Abstract
Plasmablastic lymphoma (PBL) and primary effusion lymphoma (PEL) are rare CD20-negative variants of diffuse large B-cell lymphoma. Both conditions were initially described in patients with underlying HIV infection, but cases of PBL and PEL have been more recently diagnosed in other immunocompromised states and in immunocompetent individuals. Given the rarity of these lymphomas, the management is challenging due to a lack of treatment guidelines. In addition, the response and survival outcomes of patients with PBL and PEL are poor with standard treatment approaches. In this chapter, we will review the epidemiology, pathophysiology, clinical features, diagnostic evaluation, treatment, and outcomes of patients with PBL and PEL, as well as potential novel therapeutic options.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Plasmablastic Lymphoma
Introduction
Despite its original description almost 20 years ago [1], plasmablastic lymphoma (PBL) remains a clinical and pathological challenge to the hematologist, oncologist, and pathologist providing care for these complicated patients. PBL is a rare CD20-negative lymphoma with morphological, immunophenotypical, and genomic features intermediate between aggressive diffuse large B-cell lymphoma (DLBCL) and plasma cell neoplasms [2]. The tumor cells express CD138, CD38, and IRF4/MUM1 – plasma cell markers – and lack expression of typical B-cell markers such as CD19, CD20, or PAX5. Key molecular players in the pathogenesis of PBL are MYC gene rearrangements and EBV-encoded RNA (EBER) [3]. Historically, this tumor was reported in association with HIV infection. More recently, it has become evident that PBL can also arise in immunocompetent patients. As in other high-grade B-cell lymphomas, PBL has a MYC rearrangement in about 50% of cases. Although, it is not completely understood how EBV plays a role in the pathogenesis of PBL, in most cases tumor cells are infected with EBV, usually a latency type 1. EBV-encoded RNA (EBER) has been reported as high as 80% and 50% in HIV-positive and HIV-negative PBL, respectively [4]. Despite improvements in the understanding of the biology of the disease by clinicians and pathologists, PBL carries a poor outcome and prognosis. In this chapter our efforts are to focus on the latest research in the treatment of PBL and provide a comprehensive novel therapeutic approach.
An accurate incidence of PBL is yet to be determined by large epidemiological studies. Previous studies have reported an incidence of 2–10% of HIV-associated lymphoma cases and in less than 1% of all diffuse large B-cell lymphoma (DLBCL) cases [5,6,7,8]. PBL has also been identified in other immunosuppressive states such as posttransplantation, in immunocompetent individuals, in the elderly, and in the context of other lymphoproliferative disorders, plasma cell dyscrasias, or autoimmune disorders [3, 9, 10].
Although the median age at diagnosis is in the fifth or sixth decade of life, PBL cases have been described in pediatric and elderly patients [11,12,13]. HIV-associated PBL occurs predominantly in younger men (male-to-female ratio of 7:1) with advanced stage and predilection for the oral cavity [14]. HIV-negative PBL cases were older at presentation (57 years) with a male-to-female ratio of 1.7:1 and a lower frequency of advanced stage [9, 15]. This is consistent with other case series on HIV-negative PBL [16, 17].
Based on the initial seminal report, PBL affects mainly young men with HIV infection and involves the oral cavity. Several recent case series show that the oral cavity remains a common area of involvement in HIV-associated PBL . Most patients however present with extranodal involvement, regardless of their HIV status [15]. The gastrointestinal tract is the second most common site of involvement by PBL. In addition, there are multiple case reports in which PBL has been reported in the central nervous system (CNS), skin, paranasal sinus, soft tissue, mediastinum, lungs, heart, liver, breast, and testes. Bone marrow involvement has been reported in 10–30% of patients with PBL [10, 17,18,19,20,21,22,23]. B-symptoms have been reported in 40–60% of patients with PBL [8, 15]. In 50% of cases in multiple cohorts, both HIV-negative and HIV-positive PBL patients had presented with advanced stage (i.e., stage III or IV) [8, 10, 17, 18, 22].
Diagnosis and Evaluation
PBL is morphologically characterized by a monomorphic proliferation of round- to oval-shaped cells with plasmacytoid features. A perinuclear hof is frequently seen. The background infiltrate contains small mature lymphocytes and may include apoptotic bodies, mitotic figures, and tingible body macrophages, imparting a “starry-sky” appearance [2]. Immunophenotypically, PBL demonstrates little to no expression of leukocyte common antigen (CD45) or the B-cell markers CD20, CD79a, and PAX5. However, the plasma cell markers CD38, IRF4, BLIMP-1, and CD138 seem to be almost universally expressed [2, 24]. The proliferation marker Ki-67 is almost always expressed in neoplastic cells.
EBV infection has been associated with the development of PBL [3], and EBV-encoded RNA (EBER) is frequently detected in patients with PBL. Its detection by means of fluorescent or chromogenic in situ hybridization (ISH) has become the standard for evaluating the presence of EBV genome within tumor cells. Several studies have showed detection rates of EBER at 50% or higher in PBL [10, 14, 23, 25]. Molecular testing of MYC in PBL is of importance, as MYC rearrangement or amplification can be detected in a substantial number of patients with PBL [18, 26, 27]. The most common translocation gene occurring within MYC, at about 50–60% prevalence, is the immunoglobulin heavy chain gene, c-MYC/IGH fusion, t(8;14).
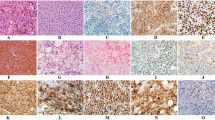
PBL should be differentiated from other CD20-negative DLBCL variants, specifically extracavitary PEL and ALK-positive DLBCL. MYC rearrangement could be helpful in distinguishing PBL from ALK-positive DLBCL, as the latter lacks MYC translocation. Also, PBL lacks rearrangements in BCL2 and BCL6, commonly seen in ALK-positive DLBCL. ALK-positive DLBCL is rarely associated with HIV, EBV, or HHV8 infections. Extracavitary forms of PEL can be difficult to differentiate from PBL. The identification of HHV8 genome in the malignant cells should suggest PEL rather than PBL in these cases. Differential features of PBL, PEL, and ALK-positive DLBCL are shown in Table 7.1. A representative case of PBL is shown in Fig. 7.1.
Immunophenotype of PBL. Immunohistochemistry with hematoxylin counterstain shows lack of expression of CD20 and CD10 and positive expression of IRF4/MUM1, Ki67, and MYC (magnification, ×400). Detection of EBER by in situ hybridization shows that neoplastic cells are positive; nuclear reactivity (×400)
The diagnosis of PBL should be established by obtaining adequate tissue for pathological evaluation. Although the staging of patients with PBL should mimic DLBCL, the data on the use of PET/CT scans are scarce. Extrapolating from other aggressive lymphomas , it follows that a PET/CT scan would be valuable at choosing a desired biopsy location, for staging and also for assessing response to therapy. For staging, current NCCN guidelines recommend PET/CT scans, bone marrow aspirate and biopsy, and a lumbar puncture [28].
Unfortunately, the prognosis of PBL continues being poor with most case series and population-based studies reporting median survival times ranging between 12 and 18 months [7, 8, 10, 18, 21, 22, 28]. Not surprisingly, patients with early stages (i.e., stage I or II) exhibit better outcomes. In addition, studies have found that patients with low or low-intermediate international prognosis index (IPI) scores had longer survival than patients with high or high-intermediate IPI scores [8, 18]. The IPI score, therefore, should be used for risk stratification and prognostic estimates in PBL patients. HIV-positive PBL patients who received antiretroviral therapy (ART) fared better when compared with patients in whom ART was not instituted or continued [17]. This has been replicated in a smaller Italian study [29]. It is possible that the induction of a virological and/or immunological response positively impacts the response and survival in HIV-infected patients. HIV-negative patients might have a worse outcome than HIV-infected individuals. This idea has been supported by case series as well as a large US population-based study [8, 15, 17]. The prognostic role of EBER expression is unclear. EBER expression was significantly associated with better OS in some studies, while no relation between EBER expression and survival was observed in others [10, 14, 15, 17, 22, 30]. The presence of MYC gene rearrangement appears consistently associated with a worse outcome in PBL [10, 18, 27].
Traditional Treatment Approach
There is not a standard treatment for PBL, and current treatment recommendations are mostly based on small case series, case reports, and experts’ opinion. Current NCCN guidelines emphasize that standard CHOP is inadequate therapy for PBL. Regimens with higher intensity such as EPOCH, CODOX-M/IVAC, and Hyper-CVAD are suggested with the use of autologous hematopoietic stem cell transplant for those with high risk and CR in first remission. However, two studies of patients with PBL treated with chemotherapy regimens more intensive than CHOP did not identify a survival benefit [18, 31]. In a large European study, higher-intensity regimens, like DA-EPOCH, ACVBP, and COPADM, did not show significantly higher CR rates compared with patients receiving CHOP therapy [17]. A recent pooled analysis suggested that infusional EPOCH might be more effective than standard CHOP [32]. In HIV-infected patients, initiation or optimization of HAART is highly recommended and should be directed by an infectious disease specialist. For these patients, appropriate antibiotic prophylaxis should be considered, especially with low CD4+ cell counts. For the small portion of PBL cases that express CD20 , rituximab should be considered in addition to chemotherapy given better outcomes seen in CD20-positive HIV-positive lymphomas treated with rituximab [33]. The use of G-CSF should be strongly considered in all patients with PBL undergoing chemotherapy. The use of CNS prophylaxis is debatable and should probably mimic recommendations for DLBCL. Radiotherapy should be used as consolidation in patients with early stage disease, similar to DLBCL, and can be considered in the palliative setting. NCCN recommends autologous SCT after achieving a first CR in a case-by-case basis. However, these recommendations come from a limited data source. After the introduction of ART, autologous SCT in HIV-positive patients with NHL has shown to be feasible [34, 35]. A small group of PBL patients was included in a recent prospective phase II multicenter trial conducted by the BMT CTN in collaboration with the AIDS Malignancy Consortium. The authors concluded that the outcomes between HIV-infected patients and controls were not significantly different, and HIV-infected patients should be considered candidates for autologous SCT if they met standard transplant criteria [36]. Both the European and American groups found in different analyses that autologous SCT might be beneficial both in the salvage setting and for consolidation after first responses [37, 38]. However, both analyses have limitations with the retrospective design, small study populations, and lack of consensus about pretransplant regimens used.
Novel Agents
Given the poor outcomes and survival of patients with PBL, novel agents have been evaluated in the treatment of PBL; however these strategies are in the context of small case reports and series. Given the plasmacytic differentiation of PBL cells, one of the agents evaluated was the proteasome inhibitor bortezomib. Bortezomib has been shown to be effective in patients with non-germinal center DLBCL, inducing higher responses and survival rates when used in combination with anthracycline-containing regimens [39]. Bortezomib alone and in combination with chemotherapy has been used with limited efficacy in HIV-positive and HIV-negative patients with relapsed PBL [40,41,42,43,44,45]. However, a case series of three previously untreated patients with PBL, two of them HIV-positive, showed efficacy with the combination of bortezomib and dose-adjusted EPOCH [46]. The experience was recently extended to two larger case series of 16 and 8 patients, respectively, in which high response rates and longer survival than expected were observed [47, 48]. In the study by Castillo and colleagues, a 5-year overall survival of 52% was reported. Likewise, a Spanish group reported three cases in which upfront bortezomib was added to CHOP [49]. Here, all three patients underwent autologous SCT at first CR. At the time of the report, two out of the three patients remained alive, one with a maximum follow-up of 22 months [50]. Moreover, in a systematic review of the use of upfront bortezomib-containing regimens in 19 patients with PBL, the study suggested an ORR of 74% as well as 3-year OS rate of approximately 60% [51].
The other novel agent of interest is the immunomodulator lenalidomide , which is largely used in the treatment of plasma cell neoplasms. The cell of origin in PBL is thought to be the plasmablast, an activated B-cell that has undergone somatic hypermutation and class switching recombination and is in the process of becoming a plasma cell. The pathogenesis of PBL is poorly understood, but inhibition of the NF-kB pathway seems to be important. This pathway might also play an important role in PBL as plasmablasts are closely related to activated B-cells, which often show NF-kB activation. Immunomodulating agents have multiple mechanisms of action, which include inhibition of angiogenesis and NF-kB downregulation, among others. Clinically, lenalidomide alone or in combination with chemotherapy has shown to induce responses in patients with PBL [40, 52,53,54,55].
Studies have shown that approximately 30% of PBL cases express the activation marker CD30 [19, 27, 56], and a recent report showed a rapid, but very short, response to brentuximab vedotin in a patient with CD30-positive relapsed PBL. Unfortunately, the patient developed complications and could not get further treatment and eventually passed away [57]. Recently, another case report showed a fast response to brentuximab vedotin in combination with lenalidomide in the fourth line setting, but the patient unfortunately passed away shortly after treatment was started [58].
Given the poor response in the frontline setting, PBL patients in the relapsed setting have very poor outcomes, short survival, and very limited options. Lenalidomide, bortezomib, and brentuximab vedotin have been used in the relapsed setting with limited success. Interestingly, one HIV-positive patient underwent autologous SCT after salvage therapy with daratumumab in combination with ifosfamide, carboplatin, and etoposide [47, 51]. That patient was alive after 2 years of follow-up.
Immune checkpoint inhibitors are of great interest in PBL. In the tumor microenvironment, PD-1 and its ligand PD-L1 perform a vital role in tumor progression and survival by escaping tumor neutralizing immune surveillance. A recent study found that PBL expresses PD-1/PD-L1 in the microenvironment and the malignant cells, particularly in EBV-positive PBL [59]. PD-L1 expression was positive in tumor cells in 22.5% of PBL cases showing a high PD-L1 score in 77% of cases compared to PD-1 which was expressed in tumor cells in 5% of PBL cases. These findings represent an important step to support further implementation of newer strategies with immunotherapy for patients with PBL who have very limited therapeutic options. Currently, a randomized phase 2 study of CDX-1127 (varlilumab) in combination with nivolumab in patients with relapsed and/or refractory aggressive B-cell lymphomas, including PBL, is ongoing (NCT03038672).
Several clinical trials that will include PBL are ongoing. DA-EPOCH-R regimen is being evaluated prospectively in untreated BL and c-MYC high-risk DLBCL patients, in which PBL is included (NCT01092182). A sequential phase I/II trial of vorinostat and chemotherapy with rituximab in HIV-related lymphoma including PBL is ongoing (NCT01193842). In the phase II portion of the trial, patients will be randomized to vorinostat plus R-DA-EPOCH or R-DA-EPOCH. In the phase I, the response rate in high-risk patients treated with vorinostat plus R-DA-EPOCH was 100% (complete 83% and partial 17%) with a 1-year event-free survival of 83% [60].
Gene therapy is another strategy being studied in HIV-associated lymphomas after frontline chemotherapy. Researchers are using peripheral blood stem cells treated with a lentivirus vector-encoding multiple anti-HIV RNAs targeted to the HIV-1 TAT/REV (SHL)-trans-active response element-chemokine cysteine-cysteine receptor 5 ribozyme-treated hematopoietic stem progenitor cells and then transferring this via SCT to patients with HIV-associated lymphoma (NCT01961063). Patients with PBL can be included in this study. The NCI group is using the same approach but with frontline R-EPOCH (NCT02337985).
An ongoing study is evaluating the therapeutic value of autologous EBV-specific CAR T-cells with CD30 as the main target (NCT01192464). Potentially, CAR T-cells can be directed against EBV antigens in patients with EBV-associated lymphomas including PBL, especially if they express CD30.
A group of reader proteins named bromodomain and extra-terminal (BET) domain has gained popularity as emerging anticancer strategy. In PBL, however it would make sense to target MYC given that >50% of patients with PBL would have MYC gene rearrangements. Yet, targeting MYC is cumbersome as it lacks a ligand-binding domain. Therefore, through epigenetics perhaps the transcriptional function of MYC can be modified. The BET family members (BRD2, BRD3, BRD4, and BRDT) comprise a class of epigenetic reader proteins, which bind acetylated lysine residues on histones to facilitate the recruitment of transcriptional elongation complexes [61]. MYC transcription depends on the assembly of these proteins. Therefore, small-molecule BET inhibitors have been proposed as a MYC pathway-targeted therapeutic. JQ1 is a small molecule inhibitor of BET, with the highest affinity for BRD4. BRD4 is a scaffolding factor that associates with acetylated chromatin to facilitate active transcription. JQ1 competitively interacts with BRD4, thus preventing BRD4 from binding to chromatin [62]. A study in DLBCL cells hypothesized that JQ1 treatment would result in decreased cell proliferation and viability in a MYC-dependent manner. The study showed that JQ1 efficiently inhibited proliferation of human DLBCL cells in a dose-dependent manner regardless of their molecular subtypes. The expression of MYC was suppressed by JQ1. Furthermore, JQ1 treatment significantly suppressed growth of DLBCL cells engrafted in mice and improved survival of engrafted mice [63]. Combining anti-PD-1 antibodies and JQ1 caused synergistic responses in mice bearing MYC-driven lymphomas [64]. Lastly, the BET inhibitor BAY 1238097 has shown strong antitumor efficacy in vivo as a single agent in two DLBCL models [65]. When DLBCL cells were treated with BAY 1238097, downregulation of EZH2 was observed. Interestingly, this led to a synergism between pharmacological inhibition of BET and EZH2, suggesting that this combination of epigenetic drugs is worth further preclinical and clinical investigation. Interestingly, BAY 1238097 decreased MYC signaling and downregulated target genes of MYC, NOTCH, and E2F, as well as members of the NF-kB/MYD88 and mTOR/AKT signaling pathways.
Recommended Treatment Approach
Our recommendation for first-line treatment of PBL is six cycles of infusional dose-adjusted EPOCH in combination with bortezomib and consideration of consolidative autologous SCT in first remission for appropriate candidates. In HIV-positive patients, ART should be started or optimized under the supervision of an infectious disease specialist with experience in the potential interactions between anticancer agents and ART. For the relapsed patient, treatment remains a challenge. Treatment with proteasome inhibitors, immunomodulators, and anti-CD30, anti-CD38, or anti-PD-1 monoclonal antibodies can be considered alone or in combination with chemotherapy. If a response is obtained, selected patients should be considered for autologous SCT.
Primary Effusion Lymphoma
Introduction
Primary effusion lymphoma (PEL) was first reported in 1989 [66]. In the initial report, a patient with a history of AIDS was diagnosed with a body cavity lymphoma of B-cell lineage that was lacking typical B-cell markers, such as CD20. In 1995, a larger case series showed seven HIV-infected individuals who presented with a malignant pleural effusion and in some cases concomitant Kaposi sarcoma (KS) [67]. DNA analysis found HHV-8 and EBV genome sequences in the neoplastic cells.
PEL is now considered a CD20-negative aggressive B-cell lymphoma and comprises 2–4% of all HIV-associated lymphomas and 0.5% of all DLBCL cases in the USA [8]. Besides association with HIV infection, PEL has also been reported in the setting of solid organ transplantation and elderly individuals. Patients with PEL can present with concurrent KS and multicentric Castleman disease. The median age at presentation is in the mid-50s [8]. However, HIV-negative cases tend to present at a later age. There is a male predominance, especially in HIV-infected individuals. There is also a higher proportion of Blacks and Hispanics among patients with PEL, when compared to DLBCL patients.
PEL typically manifests as a malignant pleural, pericardial, or peritoneal effusion without evidence of lymphadenopathy, masses, or tumors [68]. By definition, PEL is a stage IV disease. Due to the presence of effusions, patients can present with chest pain, shortness of breath, dyspnea of exertion, or abdominal distention. Constitutional symptoms are reported in about half of the patients at diagnosis. Pleural is the most commonly affected body cavity, followed by peritoneum and pericardium. Rarely, the scrotum can be affected [69]. Furthermore, there can be extracavitary PEL variants [70]. Extracavitary PEL is morphologically and genetically identical to classical PEL. The gastrointestinal tract is the commonly affected, but there have been cases of extracavitary PEL involving the skin, lungs, lymph nodes, and central nervous system.
Diagnosis and Evaluation
The diagnosis of PEL is made by demonstrating the presence of malignant lymphocytes in the affected tissue and confirming HHV-8 infection [68]. Fluid should be obtained from the effusion, which can then be prepared as cell block or cytospin. PEL cells are typically large with variable nuclear size and prominent nucleoli, and in some cases, they can resemble plasmablasts or immunoblasts. Immunophenotypic studies reveal a null lymphocyte phenotype with positive expression of CD45 but negative expression of typical B-cell markers (i.e., CD19, CD20, CD79a) or T-cell makers (i.e., CD3, CD4, CD8). PEL cells can express lymphocyte activation markers (i.e., CD30, CD71) as well as markers of plasmacytic differentiation (i.e., CD38, CD138, IRF4). The Ki67 index is typically high. In addition, concomitant HHV-8 and EBV infection is demonstrated by positive expression of HHV-8 latent nuclear antigen and EBV-encoded RNA, respectively. A representative case of PEL is shown on Fig. 7.2.
Pleural, peritoneal, and/or pericardial effusions are typically suspected by physical examination and confirmed by x-rays, computed tomography (CT) scans, or echocardiogram. It is important to evaluate all body cavities, as the number of body cavities involved might have prognostic implications [71]. Also, patients with peritoneal involvement or extracavitary PEL seem to have a worse prognosis than patients with pleural involvement [8, 71]. The prognosis of PEL remains poor. A recent US population-based study reported a median overall survival of 5 months [8]. Other case series have reported median OS ranging between 4 and 9 months. HIV, HBV, and HCV testing should be performed in all cases with PEL, and appropriate treatment for active viral infections should be started, as antiretroviral therapy (ART) seems to improve the survival of HIV-infected patients with PEL. HIV-infected PEL patients appear to have a better prognosis than HIV-negative patients [8].
Traditional Treatment Approach
The treatment for PEL is not standardized. Response rates to standard CHOP are approximately 40% with a median OS of 6 months. Other more intensive regimens such as ACVBP and dose-adjusted EPOCH have been reported in patients with PEL, but it is unclear if these regimens are associated with higher rates of response and/or survival than CHOP. Based on a recent patient-level meta-analysis showing survival benefits of EPOCH over CHOP in HIV-infected individuals with aggressive lymphoma [32], it is common practice to use EPOCH in patients with PEL. This is supported by current NCCN guidelines. To further support the use of chemotherapy in PEL, a population-based study from the USA showed that the median OS of HIV-positive PEL patients who receive chemotherapy is longer than in HIV-positive PEL patients who do not receive chemotherapy (0.7 vs. 0.4 years, respectively) [7]. However, the same study showed that only 60% of HIV-positive PEL patients actually receive chemotherapy when compared to 80–90% in HIV-positive patients with DLBCL or Burkitt lymphoma. G-CSF support should be provided to all patients with PEL. As there is negative expression of CD20, rituximab is typically not indicated; however, it can be considered in rare cases of CD20-positive PEL. ART should be instituted in HIV-infected individuals, and complete remissions have rarely been seen in PEL patients with ART alone [72, 73]. Autologous and allogeneic hematopoietic stem cell transplantation can be considered in the young and/or fit patient with relapsed and/or refractory PEL.
Novel Agents
Responses have been seen to antiviral therapy such as cidofovir, ganciclovir, or valganciclovir. Cidofovir was tested in vitro against two HHV8-positive PEL cell lines (i.e., BCBL-1 and HBL-6) [74]. Cidofovir inhibited dose-dependent cell proliferation and viability and induced apoptosis in both cell lines. Based on these results, intracavitary infusions of cidofovir were administered to three elderly patients with HHV8-positive, HIV-negative PEL. All patients tolerated cidofovir well and responded with resolution of the effusion as confirmed by x-rays or CT scans. Relapse, however, was observed in two patients. Another patient who failed two lines of chemotherapy had a durable response to intracavitary cidofovir lasting for 15 months [50]. Initial preclinical studies evaluating the role of the tumor microenvironment in PEL showed no effect of ganciclovir on cell growth in culture or in a xenograft model [75]. However, another study suggested that the combination of ganciclovir and valproate would promote lytic replication of HHV8 promoting tumor cell apoptosis without increasing viral load [76]. One case reported on the use of ganciclovir in a 31-year-old HIV-infected man with HHV8 and EBV-positive PEL [77]. Therapy consisted of ganciclovir and CHOP and induced a remission that was ongoing at 48 months after therapy. A randomized, double-blind placebo-controlled study evaluated the role of valganciclovir on suppression of HHV-8 replication and showed that valganciclovir administered daily reduced the frequency and quantity of HHV-8 replication [78]. The use of valganciclovir has also been reported in PEL patients with limited success [79], although it was successful at achieving a radiological response with clearance of HHV8 DNA after failure of bortezomib-containing therapy [80].
Preclinical data support a constitutive activation of the NF-kB pathway in PEL cells [81], suggesting that proteasome inhibitors such as bortezomib can be effective in PEL patients. Specifically, PEL cells treated with an inhibitor of IkB-alpha downregulated IL6, inducing apoptosis of PEL cells. Furthermore, a direct xenograft murine PEL model was developed, and exposure to bortezomib induced remission of PEL and prolonged the survival of NOD/SCID mice bearing PEL [82]. Transcriptome analysis revealed that bortezomib downregulated DNA replication and MYC target genes. However, the preclinical activity of bortezomib has not translated into clinical efficacy [83].
As the cell of origin on PEL is theorized to be a B-cell with plasmacytic differentiation, anti-myeloma agents such as immunomodulatory drugs (IMIDs) have been studied preclinically with evidence of efficacy against PEL cell lines [84]. In this study, clinically achievable levels of IMIDs induced an antiproliferative effect against a majority of PEL cell lines exposed and suggested that the anti-PEL effect of IMIDs involved cereblon-dependent suppression of IRF4. Clinically, there have been a few case reports suggesting activity of lenalidomide in patients with PEL. A 77-year-old man, who was felt not to be a good candidate for chemotherapy, was treated with lenalidomide 25 mg/day and experienced a decrease in his pleural effusion and tolerated lenalidomide for 18 months until the time of the report [85]. Another 80-year-old male patient obtained a complete radiologic response within 6 months of therapy with lenalidomide at a dose of 15 mg/day [86].
CD30 is frequently expressed in PEL cells [71, 87]. Targeting of PEL cells with the anti-CD30 conjugated monoclonal antibody brentuximab vedotin improved the survival of a xenograft mouse model by inhibiting proliferation and causing arrest in the G2/M cell cycle phase [88]. Similarly, CD38 is almost universally expressed in PEL [71]. However, the role of CD38 expression or inhibition in PEL cells has not been evaluated in PEL either preclinically or clinically. Targeting CD38 is of interest given the number of anti-CD38 monoclonal antibodies under development, specifically daratumumab, which is already approved by the FDA for the treatment of patients with multiple myeloma.
PEL cells have a deregulated MYC protein likely due to the activity of HHV-8-encoded latent proteins. Although MYC is considered “untargetable,” genes associated with regulation of MYC can be targeted. Specifically, bromodomain and extra-terminal (BET) bromodomain inhibitors have shown activity against PEL cells [89]. Treatment of PEL cells with BET inhibitors suppressed expression of MYC and dysregulated MYC-dependent genes inhibiting cell growth and inducing cell cycle arrest, apoptosis, and cellular senescence. In a xenograft murine model, the BET inhibitor JQ1 reduced tumor burden and improved survival of PEL-bearing mice. Furthermore, the combination of BET inhibitors and IMIDs might be synergistic against PEL cells, and the combination of JQ1 and lenalidomide increased the survival of PEL-bearing NOD/SCID mice when compared with either agent alone [84].
Several other pathways have been evaluated in preclinical cell and/or animal models. Increased PD-L1 expression was found in HHV8-associated PEL cells and also in tumor-infiltrating macrophages [90]. These findings suggest that immunotherapeutic agents with activity against the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, or atezolizumab, can be of interest for clinical development in PEL. HSP90 inhibitors have shown preclinically to be active against PEL cells [91]. Specifically, HSP90 inhibition leads to the degradation of vFLIP and IKK-gamma, as well as NF-kB downregulation, which promotes apoptosis and autophagy. Interestingly, there was synergy when a BCL2 inhibitor was added to the HSP90 inhibitor. These findings suggest the potential clinical application of HSP90 inhibitors such as tanespimycin in combination with BCL2 inhibitors such as venetoclax in PEL. IRAK1 mutations were present in virtually 100% of the cases evaluated in a preclinical study and were associated with cell survival [92]. IRAK1, along with MYD88, mediates toll-like receptor signaling. IRAK1 inhibitors are undergoing clinical development for B-cell lymphomas and could be effective in PEL. Another study reported that the hepatocyte growth factor/c-MET pathway was highly activated by HHV8. A c-MET inhibitor was able to induce cell cycle arrest and cause DNA damage, which resulted in PEL cell apoptosis and suppressed tumor progression in a xenograft murine model [93]. Targeting of the glycolytic phenotype of PEL cells by PI3K, Akt, and mTOR inhibitors showed increased cytotoxicity against PEL cells [94]. Inhibitors of the PI3K/Akt/mTOR pathway reduce lactate production and could shift cell metabolism from aerobic glycolysis toward oxidative respiration. Cytotoxic synergy was observed when combining PI3K/Akt/mTOR inhibitors with a glycolysis inhibitor. A recent study identified MALT1 as one of the main mediators of NF-kB activation in PEL cells [95]. MALT1 inhibition induced a switch from latent to lytic stages of viral infection and impacted growth and survival of PEL cells in a xenograft model.
Recommended Treatment Approach
In patients with PEL and HIV infection, we initiate or modify ART as spontaneous remission has rarely been seen with ART alone. We also recommend draining any effusions for symptomatic comfort as frequently as needed. With regard to therapy, our typical frontline approach for PEL is to use infusional EPOCH. Given the encouraging results with the addition of bortezomib to infusional EPOCH in PBL, we feel that V-EPOCH is reasonable in PEL patients as well. Daratumumab or lenalidomide in combination with chemotherapy followed by autologous SCT can be considered in selected relapsed patients.
References
Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89(4):1413–20.
Stein H, Harris N, Campo E. Plasmablastic lymphoma. In: Swerdlow S, et al., editors. WHO classification of tumours of the haematopoietic and lymphoid tissues. Lyon: IARC; 2008. p. 256–7.
Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125(15):2323–30.
Castillo JJ, Chavez JC, Hernandez-Ilizaliturri FJ, Montes-Moreno S. CD20-negative diffuse large B-cell lymphomas: biology and emerging therapeutic options. Expert Rev Hematol. 2015;8(3):343–54.
Carbone A. AIDS-related non-Hodgkin’s lymphomas: from pathology and molecular pathogenesis to treatment. Hum Pathol. 2002;33(4):392–404.
Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187–94.
Olszewski AJ, Fallah J, Castillo JJ. Human immunodeficiency virus-associated lymphomas in the antiretroviral therapy era: analysis of the National Cancer Data Base. Cancer. 2016;122(17):2689–97.
Qunaj L, Castillo JJ, Olszewski AJ. Survival of patients with CD20-negative variants of large B-cell lymphoma: an analysis of the National Cancer Data Base. Leuk Lymphoma. 2017;59:1–9.
Castillo JJ, Winer ES, Stachurski D, et al. HIV-negative plasmablastic lymphoma: not in the mouth. Clin Lymphoma Myeloma Leuk. 2011;11(2):185–9.
Morscio J, Dierickx D, Nijs J, et al. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases. Am J Surg Pathol. 2014;38(7):875–86.
Pather S, MacKinnon D, Padayachee RS. Plasmablastic lymphoma in pediatric patients: clinicopathologic study of three cases. Ann Diagn Pathol. 2013;17(1):80–4.
Vaubell JI, Sing Y, Ramburan A, et al. Pediatric plasmablastic lymphoma: a clinicopathologic study. Int J Surg Pathol. 2014;22(7):607–16.
Liu F, Asano N, Tatematsu A, et al. Plasmablastic lymphoma of the elderly: a clinicopathological comparison with age-related Epstein-Barr virus-associated B cell lymphoproliferative disorder. Histopathology. 2012;61(6):1183–97.
Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83(10):804–9.
Castillo JJ, Winer ES, Stachurski D, et al. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk Lymphoma. 2010;51(11):2047–53.
Liu M, Liu B, Liu B, et al. Human immunodeficiency virus-negative plasmablastic lymphoma: a comprehensive analysis of 114 cases. Oncol Rep. 2015;33(4):1615–20.
Tchernonog E, Faurie P, Coppo P, et al. Clinical characteristics and prognostic factors of plasmablastic lymphoma patients: analysis of 135 patients from the LYSA group. Ann Oncol. 2017;28(4):843–8.
Castillo JJ, Furman M, Beltran BE, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy. Cancer. 2012;118(21):5270–7.
Colomo L, Loong F, Rives S, et al. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol. 2004;28(6):736–47.
Liu JJ, Zhang L, Ayala E, et al. Human immunodeficiency virus (HIV)-negative plasmablastic lymphoma: a single institutional experience and literature review. Leuk Res. 2011;35(12):1571–7.
Montes-Moreno S, Gonzalez-Medina AR, Rodriguez-Pinilla SM, et al. Aggressive large B-cell lymphoma with plasma cell differentiation: immunohistochemical characterization of plasmablastic lymphoma and diffuse large B-cell lymphoma with partial plasmablastic phenotype. Haematologica. 2010;95(8):1342–9.
Schommers P, Wyen C, Hentrich M, et al. Poor outcome of HIV-infected patients with plasmablastic lymphoma: results from the German AIDS-related lymphoma cohort study. AIDS. 2013;27(5):842–5.
Teruya-Feldstein J, Chiao E, Filippa DA, et al. CD20-negative large-cell lymphoma with plasmablastic features: a clinically heterogenous spectrum in both HIV-positive and -negative patients. Ann Oncol. 2004;15(11):1673–9.
Vega F, Chang CC, Medeiros LJ, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol. 2005;18(6):806–15.
Kim JE, Kim YA, Kim WY, et al. Human immunodeficiency virus-negative plasmablastic lymphoma in Korea. Leuk Lymphoma. 2009;50(4):582–7.
Bogusz AM, Seegmiller AC, Garcia R, et al. Plasmablastic lymphomas with MYC/IgH rearrangement: report of three cases and review of the literature. Am J Clin Pathol. 2009;132(4):597–605.
Valera A, Balague O, Colomo L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol. 2010;34(11):1686–94.
NCCN Guidelines Version 1.2018. AIDS-related B-cell lymphomas. AIDS-2. Available at http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Accessed 2 Feb 2018.
Cattaneo C, Re A, Ungari M, et al. Plasmablastic lymphoma among human immunodeficiency virus-positive patients: results of a single center’s experience. Leuk Lymphoma. 2015;56(1):267–9.
Loghavi S, Alayed K, Aladily TN, et al. Stage, age, and EBV status impact outcomes of plasmablastic lymphoma patients: a clinicopathologic analysis of 61 patients. J Hematol Oncol. 2015;8:65.
Castillo JJ, Winer ES, Stachurski D, et al. Prognostic factors in chemotherapy-treated patients with HIV-associated plasmablastic lymphoma. Oncologist. 2010;15(3):293–9.
Barta SK, Lee JY, Kaplan LD, Noy A, Sparano JA. Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma. Cancer. 2012;118(16):3977–83.
Barta SK, Xue X, Wang D, et al. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood. 2013;122(19):3251–62.
Krishnan A, Molina A, Zaia J, et al. Autologous stem cell transplantation for HIV-associated lymphoma. Blood. 2001;98(13):3857–9.
Re A, Cattaneo C, Michieli M, et al. High-dose therapy and autologous peripheral-blood stem-cell transplantation as salvage treatment for HIV-associated lymphoma in patients receiving highly active antiretroviral therapy. J Clin Oncol. 2003;21(23):4423–7.
Alvarnas JC, Le Rademacher J, Wang Y, et al. Autologous hematopoietic cell transplantation for HIV-related lymphoma: results of the BMT CTN 0803/AMC 071 trial. Blood. 2016;128(8):1050–8.
Al-Malki MM, Castillo JJ, Sloan JM, Re A. Hematopoietic cell transplantation for plasmablastic lymphoma: a review. Biol Blood Marrow Transplant. 2014;20(12):1877–84.
Cattaneo C, Finel H, McQuaker G, et al. Autologous hematopoietic stem cell transplantation for plasmablastic lymphoma: the European Society for Blood and Marrow Transplantation experience. Biol Blood Marrow Transplant. 2015;21(6):1146–7.
Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113(24):6069–76.
Bibas M, Grisetti S, Alba L, et al. Patient with HIV-associated plasmablastic lymphoma responding to bortezomib alone and in combination with dexamethasone, gemcitabine, oxaliplatin, cytarabine, and pegfilgrastim chemotherapy and lenalidomide alone. J Clin Oncol. 2010;28(34):e704–8.
Bose P, Thompson C, Gandhi D, Ghabach B, Ozer H. AIDS-related plasmablastic lymphoma with dramatic, early response to bortezomib. Eur J Haematol. 2009;82(6):490–2.
Dasanu CA, Bauer F, Codreanu I, Padmanabhan P, Rampurwala M. Plasmablastic haemato-lymphoid neoplasm with a complex genetic signature of Burkitt lymphoma responding to bortezomib. Hematol Oncol. 2013;31(3):164–6.
Hirosawa M, Morimoto H, Shibuya R, Shimajiri S, Tsukada J. A striking response of plasmablastic lymphoma of the oral cavity to bortezomib: a case report. Biomark Res. 2015;3:28.
Saba NS, Dang D, Saba J, et al. Bortezomib in plasmablastic lymphoma: a case report and review of the literature. Onkologie. 2013;36(5):287–91.
Yan M, Dong Z, Zhao F, et al. CD20-positive plasmablastic lymphoma with excellent response to bortezomib combined with rituximab. Eur J Haematol. 2014;93(1):77–80.
Castillo JJ, Reagan JL, Sikov WM, Winer ES. Bortezomib in combination with infusional dose-adjusted EPOCH for the treatment of plasmablastic lymphoma. Br J Haematol. 2015;169(3):352–5.
Castillo JJ, Guerrero-Garcia T, Baldini F, et al. Bortezomib plus EPOCH is effective as frontline treatment in patients with plasmablastic lymphoma. Br J Haematol. 2018;59:1730–3.
Dittus C, Grover N, Ellsworth S, Tan X, Park SI. Bortezomib in combination with dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) induces long-term survival in patients with plasmablastic lymphoma: a retrospective analysis. Leuk Lymphoma. 2018;59:1–7.
Fernandez-Alvarez R, Gonzalez-Rodriguez AP, Rubio-Castro A, et al. Bortezomib plus CHOP for the treatment of HIV-associated plasmablastic lymphoma: clinical experience in three patients. Leuk Lymphoma. 2016;57(2):463–6.
Halfdanarson TR, Markovic SN, Kalokhe U, Luppi M. A non-chemotherapy treatment of a primary effusion lymphoma: durable remission after intracavitary cidofovir in HIV negative PEL refractory to chemotherapy. Ann Oncol. 2006;17(12):1849–50.
Guerrero-Garcia TA, Mogollon RJ, Castillo JJ. Bortezomib in plasmablastic lymphoma: a glimpse of hope for a hard-to-treat disease. Leuk Res. 2017;62:12–6.
Carras S, Regny C, Peoc’h M, et al. Dramatic efficacy of low dose lenalidomide as single agent in a patient with refractory gastric non-human immunodeficiency virus-associated plasmablastic lymphoma. Leuk Lymphoma. 2015;56(10):2986–8.
Schmit JM, DeLaune J, Norkin M, Grosbach A. A case of plasmablastic lymphoma achieving complete response and durable remission after lenalidomide-based therapy. Oncol Res Treat. 2017;40(1–2):46–8.
Sher T, Miller KC, Lee K, Chanan-Khan A. Remission induction with lenalidomide alone in a patient with previously untreated plasmablastic myeloma: a case report. Clin Lymphoma Myeloma. 2009;9(4):328–30.
Yanamandra U, Sahu KK, Jain N, et al. Plasmablastic lymphoma: successful management with CHOP and lenalidomide in resource constraint settings. Ann Hematol. 2016;95(10):1715–7.
Folk GS, Abbondanzo SL, Childers EL, Foss RD. Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn Pathol. 2006;10(1):8–12.
Holderness BM, Malhotra S, Levy NB, Danilov AV. Brentuximab vedotin demonstrates activity in a patient with plasmablastic lymphoma arising from a background of chronic lymphocytic leukemia. J Clin Oncol. 2013;31(12):e197–9.
Pretscher D, Kalisch A, Wilhelm M, Birkmann J. Refractory plasmablastic lymphoma-a review of treatment options beyond standard therapy. Ann Hematol. 2017;96(6):967–70.
Laurent C, Fabiani B, Do C, et al. Immune-checkpoint expression in Epstein-Barr virus positive and negative plasmablastic lymphoma: a clinical and pathological study in 82 patients. Haematologica. 2016;101(8):976–84.
Ramos JC, Sparano JA, Rudek MA, et al. Safety and preliminary efficacy of vorinostat with R-EPOCH in high-risk HIV-associated non-Hodgkin’s lymphoma (AMC-075). Clin Lymphoma Myeloma Leuk. 2018;18(3):180–190 e2.
Hogg SJ, Newbold A, Vervoort SJ, et al. BET inhibition induces apoptosis in aggressive B-cell lymphoma via epigenetic regulation of BCL-2 family members. Mol Cancer Ther. 2016;15(9):2030–41.
Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–73.
Trabucco SE, Gerstein RM, Evens AM, et al. Inhibition of bromodomain proteins for the treatment of human diffuse large B-cell lymphoma. Clin Cancer Res. 2015;21(1):113–22.
Hogg SJ, Vervoort SJ, Deswal S, et al. BET-bromodomain inhibitors engage the host immune system and regulate expression of the immune checkpoint ligand PD-L1. Cell Rep. 2017;18(9):2162–74.
Bernasconi E, Gaudio E, Lejeune P, et al. Preclinical evaluation of the BET bromodomain inhibitor BAY 1238097 for the treatment of lymphoma. Br J Haematol. 2017;178(6):936–48.
Knowles DM, Inghirami G, Ubriaco A, Dalla-Favera R. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73(3):792–9.
Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186–91.
Said J, Cesarman E. Primary effusion lymphoma. In: Swerdlow S, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. p. 260–1.
Nakamura Y, Tajima F, Omura H, et al. Primary effusion lymphoma of the left scrotum. Intern Med. 2003;42(4):351–3.
Pan ZG, Zhang QY, Lu ZB, et al. Extracavitary KSHV-associated large B-cell lymphoma: a distinct entity or a subtype of primary effusion lymphoma? Study of 9 cases and review of an additional 43 cases. Am J Surg Pathol. 2012;36(8):1129–40.
Castillo JJ, Shum H, Lahijani M, Winer ES, Butera JN. Prognosis in primary effusion lymphoma is associated with the number of body cavities involved. Leuk Lymphoma. 2012;53(12):2378–82.
Bower M, Newsom-Davis T, Naresh K, et al. Clinical features and outcome in HIV-associated multicentric Castleman’s disease. J Clin Oncol. 2011;29(18):2481–6.
Ripamonti D, Marini B, Rambaldi A, Suter F. Treatment of primary effusion lymphoma with highly active antiviral therapy in the setting of HIV infection. AIDS. 2008;22(10):1236–7.
Luppi M, Trovato R, Barozzi P, et al. Treatment of herpesvirus associated primary effusion lymphoma with intracavity cidofovir. Leukemia. 2005;19(3):473–6.
Staudt MR, Kanan Y, Jeong JH, et al. The tumor microenvironment controls primary effusion lymphoma growth in vivo. Cancer Res. 2004;64(14):4790–9.
Klass CM, Krug LT, Pozharskaya VP, Offermann MK. The targeting of primary effusion lymphoma cells for apoptosis by inducing lytic replication of human herpesvirus 8 while blocking virus production. Blood. 2005;105(10):4028–34.
Pereira R, Carvalho J, Patricio C, Farinha P. Sustained complete remission of primary effusion lymphoma with adjunctive ganciclovir treatment in an HIV-positive patient. BMJ Case Rep. 2014;2014:pii: bcr2014204533.
Casper C, Krantz EM, Corey L, et al. Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial. J Infect Dis. 2008;198(1):23–30.
Ozbalak M, Tokatli I, Ozdemirli M, et al. Is valganciclovir really effective in primary effusion lymphoma: case report of an HIV(−) EBV(−) HHV8(+) patient. Eur J Haematol. 2013;91(5):467–9.
Marquet J, Velazquez-Kennedy K, Lopez S, et al. Case report of a primary effusion lymphoma successfully treated with oral valganciclovir after failing chemotherapy. Hematol Oncol. 2018;36(1):316–9.
Keller SA, Schattner EJ, Cesarman E. Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood. 2000;96(7):2537–42.
Sarosiek KA, Cavallin LE, Bhatt S, et al. Efficacy of bortezomib in a direct xenograft model of primary effusion lymphoma. Proc Natl Acad Sci U S A. 2010;107(29):13069–74.
Boulanger E, Meignin V, Oksenhendler E. Bortezomib (PS-341) in patients with human herpesvirus 8-associated primary effusion lymphoma. Br J Haematol. 2008;141(4):559–61.
Gopalakrishnan R, Matta H, Tolani B, Triche T Jr, Chaudhary PM. Immunomodulatory drugs target IKZF1-IRF4-MYC axis in primary effusion lymphoma in a cereblon-dependent manner and display synergistic cytotoxicity with BRD4 inhibitors. Oncogene. 2016;35(14):1797–810.
Antar A, El Hajj H, Jabbour M, et al. Primary effusion lymphoma in an elderly patient effectively treated by lenalidomide: case report and review of literature. Blood Cancer J. 2014;4:e190.
Chan TSY, Mak V, Kwong YL. Complete radiologic and molecular response of HIV-negative primary effusion lymphoma with short-course lenalidomide. Ann Hematol. 2017;96(7):1211–3.
Michai M, Goto H, Hattori S, et al. Soluble CD30: a possible serum tumor marker for primary effusion lymphoma. Asian Pac J Cancer Prev. 2012;13(10):4939–41.
Bhatt S, Ashlock BM, Natkunam Y, et al. CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood. 2013;122(7):1233–42.
Tolani B, Gopalakrishnan R, Punj V, Matta H, Chaudhary PM. Targeting Myc in KSHV-associated primary effusion lymphoma with BET bromodomain inhibitors. Oncogene. 2014;33(22):2928–37.
Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–73.
Nayar U, Lu P, Goldstein RL, et al. Targeting the Hsp90-associated viral oncoproteome in gammaherpesvirus-associated malignancies. Blood. 2013;122(16):2837–47.
Yang D, Chen W, Xiong J, et al. Interleukin 1 receptor-associated kinase 1 (IRAK1) mutation is a common, essential driver for Kaposi sarcoma herpesvirus lymphoma. Proc Natl Acad Sci U S A. 2014;111(44):E4762–8.
Dai L, Trillo-Tinoco J, Cao Y, et al. Targeting HGF/c-MET induces cell cycle arrest, DNA damage, and apoptosis for primary effusion lymphoma. Blood. 2015;126(26):2821–31.
Mediani L, Gibellini F, Bertacchini J, et al. Reversal of the glycolytic phenotype of primary effusion lymphoma cells by combined targeting of cellular metabolism and PI3K/Akt/ mTOR signaling. Oncotarget. 2016;7(5):5521–37.
Bonsignore L, Passelli K, Pelzer C, et al. A role for MALT1 activity in Kaposi’s sarcoma-associated herpes virus latency and growth of primary effusion lymphoma. Leukemia. 2017;31(3):614–24.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Guerrero-Garcia, T.A., Castillo, J.J. (2020). Plasmablastic Lymphoma and Primary Effusion Lymphoma. In: Dittus, C. (eds) Novel Therapeutics for Rare Lymphomas. Springer, Cham. https://doi.org/10.1007/978-3-030-25610-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-25610-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25609-8
Online ISBN: 978-3-030-25610-4
eBook Packages: MedicineMedicine (R0)