Abstract
Heat shock proteins (Hsp) are primarily protecting and maintaining cell viability during stressful conditions such as thermal and oxidative challenges through protein refolding and stabilization. Hsp play an essential role to confer eye protection from disease states particularly the diseases affecting the retina. Here, we summarize the Hsp function in normal retina, and their involvement in the pathogenesis of certain retinal diseases such cancer, glaucomatous retina, retinitis pigmentosa, and retinal neurodegeneration, as well as the age-related macular degeneration. This information would provide a better understanding of Hsp function and their involvement in ocular disease pathogenesis that could be a target for therapeutic purposes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Heat shock proteins (Hsp) are molecules with highly conserved structures, that have been revealed to share in protein quality control in eukaryotes, eubacteria and archaea (Ellis 2007; Lindquist and Craig 1988). Hsp are ubiquitously expressed in various subcellular parts (Jego et al. 2013). Hsp act as stress protein because they are quickly created after exposure to the unfavorable extrinsic factors such as high temperature, cytokine releasing and hypoxia. Italy geneticist Ferruccio Ritossa detected Hsp for the first time in the salivary gland of Drosophila larvae in 1962 (Kiang and Tsokos 1998). He noticed the swelling of the Drosophila’s salivary gland after the elevation of the environmental temperature, so he named this phenomenon by heat shock response (HSR). Hsp expression increase to improve the cells resistance to adverse lethal condition(s). The Hsp were nominated according to their molecular weights because their function was obscured at the time of detection. Therefore, the 70 kDa Hsp named as Hsp70 . Hsp are six types according to molecular weight variation. They are Hsp40, Hsp60, Hsp70, Hsp90, Hsp110, and small molecule heat shock protein (small Heat Shock Proteins, sHsp) (Lindquist and Craig 1988).
In eukaryotic organisms, heat shock protein 90 represents about 1–2% of cytosolic proteins so it considered the most abundant molecular chaperone (Garcia-Carbonero et al. 2013; Singh et al. 2015; Wainberg et al. 2013). Hsp90 significantly contributes to folding, maturation, stabilization, and activation of Hsp90 client proteins in cancer and normal cells (Waza et al. 2005). Moreover, Hsp90 plays an important role in the maintenance of genome stability, cell signaling and intracellular transport in normal cells (Kaplan and Li 2012). Furthermore, Hsp90 preserves these proteins from proteasomal degradation (Kaplan and Li 2012; Lin et al. 2016). After the exposure of cells to extrinsic stressors, Hsp90 resists against stress via two ways. First, Hsp90 correct folding of protein by proper refolding and elevates the rate of the reactivated proteins. Second, Hsp90 destroys non-functional proteins by proteasomal degradation through the polyubiquitination pathway. So, Hsp90 enhances cell survival in stress conditions by maintaining the protein homeostasis (Jolly and Morimoto 2000; Parsell and Lindquist 1993).
Cancer is a critical threat to humanity. In China, it was reported 200 deaths in 2015. The highest mortality rate was recorded in lung cancer cases, gastric cancer, esophageal cancer, and liver cancer respectively (Chen et al. 2016). There is a serious necessity for modern treatments for cancer as the opposition to traditional cytotoxic and cytostatic drugs is now widespread (Sauvage et al. 2017). Cancer cells have activated improper signaling pathways and higher metabolic requirements compared to normal cells thus need a chaperone machinery to survive (Chatterjee and Burns 2017). Mainly, oncoproteins of cancer require increased levels of Hsp chaperonage for their folding, stabilization, aggregation, activation, function and proteolytic degradation (Chatterjee et al. 2016; Neckers and Workman 2012). The expressions/activities of Hsp27, Hsp70, and Hsp90 chaperones are markedly higher in cancer (Garrido et al. 2006; Jego et al. 2013).
Recent studies of the molecular mechanism of tumor incidence have been detected that Hsp90 has a pivotal role in organizing proteins in various signaling pathways which contribute to the tumor carcinogenesis. Therefore, the Hsp90 is considered as a novel important research area in the field of anti-tumor biology (Rui et al. 2018) due to its upregulation in response to oxidative stress conditions in various human tumors (Saif et al. 2013; Tukaj et al. 2017). Moreover, its ATPase activity is boosted 50X in a cancerous microenvironment (Taiyab et al. 2009). Furthermore, Hsp90 stabilizes mutant proteins that manifest throughout cell transformation, thus enabling malignant transformation (Calderwood et al. 2006). Therefore, the suppression of Hsp90 influences processes engaged at the beginning of cancer which can be considered as the “Hallmarks of Cancer” (Miyata et al. 2013; Workman et al. 2007). Consequently, suppression of Hsp90 by proteasomal degradation and inhibitors can be effective in cancer treatment (Proia and Kaufmann 2015; Venkatesan et al. 2016; Wang et al. 2016).
In this chapter, we will first describe Hsp90 in terms of its structure and function inside the cells and then concentrate on the connection of Hsp90 to cancer especially retina cancer and the effects of Hsp90 inhibitors to improve cancer therapy. We will also underline the relationships of Hsp90 to retinal neurodegeneration to understand the role of Hsp90 in the incidence of retinal diseases.
1.1 HSP90 in Normal Retina
If metabolic stress affects living cells, they react by transient elevating the expression of HSP. The cause of this denomination is due to the temperature increase was the first-reported stimulator of the Hsp reaction. In mammals, a wide range of detrimental stimuli, like ischemia and hypoxia, and damaging chemical agents, like heavy metals and metabolic poisons are the main Hsp inducers (Kiang and Tsokos 1998). Recently, 90-kDa family (HSP90 family) is one of the HSP families that have drawn the interest of many researchers. Eukaryotic cells have three types of Hsp90s: cytosolic Hsp90 with two isoforms of Hsp90α and Hsp90β, mitochondrial Trap1 (tumor necrosis receptor-associated protein 1) and Grp94 (glucose-regulated protein 94) of the endoplasmic reticulum (ER) (Chiosis 2016; Workman et al. 2007). Hsp90 is a cytosolic protein which is present in either Hsp90β form or Hsp90α form. Although separate genes encode the two isoforms of Hsp90, they are 86% symmetrical at the gene translation level (Moore et al. 1989).
Under normal physiological conditions of eukaryotic cells, Hsp90 represents about 1–2% of total cellular protein so it could be considered one of the most abundant cytoplasmic proteins. However, its expression elevates many folds under stress conditions (Scheibel and Buchner 1998). Although Hsp90 acts as a molecular chaperone-like the other Hsp, it links to a specific subset of cellular proteins, including components of the cytoskeleton, including microfilaments and microtubules (Csermely et al. 1998; Czar et al. 1996); many tyrosine and serine-threonine protein kinases, like cyclin-dependent protein kinase 4 (CDK4), v-Raf/c-Raf and v-Src/c-Src (Csermely et al. 1998; Darimont 1999; Pratt 1998); and transcription factors like steroid hormone receptors (Csermely et al. 1998; Pratt 1998; Scheibel and Buchner 1998). It seems that Hsp90 regulates the signaling capacity of these transcription factors and (Csermely et al. 1998; Pratt 1997, 1998; Ylikomi et al. 1998) and it buffers the destructive changes in the regulatory proteins caused by minor gene mutations (Rutherford and Lindquist 1998).
It has been noticed the strong mRNA expression of murine Hsp90β and Hsp90α through early mammalian ocular development (embryonic days 11.5–14.5). Moreover, Hsp90β mRNA levels still high during later embryogenesis and adulthood but Hsp90α mRNA levels decreased (Tanaka et al. 1995). On the other hand, Kojima et al. (1996) detected that the rat Hsp90α mRNA expressed from embryonic day 17 to adulthood, except around postnatal day 5.
Hsp90 immunoreactivity was detected in most layers of the retina. The strongest immunohistochemical reaction for Hsp90 was detected in the outer limiting membrane (OLM). Lesser intense reactions were observed in the outer plexiform layer (OPL), inner plexiform layer (IPL), and the inner segment (IS). The remainder of the retina staining was noticed in the outer nuclear layer (ONL), inner nuclear layer (INL), and perinuclear regions of cells of the retinal ganglion cells (RGC) and was somewhat higher than the background at the tips of the outer segment (OS), where they are interlocked with processes from the retinal pigment epithelium (RPE). The low reaction was detected in the walls of blood vessels where RGC axons stained for Hsp90 and kept the same reaction as the axons become near the optic disc. On the other hand, a high reaction was observed in the innermost row of ONL nuclei, next to the OPL and near the retina-optic nerve junction. Moreover, RPE cells situated less than 200–250 μm from the optic disc expressed intense Hsp90 reaction, especially inside the cell nuclei. In addition, Hsp90 immunoreactivity was observed in the optic nerve. Furthermore, the Hsp90 immunoreactivity had a clear perinuclear and rare nuclear reaction in the glial cells (Dean and Tytell 2001).
It was suggested that Hsp90 could be involved in the synthesis of an Hsp90-protein substrate heterocomplex (fold some), which contains at least nine accessory proteins and Hsc70 (Pratt 1997). Dean et al. (1999) detected that all retinal layers, from IS to RGC, had a positive immunoreactivity for Hsp90 and Hsc70. These results are harmonious with the functions of Hsp90 and Hsc70 being interdigitated, suggesting both proteins were part of a fold some. The plexiform layers showed the only conflict in the staining patterns. The OPL and IPL had a lower immunoreactivity for Hsc70 than for Hsp90 (Dean et al. 1999). On the other hand, many factors can influence the strength of the immunoreaction in the tissue section, containing many technical aspects of the reactions utilized to detect the immunoreaction and variances in antibody affinity for the antigen. Therefore, other more quantitative analyses must be done to measure the amount of antigen in the retina more accurately.
One functional difference between Hsc70 and Hsp90 that may attribute to their various distributions in the retina is the role of the Hsc70 and Hsp90 in keeping active steroid hormone receptors. As the retina is recognized to be susceptible to corticosteroids and thus it contains receptors (Kobayashi et al. 1998; Mirshahi et al. 1996). The receptor distribution may be related partly to the retinal distribution of Hsp90. Moreover, the plexiform layers include the synaptic connections and cell processes of the retinal cells, so it could be suggested that Hsp90 can play in keeping the structures engaged in forming synaptic junctions. There are some proofs to give a possibility that Hsp may be situated at synaptic connections between retinal cells and that Hsp can preserve synaptic functions in some eukaryotes by altering the properties of their synapses (Karunanithi et al. 1999).
Hsp70 and Hsp90 may play a pivotal part in the preserving of Müller-photoreceptor and Müller–Müller cell junctional specializations localized at the OLM. Karunanithi et al. (1999) have mentioned that the induction of Hsp and especially Hsp70 protects synaptic transmission at the Drosophila larval neuromuscular junction. Moreover, his group revealed that the process of Hsp70 induction seems to include pre- and postsynaptic modifications (Karunanithi et al. 1999).
Black et al. (1985) detected that the optic nerve has three distinct morphologic regions near the retina- optic nerve junction, the optic nerve proper (ONP), the retina-optic nerve transition region (ROT) and the optic nerve head (ONH). The pattern of distribution of Hsp90 and Hsc70 in the optic nerve was the same reported by Dean et al. (1999) and was obviously unlike the pattern detected for Hsp25. The majority of the Hsp90 immunostaining was concentrated in the juxtanuclear cytoplasm of the glial cells without any reaction in the nerve fibers.
Dean and Tytell (2001) were reported that there was no sudden elevation in Hsp90 immunoreactivity as the RGC axons entered the optic nerve head and left the retina. They assumed that Hsp25 is the highest distributed Hsp in the axons of the normal optic nerve, while Hsp90 and Hsc70 are localized mainly in the glia of the optic nerve. The functional importance of these variances in distribution stills to be investigated, but it boosts the hypothesis that each of the Hsp has characteristic roles in the different cellular structures of the optic nerve and retina (Dean and Tytell 2001).
It has been supposed that Hap90 is pivotal for neuronal polarization and axonal elongation in the retina. Neuronal function relays on the establishing of various functional and morphological differentiated domains; dendrites, soma, axon initial segment, and axon. The morphological differentiation begins with the specification of one of the initial neurites as the axon and its subsequent elongation. This process needs the dynamics and coordinated reorganization of microtubule and actin cytoskeletons (Bradke and Dotti 1999; Da Silva and Dotti 2002).
Cytoskeleton dynamic behavior is organized by microfilament and microtubule-associated proteins (ABPs or MAPs) and tubulin post-translational modifications as well (Bradke and Dotti 1999; Tapia et al. 2010). Various signaling pathways, like PI3K pathway, are engaged in axon growth and specification (Shi et al. 2003), including proteins like GSK3, Akt (Shi et al. 2004; Yan et al. 2006), Rac, GTPases Rho, Par3/Par6. The axonal specification was suggested to be because of the local accumulation of some specific proteins, that reveals a fine organizing of its synthesis and local degradation mechanisms. There are two suggested mechanisms to concentrate these proteins at the future axon tip. First, kinesins can specifically target proteins to the axon tip. In fact, KIF5 is expressed in several neurites before axon specification but appears absent from the dendrites and selectively accumulated in the emerging axon when the axon is specified (Jacobson et al. 2006). Second, protein degradation in other neurites causes axon selective accumulation of these proteins (Schwamborn et al. 2007).
In non-neuronal systems, Hsp90 proteins assist in the regulation of intracellular protein activity, including protein transport (Johnson et al. 1996). Hsp90 proteins are associated with specific serine and tyrosine kinases (Mimnaugh et al. 1995), calmodulin, actin, and tubulin (Sanchez et al. 1988). Loss of Hsp90 activity suppresses the cellular response to both v-src kinase activity, as well as the nerve growth factor (NGF)- mediated signaling pathway (Jaiswal et al. 1996; Xu and Lindquist 1993), proposing a role for Hsp90 in the organizing of these pathways. Moreover, Hsp90 protein associations can change intracellular protein activation and function (Nathan and Lindquist 1995).
Hsp90 protein and mRNA are basically expressed at high levels in neural tissue, including brain and retina (Tanaka et al. 1995). Therefore, Hsp90 proteins may be supposed to have a selective role in neural function. Bernstein et al. (1996) previously detected that Hsp90 mRNA is expressed at higher levels at the central region of the primate retina (the fovea) than the retinal periphery (Bernstein et al. 1996). Moreover, Hsp90 mRNA was detected to be selectively expressed in rodent retinal ganglion cells (RGCs) (Tanaka et al. 1995). Because of the concentration of RGCs in the fovea region of the primate retina (Shapley and Perry 1986), so, it is possible that Hsp90 has an RGC-specific or intensive function.
1.2 The Role of HSP90 in Cancer Biology
If the external temperature elevates significantly over normal body temperature, the most cells consequently respond by increasing the production of a limited class of proteins termed heat shock proteins. Inside the cells, the interactions between proteins lead to their proteolytic turnover, intracellular disposition, and folding. Hypoxia and acidosis are common conditions within tumors, and it was detected the elevated chaperone proteins expression in many types of solid tumors (Kimura et al. 1993; Santarosa et al. 1997) which may explain the ability of malignant cells to maintain homeostasis in an antagonistic environment. Chaperone proteins enhance cell resistance against environmental stressors and also consolidate tumor cells to endure the internal alterations, such as mutations of essential signaling molecules that would be fatal (Takayama et al. 2003). Chaperones can act as biochemical buffers for the genetic shakiness that is common in numerous human cancers.
The elevated heat shock protein levels detected in advanced cancers could explain the suitable cellular response to the microenvironment stressors inside the tumor such as nutrient-deprivation, acidosis, hypoxia. On the other hand, Hsp can assist tumor cells to get away from the apoptotic death that caused by the imbalanced signaling accompanied with neoplastic transformation (Takayama et al. 2003). One of the prevalent features of cancer cells is the deterioration of apoptotic signaling, which enhances tumor expansion and survival by separating them from normal regulatory factors so, tumor becomes reluctant to both chemotherapeutic drugs and host defense mechanisms (Jäättelä 1999; Sliutz et al. 1996).
It was mentioned that Hsp90 and its cochaperones modify tumor cell apoptosis. Most of this activity mediated by influences on tumor necrosis factor receptors (Vanden Berghe et al. 2003), AKT (Basso et al. 2002), and nuclear factor-nB function (Chen et al. 2002). On the other hand, Hsp90 may contribute in neoplastic transformation than suppressing apoptosis. Hsp90 is unique among all heat shock proteins as it is not needed for the biogenesis of most polypeptides (Nathan et al. 1997). On the other side, many of its client proteins or cellular substrates are labile signal transducers that have a pivotal role in tissue development, cell survival, and growth control (Pratt 1998).
The more notable Hsp90 client proteins related to cancer are receptor tyrosine kinases (EGFR, HER2, FLT3, and IGF1R), SRC family kinases (FYN, LCK, and SRC), cell cycle G2 checkpoint kinases (POLO-1, MYT1, and WEE1), serine/threonine kinases (CDK4, AKT, and RAF-1), steroid hormone receptors (androgen, glucocorticoid, progesterone, and estrogen), mutant fusion kinases (NPM-ALK and BCR-ABL), transcription factors (HIF-1, HSF-1, and p53) (Karagoz and Rudiger 2015).
1.3 Roles of HSP90 in Neurodegeneration
Hsp90 has numerous pivotal preserving roles for the functional viability and stability of cells against the mutating pressure (Chiosis 2006; Whitesell and Lindquist 2005). When protein aggregate in neurodegenerative disorders, the suppression of Hsp90 stimulates heat shock factor-1 (HSF-1) to produce Hsp40 and Hsp70, in addition to other chaperones, which in turn, enhance protein degradation (Klettner 2004; Muchowski and Wacker 2005). On the other hand, Luo et al. (2008) detected the protective effect of Hsp90 on the neuronal proteins of thumping capacity in the neurodegeneration, so enhances the gathering of toxic aggregates.
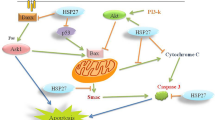
The heat shock reaction is predominately regulated by HSF-1. When the cells exposed to stressors, they normally react by stimulation of the heat shock response (HSR) associated with elevated induction of cytoprotective Hsp which reduce cytotoxicity resulting from denatured and misfolded proteins (Klettner 2004; Muchowski and Wacker 2005). The most transition events happen on the transcriptional level. The stressors start the destruction of protein by stimulation of HSF-1, which links to upstream regulatory sequences in the promoters of heat shock genes (Anckar and Sistonen 2007). The stimulation of HSF-1 begins via a multi-step pathway, including a monomer-to trimer transition, nuclear accumulation and extensive post-translational modifications (Fig. 9.1). Hsp90 regulates the function of HSF-1 (Zou et al. 1998). Under normal circumstances, Hsp90 links to HSF-1 and maintains the transcription factor in a monomeric state. Inhibition of Hsp90 or stressor releases HSF-1 from the Hsp90-HSF-1 complex, which leads to its trimerization, activation, and translocation of HSF-1 to the nucleus where it begins a heat shock reaction, revealed by the production of Hsp70 and Hsp40. Neurons have been detected to be reluctant to Hsp stimulation following heat shock (Brown 2007).
Suppression of Hsp90, heat shock or stress releases HSF-1 from the Hsp90 complex, which leads to its trimerization, activation, phosphorylation and translocation to the nucleus where it initiates a heat shock response, revealed by the production of Hsp like the chaperones Hsp70 and its activator, Hsp40
Tauopathies are neurodegenerative diseases recognized by tau protein abnormalities which lead to an accumulation of aggregated and hyperphosphorylated tau (Kosik and Shimura 2005). In Alzheimer’s disease (AD), Lau et al. (2002) supposed that tau hyperphosphorylation is a pathogenic pathway resulted from thumping stimulation of many kinases, particularly, glycogen synthase kinase-3 beta (GSK3β) and cyclin-dependent protein kinase 5 (CDK5), leading to phosphorylation of tau on pathogenic sites (Lau et al. 2002). Goedert and Jakes (2005) suggested that hyperphosphorylated tau in the AD misfolds microtubules net and synthesis of toxic tau aggregates. In a collection of tauopathies called “frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17)”, the mutation results from many transformations in human tau isoforms on chromosome 17, that lead to the accumulation of aggregated tau comparable to that in the AD (Goedert and Jakes 2005). In tauopathies, it has been mentioned that Hsp90 preserves the stability of p25 and p35, neuronal proteins that stimulate CDK5 via complex formation resulting in thumping tau phosphorylation, and Hsp90 maintains the mutant but not wild-type tau protein in tauopathies (Luo et al. 2007). Suppression of Hsp90 in mouse and cellular models of tauopathies resulted in a decrease of the pathogenic activity of these proteins and led to a time- and dose-dependent discarding of aggregated tau (Luo et al. 2007).
1.4 HSP90 Inhibition Protects Against Inherited Retinal Disease
Hsp90 is a highly conserved and copious molecular chaperone that is engaged in numerous cellular processes, containing the functional maturation of substrate proteins, which are defined as ‘clients’ (Li and Buchner 2013; Taipale et al. 2010). Many of client proteins are oncogenes, resulting in Hsp90 protruding as a pivotal target in various types of cancer treatment (Pearl et al. 2008). Post-translational modifications and nucleotide binding regulate Hsp90 function (Walton-Diaz et al. 2013). Hsp90 inhibitors connect with a high affinity to the ATP-binding pocket and block the chaperone ATPase cycle resulting in the destruction of client proteins (Li and Buchner 2013; Pearl et al. 2008). Moreover, suppression of Hsp90 function deactivates the chaperone complex with Heat Shock Factor 1 (HSF-1), leading to the activation of HSF-1 and initiation of heat shock protein expression (Zou et al. 1998). Subsequently, Hsp90 suppression can trigger a dual influence, the proteasome-mediated damage of Hsp90 client proteins and activation of HSF-1, which enhances Hsp70 and other chaperones to protect against protein aggregation and lower protein toxicity (Labbadia et al. 2011; Sittler et al. 2001).
1.5 Retinitis Pigmentosa
RP is a hereditary photoreceptor degeneration resulted from transformations in the rhodopsin gene which is considered the most prevalent origin of autosomal dominant RP. Mendes and Cheetham (2008) detected protection of the Hsp90 inhibitor 17-N-allylamino-17-demethoxygeldanamycin (17-AAG) against rhodopsin toxicity and P23H, which is deemed to be the most prevalent rhodopsin transformation in the USA. This protection depends on HSF-1 because 17-AAG could not protect mouse embryonic fibroblasts isolated from HSF-1 knock-out mice against P23H rhodopsin aggregation suggesting the dependence of protective influence on the induction of the stress response (Aguila et al. 2014).
Aguila et al. (2014) detected the enhancement of systemic administration of the blood-brain barrier permeable (HSP990), Hsp90 inhibitor, to HSF-1 and the beginning of molecular chaperone expression in vivo in the retina. A single minimum dose of HSP990 was enough to improve photoreceptor survival and visual function of a P23H rhodopsin transgenic rat model which had advanced retinal degeneration. Interestingly, this treatment lowered rhodopsin aggregation and stimulated molecular chaperones but did not influence any phototransduction component, suggesting the efficiency of Hsp90 suppression to induce the proteostasis mechanism which safeguards against misfolded proteins (Aguila et al. 2014). Inosine-5′-monophosphate dehydrogenase (IMPDH) misfolding mutations associated with RP10 is another example of using Hsp90 suppression to target photoreceptor proteostasis imbalances. In this case, Tam et al. (2010) utilized claudin 5 RNAi to temporary lower the permeability of the blood-retinal barrier and let 17-AAG to induce a preservative response in photoreceptors expressing R224P mutant IMPDH accompanied with lowering in mutant aggregated IMPDH and safeguarding the outer nuclear layer (ONL) structure (Tam et al. 2010).
Moreover, suppression of Hsp90 in another class of rhodopsin mutation (R135L) disease model was preventative, but the Hsp90 inhibition machinery was separated from HSF-1. The R135L mutation blocks binding and thumping rhodopsin endocytosis which harmfully influences vesicular traffic and causes rhodopsin hyperphosphorylation (Chuang et al. 2004).
Hsp90 suppression prohibited the mobilization of arrestin to R135L mutant rhodopsin and therefore mitigated thumping endocytosis (Aguila et al. 2014). HSF-1 null cells kept the influence of Hsp90 suppression suggesting that it was independent of HSF-1. Furthermore, a study conducted by Aguila et al. (2014) showed that rhodopsin kinase (GRK1) is a Hsp90 client protein and the influence of Hsp90 suppression on R135L rhodopsin arrest was mediated by lowering of phosphorylation upstream of R135L due to a suitable kinase deficiency (Aguila et al. 2014). This mechanism linked to the decrease of a specific client protein that is interposing an adverse influence of a genetic mutation is distinguished from the enhanced production of protective factors via the enhancement of the stress response to combat a mutational consequence. We can conclude the multiple roles of Hsp90 in the retina. Moreover, the usage of Hsp90 inhibitors has potential protective roles against various types of RP via various mechanisms.
1.6 Age-Related Macular Degeneration (AMD) and RPE Biology
AMD is a multifactorial disease including environmental, genetic, and metabolic factors. Cell death and functional anomalies in the RPE cells have a pivotal role in the AMD progress and are accompanied with elevated oxidative stress (Jarrett and Boulton 2012). Decanini et al. (2007) detected the normal expression of Hsp90 in RPE cells and its marked elevated expression during the AMD progress. Moreover, Qin et al. (2011) suggested that Hsp90 detected in necrotic RPE cells may act as an inducer for inflammatory reactions in neighboring healthy RPE. Therefore, Hsp90 suppression can prohibit the inflammatory reactions in RPE cells (Wang et al. 2010). In addition, (Wu et al. 2007) detected the ability of the Hsp90 inhibitor geldanamycin to suppress vascular endothelial growth factor expression in hypoxic RPE cells, suggesting that Hsp90 inhibitors can be a potential treatment for both neovascularization and inflammation.
In age-related macular degeneration, damage to the RPE layer leads to the death of foveal cone photoreceptors that are in charge of the sharp central vision (Kaarniranta et al. 2013). This means that RPE cells are essential in the progression of AMD. Recently, inflammasome signaling has been accompanied with the pathogenesis of AMD (Kauppinen et al. 2012; Tarallo et al. 2012). As reviewed by Harris and Rubinsztein (2011), inflammasomes and autophagy control each other, suggesting that autophagy could be in charge of the discarding of unnecessary inflammasome components. Autophagy is the cellular degradation system, particularly when proteasomal degradation has failed. It is established that proteasomal activity reduces during the aging process which is accompanied by the pathogenesis of AMD (Jung et al. 2009; Li et al. 2008). If NLRP3 inflammasome is translated after the priming signal, it is removed from the cell unless safeguarded by a protein complex including Hsp90 (Martinon 2008; Mayor et al. 2007). Mayor et al. revealed that in the obscurity of Hsp90, NLRP3 will be destructed by the proteasome (Mayor et al. 2007).
1.7 HSP90 in Glaucomatous Retina
It is well known that Hsp90 is ceaselessly expressed in mammalian cells (Park et al. 2007). Moreover, heat shock stress induces its expression in retinal Müller cells. Immunostaining for Hsp90 was detected to be stronger than Hsp70 in the glaucomatous retinas compared to the control retinas, harmonious with the fact that the apoptotic pathway is one of the key mechanisms included in glaucomatous neuropathy, and that Hsp are upregulated to suppress apoptosis and save neuronal cells. Elevated immunoreactivity for Hsp90 in glaucomatous retinas was detected at the nerve fiber layers and ganglion cell. The GFAP immunolabeling and Hsp90 were similar in both glaucomatous and normal eyes. Importantly, the retinal localization of Hsp90 was similar to GFAP, a glial cell marker, in glaucoma eyes. It is well established that retinal glial cells are stimulated with glaucomatous neuropathy (Tanihara et al. 1997). Moreover, Müller cells have been detected to generate Hsp90 under heat shock stress 32 and to produce neuroprotective cytokines like ciliary neurotrophic factor after retinal injury (Honjo et al. 2000). These cells may hold an inherent neuroprotective capability in the retina. The identical staining pattern of GFAP and Hsp90 indicated that stimulated Müller cells may be a pivotal source of the Hsp (Sakai et al. 2003).
1.8 Role of HSP90 in Prevention of Retinal Cancer
Over-expressed Hsp90 were detected in many cancer types like retinoblastoma, glioblastoma (Jiang et al. 2008) and a marked increase has been detected in tumor development (Gyrd-Hansen et al. 2004). Abundant oncogenically activated proteins like FLT3, KIT, CDKs, AKT, and HIF are accompanied with Hsp90 (Li et al. 2009). Among Hsp90 client proteins, many are engaged in the so-called “hallmarks of cancer”, first proposed by Hanahan and Weinberg (2011) and subsequently participate in tumorigenic cell stabilization comprises protein kinases. The tyrosine kinase inhibitor resistance might be treated by Hsp90 inhibition, which supposes that the drug opposition in cancer may be dependent on the Hsp90 function due to their abilities as the chaperones to protect the oncoproteins against degradation (He et al. 2013).
Survivin, a suppressor of apoptosis (IAP) gene family member, is one of the Hsp90 client proteins which is over-expressed in abundant cancers containing retinoblastoma (Sudhakar et al. 2013). Survivin shows a multifaceted role in apoptosis suppression, cell division, tumor development and cell cycle organization in different clinical cancers (Kanwar et al. 2013). Inhibition of Survivin causes mitochondrial apoptosis, suppresses cell proliferation and thus elevates the receptivity of tumor cells to chemotherapy (Trabulo et al. 2011).
Hsp90 regulates proteostasis of Survivin by refolding the unfolded/denatured protein to a native state. Hsp90-Survivin can act as a possible aim for RB cancer therapy because the percentage of Survivin and Hsp90 is detected to be high (Jiang et al. 2008). Targeting the ATP-binding pocket of Hsp90 disrupts of Hsp90/Survivin physical interaction and suppresses the downstream signaling cascade of Survivin, resulting in the ubiquitination and inhibition of Survivin signaling pathways leading to tumor growth cease (Meli et al. 2006). These detections suggest the strong possibility of targeting Hsp90-Survivin interaction as an adjuvant therapy in RB control.
2 Conclusions
Finally, we can conclude that cell stress especially heat stress is one factor that triggers the onset and progression of Hsp90 in cancer and neurodegenerative diseases where we show the significant impact of Hsp90 on tissues and retina. Thus, the different dynamics and conformational changes of Hsp90 and client proteins could be obviously a landmark for different mechanisms, binding sites and binding ability of Hsp90 for stress response due to the failure of meeting the energy demand which induced by heat stress. Therefore further understanding in the relationship between the client proteins that can be chaperoned by Hsp90 and Hsp90 inhibitors is required for future therapies and the way to control these inhibitors at a physiological level and subsequently removing the factor causing protein unfolding and degradation without affecting the homeostasis of the tissue will be a step forward in treating cancer in general and other aged related diseases of the retina specially AMD.
Abbreviations
- AMD:
-
Age-related macular degeneration
- GFAP:
-
Glial fibrillary acidic protein
- Hsp:
-
Heat shock proteins
- IMPDH:
-
Inosine-5′-monophosphate dehydrogenase
- RP:
-
Retinitis pigmentosa
- RPE:
-
Retinal pigment epithelium
References
Aguila M, Bevilacqua D, McCulley C, Schwarz N, Athanasiou D, Kanuga N, Novoselov SS, Lange CA, Ali RR, Bainbridge JW, Gias C, Coffey PJ, Garriga P, Cheetham ME (2014) Hsp90 inhibition protects against inherited retinal degeneration. Hum Mol Genet 23:2164–2175
Anckar J, Sistonen L (2007) Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol 594:78–88
Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N (2002) Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem 277:39858–39866
Bernstein SL, Borst DE, Neuder ME, Wong P (1996) Characterization of a human fovea cDNA library and regional differential gene expression in the human retina. Genomics 32:301–308
Black JA, Waxman SG, Hildebrand C (1985) Axo-glial relations in the retina-optic nerve junction of the adult rat: freeze-fracture observations on axon membrane structure. J Neurocytol 14:887–907
Bradke F, Dotti CG (1999) The role of local actin instability in axon formation. Science 283:1931–1934
Brown IR (2007) Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci 1113:147–158
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31:164–172
Chatterjee S, Burns TF (2017) Targeting heat shock proteins in cancer: a promising therapeutic approach. Int J Mol Sci 18:1978
Chatterjee S, Bhattacharya S, Socinski MA, Burns TF (2016) HSP90 inhibitors in lung cancer: promise still unfulfilled. Clin Adv Hematol Oncol 14:346–356
Chen G, Cao P, Goeddel DV (2002) TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell 9:401–410
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132
Chiosis G (2006) Targeting chaperones in transformed systems – a focus on Hsp90 and cancer. Expert Opin Ther Targets 10:37–50
Chiosis G (2016) Heat shock proteins in disease–from molecular mechanisms to therapeutics. Curr Top Med Chem 16:2727
Chuang J-Z, Vega C, Jun W, Sung C-H (2004) Structural and functional impairment of endocytic pathways by retinitis pigmentosa mutant rhodopsin-arrestin complexes. J Clin Invest 114:131–140
Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G (1998) The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther 79:129–168
Czar MJ, Welsh MJ, Pratt WB (1996) Immunofluorescence localization of the 90-kDa heat-shock protein to cytoskeleton. Eur J Cell Biol 70:322–330
Da Silva JS, Dotti CG (2002) Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci 3:694
Darimont BD (1999) The Hsp90 chaperone complex-a potential target for cancer therapy? World J Gastroenterol 5:195–198
Dean DO, Tytell M (2001) Hsp25 and −90 immunoreactivity in the normal rat eye. Invest Ophthalmol Vis Sci 42:3031–3040
Dean DO, Kent CR, Tytell M (1999) Constitutive and inducible heat shock protein 70 immunoreactivity in the normal rat eye. Invest Ophthalmol Vis Sci 40:2952–2962
Decanini A, Nordgaard CL, Feng X, Ferrington DA, Olsen TW (2007) Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol 143:607–615
Ellis RJ (2007) Protein misassembly. In: Csermely P, Vígh L (eds) Molecular aspects of the stress response: chaperones, membranes and networks. Springer New York, New York, pp 1–13
Garcia-Carbonero R, Carnero A, Paz-Ares L (2013) Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol 14:e358–e369
Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G (2006) Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle 5:2592–2601
Goedert M, Jakes R (2005) Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta 1739:240–250
Gyrd-Hansen M, Nylandsted J, Jäättelä M (2004) Heat shock protein 70 promotes cancer cell viability by safeguarding lysosomal integrity. Cell Cycle 3:1484–1485
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Harris H, Rubinsztein DC (2011) Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol 8:108–117
He S, Zhang C, Shafi AA, Sequeira M, Acquaviva J, Friedland JC, Sang J, Smith DL, Weigel NL, Wada Y (2013) Potent activity of the Hsp90 inhibitor ganetespib in prostate cancer cells irrespective of androgen receptor status or variant receptor expression. Int J Oncol 42:35–43
Honjo M, Tanihara H, Kido N, Inatani M, Okazaki K, Honda Y (2000) Expression of ciliary neurotrophic factor activated by retinal Muller cells in eyes with NMDA- and kainic acid-induced neuronal death. Invest Ophthalmol Vis Sci 41:552–560
Jäättelä M (1999) Escaping cell death: survival proteins in cancer. Exp Cell Res 248:30–43
Jacobson C, Schnapp B, Banker GA (2006) A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron 49:797–804
Jaiswal RK, Weissinger E, Kolch W, Landreth GE (1996) Nerve growth factor-mediated activation of the mitogen-activated protein (MAP) kinase cascade involves a signaling complex containing B-Raf and HSP90. J Biol Chem 271:23626–23629
Jarrett SG, Boulton ME (2012) Consequences of oxidative stress in age-related macular degeneration. Mol Asp Med 33:399–417
Jego G, Hazoumé A, Seigneuric R, Garrido C (2013) Targeting heat shock proteins in cancer. Cancer Lett 332:275–285
Jiang L, Liu X, Li B, He X, Jin Y, Li L, Gao F, Wang N (2008) Heat shock proteins and survivin: relationship and effects on proliferation index of retinoblastoma cells. Histol Histopathol 23:827–832
Johnson J, Corbisier R, Stensgard B, Toft D (1996) The involvement of p23, hsp90, and immunophilins in the assembly of progesterone receptor complexes. J Steroid Biochem Mol Biol 56:31–37
Jolly C, Morimoto RI (2000) Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst 92:1564–1572
Jung T, Catalgol B, Grune T (2009) The proteasomal system. Mol Asp Med 30:191–296
Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Vereb Z, Salminen A, Boulton ME, Petrovski G (2013) Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 9:973–984
Kanwar JR, Kamalapuram SK, Kanwar RK (2013) Survivin signaling in clinical oncology: a multifaceted dragon. Med Res Rev 33:765–789
Kaplan KB, Li R (2012) A prescription for ‘stress’–the role of Hsp90 in genome stability and cellular adaptation. Trends Cell Biol 22:576–583
Karagoz GE, Rudiger SG (2015) Hsp90 interaction with clients. Trends Biochem Sci 40:117–125
Karunanithi S, Barclay JW, Robertson RM, Brown IR, Atwood HL (1999) Neuroprotection at Drosophila synapses conferred by prior heat shock. J Neurosci 19:4360
Kauppinen A, Niskanen H, Suuronen T, Kinnunen K, Salminen A, Kaarniranta K (2012) Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells – implications for age-related macular degeneration (AMD). Immunol Lett 147:29–33
Kiang JG, Tsokos GC (1998) Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther 80:183–201
Kimura E, Enns RE, Alcaraz JE, Arboleda J, Slamon DJ, Howell SB (1993) Correlation of the survival of ovarian cancer patients with mRNA expression of the 60-kD heat-shock protein HSP-60. J Clin Oncol Off J Am Soc Clin Oncol 11:891–898
Klettner A (2004) The induction of heat shock proteins as a potential strategy to treat neurodegenerative disorders. Drug News Perspect 17:299–306
Kobayashi K, Kobayashi H, Ueda M, Honda Y (1998) Estrogen receptor expression in bovine and rat retinas. Invest Ophthalmol Vis Sci 39:2105–2110
Kojima M, Hoshimaru M, Aoki T, Takahashi JB, Ohtsuka T, Asahi M, Matsuura N, Kikuchi H (1996) Expression of heat shock proteins in the developing rat retina. Neurosci Lett 205:215–217
Kosik KS, Shimura H (2005) Phosphorylated tau and the neurodegenerative foldopathies. Biochim Biophys Acta 1739:298–310
Labbadia J, Cunliffe H, Weiss A, Katsyuba E, Sathasivam K, Seredenina T, Woodman B, Moussaoui S, Frentzel S, Luthi-Carter R, Paganetti P, Bates GP (2011) Altered chromatin architecture underlies progressive impairment of the heat shock response in mouse models of Huntington disease. J Clin Invest 121:3306–3319
Lau LF, Schachter JB, Seymour PA, Sanner MA (2002) Tau protein phosphorylation as a therapeutic target in Alzheimer’s disease. Curr Top Med Chem 2:395–415
Li J, Buchner J (2013) Structure, function and regulation of the hsp90 machinery. Biom J 36:106–117
Li Y, Wang YS, Shen XF, Hui YN, Han J, Zhao W, Zhu J (2008) Alterations of activity and intracellular distribution of the 20S proteasome in ageing retinal pigment epithelial cells. Exp Gerontol 43:1114–1122
Li Y, Zhang T, Schwartz SJ, Sun D (2009) New developments in Hsp90 inhibitors as anti-cancer therapeutics: mechanisms, clinical perspective and more potential. Drug Resist Updat 12:17–27
Lin T-Y, Guo W, Long Q, Ma A, Liu Q, Zhang H, Huang Y, Chandrasekaran S, Pan C, Lam KS (2016) HSP90 inhibitor encapsulated photo-theranostic nanoparticles for synergistic combination cancer therapy. Theranostics 6:1324
Lindquist S, Craig E (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Luo W, Dou F, Rodina A, Chip S, Kim J, Zhao Q, Moulick K, Aguirre J, Wu N, Greengard P, Chiosis G (2007) Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc Natl Acad Sci USA 104:9511–9516
Luo W, Rodina A, Chiosis G (2008) Heat shock protein 90: translation from cancer to Alzheimer’s disease treatment? BMC Neurosci 9:S7–S7
Martinon F (2008) Detection of immune danger signals by NALP3. J Leukoc Biol 83:507–511
Mayor A, Martinon F, De Smedt T, Petrilli V, Tschopp J (2007) A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol 8:497–503
Meli M, Pennati M, Curto M, Daidone MG, Plescia J, Toba S, Altieri DC, Zaffaroni N, Colombo G (2006) Small-molecule targeting of heat shock protein 90 chaperone function: rational identification of a new anticancer lead. J Med Chem 49:7721–7730
Mendes HF, Cheetham ME (2008) Pharmacological manipulation of gain-of-function and dominant-negative mechanisms in rhodopsin retinitis pigmentosa. Hum Mol Genet 17:3043–3054
Mimnaugh EG, Worland PJ, Whitesell L, Neckers LM (1995) Possible role for serine/threonine phosphorylation in the regulation of the heteroprotein complex between the hsp90 stress protein and the pp60v-src tyrosine kinase. J Biol Chem 270:28654–28659
Mirshahi M, Nicolas C, Mirshahi A, Hecquet C, d’Hermies F, Faure JP, Agarwal MK (1996) The mineralocorticoid hormone receptor and action in the eye. Biochem Biophys Res Commun 219:150–156
Miyata Y, Nakamoto H, Neckers L (2013) The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des 19:347–365
Moore SK, Kozak C, Robinson EA, Ullrich SJ, Appella E (1989) Murine 86- and 84-kDa heat shock proteins, cDNA sequences, chromosome assignments, and evolutionary origins. J Biol Chem 264:5343–5351
Muchowski PJ, Wacker JL (2005) Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 6:11–22
Nathan DF, Lindquist S (1995) Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol Cell Biol 15:3917–3925
Nathan DF, Vos MH, Lindquist S (1997) In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci USA 94:12949–12956
Neckers L, Workman P (2012) Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18:64–76
Park JW, Moon C, Yun S, Kim SY, Bae YC, Chun M-H, Moon J-I (2007) Differential expression of heat shock protein mRNAs under in vivo glutathione depletion in the mouse retina. Neurosci Lett 413:260–264
Parsell D, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496
Pearl LH, Prodromou C, Workman P (2008) The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J 410:439–453
Pratt WB (1997) The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu Rev Pharmacol Toxicol 37:297–326
Pratt WB (1998) The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med 217:420–434
Proia DA, Kaufmann GF (2015) Targeting heat-shock protein 90 (HSP90) as a complementary strategy to immune checkpoint blockade for cancer therapy. Cancer Immunol Res 3:583–589
Qin S, Ni M, Wang X, Maurier-Mahe F, Shurland DL, Rodrigues GA (2011) Inhibition of RPE cell sterile inflammatory responses and endotoxin-induced uveitis by a cell-impermeable HSP90 inhibitor. Exp Eye Res 93:889–897
Rui Z, Xiao-Yun G, Xing-Chuang X, You J, Ze-Jian H, Xiang F (2018) Progress in molecular chaperone regulation of heat shock protein 90 and cancer. Chin J Anal Chem 46:301–308
Rutherford SL, Lindquist S (1998) Hsp90 as a capacitor for morphological evolution. Nature 396:336
Saif M, Erlichman C, Dragovich T, Mendelson D, Toft D, Burrows F, Storgard C, Von Hoff D (2013) Open-label, dose-escalation, safety, pharmacokinetic, and pharmacodynamic study of intravenously administered CNF1010 (17-(allylamino)-17-demethoxygeldanamycin [17-AAG]) in patients with solid tumors. Cancer Chemother Pharmacol 71:1345–1355
Sakai M, Sakai H, Nakamura Y, Fukuchi T, Sawaguchi S (2003) Immunolocalization of heat shock proteins in the retina of normal monkey eyes and monkey eyes with laser-induced glaucoma. Jpn J Ophthalmol 47:42–52
Sanchez ER, Redmond T, Scherrer LC, Bresnick EH, Welsh MJ, Pratt WB (1988) Evidence that the 90-kilodalton heat shock protein is associated with tubulin-containing complexes in L cell cytosol and in intact PtK cells. Mol Endocrinol 2:756–760
Santarosa M, Favaro D, Quaia M, Galligioni E (1997) Expression of heat shock protein 72 in renal cell carcinoma: possible role and prognostic implications in cancer patients. Eur J Cancer 33:873–877
Sauvage F, Messaoudi S, Fattal E, Barratt G, Vergnaud-Gauduchon J (2017) Heat shock proteins and cancer: how can nanomedicine be harnessed? J Control Release 248:133–143
Scheibel T, Buchner J (1998) The Hsp90 complex – a super-chaperone machine as a novel drug target. Biochem Pharmacol 56:675–682
Schwamborn JC, Müller M, Becker AH, Püschel AW (2007) Retracted: ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity. EMBO J 26:1410–1422
Shapley R, Perry VH (1986) Cat and monkey retinal ganglion cells and their visual functional roles. Trends Neurosci 9:229–235
Shi S-H, Jan LY, Jan Y-N (2003) Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112:63–75
Shi S-H, Cheng T, Jan LY, Jan Y-N (2004) APC and GSK-3β are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol 14:2025–2032
Singh A, Singh A, Sand JM, Bauer SJ, Hafeez BB, Meske L, Verma AK (2015) Topically applied Hsp90 inhibitor 17AAG inhibits UVR-induced cutaneous squamous cell carcinomas. J Invest Dermatol 135:1098–1107
Sittler A, Lurz R, Lueder G, Priller J, Hayer-Hartl MK, Hartl FU, Lehrach H, Wanker EE (2001) Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington’s disease. Hum Mol Genet 10:1307–1315
Sliutz G, Karlseder J, Tempfer C, Orel L, Holzer G, Simon MM (1996) Drug resistance against gemcitabine and topotecan mediated by constitutive hsp70 overexpression in vitro: implication of quercetin as sensitiser in chemotherapy. Br J Cancer 74:172–177
Sudhakar J, Venkatesan N, Lakshmanan S, Khetan V, Krishnakumar S, Biswas J (2013) Hypoxic tumor microenvironment in advanced retinoblastoma. Pediatr Blood Cancer 60:1598–1601
Taipale M, Jarosz DF, Lindquist S (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11:515–528
Taiyab A, Sreedhar AS, Rao CM (2009) Hsp90 inhibitors, GA and 17AAG, lead to ER stress-induced apoptosis in rat histiocytoma. Biochem Pharmacol 78:142–152
Takayama S, Reed JC, Homma S (2003) Heat-shock proteins as regulators of apoptosis. Oncogene 22:9041–9047
Tam LC, Kiang AS, Campbell M, Keaney J, Farrar GJ, Humphries MM, Kenna PF, Humphries P (2010) Prevention of autosomal dominant retinitis pigmentosa by systemic drug therapy targeting heat shock protein 90 (Hsp90). Hum Mol Genet 19:4421–4436
Tanaka Y, Kobayashi K, Kita M, Kinoshita S, Imanishi J (1995) Messenger RNA expression of heat shock proteins (Hsp) during ocular development. Curr Eye Res 14:1125–1133
Tanihara H, Hangai M, Sawaguchi S, Abe H, Kageyama M, Nakazawa F, Shirasawa E, Honda Y (1997) Up-regulation of glial fibrillary acidic protein in the retina of primate eyes with experimental glaucoma. Arch Ophthalmol 115:752–756
Tapia M, Wandosell F, Garrido JJ (2010) Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PLoS One 5:e12908
Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, Albuquerque RJ, Hauswirth WW, Chiodo VA, Kugel JF, Goodrich JA, Ponicsan SL, Chaudhuri G, Murphy MP, Dunaief JL, Ambati BK, Ogura Y, Yoo JW, Lee DK, Provost P, Hinton DR, Nunez G, Baffi JZ, Kleinman ME, Ambati J (2012) DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 149:847–859
Trabulo S, Cardoso A, Santos-Ferreira T, Cardoso A, Simoes S, Pedroso de Lima M (2011) Survivin silencing as a promising strategy to enhance the sensitivity of cancer cells to chemotherapeutic agents. Mol Pharm 8:1120–1131
Tukaj S, Bieber K, Kleszczyński K, Witte M, Cames R, Kalies K, Zillikens D, Ludwig RJ, Fischer TW, Kasperkiewicz M (2017) Topically applied Hsp90 blocker 17AAG inhibits autoantibody-mediated blister-inducing cutaneous inflammation. J Investig Dermatol 137:341–349
Vanden Berghe T, Kalai M, van Loo G, Declercq W, Vandenabeele P (2003) Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J Biol Chem 278:5622–5629
Venkatesan N, Kanwar JR, Deepa PR, Navaneethakrishnan S, Joseph C, Krishnakumar S (2016) Targeting HSP90/Survivin using a cell permeable structure based peptido-mimetic shepherdin in retinoblastoma. Chem Biol Interact 252:141–149
Wainberg ZA, Anghel A, Rogers AM, Desai AJ, Kalous O, Conklin D, Ayala R, O’Brien NA, Quadt C, Akimov M (2013) Inhibition of HSP90 with AUY922 induces synergy in HER2-amplified trastuzumab-resistant breast and gastric cancer. Mol Cancer Ther 12(4):509–519
Walton-Diaz A, Khan S, Bourboulia D, Trepel JB, Neckers L, Mollapour M (2013) Contributions of co-chaperones and post-translational modifications towards Hsp90 drug sensitivity. Future Med Chem 5:1059–1071
Wang YQ, Zhang XM, Wang XD, Wang BJ, Wang W (2010) 17-AAG, a Hsp90 inhibitor, attenuates the hypoxia-induced expression of SDF-1alpha and ILK in mouse RPE cells. Mol Biol Rep 37:1203–1209
Wang C, Zhang Y, Guo K, Wang N, Jin H, Liu Y, Qin W (2016) Heat shock proteins in hepatocellular carcinoma: molecular mechanism and therapeutic potential. Int J Cancer 138:1824–1834
Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G (2005) 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med 11:1088
Whitesell L, Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5:761–772
Workman P, Burrows F, Neckers L, Rosen N (2007) Drugging the cancer chaperone HSP90. Ann N Y Acad Sci 1113:202–216
Wu W-C, Kao Y-H, Hu P-S, Chen J-H (2007) Geldanamycin, a HSP90 inhibitor, attenuates the hypoxia-induced vascular endothelial growth factor expression in retinal pigment epithelium cells in vitro. Exp Eye Res 85:721–731
Xu Y, Lindquist S (1993) Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc Natl Acad Sci USA 90:7074–7078
Yan D, Guo L, Wang Y (2006) Requirement of dendritic Akt degradation by the ubiquitin–proteasome system for neuronal polarity. J Cell Biol 174:415–424
Ylikomi T, Wurtz JM, Syvälä H, Passinen S, Pekki A, Haverinen M, Bläuer M, Tuohimaa P, Gronemeyer H (1998) Reappraisal of the role of heat shock proteins as regulators of steroid receptor activity. Crit Rev Biochem Mol Biol 33:437–466
Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R (1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94:471–480
Acknowledgements
We would like to thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Aboelnour, A., Noreldin, A.E., Saadeldin, I.M. (2019). Hsp90 Is a Pivotal Player in Retinal Disease and Cancer. In: Asea, A., Kaur, P. (eds) Heat Shock Protein 90 in Human Diseases and Disorders. Heat Shock Proteins, vol 19. Springer, Cham. https://doi.org/10.1007/978-3-030-23158-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-23158-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23157-6
Online ISBN: 978-3-030-23158-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)