Abstract
For a long time, the diagnosis of coronary diseases was exclusively focused on vessel lumen narrowing. Advances in the knowledge of coronary artery diseases have progressively raised awareness of changes in the vessel wall. The study of the vessel wall allows the identification of changes in early stages of diseases as well as signs of plaque vulnerability or increased risk for complications.
The techniques used to study the wall of the coronary arteries are divided into invasive and noninvasive. Intravascular ultrasound is currently widely used. It provides very good quality images of all coronary wall layers and allows the identification of plaque components, extension, and volume but mainly plaque vulnerability characteristics. Optical coherence tomography is considered by many to be the gold standard for analysis of the coronary artery wall. Its great advantage in relation to intravascular ultrasound is the excellent spatial resolution that allows the detailed analysis of the intima layer, determining its thickness and integrity and contributing significantly to defining patients at greater risk. Both of these techniques are invasive.
Magnetic resonance imaging allows safe and noninvasive coronary wall study. It assesses the thickness of the coronary wall, positive remodeling, and plaque burden in addition to other signs of plaque vulnerability such as high-intensity plaques and contrast uptake in delay enhancement techniques. Coronary artery tomography allows analysis of high-risk features such as positive remodeling, plaque component, plaque burden, and signs of plaque vulnerability. The noninvasive nature of the CT scan has increased its use in risk stratification of patients with suspected subclinical atherosclerotic disease.
In this chapter we will discuss the main characteristics of these methods of evaluation of coronary artery walls, pointing out their clinical utilities, advantages, and limitations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Coronary wall
- Plaque vulnerability
- High-risk plaque
- Intravascular ultrasound
- Optical coherence tomography
- Magnetic resonance imaging
- Coronary computed tomography angiography
Introduction
For many decades, coronary angiography was the gold standard for the study of coronary artery diseases. Angiography allows a two-dimensional assessment of the coronary lumen, a vessel silhouette. Important components of the atherosclerotic process in the coronary vessel wall are not evaluated by angiography, such as positive remodeling, plaque composition, plaque extension, or diffuse atherosclerosis. Currently, evaluating the cardiovascular risk based on diagnosing coronary stenosis by angiography is not enough for both the diagnosis and the therapeutic definition in coronary artery disease (CAD). The new paradigm is evaluating the coronary artery wall. We know that acute coronary events are caused mainly by plaques with small degrees of stenosis, but with vulnerability characteristics that are hardly diagnosed by angiography.
Several diagnostic methods have been used to evaluate the characteristics of arterial walls in order to identify early stages of CAD and features that are able to predict plaque vulnerability. Many characteristics are evaluated in coronary artery wall. Some of the most clinically valuable characteristics are plaque burden, plaque volume, plaque extension, plaque high-risk features and contents, and signs of inflammation. Currently, there is no diagnostic method capable to accurately evaluate all these characteristics at the same time. Therefore, the combination of methods to achieve a complete diagnosis is necessary. Based on the clinical history, a reasonable approach is to choose a method that provides the best answer to the clinical decision-making. In this chapter, we will cover clinically available techniques for evaluating the coronary artery wall, its related diseases, and limitations of each technique.
Invasive Diagnostic Methods to Assess Coronary Wall
In order to better evaluate the coronary atherosclerosis, new diagnostic techniques such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT) arose to complement the angiography exams. Both techniques corroborate and enrich coronary lumen assessment providing additional and valuable information regarding the coronary wall.
Intravascular Ultrasound
The first reports about the use of IVUS appeared in the end of the 1980s. Since then, its use has become increasingly commonplace and technological advances made it increasingly clinically useful. Initially, IVUS exams used gray-scale technique to assess the coronary wall characteristics. This technique had several limitations which compromised its diagnostic accuracy. Current acquisition and processing technologies such as the backscattered ultrasound waves using radio frequency allow accurate analysis of coronary wall. IVUS enables plaque composition discrimination (necrotic core, fibrous tissue, fibrofatty tissue, and dense calcium) as well as determining characteristics of high-risk plaques [1,2,3]. These diagnostic abilities allow us to use IVUS in monitoring the evolution of atherosclerosis over time and therapeutic response manifested by regression in plaque volume [4, 5].
An important clinical use of IVUS is diagnosing vulnerable plaques. Some plaque vulnerability characteristics assessed by IVUS are the thin-cap fibroatheroma (TCFA), positive remodeling, large plaque burden, and small luminal area. The PROSPECT study showed that in patients with acute coronary syndrome (ACS) plaques with vulnerability signs had increased risk of major acute coronary events (MACE) even in non-culprit lesions [6].
Another quite common clinical use of IVUS is during percutaneous coronary intervention (PCI). IVUS findings help before, during, and after procedure completion. IVUS can be used to choose the most appropriate technique and materials to be used during the PCI. It helps to confirm angiography findings and accurately reports the degree of coronary stenosis, plaque extension, plaque components, coronary ostium involvement, and vessel size. After the procedure, IVUS evaluates minimal lumen area, stent malapposition, stent edge, dissection, and tissue prolapse that may be determinant of future complications such as restenosis and stent thrombosis [2]. Metanalyses focused on comparing IVUS-guided PCI with procedures guided exclusively by angiography showed better immediate and late angiographic results (greater post-minimal lumen diameter, lower restenosis, and revascularization requirement) as well as reduction in MACE [7, 8]. Currently, there is an increasing use of IVUS during PCI, especially when implanting drug eluting stents, where the risks of thrombosis and restenosis are significantly higher if there is stent malapposition. The IVUS-guided PCI is recommended for cases with increased complexity (complex lesions, chronic occlusion, bifurcations with ostium involvement, extensive plaques, or left main artery involvement) or to assess stent failure [9].
Limitations
Despite its excellent diagnostic capacity, IVUS usage to cardiovascular evaluation in general population is restricted for its invasive nature. IVUS exams must be performed in a hemodynamic laboratory, with a trained team and executed by a highly experienced hemodynamicist. All exams use iodinated contrast and need ionizing radiation to precisely locate the target vessel or lesion to be studied. Additionally, longer examination time and extra materials increase the costs, limiting its use in the cardiologic diagnostic practice.
Concerning image quality, IVUS has some limitations evaluating plaques with a great amount of calcification, impairing the visualization behind calcium. Additionally, some plaque structures may exhibit very similar echogenicity characteristics making it impossible to differentiate them, for example, thrombi and lipid component [2]. Finally, IVUS resolution is insufficient to measure the thickness of the fibrous cap; consequently TCFA is defined based on the identification of the necrotic core compressing the lumen.
Optical Coherence Tomography
Usually OCT is considered an alternative or complement to IVUS. Its great advantage comparing to IVUS is its spatial resolution (10–15 mc vs 100–150 mcm). The excellent resolution allows OCT to evaluate the atheroma layer in detail, being the only method capable of measuring in vivo the thickness of the layer and objectively defining TCFA (<65 mcm). Additionally, OCT allows the evaluation of the integrity of the cap, classifying culprit lesions as disrupted fibrous cap (with lipid predominance or calcified nodule) and intact fibrous cap [10]. In patients with ACS, those whose culprit lesion has uninterrupted fibrous cap instead of disrupted have a better prognosis over time, with a lower incidence of death, heart attack, and hospitalization [11].
There are several plaque vulnerability features besides TCFA which can be evaluated accurately by OCT such as plaque composition, macrophages wall infiltrate, microcalcifications, neovascularization, and thrombus. All those features are usual findings in patients with ACS [12,13,14].
Like IVUS, OCT has been used in planning, guiding, and following PCI. As well as IVUS-guided procedures, using OCT showed to reduce the risk of cardiac death or acute myocardial infarction compared to the angiography-guided procedures [15]. Its excellent spatial resolution allows an excellent plaque characterization, identifying stent malapposition, stent edge, dissection, tissue prolapse, and thrombus [15, 16]. However, the assessment of lumen size after the procedure (minimal lumen area) measured by OCT is significantly lower than that measured by IVUS. This may result in a suboptimal stent expansion in OCT-guided procedures, increasing the chances of late complications such as stent thrombosis or restenosis [16]. The indications for OCT-guided PCI still need stronger randomized controlled trials to support them and should be restricted to cases with greater complexity or with stent failure suspicion [9].
Limitations
OCT imaging requires the use of contrast media to displace blood from the vessel lumen on the segment to be examined. This significantly increase the amount of contrast usually used in angiography. Another limitation imposed by red blood cells is the inability of OCT to visualize red thrombus.
The main disadvantage of OCT comparing to IVUS is the reduced image deep penetration (1–2 mm), making it impossible to fully evaluate plaque composition and the voluminous plaque necrotic core [13]. Reduced deep analysis limits the ability of assessing positive remodeling by OCT, an important plaque vulnerable feature perfectly evaluated by IVUS [13]. Recently, the advent of catheters with the two intracoronary imaging modalities IVUS and OCT promises to associate the high resolution of the OCT to evaluate the cap thickness with the best IVUS image depth for assessing the necrotic core and plaque burden. The combination of both would fully evaluate coronary plaque without dispensing the invasive approach, contrast media usage, and ionizing radiation exposure.
Noninvasive Diagnostic Methods to Assess Coronary Wall
Magnetic Resonance
The first high-resolution magnetic resonance imaging study (MRI) of human arteries was published by Martin et al. in 1995 [17]. Martin studied segments of the carotid, femoral, and aortic arteries and compared MRI findings with histological exams [17]. This study contributed significantly to developing arterial wall imaging techniques to reproduce such images in vivo and stimulated researchers to pursue studying coronary artery walls by MRI. The use of MRI for the evaluation of coronary arteries became clinically feasible after the advent of the latest-generation scanners, allowing faster acquisition sequences and better image quality. Coronary wall evaluation by MRI is safe and noninvasive, does not expose the patient to ionizing radiation, and often does not need any form of contrast medium. These features make coronary wall MRI a promising diagnostic tool for the large-scale evaluation of suspected or known CAD individuals as well as evaluating coronary involvement of inflammatory pathologies.
After a long period of technological advances with emerging new-generation scanners and improvements of MRI sequences, in 2000 Fayad et al. [18] and Botnar et al. [19] published their studies on the use of MRI for the evaluation of coronary artery wall in vivo. They used T2-weighted sequences to assess the coronary artery wall characteristics without the use of contrast media. Coronary segments with atherosclerotic plaques by angiography showed greater vessel area and wall thickness on MRI compared to normal segments in the same patients [18, 19]. These findings boosted the use of MRI to assess coronary wall features in different clinical scenarios.
Coronary MRI demonstrated to be able to identify atherosclerosis in early stages, before significant luminal reduction is established (<50% stenosis). Segments with atherosclerosis presented increased wall thickness and increased wall area compared to healthy coronary segments on angiography [20]. MRI outer vessel area and plaque burden showed good correlation with IVUS [21]. However, coronary wall thickness assessed by MRI demonstrated low correlation with IVUS [22]. Besides identifying atherosclerotic plaques, an important role of MRI is identifying high-risk plaques and their vulnerable features. These plaques are characterized by a large lipid core, positive remodeling, a thin fibrous cap, and a high-inflammatory component being prone to rupture.
In order to assess high-risk plaques, different MRI techniques are available. The measurement of vessel wall signal intensity using T1-weighted three-dimensional black-blood gradient sequences without contrast-medium injection was validated in carotid arteries. High-intensity signal (HIS) in the carotid wall was associated with a high-risk plaque, intraplate thrombus, or hemorrhage assessed by histological exams [23,24,25]. This approach was used in patients with significant coronary stenosis (>70%), and HIS plaques had a high frequency of ultrasound attenuation, low CT density, and a high incidence of transient slow flow phenomena [26, 27]. Furthermore, the location of HIS in the coronary lumen or intrawall was also related to important features of plaque vulnerability [27]. Masumoto et al. [28] compared OCT findings in patients with angina pectoris and showed that thrombus and intimal vasculature were associated with intraluminal HIS. In contrast, intrawall HIS was associated with macrophage infiltration and absence of calcification [28].
Another non-contrast MRI ability is to identify methemoglobin in coronary wall using T1-weighted black-blood sequences. Methemoglobin is visualized in acute thrombus and intraplaque hemorrhages [26, 29], an important plaque vulnerability feature present in patients ongoing acute coronary syndrome [30, 31].
These combined techniques allow non-enhanced MRI to assess coronary wall thickness, vascular remodeling, plaque burden, and intraplaque hemorrhage or thrombus. These characteristics are known as vulnerable plaque features with significant clinical impact. Patients with HIS plaques have higher probability of MACE [32].
Beyond these non-contrast techniques, MRI can also evaluate coronary artery wall through images acquired after contrast injection (gadolinium). The technique shares the same principles of late gadolinium enhancement for evaluation of myocardial fibrosis. The pathophysiological pathway is based on the theory that there is a rupture of the cell membrane and the expansion of extracellular space by collagen in the fibrosis process. Providing gadolinium is an extracellular contrast, enlarged extracellular space will increase the contrast amount in those areas and extend the contrast wash out time. Late images acquired after gadolinium injection will enhance in fibrotic regions. Histological study of carotid plaques demonstrated that contrast enhancement was associated with neovascularization of the fibrous cap, increased endothelial permeability, and infiltration of inflammatory cells [33].
Early reports using T1-weighted images demonstrated that coronary wall contrast enhancement is strongly associated with vascular remodeling and atherosclerosis severity [29, 34]. Coronary wall enhanced areas by MRI correspond predominantly to mixed plaques by computed tomography angiography (CTA) [34]. In the setting of chronic coronary artery disease, contrast uptake in the coronary wall might be associated with neovascularization, endothelial permeability, and infiltration of inflammatory cells [29, 34].
Patients with ACS had significantly greater contrast uptake in the coronary wall of culprit lesions [35, 36]. However, long-term follow-up of these patients showed that the enhancement decreased 3 months after reperfusion [35]. This finding suggests that edema and the inflammatory process present in ACS have a significant role in this phenomenon and that enhancement of the coronary wall may be a surrogate biomarker of plaque activity and/or vulnerability [35, 36].
Delayed enhancement MRI can be useful for the evaluation of coronary wall involvement in several inflammatory diseases. Puntmann et al. [37] showed that patients with systemic lupus erythematosus at clinical remission have a higher signal intensity measured by contrast noise ratio on the coronary wall compared to control subjects [37]. In another study of the same group, they demonstrated that patients with systemic lupus erythematosus presented contrast enhancement in a diffuse pattern while patients with CAD had a regional distribution, but both had contrast noise ratio values higher than control patients [38]. In Takayasu’s arteritis, the delay enhancement of the coronary wall seems to have the same distribution as segments with CAD [39].
Limitations
The image acquisition of coronary arteries is a challenge for any noninvasive imaging method, especially MRI. Some of the reasons for that lie in the fact that coronary arteries have reduced caliber and tortuous trajectory and are subjected to heart and respiratory movements. New-generation scanners with optimized sequences allow faster exams, minimizing cardiac cycle and respiratory movement artifacts while maintaining adequate image quality. Another variable that compromises coronary MRI quality is the distance between MRI coil, positioned on the thoracic wall, and the coronary arteries. The greater the distance, the lower the noise signal ratio and worse image quality. Finally, the maximum spatial resolution obtained by the MRI (0.65 × 0.65 mm) is insufficient compared to those available by the IVUS (<0.1 mm) or even CTA (0.35 × 0.35 × 0.35 mm). MRI resolution allows a satisfactory analysis only for segments with greater caliber and restricting its evaluation to the proximal or middle coronary segments [40].
Computed Tomography Angiography
Computed tomography angiography has raised as an emerging diagnostic tool in the evaluation of coronary arteries in the past years. Coronary CTA allows the evaluation of several cardiac structures in a single acquisition such as the coronary arteries lumen, coronary wall, coronary adjacent structures (pericoronary fat and pericardium), myocardial perfusion, and functional and anatomical heart structures. These evaluations are possible using a single contrast-medium injection. The overall assessment of heart structure and function became possible by the evolution of scanners and the reconstruction algorithms. New equipment with a larger number of detectors (up to 320 detectors) and dual source energy scanners significantly improved spatial and temporal resolution, as well as reducing radiation exposure and the need of larger volumes of iodinated contrast [41].
In order to evaluate coronary arteries wall, it is necessary to inject iodinated contrast through a peripheral venous. The image acquisition technique for evaluating the coronary wall follows the same protocol as conventional CTA, allowing combined analysis of coronary lumen and coronary wall (Fig. 12.1). The identification of coronary stenosis location is of great importance while interpreting CTA exams focused on coronary wall analysis, driving the examiner to the specific segment to be studied. Coronary wall analysis requires a combined interpretation, using a subjective visual analysis and objective measurements performed semiautomatically (Figs. 12.1 and 12.2). Visually, the examiner identifies several complex features such as TCFA, napkin ring signal, positive remodeling, and major cap disruption. Ideally, objective measurement should always be added, including mean plaque attenuation, determining plaque components based on its attenuation range, plaque overall volume, as well as volume of each plaque components. Objective measurements are semiautomatic by using commercially validated software [42, 43]. The combined analysis allows the characterization of plaques as non-calcified, mixed, or calcified, as well as it highlights important features of plaque vulnerability (Fig. 12.1).
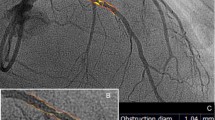
Coronary computed tomography angiography with simultaneous visualization of the three-dimensional anatomy of the heart and coronary arteries, visualization of the coronary artery lumen in curved multiplanar reconstruction, and transverse images of the coronary artery with lumen and artery wall visualization. Cross-sectional analysis of the artery allows the evaluation of the characteristics of each slice and identification of segments with lumen stenosis (A) as well as segments apparently normal to angiography (B) but with atherosclerotic involvement identified by tomography
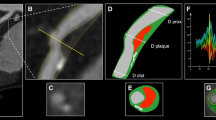
Coronary computed tomography angiography with visualization of the luminography and several transversal sections of the artery. Tomography allows semiautomatic identification of early stages of atherosclerosis, with maintenance of lumen diameter (Section 1). Different components of the plaque are discriminated by color groups, based on the density expressed in Hounsfield units (Sections 2–4). Characteristics such as positive remodeling can be demonstrated in the transversal sections (Sections 10, 11)
Although there have been technological advances, the first stages of atherosclerosis, which comprise the diffuse thickening of the intima with macrophage infiltrates and the formation of the fatty strains [44], are not visible in current clinical computed tomography scanners available [45]. However, it is well known that exactly on these early stages of atherosclerosis, the plaques are more susceptible to rupture. Positive remodeling is an early atherosclerotic feature well identified by CTA, which is commonly associated with large necrotic-core plaques, hemorrhage, and TCFA [46, 47]. Unfortunately, not all plaque disruption features can be clearly identified by CTA. However, some valuable findings such as increased plaque volume, low plaque attenuation, and positive remodeling are well documented by CTA [48, 49]. These findings are well correlated with angiographic findings [48] and are present in up to 40% of patients considered to be clinically stable, representing silent disrupted plaques. These cases are difficult to be identified by the traditional cardiovascular risk assessment and are precisely the target patient of primary cardiovascular prevention [49].
CTA demonstrated good discriminatory accuracy between lipid-rich and fibrous-rich plaques. The plaque mean attenuation assessed by CTA was validated with histology on cadaver coronaries. The tissue attenuation measurement discriminated the predominant plaque component and was able to differentiate several stages of coronary atherosclerosis [50]. Structures with lower attenuation values on CTA are predominantly composed by lipids, while higher attenuation values are associated with fibrocalcific composition [51, 52] (Fig. 12.1). Predominantly lipid or mixed plaques are more vulnerable to rupture. Plaque composition analysis by coronary CTA is considered reliable and has good correlation with gold standard methods such as IVUS and OCT [53,54,55,56]. However, defining plaques as lipid-rich or fibrous-rich based on semiautomatic attenuation measures has some pitfalls. There is some attenuation overlap among those structures and the contrast enhancement can influence attenuation values affecting the accuracy of these classifications. In order to overcome these limitations, some authors proposed that the best way to discriminate plaque components would be using quantitative histograms [51, 52].
The CONFIRM study showed that plaques classified as at early stages of coronary atherosclerosis and without significant stenosis should be treated with statins in order to prevent future cardiovascular events. Participants without CAD by CTA had no benefit on using statins [52]. Interpreting CTA focused on the coronary wall allows us to classify patients as individuals with normal coronary arteries, patients with CAD but with nonobstructive plaques and patients with CAD and obstructive plaques. Individuals with obstructive plaques obviously have worse prognosis. However, patients with nonobstructive plaques carry a worse prognosis when compared to individuals whose coronary walls are free of atherosclerosis by CTA [57, 58]. These results highlight the role of subclinical CAD on cardiovascular outcomes, requiring our attention to identifying and adequately treat these individuals. Despite usually being asymptomatic, these individuals are at higher risk compared to others with similar cardiovascular risk profile but without any coronary artery atherosclerotic involvement [57, 58]. These individuals can easily be identified by CTA, unlike via using ischemia testing methods or even angiography.
Identifying coronary plaques, even in subclinical stages, is extremely important for both therapeutic purposes and risk stratification. However, defining plaque characteristics may contain even more valuable information. A major contribution of CTA to risk stratification for ACS is identifying high-risk plaques.
TCFA plaques represent a major plaque complexity feature with high rupture probability and highly associated with ACS. Usually, those plaques have hemorrhage and calcification that can be visualized as bands separating the contrast-enhanced coronary lumen from the necrotic core on CTA [45, 52, 59, 60]. The difference between TCFA and ruptured plaques is based on the cap integrity and thrombus absence in TCFA. Incomplete visualization of the layer or cap discontinuation highly suggests rupture of a TCFA. However, CTA does not have enough spatial resolution to accurately evaluate rupture of a TCFA. This evaluation is restricted to methods with spatial resolution (<65 mic), like OCT [61]. Another plaque vulnerability feature assessed by CTA is the so-called napkin ring signal. This signal corresponds to a rim-like enhance with greater attenuation surrounding a low attenuation area. Napkin ring signal is visualized in mixed or non-calcified plaques and is associated with an increased risk of coronary events [62]. Plaques classified as TCFA by OCT usually present napkin ring signal, positive remodeling, and lower attenuation on CTA [59].
Vulnerable plaques represent an independent risk factor for future cardiac events even in clinically stable patients. Those plaques increase events risk in patients with significant coronary stenosis (>70%) [63] but also when they are identified in patients with mild CAD, with nonobstructive plaques (<50% stenosis) [64]. Several studies demonstrated that high-risk plaque features assessed by CTA (plaque burden, napkin ring signal, low attenuation, positive remodeling) are useful to refine the cardiovascular risk assessment [65,66,67]. For example, the presence of two or more high-risk plaque features is an independent predictor of all-cause mortality and ACS at a follow-up of 2 years (Hazard ratio 1.98) [68]. Among the high-risk plaque features, it is not clear which one is the most harmful. Recently, Feuchtner et al. [69] showed that in a follow-up of 8 years, low attenuation plaque and napkin ring signal were the major predictors of MACE, overcoming the degree of stenosis and other traditional risk factors. Additionally, they confirmed previous findings that a negative CTA determines a great prognosis [69].
The role of CTA plaque analysis in cardiovascular risk assessment was studied in patients with acute chest pain evaluated in the emergency department. Plaque high-risk features (positive remodeling, spotty calcium, or napkin ring sign) represented an independent risk factor for the diagnosis of ACS, regardless of the degree of coronary stenosis or traditional cardiovascular risk factors [70].
Although the main use of CTA is focused on atherosclerotic disease, CTA offers great utility in the evaluation of other coronary diseases, especially coronary involvement of systemic inflammatory diseases. Takayasu’s arteritis is one of the most frequent and shows some typical features by CTA. The disease presents coronary involvement in approximately 50% of the patients and presents one of the following presentation patterns: type 1, stenosis or occlusion in the ostia or proximal coronary segments; type 2, focal involvement of the coronary arteries with spared areas or diffuse arteritis; and type 3, coronary aneurysm [71, 72].
Other inflammatory diseases such as Kawasaki arteritis and Behçet’s disease may also present cardiac involvement, usually manifested as myocardial ischemia secondary to thrombotic aneurysms and occlusions [71]. Immunoglobulin IgG4-related arteritis rarely involves coronary arteries and presents as wall calcifications and intimal thickening [71]. Another vasculitis, the coronary periarteritis, is a rare disease that can manifest itself through inflammatory infiltration leading to intimal fibrosis and coronary aneurysm. CTA images can identify diffuse or focal nodal lesions on the coronary wall or rings with soft attenuation surrounding coronary arteries [73].
Limitations
The main negative aspects of CTA that limit its universal use as the first-line cardiovascular risk stratification tool are the need for iodinated contrast media and exposure to ionizing radiation. The advent of new-generation scanners minimized the radiation exposure to values pretty acceptable (1.5–5 msV) and decreased the need for iodinated contrast volume [41]. Besides that, CTA has some particular challenges to overcome in order to provide a good imaging quality. CTA is subject to movement artifacts secondary to respiration or heart rate variability, especially in patients with arrhythmias or very high cardiac frequencies. Again, new-generation scans virtually overcome these artifacts. Scanners with larger number of detectors increased the cover area and allow a complete volumetric acquisition of the heart in a single heart beat decreasing the time of acquisition and minimizing artifacts [61]. Despite these advances, several facilities recommend heart rate control aiming values lower than 65 beats per minute before scanning. Often it is necessary preparation using oral or injectable negative chronotropic drugs.
Despite technical advances, best spatial resolution scanners commercially available are not able to accurately characterize certain plaque components associated with higher risk such as microcalcifications, intima ulceration, and plaque hemorrhages. Identifying these require images with higher spatial resolution that are available only for invasive methods such as OCT and IVUS. The limited spatial resolution of CTA compared to IVUS and OCT may also result in overestimation of calcified plaque areas and luminal stenosis on this segment. It can also underestimate the area of low attenuation lesions such as soft tissue plaque [61].
Finally, CTA may not be accurate in distinguishing structures with similar densities due to some attenuation overlap. The dual source of energy scanners minimized this limitation, acquiring the images with tube voltages of 80 and 140 kvp, improving the capacity of differentiation between calcified and non-calcified plaques [74, 75].
References
Mehta SK, McCrary JR, Frutkin AD, Dolla WJ, Marso SP. Intravascular ultrasound radiofrequency analysis of coronary atherosclerosis: an emerging technology for the assessment of vulnerable plaque. Eur Heart J. 2007;28(11):1283–8.
Hassan A, Dohi T, Daida H. Current use of intravascular ultrasound in coronary artery disease. Clin Med Insights Ther. 2016;8:CMT.S18472.
Garcia-Garcia HM, Mintz GS, Lerman A, Vince DG, Margolis MP, van Es GA, et al. Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention. 2009;5(2):177–89.
Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352(1):29–38.
Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K, et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol. 2015;66(5):495–507.
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–35.
Parise H, Maehara A, Stone GW, Leon MB, Mintz GS. Meta-analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre-drug-eluting stent era. Am J Cardiol. 2011;107(3):374–82.
Jang JS, Song YJ, Kang W, Jin HY, Seo JS, Yang TH, et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014;7(3):233–43.
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541–619.
Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62(19):1748–58.
Yonetsu T, Lee T, Murai T, Suzuki M, Matsumura A, Hashimoto Y, et al. Plaque morphologies and the clinical prognosis of acute coronary syndrome caused by lesions with intact fibrous cap diagnosed by optical coherence tomography. Int J Cardiol. 2016;203:766–74.
Koskinas KC, Ughi GJ, Windecker S, Tearney GJ, Raber L. Intracoronary imaging of coronary atherosclerosis: validation for diagnosis, prognosis and treatment. Eur Heart J. 2016;37(6):524–35a–c.
Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59(12):1058–72.
Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111(12):1551–5.
Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8(7):823–9.
Waksman R, Kitabata H, Prati F, Albertucci M, Mintz GS. Intravascular ultrasound versus optical coherence tomography guidance. J Am Coll Cardiol. 2013;62(17 Suppl):S32–40.
Martin AJ, Gotlieb AI, Henkelman RM. High-resolution MR imaging of human arteries. J Magn Reson Imaging. 1995;5(1):93–100.
Fayad ZA, Fuster V, Fallon JT, Jayasundera T, Worthley SG, Helft G, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2000;102(5):506–10.
Botnar RM, Stuber M, Kissinger KV, Kim WY, Spuentrup E, Manning WJ. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation. 2000;102(21):2582–7.
Kim WY, Stuber M, Bornert P, Kissinger KV, Manning WJ, Botnar RM. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation. 2002;106(3):296–9.
He Y, Zhang Z, Dai Q, Zhou Y, Yang Y, Yu W, et al. Accuracy of MRI to identify the coronary artery plaque: a comparative study with intravascular ultrasound. J Magn Reson Imaging. 2012;35(1):72–8.
Gerretsen S, Kessels AG, Nelemans PJ, Dijkstra J, Reiber JH, van der Geest RJ, et al. Detection of coronary plaques using MR coronary vessel wall imaging: validation of findings with intravascular ultrasound. Eur Radiol. 2013;23(1):115–24.
Moody AR, Murphy RE, Morgan PS, Martel AL, Delay GS, Allder S, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation. 2003;107(24):3047–52.
Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111(21):2768–75.
Sun J, Underhill HR, Hippe DS, Xue Y, Yuan C, Hatsukami TS. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: a long-term time course study. JACC Cardiovasc Imaging. 2012;5(8):798–804.
Keegan J. Coronary artery wall imaging. J Magn Reson Imaging. 2015;41(5):1190–202.
Kawasaki T, Koga S, Koga N, Noguchi T, Tanaka H, Koga H, et al. Characterization of hyperintense plaque with noncontrast T(1)-weighted cardiac magnetic resonance coronary plaque imaging: comparison with multislice computed tomography and intravascular ultrasound. JACC Cardiovasc Imaging. 2009;2(6):720–8.
Matsumoto K, Ehara S, Hasegawa T, Sakaguchi M, Otsuka K, Yoshikawa J, et al. Localization of coronary high-intensity signals on T1-weighted MR imaging: relation to plaque morphology and clinical severity of angina pectoris. JACC Cardiovasc Imaging. 2015;8(10):1143–52.
Maintz D, Ozgun M, Hoffmeier A, Fischbach R, Kim WY, Stuber M, et al. Selective coronary artery plaque visualization and differentiation by contrast-enhanced inversion prepared MRI. Eur Heart J. 2006;27(14):1732–6.
Jansen CH, Perera D, Makowski MR, Wiethoff AJ, Phinikaridou A, Razavi RM, et al. Detection of intracoronary thrombus by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 2011;124(4):416–24.
Ehara S, Hasegawa T, Nakata S, Matsumoto K, Nishimura S, Iguchi T, et al. Hyperintense plaque identified by magnetic resonance imaging relates to intracoronary thrombus as detected by optical coherence tomography in patients with angina pectoris. Eur Heart J Cardiovasc Imaging. 2012;13(5):394–9.
Noguchi T, Kawasaki T, Tanaka A, Yasuda S, Goto Y, Ishihara M, et al. High-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J Am Coll Cardiol. 2014;63(10):989–99.
Kerwin WS, O’Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. 2006;241(2):459–68.
Yeon SB, Sabir A, Clouse M, Martinezclark PO, Peters DC, Hauser TH, et al. Delayed-enhancement cardiovascular magnetic resonance coronary artery wall imaging: comparison with multislice computed tomography and quantitative coronary angiography. J Am Coll Cardiol. 2007;50(5):441–7.
Ibrahim T, Makowski MR, Jankauskas A, Maintz D, Karch M, Schachoff S, et al. Serial contrast-enhanced cardiac magnetic resonance imaging demonstrates regression of hyperenhancement within the coronary artery wall in patients after acute myocardial infarction. JACC Cardiovasc Imaging. 2009;2(5):580–8.
Jansen CHP, Perera D, Wiethoff AJ, Phinikaridou A, Razavi RM, Rinaldi A, et al. Contrast-enhanced magnetic resonance imaging for the detection of ruptured coronary plaques in patients with acute myocardial infarction. PLoS One. 2017;12(11):e0188292.
Puntmann VO, D’Cruz D, Taylor PC, Hussain T, Indermuhle A, Butzbach B, et al. Contrast enhancement imaging in coronary arteries in SLE. JACC Cardiovasc Imaging. 2012;5(9):962–4.
Varma N, Hinojar R, D’Cruz D, Arroyo Ucar E, Indermuehle A, Peel S, et al. Coronary vessel wall contrast enhancement imaging as a potential direct marker of coronary involvement: integration of findings from CAD and SLE patients. JACC Cardiovasc Imaging. 2014;7(8):762–70.
Schneeweis C, Schnackenburg B, Stuber M, Berger A, Schneider U, Yu J, et al. Delayed contrast-enhanced MRI of the coronary artery wall in takayasu arteritis. PLoS One. 2012;7(12):e50655.
Kuo YS, Kelle S, Lee C, Hinojar R, Nagel E, Botnar R, et al. Contrast-enhanced cardiovascular magnetic resonance imaging of coronary vessel wall: state of art. Expert Rev Cardiovasc Ther. 2014;12(2):255–63.
Achenbach S. Coronary CT angiography-future directions. Cardiovasc Diagn Ther. 2017;7(5):432–8.
Oberoi S, Meinel FG, Schoepf UJ, Nance JW, De Cecco CN, Gebregziabher M, et al. Reproducibility of noncalcified coronary artery plaque burden quantification from coronary CT angiography across different image analysis platforms. AJR Am J Roentgenol. 2014;202(1):W43–9.
Ovrehus KA, Schuhbaeck A, Marwan M, Achenbach S, Norgaard BL, Botker HE, et al. Reproducibility of semi-automatic coronary plaque quantification in coronary CT angiography with sub-mSv radiation dose. J Cardiovasc Comput Tomogr. 2016;10(2):114–20.
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95.
Henzler T, Porubsky S, Kayed H, Harder N, Krissak UR, Meyer M, et al. Attenuation-based characterization of coronary atherosclerotic plaque: comparison of dual source and dual energy CT with single-source CT and histopathology. Eur J Radiol. 2011;80(1):54–9.
Ohayon J, Finet G, Gharib AM, Herzka DA, Tracqui P, Heroux J, et al. Necrotic core thickness and positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol. 2008;295(2):H717–27.
Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation. 2000;101(6):598–603.
Madder RD, Chinnaiyan KM, Marandici AM, Goldstein JA. Features of disrupted plaques by coronary computed tomographic angiography: correlates with invasively proven complex lesions. Circ Cardiovasc Imaging. 2011;4(2):105–13.
Bilolikar AN, Goldstein JA, Madder RD, Chinnaiyan KM. Plaque disruption by coronary computed tomographic angiography in stable patients vs. acute coronary syndrome: a feasibility study. Eur Heart J Cardiovasc Imaging. 2016;17(3):247–59.
Becker CR, Nikolaou K, Muders M, Babaryka G, Crispin A, Schoepf UJ, et al. Ex vivo coronary atherosclerotic plaque characterization with multi-detector-row CT. Eur Radiol. 2003;13(9):2094–8.
Schlett CL, Maurovich-Horvat P, Ferencik M, Alkadhi H, Stolzmann P, Scheffel H, et al. Histogram analysis of lipid-core plaques in coronary computed tomographic angiography: ex vivo validation against histology. Investig Radiol. 2013;48(9):646–53.
Marwan M, Taher MA, El Meniawy K, Awadallah H, Pflederer T, Schuhback A, et al. In vivo CT detection of lipid-rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS. Atherosclerosis. 2011;215(1):110–5.
Obaid DR, Calvert PA, Brown A, Gopalan D, West NEJ, Rudd JHF, et al. Coronary CT angiography features of ruptured and high-risk atherosclerotic plaques: correlation with intra-vascular ultrasound. J Cardiovasc Comput Tomogr. 2017;11(6):455–61.
Obaid DR, Calvert PA, Gopalan D, Parker RA, Hoole SP, West NE, et al. Atherosclerotic plaque composition and classification identified by coronary computed tomography: assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ Cardiovasc Imaging. 2013;6(5):655–64.
Wieringa WG, Lexis CP, Lipsic E, van der Werf HW, Burgerhof JG, Hagens VE, et al. In vivo coronary lesion differentiation with computed tomography angiography and intravascular ultrasound as compared to optical coherence tomography. J Cardiovasc Comput Tomogr. 2017;11(2):111–8.
Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S. Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology. 2010;257(2):516–22.
Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161–70.
Hadamitzky M, Taubert S, Deseive S, Byrne RA, Martinoff S, Schomig A, et al. Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur Heart J. 2013;34(42):3277–85.
Kashiwagi M, Tanaka A, Kitabata H, Tsujioka H, Kataiwa H, Komukai K, et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging. 2009;2(12):1412–9.
Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49–57.
Saremi F, Achenbach S. Coronary plaque characterization using CT. AJR Am J Roentgenol. 2015;204(3):W249–60.
Maurovich-Horvat P, Schlett CL, Alkadhi H, Nakano M, Otsuka F, Stolzmann P, et al. The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc Imaging. 2012;5(12):1243–52.
Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015;66(4):337–46.
Conte E, Annoni A, Pontone G, Mushtaq S, Guglielmo M, Baggiano A, et al. Evaluation of coronary plaque characteristics with coronary computed tomography angiography in patients with non-obstructive coronary artery disease: a long-term follow-up study. Eur Heart J Cardiovasc Imaging. 2017;18(10):1170–8.
Hell MM, Motwani M, Otaki Y, Cadet S, Gransar H, Miranda-Peats R, et al. Quantitative global plaque characteristics from coronary computed tomography angiography for the prediction of future cardiac mortality during long-term follow-up. Eur Heart J Cardiovasc Imaging. 2017;18(12):1331–9.
Nadjiri J, Hausleiter J, Jahnichen C, Will A, Hendrich E, Martinoff S, et al. Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. J Cardiovasc Comput Tomogr. 2016;10(2):97–104.
Tesche C, Plank F, De Cecco CN, Duguay TM, Albrecht MH, Varga-Szemes A, et al. Prognostic implications of coronary CT angiography-derived quantitative markers for the prediction of major adverse cardiac events. J Cardiovasc Comput Tomogr. 2016;10(6):458–65.
Yamamoto H, Kihara Y, Kitagawa T, Ohashi N, Kunita E, Iwanaga Y, et al. Coronary plaque characteristics in computed tomography and 2-year outcomes: the PREDICT study. J Cardiovasc Comput Tomogr. 2018;12(5):436–43.
Feuchtner G, Kerber J, Burghard P, Dichtl W, Friedrich G, Bonaros N, et al. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: a long-term follow-up study. Eur Heart J Cardiovasc Imaging. 2017;18(7):772–9.
Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64(7):684–92.
Jeon CH, Kim YK, Chun EJ, Kim JA, Yong HS, Doo KW, et al. Coronary artery vasculitis: assessment with cardiac multi-detector computed tomography. Int J Cardiovasc Imaging. 2015;31(Suppl 1):59–67.
Kang EJ, Kim SM, Choe YH, Lee GY, Lee KN, Kim DK. Takayasu arteritis: assessment of coronary arterial abnormalities with 128-section dual-source CT angiography of the coronary arteries and aorta. Radiology. 2014;270(1):74–81.
Zhou Z, Xu L, Zhang N, Wang H, Liu W, Sun Z, et al. CT coronary angiography findings in non-atherosclerotic coronary artery diseases. Clin Radiol. 2018;73(2):205–13.
Tanami Y, Ikeda E, Jinzaki M, Satoh K, Nishiwaki Y, Yamada M, et al. Computed tomographic attenuation value of coronary atherosclerotic plaques with different tube voltage: an ex vivo study. J Comput Assist Tomogr. 2010;34(1):58–63.
Barreto M, Schoenhagen P, Nair A, Amatangelo S, Milite M, Obuchowski NA, et al. Potential of dual-energy computed tomography to characterize atherosclerotic plaque: ex vivo assessment of human coronary arteries in comparison to histology. J Cardiovasc Comput Tomogr. 2008;2(4):234–42.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Marques, M.D., Lima, J.A.C. (2020). Current Imaging Approaches and Challenges in the Assessment of Coronary Artery Disease. In: Yuan, C., Hatsukami, T., Mossa-Basha, M. (eds) Vessel Based Imaging Techniques . Springer, Cham. https://doi.org/10.1007/978-3-030-25249-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-25249-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25248-9
Online ISBN: 978-3-030-25249-6
eBook Packages: MedicineMedicine (R0)