Bartke A (1965) Influence of luteotrophin on fertility of dwarf mice. J Reprod Fertil 10:93–103
Flurkey K, Papaconstantinou J, Miller RA, Harrison DE (2001) Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A 98(12):6736–6741
Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A (2003) Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci 58(4):291–296
Wiesenborn DS, Ayala JE, King E, Masternak MM (2014) Insulin sensitivity in long-living Ames dwarf mice. Age (Dordr) 36(5):9709
Heiman ML, Tinsley FC, Mattison JA, Hauck S, Bartke A (2003) Body composition of prolactin-, growth hormone, and thyrotropin-deficient Ames dwarf mice. Endocrine 20(1–2):149–154
Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M et al (1997) A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci U S A 94(24):13215–13220
Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ (2000) Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141(7):2608–2613
Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A (2001) Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav 72(5):653–660
Kinney-Forshee BA, Kinney NE, Steger RW, Bartke A (2004) Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol Behav 80(5):589–594
Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR et al (2009) Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci 64(5):522–529
Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, DiGirolamo DJ et al (2009) Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem 284(30):19937–19944
List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F et al (2014) Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology 155(5):1793–1805
Vijayakumar A, Wu Y, Buffin NJ, Li X, Sun H, Gordon RE et al (2012) Skeletal muscle growth hormone receptor signaling regulates basal, but not fasting-induced, lipid oxidation. PLoS One 7(9):e44777. https://doi.org/10.1371/journal.pone.0044777
Wu Y, Liu C, Sun H, Vijayakumar A, Giglou PR, Qiao R et al (2011) Growth hormone receptor regulates beta cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J Clin Invest 121(6):2422–2426
List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B et al (2013) The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol 27(3):524–535
Lee KY, Russell SJ, Ussar S, Boucher J, Vernochet C, Mori MA et al (2013) Lessons on conditional gene targeting in mouse adipose tissue. Diabetes 62(3):864–874
Krueger KC, Costa MJ, Du H, Feldman BJ (2014) Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Rep 3(6):1147–1158
List EO, Berryman DE, Buchman M, Parker C, Funk K, Bell S et al (2019) Adipocyte-specific gh receptor-null (AdGHRKO) mice have enhanced insulin sensitivity with reduced liver triglycerides. Endocrinology 160(1):68–80
Sun LY, Fang Y, Patki A, Koopman JJ, Allison DB, Hill CM et al (2017) Longevity is impacted by growth hormone action during early postnatal period. elife 6:pii: e24059. https://doi.org/10.7554/eLife.24059
Bartke A (2019) Growth hormone and aging: updated review. World J Mens Health 37(1):19–30
Masternak MM, Darcy J, Victoria B, Bartke A (2018) Dwarf mice and aging. Prog Mol Biol Transl Sci 155:69–83
Blackburn A, Schmitt A, Schmidt P, Wanke R, Hermanns W, Brem G et al (1997) Actions and interactions of growth hormone and insulin-like growth factor-II: body and organ growth of transgenic mice. Transgenic Res 6(3):213–222
Olsson B, Bohlooly YM, Fitzgerald SM, Frick F, Ljungberg A, Ahren B et al (2005) Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology 146(2):920–930
Palmer AJ, Chung MY, List EO, Walker J, Okada S, Kopchick JJ et al (2009) Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology 150(3):1353–1360
Katznelson L (2009) Alterations in body composition in acromegaly. Pituitary 12(2):136–142
Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ (2004) Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Hormon IGF Res 14(4):309–318
Berryman DE, List EO, Palmer AJ, Chung MY, Wright-Piekarski J, Lubbers E et al (2010) Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci 65(1):31–40
Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A (2006) Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci 61(6):562–567
Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ (2006) Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology 147(6):2801–2808
Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE et al (2014) Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY) 6(7):575–586
Reyes-Vidal CM, Mojahed H, Shen W, Jin Z, Arias-Mendoza F, Fernandez JC et al (2015) Adipose tissue redistribution and ectopic lipid deposition in active acromegaly and effects of surgical treatment. J Clin Endocrinol Metab 100(8):2946–2955
Wang Z, Al-Regaiey KA, Masternak MM, Bartke A (2006) Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci 61(4):323–331
Troike KM, Henry BE, Jensen EA, Young JA, List EO, Kopchick JJ et al (2017) Impact of growth hormone on regulation of adipose tissue. Compr Physiol 7(3):819–840
Menon V, Zhi X, Hossain T, Bartke A, Spong A, Gesing A et al (2014) The contribution of visceral fat to improved insulin signaling in Ames dwarf mice. Aging Cell 13(3):497–506
Masternak MM, Bartke A, Wang F, Spong A, Gesing A, Fang Y et al (2012) Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell 11(1):73–81
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM et al (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med 24(8):1246–1256
Li Y, Knapp JR, Kopchick JJ (2003) Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Exp Biol Med (Maywood) 228(2):207–215
Darcy J, McFadden S, Fang Y, Huber JA, Zhang C, Sun LY et al (2016) Brown adipose tissue function is enhanced in long-lived, male Ames dwarf mice. Endocrinology 157(12):4744–4753
Westbrook R, Bonkowski MS, Strader AD, Bartke A (2009) Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci 64(4):443–451
Darcy J, McFadden S, Fang Y, Berryman DE, List EO, Milcik N et al (2018) Increased environmental temperature normalizes energy metabolism outputs between normal and Ames dwarf mice. Aging (Albany NY) 10(10):2709–2722
Acknowledgements
We sincerely apologize to those whose work we did not reference due to space limitations or inadvertent omissions. This work was supported by the US National Institutes of Health (NIH) grants DK007260 (to JD), and AG019899 and AG051869 (to AB).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Darcy, J., Bartke, A. (2019). From White to Brown – Adipose Tissue Is Critical to the Extended Lifespan and Healthspan of Growth Hormone Mutant Mice. In: Guest, P. (eds) Reviews on Biomarker Studies in Aging and Anti-Aging Research. Advances in Experimental Medicine and Biology(), vol 1178. Springer, Cham. https://doi.org/10.1007/978-3-030-25650-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-25650-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25649-4
Online ISBN: 978-3-030-25650-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)