Abstract
Gankyrin (also called PSMD10, p28, or p28GANK) is a crucial oncoprotein that is upregulated in various cancers and assumed to play pivotal roles in the initiation and progression of tumors. Although the in vitro function of gankyrin is relatively well characterized, its role in vivo remains to be elucidated. We have investigated the function of gankyrin in vivo by producing mice with liver parenchymal cell-specific gankyrin ablation (Alb-Cre;gankyrinf/f) and gankyrin deletion both in liver parenchymal and in non-parenchymal cells (Mx1-Cre;gankyrinf/f). Gankyrin deficiency both in non-parenchymal cells and parenchymal cells, but not in parenchymal cells alone, reduced STAT3 activity, interleukin-6 production, and cancer stem cell marker expression, leading to attenuated tumorigenic potential in the diethylnitrosamine hepatocarcinogenesis model. Essentially similar results were obtained by analyzing mice with intestinal epithelial cell-specific gankyrin ablation (Villin-Cre;Gankyrinf/f) and gankyrin deletion both in myeloid and epithelial cells (Mx1-Cre;Gankyrinf/f) in the colitis-associated cancer model. Clinically, gankyrin expression in the tumor microenvironment was negatively correlated with progression-free survival in patients undergoing treatment with Sorafenib for hepatocellular carcinomas. These findings indicate important roles played by gankyrin in non-parenchymal cells as well as parenchymal cells in the pathogenesis of liver cancers and colorectal cancers, and suggest that by acting both on cancer cells and on the tumor microenvironment, anti-gankyrin agents would be promising as therapeutic and preventive strategies against various cancers, and that an in vitro cell culture models that incorporate the effects of non-parenchymal cells and gankyrin would be useful for the study of human cell transformation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Liver cancer is the sixth most common cancer overall (0.78 million cases, 5.6%) but ranks second as cause of death (0.74 million, 9.1%) [1]. Around 80% of liver cancer in adults is hepatocellular carcinoma (HCC), and HCC is often diagnosed at advanced stages when most curative therapies are of limited efficacy. Furthermore, HCC is resistant to conventional chemotherapy and rarely amenable to radiotherapy, leaving HCC with a very poor prognosis [2]. Although a causal relationship between chronic damage, inflammation, and carcinogenesis has been widely recognized, the exact molecular mechanism of hepatocarcinogenesis remains to be elucidated. In 2000, we discovered gankyrin as an oncoprotein overexpressed in 100% of HCCs analyzed [3]. Further studies have suggested that gankyrin is a promising molecular target for diagnosis, treatment, and prevention of almost all types of cancers besides HCC (reviewed in references [4,5,6,7,8,9]).
Isolation of Gankyrin from Hepatocellular Carcinoma
Gankyrin (Gann ankyrin-repeat protein; “Gann” in Japanese means cancer) was originally identified as an oncogene product consistently overexpressed in HCCs [3]. Independently, it was purified as the p28 component [10] or a protein bound to the S6b subunit of the 19S regulator of the 26S proteasome [11]. Thus gankyrin is also known as PSMD10 (proteasome 26S subunit, non-ATPase 10), although subsequent studies have demonstrated that gankyrin transiently binds to the 26S proteasome and works as a chaperone for the assembly of the 19S regulator [12]. Gankyrin is a small 25kD cytoplasm–nucleus shuttling protein, and highly conserved throughout evolution (~40% identity to yeast Nas6p). Structurally, gankyrin consists of seven ankyrin repeats [13]. Ankyrin repeat is a functional domain involved in protein–protein interactions.
Enhanced Degradation of RB (Retinoblastoma-Associated Protein) and p53 (Cellular Tumor Antigen p53) by Gankyrin
Gankyrin plays a key role in regulating the cell cycle [14]. Gankyrin contains the RB-recognition motif LxCxE in the C-terminal domain, and binds RB in vitro and in vivo [3]. Forced expression of gankyrin in immortalized mouse fibroblasts and human tumor cells confers growth in soft agar and tumor formation in nude mouse. Gankyrin deactivates the RB tumor suppressor pathway at multiple levels (Fig. 1a) [3]. Gankyrin binds to CDK4, competing with and displacing p16INK4A and p18INK4C, inhibitors of cyclin-dependent kinases, which results in active CDK4, hyperphosphorylation of RB, and release of the E2F transcription factor to activate DNA synthesis genes. Binding of gankyrin also increases the rate of RB ubiquitylation and degradation by the proteasome.
Interaction of gankyrin with many proteins. (a) Activities of gankyrin on cell cycle control and apoptosis. In the presence of gankyrin, CDK4 is protected from the inhibitory effect of INKs (p16 and p18). Thus, RB is hyperphosphorylated and degraded, whereas E2F transcription factors are released to trigger expression of DNA synthesis genes. More p53 is ubiquitylated by gankyrin-bound MDM2 and degraded to suppress p53-dependent apoptosis and cell cycle arrest. P phosphate, Ub ubiquitin. (b) Gankyrin is a tumor suppressor killer. Gankyrin triggers degradation of at least six tumor suppressors by ubiquitin-proteasome system (UPS), and interacts with many important molecules, facilitating carcinogenesis
When overexpressed, gankyrin inhibits apoptosis of cells that have been exposed to DNA-damaging agents [15]. This anti-apoptotic activity is due, at least partly, to increased degradation of p53. Gankyrin binds to the E3 ubiquitin ligase MDM2 in vitro and in vivo, increasing the ubiquitylation and subsequent proteasomal degradation of p53, resulting in the reduced transcription of p53-dependent pro-apoptotic genes [15]. The fact that gankyrin simultaneously binds the proteasomal S6b ATPase and RB [16] suggests that gankyrin could be a carrier of ubiquitylated proteins to the 19S regulator of the 26S proteasome to enhance their degradation.
Gankyrin as a Killer of Multiple Tumor Suppressor Proteins
In addition to the two major tumor suppressors RB and p53, gankyrin binds to other tumor suppressor proteins such as C/EBPα [17], TSC2 [18], HNF4α [19], and CUGBP1 [20], and enhances their ubiquitylation and subsequent degradation by the proteasome (Fig. 1b). Gankyrin inhibits p16 [3], PTEN [21], and FIH-1 (factor inhibiting HIF-1) [22] as well.
NF-κB/RelA is a transcription factor that is hyperactivated in many types of cancers and leads to inhibition of apoptosis. Interestingly, many studies have shown that inhibition of NF-κB in hepatocytes enhances hepatocarcinogenesis [23]. Gankyrin directly binds to NF-κB/RelA and suppresses its activity by modulating acetylation via SIRT1 [24], exporting RelA from the nucleus [25], and associating with p300 to inhibit its interaction with RelA [26].
Consisting of seven ankyrin repeats, gankyrin binds many other proteins and affects many signaling pathways, contributing to carcinogenesis. Examples include MAGE-A4 [27], IGFBP-5 [28], SHP-1 [29], ATG7 [30], Keap1/Nrf2 [31], WWP2/Oct4 [32], PI3K/Akt [33], Rac1/JNK [34], β-catenin [35], Rho-A/ROCK [21], IL-6/STAT3 [36], IL-8 [37], YAP1 [38], and hypoxia-inducible factor-1α (HIF-1α) [22, 33].
Animal Models Overexpressing Gankyrin in the Liver
To assess the oncogenic activity in vivo, we produced transgenic mice that specifically overexpress gankyrin in the hepatocytes by using the hepatitis B virus X protein (HBX) promoter and serum amyloid P component (SAP) promoter [22]. Unexpectedly, both of these transgenic lines developed hepatic vascular neoplasms (hemangioma/hemangiosarcomas), but no HCCs. Further studies suggested that this was because gankyrin binds and sequester FIH-1, which results in decreased interaction between FIH-1 and HIF-1α, resulting in increased activity of HIF-1α and vascular endothelial growth factor (VEGF) production. Using the albumin promoter, Zhao et al. [34] observed HCC in the transgenic mice, but only after diethylnitrosamine (DEN) plus carbon tetrachloride (CCl4) treatment. Recently, occurrence of spontaneous HCC was reported in transgenic zebrafish using fabp10a promoter with Tet-Off system [39].
Importance of Non-Parenchymal Cells in Carcinogenesis as Demonstrated by Gankyrin-Knockout Mice
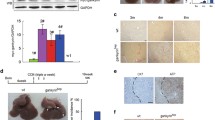
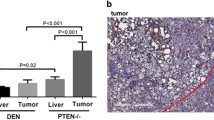
The effects of gankyrin in the tumor microenvironment were investigated by using mice with liver parenchymal cell-specific gankyrin ablation (Alb-Cre;gankyrinf/f) and gankyrin ablation both in liver parenchymal and in non-parenchymal cells (Mx1-Cre;gankyrinf/f) in the DEN hepatocarcinogenesis model (Fig. 2) [40]. Gankyrin upregulated VEGF expression in tumor cells. Gankyrin bound to Src homology 2 domain-containing protein tyrosine phosphatase-1 (SHP-1) which was mainly expressed in liver non-parenchymal cells, resulting in phosphorylation and activation of STAT3. Gankyrin deficiency in non-parenchymal cells, but not in parenchymal cells, reduced STAT3 activity, IL-6 production, and expression of cancer stem cell markers (Bmi1 and EpCAM), leading to attenuated tumorigenic potential. These results have suggested a model as shown in Fig. 3a. Essentially similar results were obtained by analyzing mice with intestinal epithelial cell-specific gankyrin ablation (Villin-Cre;Gankyrinf/f) and gankyrin ablation both in myeloid and epithelial cells (Mx1-Cre;Gankyrinf/f) in the colitis-associated cancer model [29]. Significant differences were observed in tumor numbers and sizes between Mx1-Cre;Gankyrinf/f mice and control mice, but not between Villin-Cre;Gankyrinf/f mice and control mice. Consistent with the animal models, chronic inflammation enhanced gankyrin mRNA expression in the human liver (Fig. 3b), and protein expression in non-parenchymal cells as well as hepatocytes [40]. High gankyrin expression in non-parenchymal cells was associated with enhanced IL-6 expression in HCC (Fig. 3c), and gankyrin expression in the tumor microenvironment was negatively correlated with progression-free survival in patients undergoing treatment with Sorafenib for HCCs [40]. These findings indicate important roles played by gankyrin in non-parenchymal cells as well as parenchymal cells in the pathogenesis of liver cancers and colorectal cancers.
Important roles in hepatocarcinogenesis played by gankyrin in non-parenchymal as well as parenchymal cells (Modified from reference [40]). (a) Control gankyrinf/f (GKf/f), Alb-Cre;GKf/f, and Mx1-Cre;GKf/f mice were challenged with DEN and killed after 8 months. Liver sections were examined with immunohistochemistry using gankyrin-specific antibody. Non-T non-tumorous liver tissues, T tumors. Scale bar, 50 μm. (b) Tumor number (upper) and maximal tumor sizes (diameters, lower) in GKf/f (n = 14) and Mx1-Cre;GKf/f (n = 18) mice. (c) Tumor number (upper) and maximal tumor sizes (diameter, lower) in GKf/f (n = 13) and Alb-Cre;GKf/f (n = 18) mice. (d, e) RNA was extracted from tumors of Mx1-Cre;GKf/f (d) or Alb-Cre;GKf/f (e) mice and GKf/f mice. Relative amounts of mRNA were determined by quantitative RT-PCR (qRT-PCR) and normalized to the amount of actin mRNA. The amount of each mRNA in the untreated liver was given an arbitrary value of 1.0. Data are means ± SEM (n = 5)
Inflammation and gankyrin (Modified from [40]. (a) A model of the role played by gankyrin in hepatocarcinogenesis. Chronic inflammation enhances gankyrin expression in the liver. Gankyrin binding to SHP-1 leads to enhanced IL-6 production in the tumor microenvironment. The augmented inflammatory response activates STAT3, and gankyrin upregulates the expression of VEGF in tumor cells, which eventually promote the development of HCC. (b) Liver specimens were collected using needle biopsy in 13 patients clinically suspected of non-alcoholic steatohepatitis. The expression of gankyrin mRNA in livers without inflammation or fibrosis (control, n = 5) and those with inflammation and fibrosis (chronic hepatitis, n = 8) was determined by qRT-PCR. (c) Liver specimens were collected using needle biopsy before sorafenib treatment. The mRNA levels of IL-6 in HCC were determined by qRT-PCR and compared between patients grouped according to the level of gankyrin expression in hepatic non-parenchymal cells as assessed by immunohistochemistry
Gankyrin as a Promising Therapeutic Target
Gankyrin seems to be an excellent target of cancer therapy because of the following reasons:
-
1.
Signaling interactions between cancer cells and their supporting stroma have been suggested to evolve during the course of multi-stage tumor development [41], and gankyrin promotes oncogenesis both in cancer cells and their supporting stroma [29, 40].
-
2.
Gankyrin promotes carcinogenesis both in early (initiation, promotion) and late (progression, metastasis) stages. For example, in rat HCC model, overexpression of gankyrin starts at fibrosis stage [42], and in human liver tissues, expression is progressively increased from hepatitis, cirrhosis, adenoma to HCC [43]. In many different types of cancers including those of the liver [30], colorectum [44], esophagus [45], and lung [46], high-level expression is correlated with invasion, metastasis, poor survival, and resistance to therapy.
-
3.
Gankyrin is overexpressed in most cases of HCC [3] and other types of cancers, including those of the esophagus [45], stomach [47], prostate [48], and colorectum [18].
-
4.
Ubiquitous low expression of gankyrin in normal tissues, and overexpression in almost all types of cancers including those of the brain, breast, lung, ovary, prostate, and stomach.
-
5.
Gankyrin kills multiple major tumor suppressors (Fig. 1b).
-
6.
Gankyrin can enable the hallmarks of cancer, at least five out of six original hallmarks and two out of two additional hallmarks [41]. By inhibiting gankyrin, therefore, we can target most of the pathways supporting the hallmarks therapeutically.
Experimental Anti-Gankyrin Agents
Since gankyrin is a versatile tumor suppressor killer and its activities seem to result from the binding to various partners, inhibition of the interactions is a promising strategy for controlling cancer initiation and progression. Indeed, overexpression of MAGE-A4, a gankyrin interactor of unknown function, suppressed the tumorigenic activity of gankyrin [27]. Overexpression of C-terminal portion of S6b inhibited proliferation of malignant cells (Fig. 4a, b). Although the structure of gankyrin has been clarified, research studies focusing on structure-based drug design of gankyrin are still limited. A synthetic protein GBP7.19 [49] and a small molecular drug cjoc42 [50] have recently been reported, but direct and specific gankyrin inhibitors should be investigated further.
Suppression of cell proliferation by gankyrin inhibitors. (a, b) Effects of overexpression of gankyrin-interacting S6b mutant. Human osteosarcoma U-2 OS cells were cultured in 6-cm dishes and transfected with plasmid DNAs (1 μg/dish) expressing mutant gankyrin with deletion of the two ankyrin-repeat motifs (del-GK), mutant S6b with N-terminus deletion (del-S6b), or control vector together with neo-resistance gene. After 8 days of culture in G418-containing medium, numbers of colonies were counted (a), and representative colonies were photographed under microscope (b). (c) Effects of shRNA. Human prostate cancer PC-3 cells with no wild-type p53 gene were incubated with plasmid nanoparticles expressing p53 (vlp-p53) or shRNA against gankyrin (vlp-shRNA-GK) prepared by RNTein Biotech Lab, CA. Volumes of added plasmids were adjusted with controls expressing vector alone. Four days later, surviving cell numbers were counted under microscope
Another promising strategy is an inhibition of gankyrin expression. Down-regulation of gankyrin expression by small interfering RNA (siRNA) or shRNA promotes apoptosis of tumor cells in vitro [15]. Growth of human cancer cells transplanted to nude mice is suppressed by intra-tumoral injection of siRNA [18] or adenovirus delivering shRNA against gankyrin [51]. As expected, the anti-proliferative activity of shRNA against gankyrin was enhanced by simultaneous expression of p53 in p53-deficient cancer cells (Fig. 4c).
Conclusion
Gankyrin plays important roles in non-parenchymal cells as well as parenchymal cells in the pathogenesis of liver cancers, colorectal cancers, and probably other cancers. Thus, by acting both on cancer cells and on the tumor microenvironment, anti-gankyrin agents are promising as therapeutic and preventive strategies against various cancers. As response rate of HCC to systemic chemotherapy is only 0 to 25%, blocking expression and/or function of gankyrin might be especially valuable in human HCCs. For the development of therapeutics, in vitro human cell transformation systems that incorporate the effects of non-parenchymal cells and gankyrin would be useful.
Gankyrin, a small ankyrin-repeat protein , has many activities with proteins controlling the cell cycle, transcription, apoptosis, and then many signaling pathways. How much of these activities are dependent on chaperoning the assembly of, and delivering the ubiquitylated substrates to the 26S proteasome, and how many are dependent on gankyrin being present in much smaller complexes with other proteins to control various cell processes, e.g., cell growth signaling pathways? The resolution of these questions must be answered to move toward a fuller understanding of gankyrin actions in the cell.
References
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D. M., Forman, D., & Bray, F. (2015). Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer, 136, E359–E386.
Shin, J. W., & Chung, Y. H. (2013). Molecular targeted therapy for hepatocellular carcinoma: Current and future. World Journal of Gastroenterology, 19(37), 6144–6155. https://doi.org/10.3748/wjg.v19.i37.6144.
Higashitsuji, H., Itoh, K., Nagao, T., Dawson, S., Nonoguchi, K., Kido, T., Mayer, R. J., Arii, S., & Fujita, J. (2000). Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nature Medicine, 6(1), 96–99.
Zamani, P., Matbou Riahi, M., Momtazi-Borojeni, A. A., & Jamialahmadi, K. (2018). Gankyrin: A novel promising therapeutic target for hepatocellular carcinoma. Artificial Cells, Nanomedicine, and Biotechnology, 46(7), 1301–1313.
Wang, C., & Cheng, L. (2017). Gankyrin as a potential therapeutic target for cancer. Investigational New Drugs, 35(5), 655–661. https://doi.org/10.1007/s10637-017-0474-8.
Wang, X., Jiang, B., & Zhang, Y. (2016). Gankyrin regulates cell signaling network. Tumour Biology, 37(5), 5675–5682. https://doi.org/10.1007/s13277-016-4854-z.
Iakova, P., Timchenko, L., & Timchenko, N. A. (2011). Intracellular signaling and hepatocellular carcinoma. Seminars in Cancer Biology, 21(1), 28–34. https://doi.org/10.1016/j.semcancer.2010.09.001.
Dawson, S., Higashitsuji, H., Wilkinson, A. J., Fujita, J., & Mayer, R. J. (2006). Gankyrin: A new oncoprotein and regulator of pRb and p53. Trends in Cell Biology, 16(5), 229–233.
Higashitsuji, H., Liu, Y., Mayer, R. J., & Fujita, J. (2005). The oncoprotein gankyrin negatively regulates both p53 and RB by enhancing proteasomal degradation. Cell Cycle, 4(10), 1335–1337.
Hori, T., Kato, S., Saeki, M., DeMartino, G. N., Slaughter, C. A., Takeuchi, J., Toh-e, A., & Tanaka, K. (1998). cDNA cloning and functional analysis of p28 (Nas6p) and p40.5 (Nas7p), two novel regulatory subunits of the 26S proteasome. Gene, 216(1), 113–122.
Dawson, S., Apcher, S., Mee, M., Higashitsuji, H., Baker, R., Uhle, S., Dubiel, W., Fujita, J., & Mayer, R. J. (2002). Gankyrin is an ankyrin-repeat oncoprotein that interacts with CDK4 kinase and the S6 ATPase of the 26 S proteasome. The Journal of Biological Chemistry, 277(13), 10893–10902.
Saeki, Y., Toh-E, A., Kudo, T., Kawamura, H., & Tanaka, K. (2009). Multiple proteasome-interacting proteins assist the assembly of the yeast 19S regulatory particle. Cell, 137(5), 900–913. https://doi.org/10.1016/j.cell.2009.05.005.
Krzywda, S., Brzozowski, A. M., Higashitsuji, H., Fujita, J., Welchman, R., Dawson, S., Mayer, R. J., & Wilkinson, A. J. (2004). The crystal structure of gankyrin, an oncoprotein found in complexes with cyclin-dependent kinase 4, a 19 S proteasomal ATPase regulator, and the tumor suppressors Rb and p53. The Journal of Biological Chemistry, 279(2), 1541–1545.
Iwai, A., Marusawa, H., Kiuchi, T., Higashitsuji, H., Tanaka, K., Fujita, J., & Chiba, T. (2003). Role of a novel oncogenic protein, gankyrin, in hepatocyte proliferation. Journal of Gastroenterology, 38(8), 751–758.
Higashitsuji, H., Higashitsuji, H., Itoh, K., Sakurai, T., Nagao, T., Sumitomo, Y., Masuda, T., Dawson, S., Shimada, Y., Mayer, R. J., & Fujita, J. (2005). The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell, 8(1), 75–87.
Whitby, F. G., & Hill, C. P. (2007). A versatile platform for inactivation and destruction. Structure, 15(2), 137–138.
Wang, G. L., Shi, X., Haefliger, S., Jin, J., Major, A., Iakova, P., Finegold, M., & Timchenko, N. A. (2010). Elimination of C/EBPalpha through the ubiquitin-proteasome system promotes the development of liver cancer in mice. The Journal of Clinical Investigation, 120(7), 2549–2562. https://doi.org/10.1172/JCI41933.
Qin, X., Wang, X., Liu, F., Morris, L. E., Wang, X., Jiang, B., & Zhang, Y. (2016). Gankyrin activates mTORC1 signaling by accelerating TSC2 degradation in colorectal cancer. Cancer Letters, 376(1), 83–94. https://doi.org/10.1016/j.canlet.2016.03.013.
Sun, W., Ding, J., Wu, K., Ning, B. F., Wen, W., Sun, H. Y., Han, T., Huang, L., Dong, L. W., Yang, W., Deng, X., Li, Z., Wu, M. C., Feng, G. S., Xie, W. F., & Wang, H. Y. (2011). Gankyrin-mediated dedifferentiation facilitates the tumorigenicity of rat hepatocytes and hepatoma cells. Hepatology, 54(4), 1259–1272. https://doi.org/10.1002/hep.24530.
Lewis, K., Valanejad, L., Cast, A., Wright, M., Wei, C., Iakova, P., Stock, L., Karns, R., Timchenko, L., & Timchenko, N. (2017). RNA binding protein CUGBP1 inhibits liver Cancer in a phosphorylation-dependent manner. Molecular and Cellular Biology, 37(16), e00128-17. https://doi.org/10.1128/MCB.00128-17.
Man, J. H., Liang, B., Gu, Y. X., Zhou, T., Li, A. L., Li, T., Jin, B. F., Bai, B., Zhang, H. Y., Zhang, W. N., Li, W. H., Gong, W. L., Li, H. Y., & Zhang, X. M. (2010). Gankyrin plays an essential role in Ras-induced tumorigenesis through regulation of the RhoA/ROCK pathway in mammalian cells. The Journal of Clinical Investigation, 120(8), 2829–2841. https://doi.org/10.1172/JCI42542.
Liu, Y., Higashitsuji, H., Higashitsuji, H., Itoh, K., Sakurai, T., Koike, K., Hirota, K., Fukumoto, M., & Fujita, J. (2013). Overexpression of gankyrin in mouse hepatocytes induces hemangioma by suppressing factor inhibiting hypoxia-inducible factor-1 (FIH-1) and activating hypoxia-inducible factor-1. Biochemical and Biophysical Research Communications, 432(1), 22–27. https://doi.org/10.1016/j.bbrc.2013.01.093.
Feng, G. S. (2012). Conflicting roles of molecules in hepatocarcinogenesis: Paradigm or paradox. Cancer Cell, 21(2), 150–154. https://doi.org/10.1016/j.ccr.2012.01.001.
Higashitsuji, H., Higashitsuji, H., Liu, Y., Masuda, T., Fujita, T., Abdel-Aziz, H. I., Kongkham, S., Dawson, S., Mayer, J. R., Itoh, Y., Sakurai, T., Itoh, K., & Fujita, J. (2007). The oncoprotein gankyrin interacts with RelA and suppresses NF-kappaB activity. Biochemical and Biophysical Research Communications, 363(3), 879–884.
Chen, Y., Li, H. H., Fu, J., Wang, X. F., Ren, Y. B., Dong, L. W., Tang, S. H., Liu, S. Q., Wu, M. C., & Wang, H. Y. (2007). Oncoprotein p28 GANK binds to RelA and retains NF-kappaB in the cytoplasm through nuclear export. Cell Research, 17(12), 1020–1029.
Ren, Y. B., Luo, T., Li, J., Fu, J., Wang, Q., Cao, G. W., Chen, Y., & Wang, H. Y. (2015). p28(GANK) associates with p300 to attenuate the acetylation of RelA. Molecular Carcinogenesis, 54(12), 1626–1635. https://doi.org/10.1002/mc.22235.
Nagao, T., Higashitsuji, H., Nonoguchi, K., Sakurai, T., Dawson, S., Mayer, R. J., Itoh, K., & Fujita, J. (2003). MAGE-A4 interacts with the liver oncoprotein gankyrin and suppresses its tumorigenic activity. The Journal of Biological Chemistry, 278(12), 10668–10674.
Umemura, A., Itoh, Y., Itoh, K., Yamaguchi, K., Nakajima, T., Higashitsuji, H., Onoue, H., Fukumoto, M., Okanoue, T., & Fujita, J. (2008). Association of gankyrin protein expression with early clinical stages and insulin-like growth factor-binding protein 5 expression in human hepatocellular carcinoma. Hepatology, 47(2), 493–502.
Sakurai, T., Higashitsuji, H., Kashida, H., Watanabe, T., Komeda, Y., Nagai, T., Hagiwara, S., Kitano, M., Nishida, N., Abe, T., Kiyonari, H., Itoh, K., Fujita, J., & Kudo, M. (2017). The oncoprotein gankyrin promotes the development of colitis-associated cancer through activation of STAT3. Oncotarget, 8(15), 24762–24776. https://doi.org/10.18632/oncotarget.14983.
Luo, T., Fu, J., Xu, A., Su, B., Ren, Y., Li, N., Zhu, J., Zhao, X., Dai, R., Cao, J., Wang, B., Qin, W., Jiang, J., Li, J., Wu, M., Feng, G., Chen, Y., & Wang, H. (2016). PSMD10/gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression. Autophagy, 12(8), 1355–1371. https://doi.org/10.1080/15548627.2015.1034405.
Yang, C., Tan, Y. X., Yang, G. Z., Zhang, J., Pan, Y. F., Liu, C., Fu, J., Chen, Y., Ding, Z. W., Dong, L. W., & Wang, H. Y. (2016). Gankyrin has an antioxidative role through the feedback regulation of Nrf2 in hepatocellular carcinoma. The Journal of Experimental Medicine, 213(5), 859–875. https://doi.org/10.1084/jem.20151208.
Qian, Y. W., Chen, Y., Yang, W., Fu, J., Cao, J., Ren, Y. B., Zhu, J. J., Su, B., Luo, T., Zhao, X. F., Dai, R. Y., Li, J. J., Sun, W., Wu, M. C., Feng, G. S., & Wang, H. Y. (2012). p28(GANK) prevents degradation of Oct4 and promotes expansion of tumor-initiating cells in hepatocarcinogenesis. Gastroenterology, 142(7), 1547–58.e14. https://doi.org/10.1053/j.gastro.2012.02.042.
Chen, J., Bai, M., Ning, C., Xie, B., Zhang, J., Liao, H., Xiong, J., Tao, X., Yan, D., Xi, X., Chen, X., Yu, Y., Bast, R. C., Zhang, Z., Feng, Y., & Zheng, W. (2016). Gankyrin facilitates follicle-stimulating hormone-driven ovarian cancer cell proliferation through the PI3K/AKT/HIF-1α/cyclin D1 pathway. Oncogene, 35(19), 2506–2517. https://doi.org/10.1038/onc.2015.316.
Zhao, X., Fu, J., Xu, A., Yu, L., Zhu, J., Dai, R., Su, B., Luo, T., Li, N., Qin, W., Wang, B., Jiang, J., Li, S., Chen, Y., & Wang, H. (2015). Gankyrin drives malignant transformation of chronic liver damage-mediated fibrosis via the Rac1/JNK pathway. Cell Death & Disease, 6, e1751. https://doi.org/10.1038/cddis.2015.120.
Dong, L. W., Yang, G. Z., Pan, Y. F., Chen, Y., Tan, Y. X., Dai, R. Y., Ren, Y. B., Fu, J., & Wang, H. Y. (2011). The oncoprotein p28GANK establishes a positive feedback loop in β-catenin signaling. Cell Research, 21(8), 1248–1261. https://doi.org/10.1038/cr.2011.103.
Zheng, T., Hong, X., Wang, J., Pei, T., Liang, Y., Yin, D., Song, R., Song, X., Lu, Z., Qi, S., Liu, J., Sun, B., Xie, C., Pan, S., Li, Y., Luo, X., Li, S., Fang, X., Bhatta, N., Jiang, H., & Liu, L. (2014). Gankyrin promotes tumor growth and metastasis through activation of IL-6/STAT3 signaling in human cholangiocarcinoma. Hepatology, 59(3), 935–946. https://doi.org/10.1002/hep.26705.
Bai, Z., Tai, Y., Li, W., Zhen, C., Gu, W., Jian, Z., Wang, Q., Lin, J. E., Zhao, Q., Gong, W., Liang, B., Wang, C., & Zhou, T. (2013). Gankyrin activates IL-8 to promote hepatic metastasis of colorectal cancer. Cancer Research, 73(14), 4548–4558. https://doi.org/10.1158/0008-5472.CAN-12-4586.
Pei, T., Li, Y., Wang, J., Wang, H., Liang, Y., Shi, H., Sun, B., Yin, D., Sun, J., Song, R., Pan, S., Sun, Y., Jiang, H., Zheng, T., & Liu, L. (2015). YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget, 6(19), 17206–17220.
Huang, S. J., Cheng, C. L., Chen, J. R., Gong, H. Y., Liu, W., & Wu, J. L. (2017). Inducible liver-specific overexpression of gankyrin in zebrafish results in spontaneous intrahepatic cholangiocarcinoma and hepatocellular carcinoma formation. Biochemical and Biophysical Research Communications, 490(3), 1052–1058. https://doi.org/10.1016/j.bbrc.2017.06.164.
Sakurai, T., Yada, N., Hagiwara, S., Arizumi, T., Minaga, K., Kamata, K., Takenaka, M., Minami, Y., Watanabe, T., Nishida, N., & Kudo, M. (2017). Gankyrin induces STAT3 activation in tumor microenvironment and sorafenib resistance in hepatocellular carcinoma. Cancer Science, 108(10), 1996–2003. https://doi.org/10.1111/cas.13341.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013.
Park, T. J., Kim, H. S., Byun, K. H., Jang, J. J., Lee, Y. S., & Lim, I. K. (2001). Sequential changes in hepatocarcinogenesis induced by diethylnitrosamine plus thioacetamide in Fischer 344 rats: Induction of gankyrin expression in liver fibrosis, pRB degradation in cirrhosis, and methylation of p16(INK4A) exon 1 in hepatocellular carcinoma. Molecular Carcinogenesis, 30(3), 138–150.
Jing, H., Zhang, G., Meng, L., Meng, Q., Mo, H., & Tai, Y. (2014). Gradually elevated expression of Gankyrin during human hepatocarcinogenesis and its clinicopathological significance. Scientific Reports, 4, 5503. https://doi.org/10.1038/srep05503.
He, F., Chen, H., Yang, P., Wu, Q., Zhang, T., Wang, C., Wei, J., Chen, Z., Hu, H., Li, W., & Cao, J. (2016). Gankyrin sustains PI3K/GSK-3β/β-catenin signal activation and promotes colorectal cancer aggressiveness and progression. Oncotarget, 7(49), 81156–81171. https://doi.org/10.18632/oncotarget.13215.
Ortiz, C. M., Ito, T., Tanaka, E., Tsunoda, S., Nagayama, S., Sakai, Y., Higashitsuji, H., Fujita, J., & Shimada, Y. (2008). Gankyrin oncoprotein overexpression as a critical factor for tumor growth in human esophageal squamous cell carcinoma and its clinical significance. International Journal of Cancer, 122(2), 325–332.
Wang, W. P., Sun, Y., Lu, Q., Zhao, J. B., Wang, X. J., Chen, Z., Ni, Y. F., Wang, J. Z., Han, Y., Zhang, Z. P., Yan, X. L., & Li, X. F. (2017). Gankyrin promotes epithelial-mesenchymal transition and metastasis in NSCLC through forming a closed circle with IL-6/ STAT3 and TGF-β/SMAD3 signaling pathway. Oncotarget, 8(4), 5909–5923. https://doi.org/10.18632/oncotarget.13947.
Zeng, Y. C., Sun, D., Li, W. H., Zhao, J., & Xin, Y. (2017). Gankyrin promotes the proliferation of gastric cancer and is associated with chemosensitivity. Tumour Biology, 39(6), 1010428317704820. https://doi.org/10.1177/1010428317704820.
Kim, T. D., Oh, S., Lightfoot, S. A., Shin, S., Wren, J. D., & Janknecht, R. (2016). Upregulation of PSMD10 caused by the JMJD2A histone demethylase. International Journal of Clinical and Experimental Medicine, 9(6), 10123–10134.
Chapman, A. M., & McNaughton, B. R. (2015). Synthetic proteins potently and selectively bind the oncoprotein gankyrin, modulate its interaction with S6 ATPase, and suppress gankyrin/MDM2-dependent ubiquitination of p53. ACS Chemical Biology, 10(8), 1880–1886. https://doi.org/10.1021/acschembio.5b00201.
Chattopadhyay, A., O’Connor, C. J., Zhang, F., Galvagnion, C., Galloway, W. R., Tan, Y. S., Stokes, J. E., Rahman, T., Verma, C., Spring, D. R., & Itzhaki, L. S. (2016). Discovery of a small-molecule binder of the oncoprotein gankyrin that modulates gankyrin activity in the cell. Scientific Reports, 6, 23732. https://doi.org/10.1038/srep23732.
Li, H., Fu, X., Chen, Y., Hong, Y., Tan, Y., Cao, H., Wu, M., & Wang, H. (2005). Use of adenovirus-delivered siRNA to target oncoprotein p28GANK in hepatocellular carcinoma. Gastroenterology, 128(7), 2029–2041.
Acknowledgements
We thank Dr. L. Feng (RNTein Biotech Lab, CA) for providing us with plasmid nanoparticles, Dr. R.J. Mayer (Nottingham University, UK) and Dr. J.S. Rhim (Uniformed Services University of Health Sciences, MD) for helpful suggestions, and Ms. C. Onishi for technical assistance. This work was partly supported by the grants from Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fujita, J., Sakurai, T. (2019). The Oncoprotein Gankyrin/PSMD10 as a Target of Cancer Therapy. In: Rhim, J., Dritschilo, A., Kremer, R. (eds) Human Cell Transformation. Advances in Experimental Medicine and Biology, vol 1164. Springer, Cham. https://doi.org/10.1007/978-3-030-22254-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-22254-3_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22253-6
Online ISBN: 978-3-030-22254-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)