Abstract
Electrokinetic (EK) treatment is studied for fine grained dredged soils as an innovative and cost-effective method to accelerate the dewatering and improve their mechanical behaviour. Owing to their high-water content, the dredged sediments take a very long time for the consolidation process, much more than those considered in the typical problems of geotechnical engineering.
Some electroosmotic tests in oedometer conditions on a clayey soil have been carried out at the University of Napoli Federico II in a special apparatus (special oedometer), adopting a pore fluid with different salt concentration. The results show that the addition of soluble salts in small quantities (until 8 g/l) can improve the electroosmotic consolidation of soft clay. On the contrary, excessive salinity reduces the efficiency of electroosmotic dewatering. The optimal salinity content has been then determined. At the end of the EK tests, some triaxial tests have been performed on the treated specimens in order to analyse the effectiveness of the EK treatment in the improvement of the soil mechanical properties.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Every year the dredging activities produce a large amount of sediments that must properly be managed. These sediments can be considered as a resource to be reused in civil fields. Owing to their proprieties (under-consolidated, with an extremely high water content, sometimes contaminated) dredged sediments need treatments as dewatering, decontamination and stabilization before they can be reused in civil works. The electrokinetic (EK) treatments represent (Lockhart 1983; Flora et al. 2017) a possible technique to induce a water flow without hydraulic gradients. The application of an electric gradient in a soil volume, in fact, induces a water flow that can be related to the applied electric gradient with a flow rule similar to the one caused by a hydraulic gradient (Darcy’s equation). The form of such an equation is:
In which, i.e. is the electrical potential gradient (V/m); ke is the electroosmotic permeability (m2/sV); \( A_{c} \) the area of the cross section (m2). The electrical potential gradient can be expressed as \( \Delta \phi \)/ΔL where \( \Delta \phi \) is the applied potential difference (V) and ΔL is the distance between the electrodes.
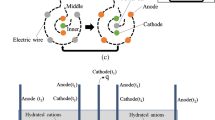
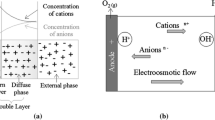
The applied electrical potential difference induces ion migration within the fluid phase: the positively charged ionic species move through the medium to the cathode (negative pole), while the negatively ones move through the porous medium to the anode (positive pole). As the ions migrate, they pull the liquid with them and resulting in an electroosmotic flow. The flow starts in the diffuse double layer, where there are significantly more cations than anions due to the proximity of the negatively charged surface of clayey particles. The electric field can also trigger the transportation of the colloidal state: the negatively charged clayey particles (colloidal particles) migrate towards the anode (electrophoresis). All the electrochemical processes mentioned above induce a significant irreversible change in the physico-chemical and mechanical properties of the treated soil.
A theoretical explanation of electroosmotic consolidation has been presented by Esrig (1968), based upon the development of pore water pressures resulting from the application of a uniform potential field. The nature of the pore pressure developed depends on the boundary conditions at the anode and cathode sides. The usual boundary conditions in electroosmotic consolidation are anode “closed” and cathode “open”. In this case, water is not replaced at the anode resulting in negative pore pressure. With anode and cathode “open” (free access to water), if the water at the anode is replenished, no excess pore water pressure is generated. It is well known (Hamir et al. 2001; Acar et al. 1994; Alshawabkeh and Acar 1996) that even with both anode and cathode open (without water replenishment), excess pore water pressure is still generated because the induced change in the ion concentration and pH within the specimen.
In literature, many experimental works (Bjerrum et al. 1967; Casagrande 1948; Flora et al. 2016; Lo et al. 1991; Lockhart 1983; Reddy et al. 2006) verified the effectiveness of EK treatment both to accelerate the water drainage and to improve the strength and stiffness of clayey soils. These studies showed that the effectiveness of the EK treatment is linked not only to the mineralogical nature of the soil, but also to the applied potential, the type of electrodes, and the chemical composition of the interstitial fluid.
Several theories have been developed to explain electroosmotic transport of water in clay soils. Of these, the Helmholtz–Smoluchowski theory is one of the earliest and the most widely used (Mitchell 1993). It is based on an electrical condenser analogy that assumes that the soil capillaries have charges of one sign on or near the surface of the wall and countercharges concentrated in a layer in the liquid a small distance from the wall. The mobile shell of counterions is assumed to drag water through the capillary by plug flow, resulting in a high-velocity gradient between the two plates of the condenser. The balance between the electrical force causing water movement and friction between the liquid and the wall controls the rate of water flow.
According to the Helmholtz-Smoluchowski model, the electroosmosis coefficient (ke, Eq. 1) can be expressed as (Mitchell 1993):
in which \( \upzeta \)(V) is the soil zeta potential (negative in clayey soils); ε (F/m) is the dielectric constant of the pore fluid; \( \eta \)(Pa s) the dynamic viscosity of the fluid; n the porosity of the soil.
Based on the H-S model defined by Eq. (2), since the permittivity and the viscosity of pore water are approximately constant over a fairly large range of salinity, ke is controlled primarily by the zeta potential and porosity of the soil. In particular, the zeta potential of aqueous colloidal suspensions is a function of the solid surface charge, electrolyte concentration and pH. It decreases (becomes less negative) with the increase of the solution ionic concentration (salinity).
It should be noted that all the successful applications of the electrokinetic treatment involved soils of low salinity (the salt content in the pore water was less than 2 g NaCl/l or the equivalent). However, for clays with a high salt content in the pore water, such as marine sediments, limited data reported in the literature suggest that the high salinity can significantly decrease the electroosmotic flow in soil (e.g., Casagrande 1949; Gray and Mitchell 1967; Lockhart 1983; Mitchell 1993).
Lockhart (1983) found that the optimum dewatering results on a kaolin were achieved at a moderate pore fluid salinity (0.59 g/l) rather than at a low salinity (0.059 g/l) or pure water.
Mohamedelhassan and Shang (2002) also found an optimum salinity for the marine sediment tested, which in their tests corresponded to about 8 g/l of NaCl.
In this paper, the authors describe a series of electroosmotic consolidation laboratory tests conducted to investigate the influence of the salt on the dewatering of a soft clay and the mechanical behaviour of some treated soils.
2 Material and Experimental Program
The experimental activity has been carried out on a soil identified as a high plasticity soil (Fig. 1). Its mineralogical composition was evaluated by XRD analysis (Fig. 2): the main crystalline phases are quartz (Q), vermiculite (V) and calcite (C) with traces of halloysite (H).
All the laboratory tests have been carried out on remoulded specimens, obtained by drying the soil in stove at 105 °C and then mixing it with distilled water with the addition of salt at different concentrations (Table 1) obtaining a sludge with an initial water content w ~ 1.4 wL (with wL the liquid limit).
Four electroosmotic tests have been carried out in a special oedometer (Fig. 3) and at the end of those tests some triaxial tests have been carried out on treated specimens retrieved from the apparatus.
3 Apparatus
The electroosmotic tests have been carried out in a floating special oedometer (Flora et al. 2017; Gargano et al. 2018) designed to allow large displacements (maximum specimen height H = 25 cm, internal diameter D = 6.9 cm) as expected for very soft soils. The device (Fig. 3) is capable to apply to the soil different combinations of mechanical (M) and electrical loads (EK). In the apparatus two hollow pistons are connected to conductive porous stones placed at the top and the bottom base of the specimen: in the electroosmotic tests, the upper (anode) and the lower (cathode) end plates are connected to a (DC) power supply, operating under constant voltage (ΔФ). An LVDT is used to measure the settlements while a balance is used to measure the water expelled by the specimen during the time. All the tests have been carried out under the double-way drainage conditions, in the EK tests, water is not replenished at the anode.
One test (named SE-M1, Table 1) has been carried out applying only a mechanical load (σ’v = 1 kPa), without the application of the electrical field. Four electrokinetic (SE-EK) tests (Table 1) have been performed under different salt concentrations (from 0.2 - that is the salt concentration of the tap water - to 30 g/l). During the application of the electric field, the intensity of the electric current (i) was measured.
4 Experimental Results
4.1 Consolidation Tests in the Special Oedometer
Volume of expelled water and coefficient of electroosmotic permeability
The results obtained in the special oedometer are shown in Fig. 4 in terms of expelled water.
This measurement has been chosen, instead of the specimen settlements, because of the unreliability of the LVDT data, affected by the big amount of gas produced by the electrolysis phenomenon (EK tests). Because in the mechanical test (with no electric gradient) the water is expelled from both specimen sides, the total expelled water has been quantified via the settlement measurements. In the EK tests, the water flow goes from the top to the bottom of the cylinder and it is collected in the balance.
It can be noted that, at the same stress level, the application of an electric field (tests SE-EK2, SE-EK3, SE-EK4, SE-EK5, Fig. 4) enhances the consolidation reducing the time needed to end it and producing a major volume of expelled water respect to the case without the current (SE-M1, Fig. 4) Furthermore, the different concentration of salt in the pore fluid leads to a different behaviour during the application of the current field. The salt concentration seems to affect the velocity of consolidation only slightly, while it is strictly connected to the quantity of the removed water. The lower the salt concentration the higher the quantity of water removed.
The coefficient of electroosmotic permeability has been calculated from the Eq. (1) and plotted against the pore fluid salinity in Fig. 5. The variation of ke with the soil porosity and pore fluid salinity was studied by previous researchers (Mohamedelhassan and Shang 2002) whose findings are also shown in Fig. 5. Overall, the trend of the results of this study is consistent with the results of the previous researchers. The Fig. 5 shows that ke/n (that is the ratio between the coefficient of electroosmotic permeability and the porosity) decreases with the salinity when the pore fluid salinity is greater than 8 g NaCl/l, which is consistent with the H-S model (Eq. 2), since the zeta potential (Eq. 2) decreases as the salinity increases. However, the H-S model cannot explain the trend for the salinity range from 0.2 to 8 g NaCl/l.
Therefore, a soil with a very low pore fluid salinity does not necessarily yield the highest electroosmotic permeability, but a soil with a high pore fluid salinity does not always give the lowest electroosmotic permeability. The current results are in general agreement with the results obtained by Lockhart (1983), who reported a better dewatering performance in a soil sample with pore fluid salinity of 0.59 g NaCl/l compared with 0.059 g NaCl/l and pure water.
Current intensity and electrical conductivity
The current intensity measured during the EK tests is shown in Fig. 6: it reaches its maximum value after 100–200 min and then it starts to decrease reaching its minimum at the end of the electroosmotic dewatering. The higher the salt concentration, the higher the current intensity.
Figure 7 shows the electrical conductivity of the soil samples plotted against the pore fluid salinity. The variation of the conductivity of the soil pore fluid (water) with the salinity is known (from Keller and Frischknecht 1966), the conductivity of the soil particles has been assumed equal to that of a clay with similar geotechnical properties (Mohamedelhassan and Shang 2002), the conductivity of the soil sample has been evaluated from the current intensity with Ohm’s law at the beginning of tests and at peak. The figure indicates that the conductivity of the soil sample is proportional to the pore fluid salinity and is located between the conductivity of the soil particles and the pore fluid.
4.2 Improvement of Mechanical Properties Due to EK Consolidation
The effect of the electric field on the overall mechanical behaviour of the specimens has been analysed by means of some triaxial tests (Isotropically Consolidated Undrained CIU tests) carried out on specimens retrieved in the middle part of the special oedometric device, at the end of mechanical (M) and electrokinetic (EK) tests. The specimens have been isotropically consolidated into the triaxial device applying a confining pressure (σ’c) equal or similar to the vertical stress applied during the tests, thus only slightly modifying the initial stress state (that in the oedometer is not isotropic). After the consolidation, an undrained shearing phase has been applied to all the specimens to failure.
The following comparisons (Table 2) have been made among results of triaxial tests on specimens at the same pore fluid salinity (0.2 g/l), from previous researches (SE-M2, SE-EK6, SE-M3, SE-EK7), where the confining pressure is equal to 15 or 30 kPa (Flora et al. 2017), and the current ones (SE-M1, SE-EK2) where the confining pressure is equal to 5 kPa.
The results of the CIU tests are plotted in Fig. 8 in terms of q-εa (Fig. 8a) (where q is the deviatoric stress and εa the axial strain) and pore water pressure increment (Δu), versus the axial strains (Fig. 8b). The mechanical behaviour of the electrically treated specimens differs from the one having undergone only a mechanical stress path. At the same confining stress, the treated specimens always show a higher deviatoric stress (from 2.4 to 3.3 times higher) than the mechanical ones (Fig. 8a). The specimens SE-EK6 and SE-EK7 show a dilatant tendency with excess pore water pressures (Fig. 8b) that become negative for the test SE-EK7 at larger strains.
The overall effectiveness of the EK treatment has been analysed in Fig. 9 in terms of measured undrained cohesion versus the applied confining stresses (σ’c): it can be clearly noted that the EK treated specimens have a higher shear strength.
5 Conclusions
The paper has investigated on some effects of the electroosmotic treatment of a fine-grained dredged soil. The experimental results show that the application of an electrical gradient has a remarkable effect on the mechanical behaviour of the soil. Furthermore, the electroosmotic consolidation induces a dewatering strictly connected to the salinity of the pore fluid: the lower the salt concentration the higher the quantity of water removed.
Further investigations are in progress to assess the effect of the pore fluid salinity on the mechanical behavior of the treated soil.
References
Acar YB, Hamed J, Alshawabkeh AN, Gale RJ (1994) Removal of cadmium (II) from saturated kaolinite by application of electrical current. Geotechnique 44(2):239–254
Alshawabkeh AN, Acar YB (1996) Electro-kinetic remediation I: theoretical model. J Geotech Eng Div ASCE 122(3):186–196
Bjerrum L, Moum J, Eide O (1967) Application of electro-osmosis to a foundation problem in Norwegian quick clay. Geotechnique 17:214–235
Casagrande L (1948) Electro-osmosis. Proceedings, 2nd International Conference on Soil Mechanics and Foundation Engineering, Rotterdam 1, pp 218–223
Casagrande L (1949) Electro-osmosis in soils. Géotechnique 1(3):159–177
Esrig MI (1968) Pore pressure, consolidation and electrokinetics. J Soil Mech Found Div ASCE 94(SM4):899–922
Flora, A, Gargano, S, Lirer, S, Mele, L (2016) Effect of Electro-kinetic consolidation on fine grained dredged sediments. In: VI national conference of researchers in geotechnical engineering (CNRIG), Italy, procedia engineering, vol 158, pp 3–8
Flora A, Gargano S, Lirer S, Mele L (2017) Experimental evidences of the strengthening of dredged sediments by electroosmotic consolidation. Geotech Geol Eng 35(6):2879–2890
Gargano S, Lirer S, Flora A (2018) Analysis of the coupled electro-osmotic and mechanical consolidation in clayey soils. Proceedings of the Institution of Civil Engineers - Ground Improvement. https://doi.org/10.1680/jgrim.18.00010
Gray DH, Mitchell JK (1967) Fundamental aspects of electroosmosis in soils. J Soil Mech Found Div ASCE 93(6):209–236
Hamir RB, Jones CJFP, Clarke BG (2001) Electrically conductive geosynthetics for consolidation and reinforced soil. Geotext Geomembr 19:455–482
Jones CJ, Lamont-Black J, Glendinning S (2011) Electrokinetic geosynthetics in hydraulic applications. Geotext Geomembr 29:381–390
Keller GV, Frischknecht FC (1966) Electrical methods in geophysical prospecting. Pergamon Press, Oxford 517ss
Lo KY, Ho KS, Inculet II (1991) Electro-osmotic strengthening of soft sensitive clays. Can Geotech J 28:62–73
Lockhart NC (1983) Electroosmotic dewatering of clays, II. Influence of salt, acid and flocculants. Colloids Surf 6:253–269
Mitchell JK (1993) Fundamentals of soil behavior, 2nd edn. Wiley, New York
Mohamedelhassan E, Shang JQ (2002) Feasibility assessment of electro-osmotic consolidation on marine sediment. Ground Improv 6(4):145–152
Reddy KR, Urbanek A, Khodadoust AP (2006) Electroosmotic dewatering of dredged sediments: bench-scale investigation. J Environ Manage 78:200–208
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Gargano, S., Lirer, S., Flora, A. (2020). Mechanical and Physical Effects of Electroosmotic Dewatering of Clayey Soils. In: Calvetti, F., Cotecchia, F., Galli, A., Jommi, C. (eds) Geotechnical Research for Land Protection and Development. CNRIG 2019. Lecture Notes in Civil Engineering , vol 40. Springer, Cham. https://doi.org/10.1007/978-3-030-21359-6_41
Download citation
DOI: https://doi.org/10.1007/978-3-030-21359-6_41
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21358-9
Online ISBN: 978-3-030-21359-6
eBook Packages: EngineeringEngineering (R0)