Abstract
Intrahepatic cholangiocarcinoma (ICC) is an aggressive primary hepatic malignancy. While standard therapy for localized disease involves radical resection with regional lymphadenectomy, percutaneous ablative techniques including radiofrequency ablation (RFA) and microwave ablation (MWA) have been used in patients with unresectable recurrent ICC. In addition, limited data are available on the use of ICC for patients with early stage ICC. These treatments have fewer complications compared with hepatectomy.
Several studies have reported optimal long-term survival following ablative treatments. However, current research on these treatments in ICC patients is still limited. Research on treatment indications, comparisons of outcomes following surgical resection versus ablation, and the differences in treatment efficacy between RFA and MWA are relatively immature compared to the evidence available for patients with hepatocellular carcinoma. Nevertheless, emerging data suggest percutaneous ablation is safe, feasible, and an important component in the multidisciplinary care of patients with ICC. In this chapter, we review the technique, mechanism of action, indications, and outcomes of percutaneous ablation for ICC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a primary adenocarcinoma originating from the intrahepatic biliary tree and is the second most common primary liver cancer after hepatocellular carcinoma (HCC) [1]. The incidence of ICC is increasing worldwide according to the recent reports and its development is known to be associated with certain predisposing genetic and environmental factors [2]. Because ICC is often diagnosed at an advanced stage and exhibits aggressive tumor biology, the long-term survival outcomes of patients with ICC remain poor [3]. Among possible treatments for patients with ICC, surgical resection is the only established treatment that may provide long-term survival in well-selected patients, especially when the tumor is completely resected with a negative surgical margin [4, 5]. However, the majority of patients are not candidates for curative-intent surgery due to advanced disease at the time of diagnosis [6]. In addition, tumor recurrence and metastasis are still common even among patients who are able to undergo radical resection.
Image-guided percutaneous ablation is a minimally invasive therapy, which can result in local destruction of multiple types of liver malignancies [7,8,9]. Although ablative techniques have been well established in the treatment of HCC and isolated liver metastases, demonstrating efficacy even in large liver tumors via stereotactic placement of multiple radiofrequency probes [10], only limited data are currently available on the use of ablation in ICC. In addition, for recurrent ICC after initial curative resection, the use of repeat hepatectomy is usually limited by poor liver remnant function or multifocal recurrent diseases. In addition to systemic chemotherapy, these patients may be treated with locoregional therapies such as external beam radiation (XRT), radiofrequency ablation (RFA), microwave ablation (MWA), and radioactive implants (RIs) [11, 12].
This chapter reviews the technique, mechanism of action, indications, and outcomes of percutaneous ablation for ICC, highlighting the currently available evidence.
Indications for Percutaneous Ablation for ICC
There is limited research for which to base guidelines on the indications for percutaneous ablation in ICC. In clinical practice, patients with ICC who are not suitable for resection or who have developed relapse after resection are often considered for percutaneous thermal ablation. However, it is not uncommon for patients with ICC to undergo ablation based on a presumptive diagnosis of HCC, for which the use of curative-intent ablation is more established. Since the accurate histopathological evaluation of ablated tumors is usually not possible, this limitation must be considered when evaluating research on ablation for ICC. Percutaneous thermal ablation is commonly used in HCC patients who have a tumor within the Milan criteria, either as a curative-intent treatment or as a bridge to transplantation [13]. However, some authors have reported that the indications for percutaneous ablation should be less stringent: less than 5 nodules, each <5 cm in size, Child-Pugh class A or B liver function, prothrombin time <17 seconds, platelet count >45 cells ×109/L, and no evidence of macrovascular invasion and/or extrahepatic distant metastases [14]. However, treatment guidelines for the use of percutaneous thermal ablation in ICC are not comprehensive and immature. Zhang et al. reported that ablation should be considered based on the following criteria: histopathologically proven ICC, primary or recurrent tumor after surgery, maximum tumor size <5 cm, tumor number <3. Whether additional indications beyond this standard are also suitable for ablation is unknown [15].
Therapeutic Mechanism of and Equipment for RFA and WMA
Both RFA and MWA result in cytotoxic destruction of cancer cells via direct thermal injury. The ablation procedures involve placing needles (electrodes/antennas) directly into the targeted tumors. It aims to increase the temperature between 60 and 100 °C in the tumor tissues, which can lead to coagulation necrosis of the tumor while avoiding charring and vaporization of tissues [16,17,18]. In addition, thermal ablation technology is designed to destroy tumors without disrupting adjacent liver structures. These treatments have achieved acceptable outcomes in previous studies of liver tumors [12, 14, 19].
RFA
A large body of literature exists on the use of RFA for HCC and liver metastases. During the process of ablation, the needle is placed directly into the targeted tumor, and one or more electrodes are deployed from the tip of the needle to the tumor tissues. The heat and the friction generated by the radio energy through the ion produced by the needle generate heat and destroy the tumor tissues. A miniature thermometer coupled to the tip of the electrode allows continuous monitoring of tissue temperature. The power is automatically adjusted to keep the target temperature constant. As tissue temperature increases above 60 °C, cancer cell death occurs almost instantaneously [20].
Multiple ablations can overlap to reduce the chance of residual disease and/or local recurrence following ablation. The size of the ablated area depends mainly on the size of the electrode needle, the temperature generated in the tissues and the duration of the energy applied. A sharp boundary separates dead tissue and unaffected surrounding tissue [20,21,22].
MWA
MWA is an alternative method of inducing tissue thermal coagulation. Microwave magnetic fields make surrounding molecules rotate at high speed and frictional heating, resulting in tissue coagulation, dehydration, and necrosis. It involves placing needle electrodes directly into the targeted tumors. Each ablation produces a hyperechoic region surrounding the needle. Unlike RFA, MWA does not need to use a retractable tip that results in a tendency to be more elliptical and requires more courses of treatment for larger tumors. On the other hand, treatment sessions are usually shorter than that for RFA because an ablation is produced in 60 seconds with microwave therapy [23].
Currently, MWA is performed usually using a cooling shaft system that produces a maximum power of 100 W at 2450 MHz [24], while the conventional setting for ablation is 60–100 W output power, 120–300 seconds. If the hyperechoic microbubbles produced by heat do not completely cover the entire tumor, extended microwave emission is required until the desired ablative range is reached. After MWA treatment, needle burning is needed to prevent tracking of tumor cells [12, 15, 25].
Survival Outcomes after RFA for ICC
In previous studies, the technical success rate (i.e., complete ablation without local progression for at least 1 month) defined by the Interventional Radiology Reporting Standard [26] has been reported to be between 80% and 100% in ICC. However, local tumor progression rate after RFA was relatively high, which was reported to range from 8% to 50% [27,28,29,30,31,32,33], and the pooled rate in a meta-analysis was reported to be 21% (95% confidence interval [CI], 13–30%) [34]. The incidence of major complications observed after RFA was reported to be between 3.9% and 27% [14, 19, 29,30,31,32, 35].
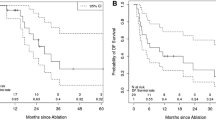
In a meta-analysis on RFA for ICC, the pooled 1-, 3-, and 5-year survival rates were 82% (95% CI, 72–90%), 47% (28–65%) and 24% (11–40%), respectively. These results were comparable to the outcomes recently estimated using the SEER database [26, 34]. Amini et al. reported that in a review of 1232 patients who were selected from the SEER database, only 64 (5.2%) patients underwent ablative therapy alone. Interestingly, they noted that the median survival of patients who were treated with ablation therapy was 20 months, which was worse than the outcomes of patients who were treated with resection but better than the outcomes following radiation therapy alone [26]. A review from Shindoh et al. reported that although the outcomes mentioned above were likely to be influenced by the differences in the baseline characteristics of the patients in each group, RFA might confer a modest survival advantage compared with other nonsurgical treatment options [36].
More recently, an original article reported by Takahashi et al. demonstrated that the median overall survival after ICC ablation was 23.6 months (range: 7.4–122.5 months), and the estimated 1-, 3-, and 5-year survival rates were 95% (95% CI: 86–100%), 40% (21–76%) and 32% (15–70%), respectively. The median disease-free survival was 8.2 months (range: 1.1–70.4 months) [12]. Another study reported that an increased tumor stage was associated with worse outcomes following RFA. The use of RFA was associated with a significantly prolonged survival compared with no local therapy in patients with stage I disease (2.1 vs. 0.7 years, P = 0.012), whereas patients with stage IV disease demonstrated no survival benefit from RFA [11]. Of note, all patients who were diagnosed as having ICC from 2004 to 2015 in the National Cancer Database (NCDB) were analyzed in this article, and the tumor staging was according to the seventh edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system of ICC [37]. Figure 9.1 shows the features of an ICC tumor before and after ablation on contrast-enhanced magnetic resonance imaging (MRI).
A 59-year-old female patient who underwent left lateral lobectomy of the liver for a histopathologically proven ICC. Two years after the operation, a 1.6 cm recurrent lesion in the right lobe was identified by MRI (a). The nonenhancing area completely enveloped the ablated tumor at 2months after the ablation (b)
RFA Versus MWA in the Treatment of ICC
Compared with RFA, MWA may have several distinct advantages including less dependence on tissue conductivity, shorter ablation time, higher intratumoral temperature, and larger ablation area and homogeneity [35, 38, 39]. Up to now, only two original studies reported by Zhang et al. [15] and Yu et al. [25] have described the relatively detailed procedures and outcomes of MWA in ICC. There has been no report to compare the outcomes following RFA versus MWA within any independent study. The comparison of outcomes of these two procedures from 5 studies using either RFA or MWA for ICC is listed in Table 9.1.
Among these studies, a meta-analysis by Han et al. included 7 observational studies that comprised 84 ICC patients [27,28,29,30,31,32,33] through a comprehensive literature search on Ovid MEDLINE and EMBASE to identify the studies that reported data of overall survival, local tumor progression, and complications after RFA. The pooled 1-, 3-, and 5-year overall survival rates were 82% (95% CI: 72–90%), 47% (28–65%) and 24% (11–40%), respectively, as above-mentioned [34]. In an article by Zhang et al., a total of 107 patients with 171 ICC tumors (≤5 cm in size, tumor number≤3) underwent MWA. The median follow-up after MWA was 20.1 months (2.8–63.5 months). The median progression-free survival (PFS) after MWA was 8.9 months; and the PFS rates at 6, 12, 18, and 24 months after the treatment were 67.4%, 41.5%, 18.2%, and 8.7%, respectively. The median overall survival was 28.0 months; and the overall survival rates at 1, 3, and 5 years after the treatment were 93.5%, 39.6%, and 7.9%, respectively [15]. In these two articles, the reported 1-year overall survival rate following RFA was lower than that after MWA , while RFA had a higher 3- and 5-year overall survival rates than MWA.
Complications Following Percutaneous Ablation
There are fewer reports on complications associated with percutaneous thermal ablation for ICC compared to HCC. In general, complications are classified as minor and major according to the clinical practice guidelines proposed by the Society of Interventional Radiology (SIR) [27]. Complications that require additional therapy, cause prolonged hospital stay, lead to permanent adverse sequelae, or result in death are evaluated as major complications. Others are considered as minor complications.
The following data are obtained by pooling 14 original studies about ICC [3, 12, 14, 15, 19, 27,28,29,30,31,32,33, 35, 40]. In 380 patients who were treated with percutaneous thermal ablation, major complications were registered in 5% (19/380) of patients. The mortality rate was 0.26% (1/380). Major complications included abdominal bleeding (1/380, 0.26%), needle-track cancer seeding (1/380, 0.26%), large biloma (2/380, 0.52%), biliary stricture (1/380, 0.26%), biliary fistula (1/380, 0.26%), pleural effusion with symptoms of dyspnea (3/380, 0.79%), hepatic failure (1/380, 0.26%), and liver abscess (9/380, 2.37%). One patient died of hepatic sepsis at 3.3 months after ablation despite percutaneous drainage and antibiotic therapy [29].
The minor complications included asymptomatic pleural effusion, mild bile duct dilation with or without jaundice, gallbladder wall thickening, a small amount of pleural effusion around the ablated area, small hematomas, minimal to moderate pain and fever, and increase in aminotransaminases. All minor complications resolved with conservative treatment. However, since the reported overall incidence of complications following ablation is low, percutaneous thermal ablation is generally considered safe for patients with ICC.
Ablation Versus Surgical Resection for ICC
Surgical resection is considered the first-line treatment for patients with localized ICC. The goals of surgery include achieving a margin-negative hepatic resection and performing a porta hepatis lymphadenectomy. However, the majority of patients present with advanced disease at diagnosis, and only about 30% of patients may be eligible for liver resection [41]. Surgical resection has been reported to provide a 5-year overall survival of 22–60% depending on specific clinicopathologic criteria [3, 4]. However, tumor recurrence rates after resection are high, ranging between 44% and 70% at 5 years after surgery [42, 43].
In most previous studies, the outcomes following ablative therapies were mainly investigated among patients who had unresectable ICC or recurrent ICC after initial surgery [3, 12, 14, 15, 19, 23, 27,28,29,30,31,32,33,34,35, 40, 44]. Prognostic factors associated with ablation treatment included tumor size, nodal invasion, and tumor differentiation. Given the different indications for treatment among patients receiving surgery or ablation, it is difficult to directly compare their long-term outcomes. There is only one original article, which has compared the outcomes of repeat hepatic resection versus thermal ablation for recurrent ICC [14]. Median survival time after repeated hepatic resection and thermal ablation therapy was 20.3 and 21.3 months, respectively. The 1-, 2-, and 3-year overall survival rates were 83.8%, 38.0% and 17.1% after repeated hepatic resection, and 69.8%, 37.3% and 20.5% after thermal ablation therapy (Table 9.2). Overall survival rates did not differ significantly between the two groups (p = 0.996), especially in patients with tumors less than 3 cm in size [14], suggesting that although ablation might be effective in selected patients with recurrent ICC, its indication should be limited according to tumor size [40].
Tumor size is an important factor associated with the therapeutic outcomes of ablation. The length of hospital stay, treatment cost, and risk of complications tend to be less with ablation than with hepatic resection. The incidence of major complications is also higher for hepatectomy compared to thermal ablation (46.9% vs. 3.9%) [14]. Post-ablation mortality is rare, whereas the perioperative mortality rates following resection of ICC range from 1.2% to over 7% [4, 45, 46]. Other studies have suggested that the overall survival rate after ablation for ICC is significantly higher compared to conservative treatments and comparable to that after radical resection in well-selected patients [47,48,49,50]. These results suggest that ablation may represent a less invasive alternative to surgical resection and is safe and effective for patients with recurrent ICC. While additional research is needed, ablation therapy may be considered a first-line treatment for selected patients with small recurrent ICC.
An important limitation of ablative techniques is the omission of regional, lymph node dissection. While not routinely performed for HCC, lymphadenectomy is an important component of accurate staging and locoregional control for patients with ICC. While less important for patients with recurrent ICC, the inability to perform lymph node evaluation limits the current application of percutaneous ablation to patients with otherwise resectable de novo ICC.
Combined Therapy
Because of the advanced stage at which most patients with ICC present, only a small proportion are suitable for radical surgical resection or complete therapeutic ablation. Combined multimodality therapy is an alternative approach to overcome some of these limitations. Unlike HCC, ICC has poor vascularity with a fibrotic characteristic, which leads to a limited survival benefit following transarterial chemoembolization (TACE) [54]. On the other hand, percutaneous thermal ablation in combination with TACE may provide improved outcomes. TACE can effectively decrease heat dispersion during thermal ablation by occluding bloodstream and consequently promote tumor ruin [55]. Meanwhile, thermal ablation may decrease the required chemotherapy dose of TACE and accordingly lessen side reaction and may also expand the ablation area and prolong progression-free survival. A study on microwave ablation combined with TACE for ICC demonstrated improved results compared to either TACE or ablation alone [56, 57].
Satellite lesions are often present in patients with ICC, which may preclude the ability to perform radical resection. Local thermal ablation combined with surgical resection is an option for patients with initially unresectable ICC. Although there is no sufficient data about ICC specifically, several studies in HCC have reported encouraging results. In a study by Choi et al., 53 patients with multifocal HCC received combined intraoperative RFA with hepatic resection. The cumulative survival rates at 1, 2, 3, 4, and 5 years were 87%, 83%, 80%, 68%, and 55%, respectively. Patients with smaller resected tumors (≤5 cm) demonstrated better survival results compared with those with larger tumor (P = 0.004). No procedure-related deaths occurred. They reported hepatectomy-related complications in 4 patients (4/53, 8%) and RFA-related complication only in 1 patient (1/53, 2%) [58]. However, current data on multimodality treatment in ICC, particularly percutaneous thermal ablation combined with surgical resection or other locoregional treatments are still lacking.
In clinical practice, patients with recurrent, metastatic, or unresectable ICC are often treated with systemic chemotherapy first. This approach prioritizes early systemic therapy for biologically aggressive cancer, ensures the absence of rapidly progressive disease, and potentially downsizes liver disease enabling the use of locoregional treatments. Percutaneous ablation, like other locoregional therapies, is most often considered in these patients who have demonstrated favorable tumor biology in order to optimize locoregional control and facilitate chemotherapy-free time.
Summary
Although percutaneous thermal ablation for ICC has been shown to have several distinct advantages, such as minimally invasiveness, easy to perform, repeatability, and cost-effectiveness [19], data for its efficacy remain limited [11]. Indeed, while the indications for ablation in HCC are well established (solitary lesion ≤5 cm, or no more than 3 lesions and each ≤3 cm), there remain no formal guidelines for the indications for percutaneous thermal ablation of ICC.
Based on the outcomes of retrospective data, percutaneous ablation appears safe and associated with acceptable locoregional control and survival outcomes for patients with recurrent or unresectable ICC. While ablation may be appropriate for some select patients with early stage disease, the inability to perform regional lymph node dissection prevents the wider adoption of ablation for patients with otherwise resectable disease. Although more high-quality data are needed, including prospective multicenter trials, percutaneous ablation is an important component of the multimodality treatment of patients with ICC, particularly at high-volume centers equipped with experienced multidisciplinary teams.
References
Lubezky N, Facciuto M, Harimoto N, Schwartz ME, Florman SS. Surgical treatment of intrahepatic cholangiocarcinoma in the USA. J Hepatobiliary Pancreat Sci. 2015;22(2):124–30.
Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–7.
Kamphues C, Seehofer D, Eisele RM, Denecke T, Pratschke J, Neumann UP, et al. Recurrent intrahepatic cholangiocarcinoma: single-center experience using repeated hepatectomy and radiofrequency ablation. J Hepatobiliary Pancreat Sci. 2010;17(4):509–15.
Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96.
Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208(1):134–47.
Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48(1):308–21.
McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Investig Radiol. 1990;25(3):267–70.
Rossi S, Fornari F, Pathies C, Buscarini L. Thermal lesions induced by 480 KHz localized current field in Guinea pig and pig liver. Tumori. 1990;76(1):54–7.
Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249(1):20–5.
Widmann G, Schullian P, Haidu M, Bale R. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc Intervent Radiol. 2012;35(3):570–80.
Kolarich AR, Shah JL, George TJ Jr, Hughes SJ, Shaw CM, Geller BS, et al. Non-surgical management of patients with intrahepatic cholangiocarcinoma in the United States, 2004–2015: an NCDB analysis. J Gastrointest Oncol. 2018;9(3):536–45.
Takahashi EA, Kinsman KA, Schmit GD, Atwell TD, Schmitz JJ, Welch BT, et al. Thermal ablation of intrahepatic cholangiocarcinoma: safety, efficacy, and factors affecting local tumor progression. Abdom Radiol. 2018;43(12):3487–92.
Xu Y, Shen Q, Liu P, Xu Z, Wu P, Lu Z, et al. Microwave ablation for the treatment of hepatocellular carcinoma that met up-to-seven criteria: feasibility, local efficacy, and long-term outcomes. Abdom Radiol. 2017;27(9):3877–87.
Zhang SJ, Hu P, Wang N, Shen Q, Sun AX, Kuang M, et al. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2013;20(11):3596–602.
Zhang K, Yu J, Yu X, Han Z, Cheng Z, Liu F, et al. Clinical and survival outcomes of percutaneous microwave ablation for intrahepatic cholangiocarcinoma. Int J Hyperth. 2018;34(3):292–7.
Dodd GD 3rd, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000;20(1):9–27.
Cline HE, Hynynen K, Watkins RD, Adams WJ, Schenck JF, Ettinger RH, et al. Focused US system for MR imaging-guided tumor ablation. Radiology. 1995;194(3):731–7.
Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1(2):93–122.
Xu HX, Wang Y, Lu MD, Liu LN. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol. 2012;85(1016):1078–84.
Lin SM. Recent advances in radiofrequency ablation in the treatment of hepatocellular carcinoma and metastatic liver cancers. Chang Gung Med J. 2009;32(1):22–32.
Hansler J, Frieser M, Tietz V, Uhlke D, Wissniowski TT, Bernatik T, et al. Percutaneous ultrasound-guided radiofrequency ablation (RFA) using saline-perfused (wet) needle electrodes for the treatment of hepatocellular carcinoma–long-term experience. Ultraschall Med. 2007;28(6):604–11.
Plasencia Martinez JM. Pulmonary radiofrequency ablation (Part 1): current state. Radiologia. 2015;57(4):275–86.
Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology. 2009;251(3):933–40.
Kuang M, Lu MD, Xie XY, Xu HX, Mo LQ, Liu GJ, et al. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna–experimental and clinical studies. Radiology. 2007;242(3):914–24.
Yu J, Liang P, Yu X, Liu F, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79(1):124–30.
Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 Suppl):S377–90.
Chiou YY, Hwang JI, Chou YH, Wang HK, Chiang JH, Chang CY. Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J Med Sci. 2005;21(7):304–9.
Carrafiello G, Lagana D, Cotta E, Mangini M, Fontana F, Bandiera F, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33(4):835–9.
Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196(2):W205–9.
Kim JH, Won HJ, Shin YM, Kim PN, Lee SG, Hwang S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol. 2011;80(3):e221–5.
Fu Y, Yang W, Wu W, Yan K, Xing BC, Chen MH. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2012;23(5):642–9.
Haidu M, Dobrozemsky G, Schullian P, Widmann G, Klaus A, Weiss H, et al. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Intervent Radiol. 2012;35(5):1074–82.
Butros SR, Shenoy-Bhangle A, Mueller PR, Arellano RS. Radiofrequency ablation of intrahepatic cholangiocarcinoma: feasability, local tumor control, and long-term outcome. Clin Imaging. 2014;38(4):490–4.
Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol. 2015;26(7):943–8.
Yu MA, Liang P, Yu XL, Cheng ZG, Han ZY, Liu FY, et al. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol. 2011;80(2):548–52.
Giorgio A, Gatti P, Matteucci P, Giorgio V. Ablative therapies for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2018;7(3):192–4.. 37
Farges O, Fuks D, Le Treut YP, Azoulay D, Laurent A, Bachellier P, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma. Cancer. 2011;117(10):2170–7.
Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236(1):132–9.
Vroomen L, Petre EN, Cornelis FH, Solomon SB, Srimathveeravalli G. Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney, and bone: what are the differences? Diagn Interv Imaging. 2017;98(9):609–17.
Giorgio A, Calisti G, DE Stefano G, Farella N, DI Sarno A, Amendola F, et al. Radiofrequency ablation for intrahepatic cholangiocarcinoma: retrospective analysis of a single-center experience. Anticancer Res. 2011;31(12):4575–80.
Park HM, Yun SP, Lee EC, Lee SD, Han SS, Kim SH, et al. Outcomes for patients with recurrent intrahepatic cholangiocarcinoma after surgery. Ann Surg Oncol. 2016;23(13):4392–400.
Ercolani G, Vetrone G, Grazi GL, Aramaki O, Cescon M, Ravaioli M, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010;252(1):107–14.
Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153(6):811–8.
Joo I, Lee JM, Yoon JH. Imaging diagnosis of intrahepatic and Perihilar cholangiocarcinoma: recent advances and challenges. Radiology. 2018;288(1):7–13.
Lang H, Sotiropoulos GC, Sgourakis G, Schmitz KJ, Paul A, Hilgard P, et al. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg. 2009;208(2):218–28.
Jonas S, Thelen A, Benckert C, Biskup W, Neumann U, Rudolph B, et al. Extended liver resection for intrahepatic cholangiocarcinoma: a comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification. Ann Surg. 2009;249(2):303–9.
Wu ZF, Wu XY, Zhu N, Xu Z, Li WS, Zhang HB, et al. Prognosis after resection for hepatitis B virus-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21(3):935–43.
Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB. 2016;18(1):79–87.
Spolverato G, Yakoob MY, Kim Y, Alexandrescu S, Marques HP, Lamelas J, et al. The impact of surgical margin status on long-term outcome after resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2015;22(12):4020–8.
Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large-scale observational study. Gut Liver. 2009;3(4):298–305.
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–95.
Saiura A, Yamamoto J, Kokudo N, Koga R, Seki M, Hiki N, et al. Intrahepatic cholangiocarcinoma: analysis of 44 consecutive resected cases including 5 cases with repeat resections. Am J Surg. 2011;201(2):203–8.
Saxena A, Chua TC, Sarkar A, Chu F, Morris DL. Clinicopathologic and treatment-related factors influencing recurrence and survival after hepatic resection of intrahepatic cholangiocarcinoma: a 19-year experience from an established Australian hepatobiliary unit. J Gastrointest Surg. 2010;14(7):1128–38.
Kim JH, Yoon HK, Sung KB, Ko GY, Gwon DI, Shin JH, et al. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer. 2008;113(7):1614–22.
Seki T, Tamai T, Nakagawa T, Imamura M, Nishimura A, Yamashiki N, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. 2000;89(6):1245–51.
Yang GW, Zhao Q, Qian S, Zhu L, Qu XD, Zhang W, et al. Percutaneous microwave ablation combined with simultaneous transarterial chemoembolization for the treatment of advanced intrahepatic cholangiocarcinoma. Onco Targets Ther. 2015;8:1245–50.
Zhao Q, Qian S, Zhu L, Qu XD, Zhang W, Yan ZP, et al. Transcatheter arterial chemoembolization with gemcitabine and oxaliplatin for the treatment of advanced biliary tract cancer. Onco Targets Ther. 2015;8:595–600.
Choi D, Lim HK, Joh JW, Kim SJ, Kim MJ, Rhim H, et al. Combined hepatectomy and radiofrequency ablation for multifocal hepatocellular carcinomas: long-term follow-up results and prognostic factors. Ann Surg Oncol. 2007;14(12):3510–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Qian, G., Zhang, J., Shen, F. (2019). Percutaneous Ablation. In: Pawlik, T., Cloyd, J., Dillhoff, M. (eds) Intrahepatic Cholangiocarcinoma. Springer, Cham. https://doi.org/10.1007/978-3-030-22258-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-22258-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22257-4
Online ISBN: 978-3-030-22258-1
eBook Packages: MedicineMedicine (R0)