Abstract
Dengue virus (DENV) and Zika virus (ZIKV) are a cause of significant morbidity and mortality worldwide within specific regions of the world where they are endemic and susceptible to the occurrence of epidemics. The interaction between these viruses and other host factors (especially the immunocompetence) seems to influence the expression and severity of these conditions. The various clinical forms of both DENV and ZIKV are still being characterized phenotypically, serologically, and genetically together with the pathophysiological mechanisms leading to their protean clinical manifestations and complications. Arthralgias involving small and large joints occur frequently in DENV-affected patients, but typical arthritis is seldom found; accordingly, a physical exam of joints is often unremarkable. Joint pain may precede, appear simultaneously, or subsequently to the onset of fever, and might be masked by more prominent symptoms like backache, large-bone pain, and myalgias. The arthritic phenotype of DENV infection is still unknown, but it seems monoarticular, short-lived, and responsive to symptomatic treatment, whereas the articular manifestations of ZIKV infection are less severe. ZIKV is more likely to induce neurological complications and congenital abnormalities. Coinfection of DENV and ZIKV with chikungunya virus seems to lead to protracted arthritis manifestations and may be associated to a higher morbidity and mortality.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dengue
- Zika virus
- Arthritis phenotype
- Autoimmune diseases
- Pathophysiology
- Diagnosis
- Pregnancy
- Management
- Prevention
Introduction
Arboviruses cause diseases that occur epidemically, and many have a similar clinical expression at presentation. The genus Flavivirus of the family Flaviviridae consists of more than 70 members and includes dengue virus (DENV) and Zika virus (ZIKV). Until recently these viruses have not been directly implicated as a cause of chronic inflammatory arthritis or autoimmune diseases (AID) such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE). However, they may either initiate rheumatic manifestations or trigger some AID via a variety of mechanisms or worsen an established AID. There are case reports and epidemiological studies that have established a relationship between DENV and ZIKV infection with the occurrence of arthritis and AID. Co-infection with chikungunya (CHIKV) may cause severe manifestations that in some cases may be fatal. These viruses cause their effects through numerous mechanisms interacting with host factors such as age, gender, genetics, previous infectious history, and immunocompetency.
ZIKV infection has been associated with complications like congenital microcephaly and fetal losses among women infected during pregnancy, as well as severe neurologic complications. Their syndromic expression can be either febrile (DENV or chikungunya) or exanthematic (ZIKV). Frequent symptoms at presentation are headaches, myalgias, arthralgias as occurring frequently in DENV infection (break-bone fever), arthritis in those with CHIKV co-infection, or both in those with ZIKV infection. Both DENV and ZIKV infections may overlap with other viral or bacterial infections, autoimmune diseases, or chronic conditions (e.g., diabetes mellitus, chronic heart failure, etc.) making the differential diagnosis very challenging. Hence, the practicing rheumatologist shall be equipped with appropriate knowledge of implicated viruses. Even though it is not an easy task, suspecting them on clinical grounds in individuals presenting with either typical or atypical clinical manifestations or living or coming from geographic areas where DENV and ZIKV are endemic is crucial [1].
Epidemiology
Currently five human epidemic mosquito-borne arboviruses, yellow fever viruses, DENV, West Nile virus, CHIKV, and ZIKV, have emerged in both hemispheres during recent centuries. However, this has not been the case for other mosquito-borne arboviruses (Japanese encephalitis virus, St. Louis encephalitis virus, Murray Valley encephalitis virus, Usutu virus, Spondweni virus, O’nyong-nyong virus, and Rift Valley fever virus) that have only emerged in specific regions [2]. At least in 215 countries/territories, arboviral diseases are a global public health threat and are potentially suitable for the most important arboviral disease vectors with more than half of these regions reporting cases, and the increasing number of reports highlights the expansion of their common transmission vectors [3]. Dengue is widespread throughout the tropics, with risk factors influenced by local spatial variations of rainfall, temperature, relative humidity, degree of urbanization, and quality of vector control services in urban areas. Before 1970, only nine countries had experienced severe dengue epidemics. Today, the disease is endemic in more than 100 countries in World Health Organization regions (WHO’s): WHO’s African, Americas, Eastern Mediterranean, South-East Asia, and Western Pacific regions. The Americas, South-East Asia, and Western Pacific regions are the most seriously affected. It is likely that the actual numbers of dengue cases are underreported, and many cases are misclassified. WHO reported in 2018 that a recent estimate indicates that 390 million dengue infections occur every year (95% credible interval 284–528 million), of which 96 million (67–136 million) manifest clinically (with any severity of disease), and that another study estimates that 3.9 billion people in 128 countries are at risk of infection with dengue viruses, figures highlighting the overwhelming epidemiological and economic burden in endemic countries [4]. ZIKV continues to spread geographically to and within areas where competent vectors are present [5]. As of March 10, 2018, there were 84 countries, territories, or subnational areas with evidence of vector-borne ZIKV transmission and 64 countries, territories, or subnational areas where the competent vector is established but with no documented past or current ZIKV transmission; 13 countries have reported evidence of person-to-person transmission of ZIKV. Thirty-one countries or territories have reported ZIKV-related complications including microcephaly and central nervous system (CNS) malformations suggestive of congenital infection, and 23 countries or territories have reported an increased incidence of Guillain-Barré syndrome (GBS) and/or laboratory confirmation of a ZIKV infection among GBS reported cases [5] (Figs. 12.1, 12.2, and 12.3).
Dengue risk in the Americas and the Caribbean. (1) Risk areas are shown on a national level except for where evidence exists of different risk levels at subnational regions. Areas that are too small to be seen on the regional maps are labeled in dark blue or light blue depending on their risk categorization. (2) Jentes et al. [66]. (Source: Centers for Disease Control and Prevention. Chapter 3. Infectious Diseases Related to Travel. Available at https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/dengue)
Dengue risk in Africa and the Middle East. (1) Risk areas are shown on a national level except for where evidence exists of different risk levels at subnational regions. Areas that are too small to be seen on the regional maps are labeled in dark blue or light blue depending on their risk categorization. (2) Jentes et al. [66]. (Source: Centers for Disease Control and Prevention. Chapter 3. Infectious Diseases Related to Travel. Available at https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/dengue)
Dengue risk in Asia and Oceania. (1) Risk areas are shown on a national level except for where evidence exists of different risk levels at subnational regions. Areas that are too small to be seen on the regional maps are labeled in dark blue or light blue depending on their risk categorization. (2) Jentes et al. [66]. (Source: Centers for Disease Control and Prevention. Chapter 3. Infectious Diseases Related to Travel. Available at https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/dengue)
DENV and ZIKV Transmission
The knowledge of transmission of DENV and ZIKV to humans is of paramount importance to assess and then to apply preventive and control measures with consideration given to the various modes of transmission of ZIKV. Aedes (Ae.) aegypti mosquito is the primary vector of DENV that is transmitted to humans through the bites of infected female mosquitoes. After virus incubation of about 4–10 days, the infected mosquito is capable of transmitting the virus for the rest of its life. Infected symptomatic or asymptomatic humans are the main carriers and multipliers of the virus, serving as a source of the virus for uninfected mosquitoes. Patients who are already infected with the DENV can transmit the infection (for 4–5 days to a maximum of 12 days) via Ae. mosquitoes after their first symptoms appear. The Ae. aegypti mosquito lives in urban habitats and breeds mostly in man-made containers. Unlike other mosquitoes, Ae. Aegypti is a daytime feeder; its peak biting periods are early in the morning and in the evening before dusk. Female Ae. Aegypti bites multiple people during each feeding period. Ae. albopictus , a secondary dengue vector in Asia, has spread to North America and more than 25 countries in the European Region, largely due to the international trade in used tires (a breeding habitat) and other goods (e.g., lucky bamboo). Ae. albopictus is highly adaptive and, therefore, can survive in cooler temperate regions of Europe. Its spread is due to its tolerance to temperatures below freezing, hibernation, and ability to shelter in microhabitats. ZIKV is primarily transmitted by the bite of the same mosquito that transmits DENV, CHIKV, and yellow fever in tropical and subtropical regions. These mosquitoes usually bite during the day, peaking during early morning and late afternoon/evening. Other transmission forms of ZIKV are maternal-fetal, sexual contact (vaginal, anal, and oral), blood products transfusion, organ transplantation, and laboratory exposure. ZIKV RNA has been detected in blood, urine, semen, saliva, female genital tract secretions, cerebrospinal fluid, amniotic fluid, and breast milk. After ZIKV infection ZIKV RNA may be detected after long periods of time [6]. It is possible that body fluids such as sweat or tears of patients with ZIKV disease could be infectious while the index patient’s viral load is very high.
The Changing Nature of Global Health and the Influence of Environmental Changes on the Spread of DENV and ZIKV
Humans have influenced wild habitats by interacting and evolving with wild animals and plants; consequently, contemporary arthropods are frequently exposed to the modern human environment, domestic animals, and livestock to which they rapidly adapt. This adaptive process defined as domestication makes populations vulnerable to the threat of successive arbovirus epidemics. Most of the arboviruses are zoonotic, i.e., they infect a wide variety of arthropods, animals including birds in their sylvatic habitats, and humans as incidental hosts. Arboviruses have progressed to developed balanced relationships with the sylvatic hosts over many years which explains why morbidity and mortality are rarely observed in sylvatic animals when they are infected by arboviruses and is contrary to what happens with humans where infections by sylvan arboviruses are generally rare and balanced relationships have not been established; as a consequence, they will exhibit significant morbidity and mortality after infection by sylvan arboviruses. With the exception of epidemic arboviruses such as DENV, ZIKV, and CHIKV, human infections are generally not essential to maintain the arbovirus. Several factors have contributed to recent emergence and re-emergence of DENV, ZIKV, and CHIKV: increases in population density, development of global transportation systems, increased exposure frequency of humans to mosquitoes, and global mobility of humans. Other rapid changes like an increased need of agricultural capacity, deforestation, and animal husbandry have also been implicated.
Pathophysiology and Immune Response
Viruses may cause arthritic manifestations by different mechanisms: (1) direct invasion (e.g., rubella), (2) immune complex formation (e.g., hepatitis B infection, alphaviruses, hepatitis C), or (3) by latent viruses and immune dysregulation (e.g., lentivirus infection). Viruses may initiate or precipitate rheumatic symptoms through mechanisms that depend on host factors (age, gender, genetics, infectious history, and immune response) and virus-related factors (virulence, etc.).
DENV and ZIKV are enveloped, positive-sense, single-stranded RNA viruses with a genome of approximately 10.7 kb in length that encodes a polyprotein with three structural proteins [capsid (C)-premembrane (prM)-envelope (E)] at the N terminus and seven nonstructural proteins (NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5) at the C terminus flanked by 5′ and 3′ untranslated regions (UTRs) [7]. There are four closely related but serologically distinct DENV types of the genus Flavivirus , called DENV-1, DENV-2, DENV-3, and DENV-4. There is transient cross-protection among the four types, which weakens and disappears over the months following infection; therefore, individuals living in a dengue-endemic area with all types co-circulating are at risk for infection with any and all DENV types. The virus-host interaction will determine the immune response. It is clear that there are DENV lineages that are more virologically and epidemiologically fit than others and are thus associated with more severe manifestations (DHF/DSS), whereas on the host side a prior DENV infection is the primary culprit associated with a more severe clinical picture. Although this also may apply to ZIKV infection where most infected people are asymptomatic or only develop a mild self-limiting febrile disease, there are fetal infection and congenital ZIKV syndrome and in adults ZIKV infection may induce Guillain-Barre syndrome (GSB), but unlike DENV, ZIKV is characterized by multiple modes of sexual transmission. Central to understanding mechanisms of viral immunity and pathogenesis is the knowledge of viral entry receptors and cellular tropism. Although the E protein has been known to mediate receptor binding and fusion, the precise identity of entry receptors for DENV and ZIKV in humans remains uncertain. DENV appears to use multiple cell surface molecules for binding to and infecting target cells, depending on the cell type. Several candidate molecules—including glycosaminoglycans; C-type lectins; dendritic cell-specific ICAM3-grabbing nonintegrin (DC-SIGN) and liver/lymph node-specific ICAM3-grabbing integrin (L-SIGN) (33–35); mannose receptor; the phosphatidylserine receptors T cell immunoglobulin and mucin domain (TIM) and Tyro3, Axl, and Mertk (TAM); and the phospholipid receptor CD300a—have been proposed to serve as receptors for DENV based primarily on in vitro studies with cell lines and primary human cells. One TAM family member, Axl, has also been implicated as a ZIKV entry receptor in studies with cell lines and primary human cells. Once the virus has invaded the cells, a short course of illness and self-limiting febrile symptoms in most DENV and ZIKV cases implicate a key role for the innate immune system in controlling DENV and ZIKV infections. The interferon system, comprising type I interferons (IFN-α, β), type II interferon (IFN-γ), and type III interferons (IFN-λ1–4), is the primary mechanism by which the innate immune system defends against viruses. Several lines of evidence indicate that the type I interferon system is the central mediator of protection against DENV and ZIKV. Mouse models of experimental DENV and ZIKV infection have shown that the interferon system is essential and more important than T and B cell-dependent immunity in controlling DENV infection in mice. All flaviviruses studied to date must evade the type I interferon system-mediated antiviral defense in order to replicate and cause disease in vertebrate hosts; thus, DENV and ZIKV employ multiple viral mechanisms to antagonize both type I interferon induction and type I interferon signaling, underscoring the importance of the type I interferon system in anti-DENV/ZIKV immunity. An innate immune response is triggered by the virus in infected primary human fibroblasts. Type 1 and type 2 interferons trigger the inhibition of Zika viral replication. At the molecular level, TLR3 recognizes the double-stranded RNA. Initial investigations suggested that at the cellular level the virus induces autophagosome formation to promote replication and may trigger apoptosis to foster viral dissemination.
Immune Cross-Reactivity Between Dengue and Zika Viruses
Serologic interpretation can be difficult in individuals who have resided in dengue-endemic areas, because of the significant serologic cross-reactivity between Zika virus and other flaviviruses, especially dengue viruses 1 through 4. Preexisting dengue antibodies due to past symptomatic or asymptomatic infection may yield false-positive Zika antibody results. Similarly, Zika virus antibodies also cross-react with DENV antibodies and may yield false-positive DENV antibody results. The four DENV serotypes (DENV-1, DENV-2, DENV-3, and DENV-4) and ZIKV are antigenically related mosquito-borne flaviviruses. The cross-reactivity between DENV and ZIKV has raised questions about cross-neutralization and concerns of cross-enhancement, yet few data exist characterizing the long-term antibody response. The extensive immunological cross-reactivity observed between ZIKV and other flaviviruses has practical implications for making serological diagnoses. Current serological methods are generally believed to not be sufficiently specific to discern a ZIKV from DENV infection in the setting of secondary flavivirus exposure [8]. To address the degree and nature of cross-reactivity observed between DENV and ZIKV in serial specimens collected from study participants of three countries (Nicaragua, Sri Lanka and Thailand), Montoya et al. observed that among acute DENV infections and in the presumed absence of prior ZIKV exposure, cross-reactivity to ZIKV was observed following DENV infection, but DENV titers were consistently higher at all convalescent time points, a pattern that was observed in the antibody titers from all three countries, whereas that in acute ZIKV infections, a similar pattern was observed; DENV cross-reactivity was observed following ZIKV infection, but ZIKV titers were consistently higher at all convalescent time points [9]. Importantly, similar results were found when comparing ZIKV infections in the DENV-exposed and DENV-naive patients. ZIKV lies outside the DENV serocomplex, and the measurement of neutralizing antibody titers in convalescence can distinguish ZIKV and DENV infections when all viruses are analyzed simultaneously under similar testing conditions. For example, a patient with a ZIKV infection will be counseled and monitored differently than a patient with a DENV infection, given differences in common modes of transmission (e.g., sexual, maternofetal) and potential clinical complications. Given the antigenic similarity between ZIKV and DENV, the cross-reactivity of ZIKV and DENV B cell responses is not fully understood in the context of natural human infections. Andrade et al. used a novel ELISPOT-based assay designated Quad-Color Fluorospot that allows investigation of the DENV serotype specificity vs. cross-reactivity of the memory B cell (MBC) population at a single-cell level, adding a fifth color to include ZIKV [10]. They analyzed a unique set of peripheral blood mononuclear cells from the Nicaraguan Pediatric Dengue Cohort Study. Samples were collected ~2 weeks and several months after RT-PCR-confirmed ZIKV infection from children who were previously DENV-immune or DENV-naïve, and they also included a set of DENV patients who were ZIKV-naïve. Preliminary results showed that despite the antigenic similarity between DENV and ZIKV, MBCs from ZIKV-infected subjects were highly specific to ZIKV, with lesser cross-reactivity to DENV [10].
Diagnosis
Reverse-transcriptase polymerase chain reaction (RT-PCR) in serum is the main test for detection of viral nucleic acid of Zika, chikungunya, and dengue during the initial viraemic phase. The detection of Zika RNA in serum is limited to the first 5 days of the disease. Urine may be the specimen of choice to enlarge the window of detection of DENV and ZIKV after viremia has faded: PCR positivity is possible for a longer window, and higher viral loads facilitate virus typing. In dengue, ELISA can detect NS1 antigen in the acute phase, but this test was not yet available for Zika. Because viremia is short lived, a negative RT-PCR does not rule out Zika infection and serologic tests should be performed. Typically, IgM antibodies last for 2–12 weeks. In patients with clinical symptoms, the serum should be collected 4 days after disease onset and tested for Zika, chikungunya, and dengue. The applicability of IgM might depend on the clinical situation; the duration of anti-Zika IgM has not yet been established, and there are initial indications that anti-Zika IgM might be useful in diagnosing congenital Zika syndrome [11]. The sensitivity and specificity of IgM and IgG tests are poorly established, and there is strong cross-reactivity between ZIKV, DENV, and other flaviviruses. Plaque reduction neutralization tests (PRNT) can measure virus-specific neutralizing antibodies and may be able to determine the cause of the primary infection with high specificity and clarify cross-reacting results; however, PRNT is expensive and very labor intensive.
Clinical Manifestations Related to DENV and ZIKV Infections Including Arthritis and AID
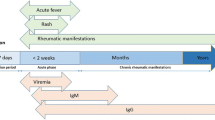
It is estimated that over 390 million DENV infections occur yearly with 96 million being clinically apparent [12]. Rheumatic manifestations dominate the initial clinical manifestations of DENV infection. Adult DENV-infected patients have a higher likelihood of being symptomatic than children. The incubation period of DENV infection ranges from 3 to 14 days; symptoms typically develop between 4 and 7 days after the bite of an infected mosquito (Fig. 12.4). DENV infection consists of three phases: (1) a febrile phase, (2) a critical phase, and a (3) recovery phase [13]. In 1997, the World Health Organization (WHO) published a classification scheme with three categories of symptomatic DENV infection, dengue fever (DF), dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS), that was revised in 2009 by the same organization to introduce the following categories: (1) dengue without warning signs, (2) dengue with warning signs, and (3) severe dengue [13, 14]. The diagnosis of DENV infection should be suspected in febrile individuals with fever, headache, nausea, vomiting, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, positive tourniquet test, leukopenia, and a relevant epidemiologic exposure (residence in or travel within the past 2 weeks to an area with mosquito-borne transmission of dengue virus infection). ZIKV incubation’s period is typically 2–14 days between mosquito bite and onset of clinical manifestations [15]. Clinical manifestations of ZIKV infection occur in 20–25 percent of individuals who usually develop a mild illness with symptoms subsiding within 2–7 days. Severe disease requiring hospitalization is uncommon, and case-fatality rates are reported to be low. It seems that symptomatic infection has been described more frequently among women and patients <40 years in one study; however, neither female sex nor age was associated with an increased prevalence of infection [16]. Immunity to reinfection occurs following primary infection. Symptoms and signs of ZIKV infection typically include acute onset of low-grade fever (37.8–38.5 °C), pruritic rash (erythematous macules and papules on the face, trunk, extremities, palms, and soles), arthralgia (notably in the small joints of the hands and feet), and non-purulent conjunctivitis; clinical illness is consistent with Zika virus disease if two or more of these symptoms are present. Other commonly reported clinical manifestations are myalgia, headache, dysesthesia, retro-orbital pain, and asthenia. Relapse of symptoms in the absence of repeat exposure has been described. In children, ZIKV infection includes intrauterine infection (vertical transmission during pregnancy), intrapartum infection (vertical transmission at the time of delivery), and postnatal infection (transmission via mosquito bites). In general, clinical manifestations in infants and children with postnatal infection are similar to the findings seen in adults with ZIKV infection; however, arthralgia is difficult to detect in infants and young children and very importantly no developmental complications have been observed in otherwise healthy children with postnatal ZIKV infection. ZIKV infection has also been associated with congenital microcephaly and fetal losses among women infected during pregnancy, as well as neurologic complications. Table 12.1 shows salient features comparing DENV versus ZIKV infection.
Relative sensitivity of detection of dengue virus nucleic acid, antigen, and IgM. (1) DENV RNA and NS1 are detectable during the first week of illness. Anti-DENV IgM is detectable starting approximately 5 days after illness onset. Although most cases only have detectable IgM anti-DENV for 14–20 days after illness onset, in some cases it may be detectable for up to 90 days. Detection of anti-DENV IgG is neither sensitive nor specific in identifying patients with dengue. Abbreviations: DENV dengue virus, NS1 nonstructural protein 1. (Source: Centers for Disease Control and Prevention. Chapter 3. Infectious Diseases Related to Travel. Available at https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/dengue)
Acute Manifestations Related to DENV and ZIKV Infection
Rheumatic manifestations are a major feature of DENV often overshadowed by other clinical features such as biphasic fever, skin rash, conjunctival involvement, pharyngitis, headache, vomiting, photophobia and orbital pain, lymphadenopathy, leukopenia, hepatosplenomegaly, and in severe cases DHF and/or DSS. Typically, DENV involves muscles, tendons, joints, and bones. Polyarthralgia is often present but eclipsed by intense backache and pain in the long bones. Severe myalgia is common and creatinine phosphokinase levels may be raised. Apart from joint and bone tenderness, there is little to find on joint examination. The diagnosis may be suggested by an acute viral type illness in a person from a DENV endemic area that requires serological confirmation [17]. While the basic constellation of symptoms including fever and rash is common to many arboviruses, including DENV, ZIKV, and CHIKV, there are some differences in symptomatology: conjunctivitis has been linked more commonly with ZIKV, severe arthralgias occur more commonly with DENV and CHIKV, and more prolonged arthralgias and rheumatological symptoms characterize CHIKV. Arthralgias occur frequently in DENV-affected patients (60–80%), but typical arthritis is seldom found; however, given the high frequency of DENV infection and its life-threatening consequences including 500,000 reported cases of DHF and DSS, its arthritic phenotype is still unknown. Patients experiencing various clinical forms of DENV infection behave phenotypically different. Few cases have been reported presenting possible DENV-related arthritis. Patil et al. reported the case of a 28-month-old Indian boy with fever of 5 days duration and black stools the day prior to admission with petechial lesions over the trunk and abdomen and an erythematous rash on palms and soles, tachycardia, a wide pulse pressure (50 mm Hg), and hepatomegaly. A diagnosis of DENV infection was made on a positive NS1 antigen and positive DENV IgM. He was treated as per standard WHO protocol with improvement and discharged home. On the 5th day, the patient was readmitted with a diffusely swollen right knee with restricted movements (radiograph of the right knee revealed widened joint space with normal surrounding structures). He had anemia, thrombocytosis, and ESR of 120 mm; ANA and CHIKV IgM antibodies were negative. Arthrocentesis of the right knee revealed turbid fluid with only five lymphocytes per mm3 without any microorganism growth on cultures. Viral examination of the fluid was not performed and the Mantoux test was negative. A provisional diagnosis of DENV arthritis was considered against post-viral reactive arthritis because this patient did not have involvement of a hip joint. He was then treated with oral acetaminophen and at follow-up after 2 weeks of discharge was afebrile and playful without pain or swelling in the right knee [18].
Jayamali et al. from National Hospital of Sri Lanka in Colombo described a 14-year-old Sri Lankan girl who complained of right buttock and hip pain of 3 weeks’ duration with confirmed DENV infection 10 days prior to the onset of symptoms [19]. Before the development of fever, arthralgia, myalgia, and headache approximately 5 weeks earlier, she was in good health. A nonstructural protein 1 (NS1) antigen test for DENV had been positive, and laboratory investigations were compatible with DENV. She was treated and had an uneventful recovery and was discharged after 6 days. Ten days after the onset of fever (4 days after the discharge), her right-sided buttock and hip pain recurred and needed to be readmitted to receive nonsteroidal anti-inflammatory drugs (NSAIDs) and steroids with symptoms improvement and discharged home. Due to the persistence and worsening of her symptoms, she was readmitted. At exam, she did not have small or large joint pain involvement or swelling, and there was no history of enthesitis. She did not have red eyes, dysuria, skin eruptions, a diarrheal illness, or sore throat. There was no past history of joint pains, recurrent oral ulceration, or photosensitive rashes as well as no history of bloody diarrhea suggestive of inflammatory bowel disease. Her family history was unremarkable for arthritis or AID. There was no past or contact history of tuberculosis. A typical right sacroiliitis was demonstrated by radiograph with joint space widening and reactive bone changes, and MRI of her pelvis and sacroiliac joint confirmed acute sacroiliitis. ESR was elevated at 110 mm and her C-reactive protein was normal. DENV IgM on admission was positive. Human leukocyte antigen-B27, rheumatoid factor, antinuclear antibody, CHIKV antibody, hepatitis serology, Brucella serology, and tuberculin skin tests were all negative. She was treated with diclofenac sodium 50 mg every 8 hours and acetaminophen. She gradually improved with NSAIDs and her ESR reduced to 70 from 110 after 1.5 weeks of treatment, and physiotherapy was arranged at the local hospital as well as follow-up at regular intervals.
During the epidemic of 2006–2007 in Sri Lanka, Kularatne et al. compared the clinical and laboratory features of CHIK and DENV confirmed cases based on serology at the General Hospital, Peradeniya, Sri Lanka [20]. During the study period, 54 serology confirmed patients with fever were included, of them, 21 patients had CHIKV infection, whereas 20 had DENV infection and 3 co-infections. The mean age of patients with CHIKV fever was 45 years (range 21–74 years), and patients with DENV fever was 30 years (range 15–63 years) (p = 0.005). Sixteen (70%) of CHIKV fever patients were females, while 15 (71%) of those with DENV fever were males (p = 0.007). Arthralgia was common to both groups (p = 0.155), while headache and a bleeding tendency were observed more in patients with DENV fever. Of CHIKV cases 12 (57%) developed acute arthritis compared with none in the DENV group (p = 0.001), lasting mean 6 days (range 1–14 days) and was a pathognomonic sign. Other clinical and laboratory features of patients with CHIKV and DENV were similar. In contrast to this study, Bhaskar et al. described the pattern of MSK manifestations among adults older than 18 years old with a confirmed serological diagnosis of DENV infection between April 2008 and December 2010 in Chennai, India. The study cohort included a total of 146 patients of whom 82 were men and 64 women with MSK manifestations occurring in 18 (12.3%) patients with a median symptom duration from onset to a resolution of symptoms of 5 days (range, 2–12 days). Only two patients had arthritis (one in the knee and one in the ankle with swelling and joint line tenderness without effusion) [21]. It seems that there are some features characterizing patients with CHIKV mono-infection and DENV + CHIK co-infection from those with DENV mono infection: high VAS score, morning stiffness, arthralgias, and restriction of joint movements. Patients with DENV mono infection had bone pains and myalgias in addition to joint pains; however, restriction of joint movements is only observed in 13.2% as compared with 100% of mono CHIKV or dual infection [22]. Between 2005 and 2010 individuals with a febrile disease from Peru, Bolivia, Ecuador, and Paraguay were enrolled in an outpatient passive surveillance study that aimed to estimate and compare the prevalence of non-hemorrhagic clinical manifestations of DENV infection by serotype. Detailed information on clinical signs, symptoms, and demographics were obtained. DENV infection was confirmed in patient sera with polyclonal antibodies in a culture-based immunofluorescence assay, and the infecting serotype was determined by serotype-specific monoclonal antibodies. Differences in the prevalence of individual and organ-system manifestations were compared across DENV serotypes. One thousand seven hundred and sixteen individuals were identified as being infected with DENV-1 (39.8%), DENV-2 (4.3%), DENV-3 (41.5%), or DENV-4 (14.4%). When all four DENV serotypes were compared with each other, individuals infected with DENV-3 had a higher prevalence of MSK and gastrointestinal manifestations, whereas individuals infected with DENV-4 had a higher prevalence of respiratory and cutaneous manifestations [23]. Similarly, Oliveira et al. examined the presence of arthralgia and/or objective arthritis among 251 patients with clinical and serological diagnosis (specific IgM detection by enzyme immunoassay) of exanthematous viral diseases; arthralgias but not arthritis were more common in patients with DENV (49%) and rubella (38.2%) than in those with human parvovirus (30%) and measles (28.1%), and except for measles cases, joint complaints were more prevalent in adults older than 15 years of age [24].
Other arboviruses like ZIKV and CHIKV circulate along with DENV and coexist in several endemic countries putting people exposed to them at high risk of developing viral-related arthritis, AID, or complications of a preexisting condition. Since 2015, Brazil has experienced a major public health crisis caused by the ZIKV, which is now considered endemic in all Brazilian states and is spreading widely in South and Central America and now threatens the USA and Europe. ZIKV and CHIKV share similar acute clinical presentation that may resemble commonest rheumatic disease manifestations. They may also complicate the clinical status of a rheumatic disease. Roimicher et al. from Brazil reported the case of a 53-year-old woman with a 4-year diagnosis of RA on clinical remission receiving stable dosages of prednisone (5 mg), etanercept (50 mg weekly), and methotrexate (20 mg weekly) 4 months before the development of fever (37.8 °C); a maculopapular rash on the face, trunk, and limbs; bilateral conjunctivitis; and polyarthritis involving the fingers, wrist, right knee, and ankles. ETN and MTX were suspended, and blood and knee synovial fluid (SF) were collected for molecular testing for ZIKV and CHIKV. Only ZIKV was identified in both samples by real-time PCR. The fever lasted 2 days, conjunctivitis 4 days, and rash and arthritis showed improvement on the 5th day. At day 7, all symptoms had disappeared, and a follow-up blood sample was negative for ZIKV by molecular test. DENV assays performed with a sample of that day were negative for IgM and positive for IgG (consistent with a prior DENV infection). Thirteen days after her first visit, the patient returned to the clinic, complaining of mild arthralgia and minor effusion of the right knee. New blood and SF samples were collected and tested for ZIKV and CHIKV. Again, only ZIKV was found in the SF, but it remained negative in the blood. ZIKV RNA fragments (843 base pairs) isolated from the patient’s blood and SF samples were subjected to PCR sequencing, and no differences were found between them [25]. Arthralgias with a median duration of 3.5 days (range 1–14 days) have also been described at a varying rate (ranging from 14% to 65%) in previous outbreaks of ZIKV in Indonesia, Micronesia, French Polynesia, and Brazil, but none of these case series have reported longer-term rheumatological sequelae [26].
Another well-documented case demonstrated what can happen between the interaction of a healthy host and a concomitant ZIKV and CHIKV infection that may lead to MSK sequelae. Cherabuddi et al. described a 40-year-old woman who has travelled from the USA to Bogota, Colombia, for 7 days spending time outdoors in both urban and rural areas and had had mosquito bites. During her stay she was asymptomatic but on day 3 upon returning to the USA developed scalp itchiness and fatigue, low-grade fever, and back pain on day 4. On day 5, she presented to the outpatient infectious diseases clinic with an erythematous scalp, a pruritic maculopapular rash on face and trunk that rapidly spread over the entire body. Her wrist and ankle joints became very painful and swollen, and she developed conjunctival redness. Saliva, serum, and urine samples were sent to the Florida State Laboratory as she fulfilled criteria for ZIKV testing. Reverse transcription–polymerase chain reaction (RT–PCR) tests for the viral genomic RNAs (vRNAs) of ZIKV, CHIKV, and DENV 1,2,3,4, and ELISA tests for ZIKV, CHIKV, and DENV IgM antibodies were performed, as well as an IgG assay for DENV. All the tests from the Florida State Lab were negative, with the exception of a positive RT-PCR assay for ZIKV vRNA. On day 9, she continued to have severe fatigue, worsening joint pain, and swelling, and because the DENV RT-PCR was negative, she was started on ibuprofen. The rash was significantly better but persisted on her torso and legs for another week. On day 16, she returned to work, though fatigue and joint discomfort persisted. Two months after the initial infection, she continued to experience severe arthralgias on wrists bilaterally and the plantar surface of the left foot and at the orthopedic clinic was noted to have tenderness of the second and third left metatarsal heads 3 months after the initiation of her illness. Radiographs of the left foot revealed no fractures or soft-tissue swelling, and thus she was recommended to wear a brace for 3 weeks. Five months after illness onset, she continued to have persistent pain, and an MRI study of the left foot showed trace fluid in the intermetatarsal bursae between the first and second metatarsal heads and second and third metatarsal heads. Persistence of symptoms prompted a re-evaluation of the viral isolation studies, which were initially terminated 9 days’ post-inoculation upon isolation of ZIKV. It was noted that a second virus was present that displayed cytopathic effects (CPE) more consistent with findings expected for alphaviruses: lytic infection/apoptosis of infected. As described in CHIKV vRNA was detected in spent-cell culture media by using the CDC real-time RT-PCR for detection of CHIK virus. These findings highlight the need to consider CHIKV co-infection in patients with prolonged rheumatological symptoms after diagnosis with ZIKV and the usefulness of cell culture as an amplification step for low-viremia blood and other samples [27].
A 30-year-old man with SLE diagnosed at age 9, class III/IV lupus nephritis from 2007, and common variable immunodeficiency from 2014 who has become infected with both ZIKV and CHIKV during the 2016 outbreak in Rio de Janeiro, Brazil, with a protracted, severe disease, leading to a fatal outcome was documented by Silva et al. who initially presented with intense wrist and right ankle arthritis but no clinical manifestations of nephropathy; nevertheless, laboratory results showed a slight renal impairment with laboratory features showing anemia, leukocytosis with neutrophilia, lymphopenia, and elevated CRP. He was on prednisone, hydroxychloroquine, cyclosporine, colchicine, and prophylactic azithromycin. Previously he received irregular treatment with immunosuppressives and rituximab (2008–2010). Gonococcal infection was suspected, and ceftriaxone with azithromycin was used in addition to prednisone dose reduction and cyclosporine withdrawal. Blood cultures were negative and synovial fluid was not accessible by needle aspiration. Ultrasonography showed severe inflammatory joint disease with a high inflammatory response in his right ankle for which a synovial biopsy was indicated that showed fibro-adipose overgrowth, hypervascularization, and granulation with mono- and polymorphonuclear infiltrate and fibrin deposition. No bacterial, fungal, or Mycobacterium tuberculosis infections were detectable by direct examination or culture. Severe tenosynovitis was considered, and MRI of wrists and right ankle was performed 278 days post-symptom onset that showed bilateral wrists synovitis and tenosynovitis bilaterally. Coronal images demonstrated edema and excessive fluid in the carpus and radioulnar joints and in flexor and extensor compartments. A sagittal MRI diffusion-prepared weighted sequence with fat suppression image of the right ankle showed synovial overgrowth within the tibiotalar and intertarsal joints. Signs of plantar fasciitis and hyperintense abnormality were also observed (edema pattern). Patient’s mother and sister had a history of non-laboratory proved CHIKV and ZIKV infection, respectively. ZIKV RNA and virus particles were detected in synovial tissue, blood, and urine and CHIKV RNA in serum sample, at the time of the diagnosis. Low level of IgG anti-DENV was also demonstrated. During the follow-up, ZIKV RNA persisted for 275 days post-symptom onset. The patient evolved with severe arthralgia/arthritis and progressive deterioration of renal function. Fatal outcome occurred after 310 days post-ZIKV and CHIKV co-infection onset. The data suggests a correlation between immunodeficiency and prolonged ZIKV RNA shedding in both blood and urine with progressive disease [28].
Contrary to the previous reports, Read et al. analyzed 7191 children enrolled in the Sentinel Enhanced Dengue and Acute Febrile Illness Surveillance System living in Puerto Rico on or before December 31, 2016, of whom 351 participants had a confirmed ZIKV infection; of them, 25 were infants (7.1%), 69 children (19.7%) aged 1–4 years, 95 (27.1%) aged 5–9 years, and 162 (46.1%) aged 10–17 years. Most patients (260 or 74.1%) presented for evaluation of ZIKV infection at fewer than 3 days after the onset of symptoms, 340 (96.9%) were discharged to home after evaluation, and 349 (99.4%) had fever, 280 (79.8%) had a rash, 243 (69.2%) had facial or neck erythema, 234 (66.7%) had fatigue, 223 (63.5%) had headache, 212 (60.4%) had chills, 206 (58.7%) had pruritus, and 204 (58.1%) had conjunctival hyperemia, but none of these patients developed ZIKV-related arthritis [29].
Chronic Manifestations
DENV and ZIKV have not been yet directly implicated as a cause of AID, but few case reports have signaled a possible association linking these viruses with AID. To investigate this risk, Li et al. conducted a population-based cohort study examining the Taiwan National Health Insurance Research Database that included 12,506 newly diagnosed DENV patients and 112,554 control subjects matched by age, gender, income, urbanization, and comorbidities between 2000 and 2010 with both cohorts being followed for a 3-year period to determine the incidence of AID. A Cox-proportional hazards regression analysis was applied to calculate the risk of AID between both groups. The DENV group showed an overall increased risk for 21 autoimmune diseases, with an adjusted hazard ratio (aHR) of 1.88 (95% confidence interval [CI], 1.49–2.37, p < 0.001). Compared with the control group, the DENV group had higher risks of Reiter’s syndrome (as used by the authors) (aHR 14.03, 95% CI 1.63–120.58), multiple sclerosis (aHR 11.57, 95% CI 1.8–74.4), myasthenia gravis (aHR 5.35, 95% CI 1.43–20.02), autoimmune encephalomyelitis (aHR 3.8, 95% CI 1.85–7.8), systemic vasculitis (aHR 3.7, 95% CI 1.11–12.28), SLE (aHR 3.5, 95% CI 1.85–6.63), and primary adrenocortical insufficiency (aHR 2.05, 95% CI 1.25–3.35) [30].
Similarly, Monsalve et al. established an association between a ZIKV infection with Guillain-Barré syndrome (GBS) and with idiopathic thrombocytopenic purpura (ITP) in a small case-control study where the case group consisted of 29 Colombian patients with GBS associated with ZIKV infection, 13 patients with ZIKV and other neurological syndromes, and 53 patients with ZIKV without neurological conditions, AID, or first-degree relatives with AID, whereas the control group was composed of 100 healthy individuals with no evidence of ZIKV disease and without clinically AID. The association between rheumatic and thyroid autoimmunity in patients with ZIKV disease was evaluated using a panel of 14 autoantibodies related to these conditions. They have also performed a literature review on ZIKV infection and the presence of GBS and ITP. In contrast to what has been reported in the previous much larger study with DENV patients, Monsalve et al. found a lack of association of rheumatoid and thyroid autoimmunity with ZIKV disease. At the time of their literature review, 272 cases of GBS related to ZIKV were retrieved with the majority of these patients showing electrophysiological findings indicating acute inflammatory demyelinating polyneuropathy as the most frequent sub-phenotype (75.7%) and 24 cases of ITP in patients with ZIKV disease. Although a few fatal cases have been observed, most of the reported patients responded well to immunomodulatory treatment. They also speculated that molecular mimicry could be one of the mechanisms incriminated in the development of autoimmunity in ZIKV-induced diseases [31].
Several case reports have incriminated both DENV and ZIKV in an SLE phenotype. An association has been described by Zea-Vera et al. by a case report of ITP exacerbation with ANA positivity induced by ZIKV in a 30-year-old Colombian woman with prior history of ITP who presented with 2 days of headache, arthralgia, myalgia, and low-grade fever and a generalized erythematous rash. At the 4th day of symptoms, platelets dropped to 9 × 109/L without hemorrhagic manifestations that recovered to 30 × 109/L in 24 hours. They ruled out DENV as well as other viral infections. ZIKV was evaluated in serum and urine samples by a real-time reverse-transcriptase polymerase chain reaction that was positive in urine but negative in serum confirming a recent ZIKV infection with urinary tract virus excretion at 7th day after disease onset [32]. Talib et al. also reported a rare case of DENV triggering SLE and lupus nephritis (LN) during an outbreak of DENV during December 2012 in Maharashtra, India. DENV diagnosis was confirmed by the presence of NS-1 antigen during the first few days of fever. Eight weeks later, a kidney biopsy revealed lupus nephritis [focal proliferative and segmental sclerosis (stage IIIC)] [33]. Similarly, Rajadhyaksha and Mehra reported a case of a 22-year-old woman who presented with high-grade fever, skin rash, breathlessness, retro-orbital pain, abdominal pain, arthralgias, and myalgias for 10 days after DENV infection that evolved onto SLE and LN [34]. She tested positive for DENV IgM and received supportive treatment and was subsequently discharged. Four weeks later she developed recurrent fever, arthralgia, rash, and anasarca and found to have SLE and active LN (renal biopsy showed diffuse proliferative glomerulonephritis) with positive ANA, increased anti-dsDNA titers, and low complement levels. She responded to steroids and immunosuppressants. It is thought that DENV incites antibody production, which if excessive causes deposition of viral antigen-antibody immune complexes, leading to renal tubular damage and glomerulonephritis in susceptible individuals. DENV infection and SLE share common manifestations: fever, fatigue, arthralgia, rashes, leukopenia, thrombocytopenia, and serositis. Other cases of DENV infection either inducing or complicating a pre-existing SLE condition have also been reported; DENV infection may also mimic a lupus flare [35,36,37,38,39]. The misinterpretation of DENV infection serology may lead to the delay of the diagnosis of SLE [40, 41]. Zainal et al. have suggested that sera of patients with SLE may contain IgG together with other types of antibodies that can cross-neutralize DENV that may explain the rarity of severe dengue in individuals with SLE [42]. A variety of factors have been associated with macrophage activation syndrome (MAS): infections, drugs, and AID (SLE or systemic onset juvenile idiopathic arthritis). Morel et al. from Paraguay reported three pediatric cases that have developed AID related to a DENV infection. One was an 8-year-old boy who presented with confirmed DENV infection with persistent fever, proteinuria, hypoalbuminemia, leukopenia, thrombocytopenia, hypocomplementemia, and normal C3 and C4 levels, negative ANA, and dsDNA and lupus anticoagulant but IgM anticardiolipin antibody positivity. This patient improved without specific treatment. The other two patients, a 3-year-old boy and a 3-month-old boy developed MAS requiring intravenous bolus of methylprednisolone with clinical improvement and subsequent hospital discharge [43, 44].
Whether a DENV infection may worsen or not, an existing AID is always a consideration. Colman et al. in Paraguay [45] and Agüero et al. [46] in NW Argentina have addressed this question, the first by a retrospective, longitudinal observational study of patients with AID and DENV infection from February 2007 to February 2012. They examined baseline AID, AID activity, treatment, clinical classification of DENV severity, and patient outcomes during the acute phase of infection, 15–30 days post-infection, and 3 months after infection. They included 22 patients with SLE, rheumatoid arthritis, scleroderma, spondyloarthropathy, vasculitis, and anti-synthetase syndrome. Patient’s AID activity at baseline was categorized as no activity (n = 8), low (n = 11), and moderate (n = 3). Sixteen patients were taking immunosuppressants and 16 corticosteroids, 3 of them at low doses. Half of the patients with DENV infection were classified as without alarm symptoms (n = 10) and the other half with alarm symptoms (n = 11). Only one patient had severe DENV infection. Eighteen patients (81%) had complete resolution of infection without worsening of baseline autoimmune disease, one had disease reactivation, and one had new organ involvement, which was a cerebrovascular accident in a RA patient. Complications related to DENV infection included thrombocytopenia with mucosal bleeding in a SLE patient with a favorable outcome and a central nervous system hemorrhage in a rheumatoid arthritis patient who died. Evaluation of AID 15–30 days post-infection revealed no activity in nine patients, baseline activity in nine patients, and exacerbation in one patient. Sixteen patients had been followed up at 3 months of which seven had no activity, seven had baseline activity, and two had SLE exacerbation: one hematologic and one cutaneous [45]. And the second study from Agüero et al. (February–May 2009) aimed to describe the clinical and biochemical features of consecutive patients with rheumatic diseases at three specific periods (pre-, during, and post-DENV infection). Demographic, clinical, biochemical, and overall assessment of rheumatic disease activity were ascertained as well as the use of medications. Eleven patients (nine women) were included: six with RA, two with SLE, and one with psoriatic arthritis, dermatopolymyositis, and ankylosing spondylitis, respectively, with an average age of 47 years. At the beginning of the DENV infection, all patients had fever and headache, and 90% exhibited leukopenia. Only the patient with SLE changed her clinical status during the DENV. There were no serious DENV events, but one patient had self-limited hepatitis. Glucocorticoids and hydroxychloroquine were not suspended [46]. Colman and Agüero had examined in their patients with an AID and concurrent DENV infection whether the intake of commonly used drugs to treat AID may predispose to severe forms of DENV infection or to lead to fatal outcomes. Currently the answer to this question has not been fully addressed yet by large longitudinal prospective studies including appropriate patient samples. However, these two small studies revealed that the vast majority of patients with AID did not have a worsening or reactivation after DENV infection from their baseline clinical status and the drugs used to treat them were not implicated. However, the refined study from de Abreu et al. analyzed the clinical profile and outcomes of patients with SLE and RA with primary DENV infection diseases reported to the Brazilian Health Information System with two aims: one to describe the clinical characteristics of RA/lupus patients who had dengue infection and one to compare RA/SLE patients with or without dengue for hospitalization rates after index dengue diagnosis for dengue-exposed or matching date for dengue-unexposed [47]. Sixty-nine SLE and 301 RA patients with DENV infection were included. In the RA/SLE with DENV case series, hospitalization was found in 24.6% of lupus subjects and of 11.2% of RA subjects. It differed by geographic region (p = 0.03), gender (p = 0.05), and use of azathioprine (p = 0.02). Dengue was the most frequent reason for hospitalization (43.0%). Hospitalization due to DENV was noted in 12 (42.9%) dengue-exposed patients (p = 0.02), while rheumatoid arthritis was reported as the cause of hospitalization in 22.2% of dengue-unexposed (p = 0.005). Five deaths were reported among the DENV-exposed and none among DENV-unexposed. Bacterial infection was the most frequent cause of death. DENV exposure was associated with an increased risk of hospitalization outcome in RA and SLE patients (RR = 6.2; 95% CI: 2.99–12.94). Comparing RA/SLE patients with or without DENV, DENV-exposed patients had an increased rate of hospitalization and death [47].
In patients with AID, a potential complication of immunosuppressive therapy is reactivation of pathogenic viruses that have remained latent (e.g., varicella-zoster virus, hepatitis B and C, Epstein-Barr virus) which have been more frequently seen in immunocompromised patients (RA or SLE) or in those on biologics. As numerous patients receive biologics while vacationing in countries where DENV is endemic, Deligny et al. conducted a survey among individuals who were experiencing a DENV infection and were on biologics; they described a case-series of eight patients of whom six were on anti-TNF agents and two on rituximab for a rheumatic condition. None of these patients experienced a severe DENV infection while on these agents [48]. A different approach was followed by Wu et al. [49] who studied the immunomodulatory effects of leflunomide in DENV-stimulated monocyte-derived dendritic cells (mo-DCs) and showed that leflunomide at therapeutic concentrations inhibited cytokine and chemokine production from DENV-infected mo-DCs by suppressing mo-DC maturation via downregulating of the expression of both CD80 and CD86. Leflunomide also inhibited DENV-induced mo-DC migration and mo-DC response to chemoattractants CCL19 and CCL21. Inhibition of mo-DC migration was likely due to the suppression of CCR7 expression on mo-DCs. These events were associated with the suppression of nuclear factor kappa B and activator protein-1 signaling pathways by leflunomide. Of note, only two patients in Colman et al. [45] and none in Agüero et al. [46] studies had taken leflunomide at the time of their DENV infection. The significance of leflunomide exposure among DENV-infected patients is uncertain, and we cannot conclude that this drug may have mitigated the clinical expression of DENV infection in those who have taken it [49].
Numerous other conditions have been related to DENV and ZIKV infection. Among them, neurological syndromes (involving both the peripheral and the CNS) are a hallmark of ZIKV infection. Mancera-Páez et al. described a 24-year-old woman from Cúcuta, Colombia, who developed the simultaneous occurrence of Guillain-Barré syndrome (GBS), plus MRI-demonstrated transverse myelitis (TM) and acute disseminated encephalomyelitis (ADEM+GBS) after an acute ZIKV infection confirmed by serum reverse transcriptase-polymerase chain reaction (RT-PCR) and convalescent ZIKV IgG antibodies. Interestingly, she had preexisting immunity against CHIKV and DENV. This patient survived with residual flaccid paraparesis after intensive care treatment, respiratory support, steroids, and intravenous immunoglobulin. The authors reviewed 19 cases of ZIKV-associated TM, encephalitis, and ADEM, and they occurred after a mean latent period of 10.5 days (range 1–96) post-infection. Although GBS and ADEM are usually considered post-infectious and associated with the development of antibodies against peripheral nerve and CNS epitopes, the authors speculated that the case of ADEM+GBS is para-infectious, induced by acute ZIKV neurotropism boosted by active immunity against other arboviruses [50]. DENV neurological complications seem to be sporadic and include meningitis, encephalitis, stroke, acute disseminated encephalomyelitis, and GBS [51]. The latter has been described in a 60-year-old Sri Lankan man who presented with a history of fever, arthralgia, and generalized malaise of 2 days duration with leukopenia, thrombocytopenia, and positive NS1 antigen and DENV IgM. He had weakness of both lower limbs, which progressed in an ascending pattern to involve upper limbs and neck muscles and to require assisted ventilation. Electromyography confirmed a demyelinating polyneuropathy, and cerebrospinal fluid showed albumin cytological dissociation. He was treated with intravenous immunoglobulins and made an uneventful recovery. Another neurological rare entity associated to DENV infection was longitudinal extensive transverse myelitis in a 15-year-old boy who presented with symptoms of transverse myelitis that developed 4 weeks after fever. MRI confirmed the diagnosis of longitudinally extensive transverse myelitis involving dorso-lumbar cord. After 6 weeks of corticosteroids and supportive management including physiotherapy, he recovered almost completely with minimal residual neurological deficit. Complications of possible CNS vasculitis and cranial nerve palsy due to DENV infection have been also described. One is a 53-year-old previously healthy Singhalese woman who developed acute-onset slurring of speech and ataxia with altered sensorium 1 day after recovery from a critical period of DHF with investigations revealing encephalopathy with brainstem ischemic infarctions considered to be compatible with CNS vasculitis. She was treated successfully with intravenous steroids and had a full functional recovery. The second patient was a middle-aged Singhalese woman who had otherwise uncomplicated DENV infection and developed binocular diplopia on day 4 of fever. The ocular examination revealed a convergent squint in the left eye with lateral rectus palsy but no other neurological manifestation [52,53,54]. Another infrequent DENV-associated manifestation is necrotizing scleritis that was described in a 60-year-old Japanese female with positive IgM and IgG for DENV infection who presented by slit lamp examination of her left eye conjunctival and scleral injection, elevation of the entire circumference of the sclera, and bulging of the sclera on the nasal upper side with a patch of avascular episcleral tissue. Additional systemic examinations identified no autoimmune diseases. She received intensive systemic and topical steroids during the initial acute phase that was tapered off over the ensuing 15 months as scleritis gradually declined. Overall there was no recurrence of active scleritis, but gradual thinning of the sclera continued to occur during the 18-year follow-up [55].
Assessment and Management of Pregnancy in a Possible Scenario of an Autoimmune Condition With ZIKV Infection
Pregnancy has long been considered a high risk for women with SLE and in other AID. The relationship between ZIKV and pregnancy related-complications is well established; thus, appropriate assessment and management of both conditions require deep knowledge on how ZIKV may impact both pregnancy and fetal outcomes given the insidious nature of ZIKV infections and its devastating consequences on fetal development. The practicing rheumatologist should know that the diagnosis approach is different in pregnant compared with nonpregnant individuals as ZIKV RNA persists approximately three times longer in a pregnant woman’s serum and because of the offspring’s risk of major CNS anomalies with congenital infection, even in an asymptomatic mother. The risk for vertical transmission exists throughout pregnancy and in offspring of both symptomatic and asymptomatic mothers, and the frequency of birth defects resulting from vertical transmission is also uncertain, but the greatest risk of serious fetal/newborn sequelae during exposure to ZIKV infection seems higher on the first or second trimester, but serious fetal/newborn sequelae may also occur on a third-trimester infection. The severity of maternal symptoms and signs, maternal virus load, and preexisting DENV antibodies do not appear to be predictors of infant outcome. Major findings of 14 studies with adequate radiological assessment of suspected or confirmed Zika virus-infected fetuses found that the most common abnormalities among 66 fetuses were ventriculomegaly (33%), microcephaly (24%), and intracranial calcifications (27%) [56]. In the context of a planned pregnancy, a patient with an AID preconception assessment should include:
-
1.
Assessment of disease activity and major organ involvement.
-
2.
Presence of a hypercoagulable state and any other comorbidity that may impact on pregnancy outcomes.
-
3.
Obstetric outcomes should be reviewed, with particular attention paid to history of small for gestational age fetus, preeclampsia, stillbirth, miscarriage, and preterm birth.
-
4.
It will be wise to determine maternal antibody status including antiphospholipid (aPLs), anti-Ro, and anti-La antibodies.
ZIKV and DENV may induce autoantibody production with certain autoantibodies that will increase obstetric risks (recurrent pregnancy loss, stillbirths, preeclampsia, and neonatal lupus). Low-dose aspirin has been given to pregnant women to reduce the risk of preeclampsia and its sequelae (e.g., fetal growth restriction) regardless of the presence of aPLs; however, caution should be exerted in ZIKV-infected patients as DENV may co-exist. Maternal treatment of ZIKV is similar to those without pregnancy. The use of NSAIDs should be avoided until DENV infection has been ruled out to reduce the risk of hemorrhage and also be avoided in pregnant women ≥32 weeks of gestation to minimize risk for premature closure of the ductus arteriosus.
Women who are already pregnant or planning a pregnancy shall be advised to follow specific protective measures:
-
1.
Avoid travel to a ZIKV-affected area (Fig. 12.5).
-
2.
Avoid sex with a partner who may be infected with ZIKV or who has recently travelled to a ZIKV-affected area (use a barrier method of birth control every time).
-
3.
Adherence to workplace safety rules (if working at health-care setting).
-
4.
Blood donation has to be delayed for at least 4 weeks and umbilical cord blood donation avoided.
-
5.
Those women who are about to get pregnant with donated sperm shall discuss with the appropriate experts.
Zika travel recommendations by traveler type and country category. (1) These countries have a potential risk of Zika, but we do not have accurate information on the current level of risk. As a result, detection and reporting of new outbreaks may be delayed. (2) Because Aedes aegypti mosquitoes (the mosquitoes that most commonly spread Zika) are present in these countries, Zika has the potential to be present, along with other mosquito-borne infections. Detection and reporting of cases and outbreaks may be delayed. (3) No Aedes aegypti mosquitoes (the mosquitoes that most commonly spread Zika) have been reported in these countries. However, other Aedes species mosquitoes have been known to spread Zika, and these may be present. (Source: Centers for Disease Control and Prevention. Zika Travel Information. Available at https://wwwnc.cdc.gov/travel/page/zika-travel-information)
Transmission of Zika virus through breastfeeding has not been described, although the virus has been detected in breast milk. Women with Zika virus exposure may breastfeed. Recent reports have highlighted that chloroquine (CQ) is capable of inhibiting ZIKV endocytosis in brain cells, but this use is not indicated [57].
Prognosis
Morbidity and mortality of DENV are recognized to be high. Stanaway et al. estimated DENV mortality, incidence, and burden for the Global Burden of Disease Study 2013 by modelling incidence from officially reported cases and adjusted the raw estimates for under-reporting based on published estimates of expansion factors. They analyzed 1780 country-years of mortality data from 130 countries, 1636 country-years of dengue case reports from 76 countries, and expansion factor estimates from 14 countries. Their estimates were as follows: 9221 dengue deaths per year between 1990 and 2013, increasing from a low of 8277 (95% uncertainty estimate 5353–10,649) in 1992 to a peak of 11,302 (6790–13,722) in 2010. This yielded a total of 576,900 (330,000–701,200) years of life lost to premature mortality attributable to dengue in 2013. The incidence of dengue increased greatly between 1990 and 2013, with the number of cases more than doubling every decade, from 8.3 million (3.3 million–17.2 million) apparent cases in 1990 to 58.4 million (23.6 million–121.9 million) apparent cases in 2013. When accounting for disability from moderate and severe acute dengue, and post-dengue chronic fatigue, 566,000 (186,000–1,415,000) years lived with disability were attributable to dengue in 2013. Considering fatal and non-fatal outcomes together, dengue was responsible for 1.14 million (0.73 million–1.98 million) disability-adjusted life-years in 2013 [58]. Shepard et al., using the latest DENV incidence estimates from the Institute for Health Metrics and Evaluation’s Global Burden of Disease Study 2013 and other data sources to assess the economic burden of symptomatic DENV cases in the 141 countries and territories with active DENV transmission, have estimated cases and costs by setting, including the non-medical setting, for all countries and territories from the scientific literature and regressions performed [59]. Their global estimates suggested that in 2013 there were a total of 58.4 million symptomatic DENV infections (95% uncertainty interval [95% UI] 24 million–122 million), including 13,586 fatal cases (95% UI 4200–34,700), and that the total annual global cost of DENV illness was US$8.9 billion (95% UI 3.7 billion–19.7 billion). The global distribution of DENV cases is 18% admitted to hospital, 48% ambulatory, and 34% non-medical. Similarly, on May 5, 2016, the WHO re-profiled ZIKV as a serious disease 1 year after the ZIKV outbreak in Brazil as a serious condition with enormous medical, ethical, and economic implications [60, 61].
Vaccine Development
Vaccine development is underway to protect from ZIKV. Several inactivated vaccine candidates have been found to induce detectable neutralizing antibodies in phase I trials [62]. The tetravalent dengue vaccine (CYD-TDV) was licensed in several Latin American countries and Southeast Asia but not in the USA beginning in 2015, and it was approved for use in Europe in 2018. CYD-TDV should be administered only to individuals with a history of previous dengue virus infection or laboratory evidence of previous dengue virus infection. In December 2017, the WHO issued a statement indicating that the vaccine is protective against severe DENV for individuals with seropositive DENV at the time of first vaccination but the risk of severe DENV is significantly increased for individuals with seronegative DENV at the time of first vaccination [63]. In April 2018 WHO advised that the vaccine should not be used until prior dengue infection can be confirmed at the time of administration [64].
Prevention
Until vaccines become completely efficacious and safe, avoiding mosquito bites continues to be the most important step and limiting travel to endemic areas when possible, control of mosquito populations, and together with the measures delineated to prevent ZIKV transmission in order to avoid its impact on fetal outcomes [65].
Decision-Making for the Practicing Rheumatologist
Urban crowding, ceaseless international travel, and immigration, human behaviors causing perturbations in ecologic balance will lead to innumerable infectious agents to emerge. The burden of both DENV and ZIKV infections remains underestimated and undermanaged and are associated to considerable suffering, increased health-care costs, disability, and mortality. In response to the threads and shared features of arboviral diseases, integrative medicine and innovative research to expand the understanding of the complex ecosystems in which these viruses evolve to initiate epidemics and to solve world pandemics need combined efforts that include the rheumatologist. Its unique training and perspective in diagnosing and managing complex conditions will be of help to diagnose, manage, and diminish the suffering and economic burden of viral-related arthritis. A combined clinic including the rheumatologist and infectious disease specialist may also be of benefit as well as training physicians in both specialties. In the event of a patient with fever and joint pain, the rheumatologist’s suspicion of DENV and ZIKV infection is of paramount importance, and the history intake shall always include:
-
1.
Ascertainment of a recent travel to an endemic area
-
2.
Immunocompetency (e.g., RA or SLE)
-
3.
Use of disease-modifying antirheumatic drugs (traditional and biologics including small-molecules)
-
4.
Demographic factors such as age and sex
-
5.
Sexual history and pregnancy status
-
6.
Exposure history such as work-related activities and those including different modes of transmission of ZIKV as described
Once DENV and ZIKV have been considered as a possible diagnosis, looking for the presence of the following signs and manifestations is mandatory:
-
1.
Arthralgia versus arthritis. If present, pattern of arthritis
-
2.
Fever
-
3.
Rash
-
4.
Constitutional symptoms
-
5.
Organ involvement
After DENV and ZIKV have been highly regarded as the cause of a patient’s illness, the rheumatologist shall collaborate with an infectious disease specialist and adhere to serologic algorithms to screen and diagnose these conditions. The rheumatologist shall never avoid synovial fluid and biopsy together with body fluids analysis (e.g., urine) if indicated. DENV and ZIKV infection management is mainly supportive, and the rheumatologist must exercise judicious decision-making. The clinical approach to a patient with high suspicion of either DENV or ZIKV is depicted in Table 12.2. Epidemiological surveillance to examine whether DENV and ZIKV are putative factors inducing either chronic arthritis or AID is necessary.
References
Rigau-Pérez JG. The early use of break-bone fever (Quebranta huesos, 1771) and dengue (1801) in Spanish. Am J Trop Med Hyg. 1998;59(2):272–4.
Gould E, Pettersson J, Higgs S, Charrel R, de Lamballerie X. Emerging arboviruses: why today? One Health. 2017;4:1–13. https://doi.org/10.1016/j.onehlt.2017.06.001. eCollection 2017 Dec. Review.
Leta S, Beyene TJ, De Clercq EM, Amenu K, Kraemer MUG, Revie CW. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int J Infect Dis. 2018;67:25–35. https://doi.org/10.1016/j.ijid.2017.11.026. Epub 2017 Nov 28.
World Health Organization. Dengue and severe dengue; 13 September 2018. Accessed online at https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
World Health Organization. Situation Report Zika Virus Microcephaly Guillain-Barré Syndrome 10 March 2017 Data as of 9 March 2017. Accessed online at http://apps.who.int/iris/bitstream/handle/10665/254714/zikasitrep10Mar17-eng.pdf;jsessionid=BCE3098CD567B55EAAFDA1F9878FE731?sequence=1.
Song BH, Yun SI, Woolley M, Lee YM. Zika virus: History, epidemiology, transmission, and clinical presentation. J Neuroimmunol. 2017;308:50–64.
Ngono AE, Shresta S. Immune response to Dengue and Zika. Annu Rev Immunol. 2018;26(36):279–308.
Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–9.
Montoya M, Collins M, Dejnirattisai W, Katzelnick LC, et al. Longitudinal analysis of antibody cross-neutralization following Zika Virus and Dengue Virus Infection in Asia and the Americas. J Infect Dis. 2018;218(4):536–45.
Andrade PE, Coloma J, Michlmayr D, Balmaseda AL, Harris E. Zika and dengue virus specific and cross-reactive memory B cell responses. J Immunol. 2017;98(1 Suppl):214.
Cordeiro MT, Pena LJ, Brito CA, et al. Positive IgM for Zika virus in the cerebrospinal fluid of 30 neonates with microcephaly in Brazil. Lancet. 2016;387:1811–2.
Bhatt S, Gething PW, Brady OJ, Messina JP, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504.
World Health Organization and the Special Programme for Research and Training in Tropical Diseases (TDR). Dengue guidelines for diagnosis, treatment, prevention and control: new edition. Publication date: 2009. Accessed online at http://www.who.int/rpc/guidelines/9789241547871/en/.
World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. Geneva: WHO; 1997. Accessed online at http://apps.who.int/iris/bitstream/10665/41988/1/9241545003_eng.pdf.
Centers for Disease Control and Prevention. Zika Virus Disease Q & A. Accessed at: https://www.cdc.gov/zika/about/questions.html.
Lozier MJ, Burke RM, Lopez J, Acevedo V, et al. Differences in prevalence of symptomatic Zika virus infection, by age and sex-Puerto Rico, 2016. J Infect Dis. 2018;217(11):1678–89.
Adebajo AO. Dengue arthritis. Br J Rheumatol. 1996;35:909–10.
Patil MM, Akki AS. Dengue arthritis in a child. Indian Pediatr. 2013;50(5):523–4.
Jayamali WD, Herath HMMTB, Kulatunga A. A young female presenting with unilateral sacroiliitis following dengue virus infection: a case report. J Med Case Rep. 2017;11(1):307.
Kularatne SA, Gihan MC, Weerasinghe SC, Gunasena S. Concurrent outbreaks of Chikungunya and Dengue fever in Kandy, Sri Lanka, 2006-07: a comparative analysis of clinical and laboratory features. Postgrad Med J. 2009;85(1005):342–6.
Bhaskar E, Sowmya G, Moorthy S. Musculoskeletal manifestations of dengue fever: is there a changing pattern? J Clin Rheumatol. 2012;18(2):102–3.
Londhey V, Agrawal S, Vaidya N, Kini S, Shastri JS, Sunil S. Dengue and Chikungunya virus co-infections: the inside story. J Assoc Physicians India. 2016;64(3):36–40.
Halsey ES, Marks MA, Gotuzzo E, Fiestas V, Suarez L, Vargas J, Aguayo N, Madrid C, Vimos C, Kochel TJ, Laguna-Torres VA. Correlation of serotype-specific dengue virus infection with clinical manifestations. PLoS Negl Trop Dis. 2012;6(5):e1638.
de Oliveira SA, Camacho LA, Bettini LR, Fernandes DG, Gouvea NA, Barros RA, Setúbal S, Siqueira MM. [The joint manifestations of exanthematous viroses]. Rev Soc Bras Med Trop. 1999;32:125–30.
Roimicher L, Ferreira OC Jr, Arruda MB, Tanuri A. Zika virus in the joint of a patient with rheumatoid arthritis. J Rheumatol. 2017;44(4):535.
Duffy MR, Chen TH, Hancock WT, Powers AM, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43.
Cherabuddi K, Iovine NM, Shah K, White SK, Paisie T, Salemi M, Morris JG Jr, Lednicky JA. Zika and Chikungunya virus co-infection in a traveller returning from Colombia, 2016: virus isolation and genetic analysis. JMM Case Rep. 2016;3(6):e005072.
Silva KR, Bica BERG, Pimenta ES, Serafim RB, et al. Fatal human case of Zika and Chikungunya virus co-infection with prolonged viremia and viruria. Diseases. 2018;6(3):53.
Read JS, Torres-Velasquez B, Lorenzi O, Rivera Sanchez A, et al. Symptomatic Zika virus infection in infants, children, and adolescents living in Puerto Rico. JAMA Pediatr. 2018;172(7):686–93.
Li HM, Huang YK, Su YC, Kao CH. Increased risk of autoimmune diseases in dengue patients: a population-based cohort study. J Infect. 2018;77(3):212–9. https://doi.org/10.1016/j.jinf.2018.03.014. Epub 2018 May 7.
Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Rodríguez Y, Ramírez-Santana C, Anaya JM. Zika virus and autoimmunity. One-step forward. Autoimmun Rev. 2017;16(12):1237–45. https://doi.org/10.1016/j.autrev.2017.10.008. Epub 2017 Oct 14. Review.
Zea-Vera AF, Parra B. Zika virus (ZIKV) infection related with immune thrombocytopenic purpura (ITP) exacerbation and antinuclear antibody positivity. Lupus. 2017;26:890–2.
Talib SH, Bhattu S, Bhattu R, Deshpande S, Dahiphale D. Dengue fever triggering systemic lupus erythematosus and lupus nephritis: a case report. Int Med Case Rep J. 2013;6:71–5.
Rajadhyaksha A, Mehra S. Dengue fever evolving into systemic lupus erythematosus and lupus nephritis: a case report. Lupus. 2012;21(9):999–1002.
Verdolin LD, Borner AR, Mussi H, Gismondi RA, Schau B, Ramos RC. Rhabdomyolysis associated with dengue fever in a lupic patient. Rev Bras Reumatol. 2014;54(4):318–21.
Harris VK, Danda D, Murali NS, Das PK, Abraham M, Cherian AM, Chandy M. Unusual association of Kikuchi’s disease and dengue virus infection evolving into systemic lupus erythematosus. J Indian Med Assoc. 2000;98(7):391–3.
Kumar S, Iuga A, Jean R. Cardiac tamponade in a patient with dengue fever and lupus nephritis: a case report. J Intensive Care Med. 2010;25(3):175–8.
de Souza SP, de Moura CG. Dengue mimicking a lupus flare. J Clin Rheumatol. 2010;16(1):47–8.
Chen WH. An unusual transitory increase of lupus anticoagulant in dengue virus infection complicated with cerebral ischaemia. J Infect. 2006;52(3):e87–91.
Santosa A, Poh Z, Teng GG. Delayed diagnosis of systemic lupus erythematosus due to misinterpretation of dengue serology. Scand J Rheumatol. 2012;41(1):77–9.
Wiwanitkit V. Delayed diagnosis of systemic lupus erythematosus due to misinterpretation of dengue serology: comments on the article by Santosa et al. Scand J Rheumatol. 2012;41(5):410–1.
Zainal N, Tan KK, Johari J, Hussein H, Wan Musa WR, Hassan J, Lin YS, AbuBakar S. Sera of patients with systemic lupus erythematosus cross-neutralizes dengue viruses. Microbiol Immunol. 2018;62(10):659–72.
Morel Z, Ramírez A. Autoimmune response in children with dengue. Case reports. Reumatol Clin. 2014;10(4):257–9.
Palacios-Cuervo F, Calderón-Rivera A, Espinal-Reyes F, Canelo-Aybar C. Autoimmunity in dengue: literature review. Reumatol Clin. 2016;12(3):173–4.
Colman N, Ojeda A, Yinde Y, Aquino A, Duarte M. Impacto de la infeccion por Dengue en pacientes con enfermedades autoinmunes. Revisión de casos. Rev Par Reumatol. 2017;3(1):20–4.
Agüero S, Toloza S, Lejtman N, Orellana Barrera S, Guaraz G. Manifestaciones clínicas y bioquímicas asociadas a infección por virus del dengue en pacientes con enfermedades reumáticas durante un brote epidémico en la provincia de Catamarca, República Argentina. Rev Arg Reumatol. 2011;22(3):14–28.
de Abreu MM, Maiorano AC, Tedeschi SK, Yoshida K, Lin TC, Solomon DH. Outcomes of lupus and rheumatoid arthritis patients with primary dengue infection: a seven-year report from Brazil. Semin Arthritis Rheum. 2018;47(5):749–55.
Deligny C, de Bandt M, Dehlinger V, Numéric P, Cabié A, Lombard F, Polomat K, JeanBaptiste G, Arfi S. Dengue fever in patients under biologics. J Clin Virol. 2014;61(3):442–3.
Wu WL, Ho LJ, Chen PC, Tsai YT, Hsu ST, Chang DM, Lai JH. Immunosuppressive effects and mechanisms of leflunomide in dengue virus infection of human dendritic cells. J Clin Immunol. 2011;31(6):1065–78.
Mancera-Páez O, Román GC, Pardo-Turriago R, Rodríguez Y, Anaya JM. Concurrent Guillain-Barré syndrome, transverse myelitis and encephalitis post-Zika: a case report and review of the pathogenic role of multiple arboviral immunity. J Neurol Sci. 2018;395:47–53.
Li GH, Ning ZJ, Liu YM, Li XH. Neurological manifestations of dengue infection. Front Cell Infect Microbiol. 2017;7:449.
Dalugama C, Shelton J, Ekanayake M, Gawarammana IB. Dengue fever complicated with Guillain-Barré syndrome: a case report and review of the literature. J Med Case Rep. 2018;12(1):137.
Malik S, Saran S, Dubey A, Punj A. Longitudinally extensive transverse myelitis following dengue virus infection: a rare entity. Ann Afr Med. 2018;17(2):86–9.
Herath HMM, Hewavithana JS, De Silva CM, Kularathna OAR, Weerasinghe NP. Cerebral vasculitis and lateral rectus palsy - two rare central nervous system complications of dengue fever: two case reports and review of the literature. J Med Case Rep. 2018;12(1):100.
Kamoi K, Mochizuki M, Ohno-Matsui K. Dengue fever-associated necrotizing scleritis: a case report with long-term follow-up. Medicine (Baltimore). 2018;97(32):e11875.
Vouga M, Baud D. Imaging of congenital Zika virus infection: the route to identification of prognostic factors. Prenat Diagn. 2016;36(9):799–811.
Olafuyi O, Badhan RKS. Dose optimisation of chloroquine by pharmacokinetic modelling during pregnancy for the treatment of Zika virus infection. J Pharm Sci. 2019;108(1):661–73. pii: S0022-3549(18)30689-0.
Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, et al. The global burden of dengue: a analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16(6):712–23.
Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016 Aug;16(8):935–41.
World Health Organization. Emergencies: one year into the Zika outbreak: how an obscure disease became a global health emergency. Accessed at: http://www.who.int/emergencies/zika-virus/articles/one-year-outbreak/en/index5.html.
Plourde AR, Bloch EM. A literature review of Zika virus. Emerg Infect Dis. 2016;22(7):1185–92.
Tebas P, Roberts CC, Muthumani K, Reuschel EL, et al. Safety and immunogenicity of an anti-Zika virus DNA vaccine - preliminary report. N Engl J Med. 2017; https://doi.org/10.1056/NEJMoa1708120.
World Health Organization. Dengue vaccine: WHO position paper, July 2016 - recommendations. Vaccine. 2017;35(9):1200–1.
World Health Organization. Questions and answers on dengue vaccines; 20 April 2018. Accessed at: https://www.who.int/immunization/research/development/dengue_q_and_a/en/.
Eppes C, Rac M, Dunn J, Versalovic J, et al. Testing for Zika virus infection in pregnancy: key concepts to deal with an emerging epidemic. Am J Obstet Gynecol. 2017;216(3):209–25.
Jentes ES, et al. Evidence-based risk assessment and communication: a new global dengue-risk map for travellers and clinicians. J Travel Med. 2016;23(6)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Toloza, S.M.A., Agüero, S.E. (2019). Arthritis Associated with Alphavirus Infections: Dengue and Zika. In: Espinoza, L. (eds) Infections and the Rheumatic Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-23311-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-23311-2_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23310-5
Online ISBN: 978-3-030-23311-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)