Abstract
Many Cladocera species under unfavourable conditions produce resting eggs that can survive for years in deep diapause. Resting eggs form egg banks at the bottom sediments, which serve as a source of genetic diversity and replenish the population after periods of decline. Despite the obvious importance of resting eggs for the ecosystem functioning, studies assessing the sensitivity of resting eggs to different toxicants are scarce. We reviewed published data on the sensitivity of resting eggs to the effect of heavy metals, organic pollutants and ionizing radiation. Analysis shows that the effects of toxicants of different types on resting eggs will have different environmental consequences. Egg banks may suffer from prolonged contact of dormant eggs with heavy metals. However, the ecological relevance of these effects is low, since the effective concentrations of toxicants must be very high. In addition, the effect of heavy metals on resting eggs is not transmitted to hatchlings from exposed eggs. Taking into account high toxicity of heavy metals to active animals, we assume that the toxic effect of heavy metals is critical for active zooplankters and relatively safe for resting stages. Accumulation of artificial radionuclides in bottom sediments can have a significant impact on aquatic ecosystems through chronic effects both on survival of resting eggs and on the life cycle parameters of animals hatched from irradiated eggs. Resting eggs during reactivation are more sensitive to the effect of ionizing radiation. Pesticides and complex chemical compounds produce similar toxic effects on both resting eggs and life history parameters of hatchlings from exposed resting eggs. The bottom line is that to predict the effect of contamination of bottom sediments by different pollutants on the ecosystem structure and functioning, it is highly important to investigate the viability of the resting eggs under a wide range of concentrations of various toxicants and different durations of direct contact of eggs with toxicants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cladocerans play a vital role in aquatic food webs, transferring organic carbon from primary producers to fish or invertebrate predators. Under favourable conditions, they reproduce by parthenogenesis, which allows them to increase in numbers quickly and control the lower food web level (Lampert et al. 1986). Under adverse conditions, many species of Cladocera produce resting eggs (Alekseev et al. 2007). The production of resting eggs is the strategy to survive either seasonal or occasional unfavourable conditions (Radzikowski 2013). Usually in natural habitats, cladocerans produce diapausing egg at the end of the growing season (Alekseev et al. 2007). Diapausing eggs produced in different years are accumulated at the bottom sediments, where they form the egg bank. The bank of dormant eggs is important for ecology and evolution of zooplankton. It is a source of genetic diversity; natural populations can recover from the egg bank, which supports the stability of zooplankton communities (Brendonck and De Meester 2003; Hairston 1996).
Resting eggs accumulated in the sediments can be exposed to various unfavourable factors. However, dormant organisms can stay alive in the resting form for a long time and demonstrate high tolerance to different unfavourable factors. The resistance of dormant forms to abiotic factors, such as desiccation or freezing and salinity stress, has been well characterized in recent decades. Radzikowski (2013) reviewed the survival of planktonic animals under adverse abiotic factors and reported longevity of dormant forms of selected aquatic invertebrates. For example, dry resting eggs of Daphnia magna can tolerate extreme temperatures of -84 °C and 110 °C during several hours. Nielsen et al. (2012) demonstrated that resting eggs of freshwater zooplankton are able to survive without decline of hatching success exposure to salt concentration of 13.5 g/l of sea salt for a period of 22 months. The maximal reported age of viable dormant forms of D. pulicaria and D. galeata mendotae was 125 years (Radzikowski 2013) and of the copepod Diaptomus sanguieneus was 322 years (Hairston Jr. et al. 1995).
In addition to natural factors that affect resting eggs in the bottom sediments, they can be influenced by anthropogenic factors. Taking into account high resistance of resting eggs to adverse conditions, it is not surprising that they tolerate high concentrations of toxic substances (e.g. Jiang et al. 2007; Alekseev et al. 2010; Raikow et al. 2007a, b). At the same time, bottom sediments accumulate compounds that enter the water body, including toxic ones. As a result, concentrations of toxicants in the sediments may exceed those in the water column and pose a certain threat to bottom biota (e.g. Avila-Perez et al. 1999; Jiang et al. 2007).
Some studies also demonstrated that toxic compounds not only directly affected resting eggs (e.g. the efficiency of reactivation) but also influenced life cycle parameters of active animals hatched from eggs exposed to toxicants (e.g. Navis et al. 2013; Rogalski 2015). It is very important to understand whether the contact with a toxicant during the diapause will affect the parameters of growth and reproduction of animals hatched from exposed eggs.
In this chapter, we review published studies and present our results on the effects of various anthropogenic pollutants on the resting eggs of zooplankters. Pollutants that we cover in this review are heavy metals, pesticides and other complex chemical compounds, and radioactive materials. After a brief review, we discuss environmental relevance of the effects observed and suggestions for future studies.
2 The Effects of Heavy Metals and Organic Pollutants on Resting Eggs and Hatchlings from Exposed Eggs
Heavy metals are of high ecological importance. They demonstrate variable toxicity to active Cladocera species, and, hence, they are very often used as model toxicants in toxicological research (Wong and Wong 1990; Wong et al. 1991). Alekseev et al. (2010) showed that resting eggs of Moina macrocopa remained viable after 100 days of incubation in the solution with a copper concentration of 500 mg/l. Experiments with resting eggs of Acartia pacifica showed reduced hatching success of resting eggs after exposure to 348 mg/kg of copper and 6.8 mg/kg of cadmium (Jiang et al. 2007). Zinc concentrations of up to 5 mg/l did not affect the survival of resting eggs of Artemia parthenogenetica (Sarabia et al. 2008).
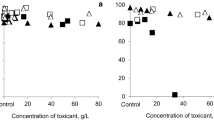
In experiments with resting eggs of M. macrocopa, we observed resistance of resting eggs to higher concentrations of heavy metals (up to 60–70 g/l) than was demonstrated previously (Lopatina et al. 2017; Oskina et al. 2018). First, we tried to determine the critical concentrations of heavy metals that are lethal for resting eggs after one-month exposure of eggs to a wide range of concentrations of heavy metals dissolved in water, but survival and hatching success of resting eggs were not affected. We used concentrations of heavy metals (from the background level to 60–70 g/l) that far exceeded toxic thresholds of these metals for active animals. When the exposure to toxicants was prolonged, we observed some adverse effects. In the experiment with contaminated artificial sediment, hatching of resting eggs was completely suppressed after 8-month exposure to high concentrations of copper (34 and 67 g/kg). None of the other treatments with sediment contaminated by different concentrations (from the background level to 60–70 g/l) of cadmium, zinc or nickel affected survival of resting eggs.
Our experiments showed that resting eggs could tolerate high concentrations of heavy metals. Recently, it was shown that resting eggs of cladoceran Daphnia are good biosorbent of heavy metals (Sacmaci et al. 2014). Thus, most probably protective structures of the ephippium are able to absorb high concentrations of ions of heavy metals and protect enveloped embryos from toxic exposure. However, the toxic effect of heavy metals on reactivation of eggs was manifested when we extended the duration of contact between eggs and toxicants. With the increased exposure time, the viability of resting eggs was reduced under the effect of copper, which was the most toxic heavy metal of the selected toxicants. If the viability of resting eggs is determined by the diffusion of a toxicant through the protective wall of the ephippium, the toxic effect of copper should be manifested first, as this metal is the most toxic of the selected heavy metals. We assume that longer contact with other heavy metals will also result in unfavourable effects. Indeed, Rogalski (2015) demonstrated that the concentration of heavy metals in the bottom sediments of the lake had a significant effect on the hatching success of Daphnia resting eggs extracted from the sediment core. However, at least for the period of up to 8 months, resting eggs protected offspring from the effect of heavy metals.
Raikow et al. (2006) examined the acute toxicity of the biocide SeaKleent (menadione), which is used for chemical treatment of residual material in ship ballast tanks, on resting eggs of several zooplankton species. SeaKleen was toxic to resting eggs of all the tested taxa. D. mendotae resting eggs encased in protective ephippia were the least sensitive, as indicated by 90% mortality of organisms after 24-h exposure to an 8.7 g/l concentration of the toxicant. SeaKleen induced teratogenic effects in D. mendotae and Artemia sp. In a study of the acute toxicity of glutaraldehyde and sodium hypochlorite for the resting eggs of D. mendotae and Artemia sp., Raikow et al. (2007a, b) demonstrated that glutaraldehyde was toxic to resting eggs of Artemia sp., while D. mendotae displayed erratic responses to glutaraldehyde. Sodium hypochlorite was similarly toxic to resting eggs of Artemia sp. and D. mendotae, which displayed LC90 of 86.5 ± 3.0 and 78.3 ± 1.6 mg/l, respectively.
Most et al. (2015) demonstrated the stimulating effect of organic pollutants on the hatchability of resting eggs of D. longispina. However, the offspring hatched from the eggs showed increased mortality and developmental anomalies associated with the presence of toxicants in the water. These results are difficult to interpret due to short exposure times and the presence of toxicants not only during the resting period but also at the time of reactivation of eggs. We proposed (Lopatina et al. 2017; Oskina et al. 2018) that variable resistance of resting eggs to heavy metals could be explained by methodological differences between studies. In preliminary experiments, we observed high mortality of juveniles hatched from resting eggs exposed to high concentrations of heavy metals. However, when resting eggs were thoroughly washed, no mortality of hatchlings was observed. We assumed that mortality of animals was associated with the diffusion of heavy metals bound to the surface of resting eggs to the culture medium. When we excluded the effect of water pollution by toxicants during the reactivation of eggs, we observed their high resistance to heavy metals. In most of the studies that we cited before, the method sections did not specify whether the resting eggs had been washed after exposure to toxicants. In general, standardization of techniques is considered as one of the key problems in studying the sensitivity of resting eggs of zooplankton to the effect of toxic compounds (Luc Brendonck, pers. communication).
We also found (Lopatina et al. 2017, Oskina et al. 2018) that parameters of the life cycle (life span, juvenile growth rate, number of hatched clutches, fecundity) of females hatched from resting eggs that had been exposed to heavy metals in a wide range of concentrations did not differ from those of the group of control animals. Taking into account high susceptibility of active cladocerans to the toxic effect of heavy metals, we assumed that when a toxicant penetrated through the protective ephippia, it caused mortality of encapsulated eggs. When resting eggs remained viable, hatched offspring did not differ from the control group.
The effects of heavy metals on performance of hatchlings from resting eggs exposed to toxicants should be different from the effects of toxic compounds that can modify life cycle parameters. For example, exposure to pesticides (carbaryl and fenoxycarb) affected both the reactivation of exposed resting eggs of D. magna and the survival and life cycle parameters of hatchlings from these eggs (Navis et al. 2013). Later, in the study of the sensitivity of resting eggs of D. magna at different stages of embryonic development to fenoxycarb, the authors showed that ephippium did not protect eggs against the pesticide (Navis et al. 2015). The concentration of the pesticide in the embryo tissues in the resting egg did not differ from that in an embryo devoid of the protective shell. Hence, the lethal concentration of fenoxycarb for resting eggs was only twice as high as that for living Daphnia. Thus, studies demonstrate that sensitivity of resting eggs to different toxicants varies considerably. Estimating the toxicity of heavy metals, organic substances and low-molecular-weight compounds, Alekseev et al. (2010) suggested that low-molecular-weight compounds were more toxic to resting eggs. Assessment of the toxicity of various compounds for resting eggs should probably require a detailed study of the structure of the ephippium and modelling of the interactions of toxicants with these protective structures.
We can summarize that resting eggs are quite resistant to the effect of heavy metals. Studies demonstrated that the survival of eggs was affected by both the duration of contact with heavy metals and their concentrations (Jiang et al. 2007; Rogalski 2015). The effect of heavy metals on resting eggs is mainly manifested as increased mortality of eggs. Performance of hatchlings from resting eggs exposed to heavy metals is usually not affected. Effects of pesticides and complex chemical compounds are observed at lower concentrations. Not only do such toxicants decrease the survival of resting eggs, but they also affect the parameters of the life cycle of hatchlings.
3 The Effect of Ionizing Radiation on Resting Eggs and Hatchlings from Exposed Eggs
Data on the effect of ionizing radiation on aquatic invertebrates are fragmentary and incomplete (Dallas et al. 2012). Over the past century, the level of background radioactivity has increased due to various anthropogenic activities (United Nations Scientific Committee on the Effect of Atomic Radiation 2010). Artificial radionuclides enter aquatic ecosystems due to discharges from nuclear-power facilities, washouts from water catchment areas and nuclear fall-outs (Van der Stricht and Janssens 2010). Bottom sediments accumulate various anthropogenic pollutants including radionuclides (e.g. Kansanen et al. 1991), which expose benthic dwellers to adverse effects.
Numerous experimental data demonstrated adverse effects of ionizing radiation on active cladocerans (e.g. Alonzo et al. 2008; Massarin et al. 2010; Sarapultseva and Gorski 2013). Recent studies have also demonstrated the transgenerational effects of parental exposure to ionizing radiation on survival and reproduction of exposed females of D. magna and their offspring (Sarapultseva and Dubrova 2016).
Studies of the effect of ionizing radiation on resting eggs of cladocerans are scant. The ability of resting eggs of Daphnia sp. to endure the conditions of outer space was tested at the International Space Station (ISS) (Novikova et al. 2011). Despite the high-temperature differences and the impact of ionizing radiation (absorbed dose was equal to 2–3 Gy), part of the resting eggs retained the ability for reactivation (Novikova et al. 2011). It was noted that radiosensitivity correlated positively with the rates of metabolic processes, which resulted in the tolerance to the effect of radiation of dormant eggs of aquatic invertebrates (Eisler 1994) or animals in cryptobiosis (Watanabe 2006). Thus, we should expect relatively high tolerance of resting eggs to the effect of radiation. Some anhydrobiotic invertebrates show extremely high tolerances against radiation. A tardigrade, Macrobiotus areolatus, tolerates exposure to 5500 Gy of X-ray (May et al. 1964). LD50 for dry Artemia cysts can be as high as 5000 Gy of Co60 gamma radiation (Iwasaki 1964).
Active invertebrates can also tolerate high doses of radiation. Some bdelloid rotifers were able to tolerate doses of up to 600 Gy (Gladyshev and Meselson 2008). Gladyshev and Meselson (2008) suggested that the radiation resistance of bdelloid rotifers was a consequence of their ability to survive the desiccation. The damage incurred in desiccation episodes includes the DNA breakage that is repaired upon rehydration. Thus, these species have developed effective mechanisms of DNA repair that can be activated to minimize the unfavourable effects of radiation.
Recently, we determined (Zadereev et al. 2017) the dose-response relationship for the effect of gamma radiation on hatching success of resting eggs of M. macrocopa. The absorbed dose of 200 Gy was lethal to resting eggs. Gamma irradiation in the range of doses from the background level to 100 Gy had no effect on the survival of resting eggs. Even though survival and hatching of resting eggs was not affected by doses below 100 Gy, the effect of radiation was manifested in hatched neonates at individual and population levels. The number of clutches and net reproductive rate (R0) of hatchlings from eggs exposed to radiation were the strongly affected parameters in experiments with individual females. The number of clutches per female was drastically reduced for females hatched from eggs exposed to 80–100 Gy. The effective dose that induced 50% R0 reduction was 50 Gy.
Life cycle parameters of active animals had different sensitivities to the irradiation of resting eggs. Life span and growth rate of juvenile females were relatively non-sensitive parameters. The proportion of males in the progeny was too variable a parameter to be used as a sensitive endpoint. Reproduction parameters (the net reproductive rate, fecundity) of active animals were the most sensitive parameters of life cycle in response to the effect of ionizing radiation on animals during the resting stage. These findings are similar to the results of several experiments with active animals exposed to ionizing radiation. For example, the size of clutches hatched by D. magna decreased at radiation doses greater than 0.1 Gy. At the same time, neither survival nor somatic growth rate of females were affected by radiation doses of 1–2 Gy (Gilbin et al. 2008). Sarapultseva and Dubrova (2016) also demonstrated that the fecundity of Daphnia significantly decreased at a dose of 0.1 mGy or higher while the survival was significantly compromised only for Daphnia exposed to 1 and 10 mGy of acute γ-rays. Moreover, Alonzo et al. (2016) modelled population responses to chronic external gamma radiation in 12 laboratory species (including aquatic and soil invertebrates, fish and terrestrial mammals) and found that the net reproductive rate showed the lowest EDR10 (effective dose rate inducing 10% effect) in all species.
While diapausing eggs can tolerate high doses of radiation, there is a knowledge gap on the sensitivity of resting eggs to the effect of radiation during hatching. When placed in favourable conditions, the resting egg hatches in several days. During this short period, the embryo should be very sensitive to the effect of radiation, as aquatic organisms exhibit increased sensitivity to radiation during active metabolism (Donaldson and Foster 1957). Indeed, we observed higher sensitivity of resting eggs when we exposed them to gamma radiation during the reactivation. The survival rate of resting eggs was decreased at the dose of 100 Gy, the growth rate of juveniles sharply decreased for hatchlings from eggs exposed to doses 24 Gy or higher, and reproduction parameters dramatically decreased for animals hatched from eggs exposed to doses of 64 Gy or higher (Lopatina et al. 2018). We can compare sensitivity of resting eggs of M. macrocopa to the effect of gamma radiation during deep dormancy and during reactivation. Neither the survival of resting eggs nor life span or growth rate of hatchlings from eggs irradiated in the deep dormancy were affected by the doses of up to 100 Gy (Zadereev et al. 2016; Zadereev et al. 2017). The reproductive parameters of females that hatched from irradiated eggs dramatically decreased at the exposure doses of 80 and 100 Gy. Thus, hatchlings from resting eggs of M. macrocopa exposed to gamma radiation during the reactivation demonstrated higher sensitivity (Lopatina et al. 2018).
There are fragmentary data on the irradiation of resting stages of zooplankton during reactivation. For example, wet cysts of Artemia (initial stage of reactivation) were more than twice radiosensitive to an irradiation dose of 500 Gy compared with dry cysts (Donaldson and Foster 1957). Similar results were obtained for other aquatic animals. For example, in fish of the genus Oryzias, chromosomal aberrations were found when eggs at their early stages of development (immediately after fertilization) were exposed to beta- (more than 0.19 Gy) and gamma (more than 0.58 Gy) radiation (Suyama et al. 1981). The mortality of eggs of mollusc Milax nigricans in the stage of two blastomeres increased at a dose of gamma radiation equal to 0.2 Gy and became absolute at an exposure dose of 0.8 Gy (Zampi and Focardi 1973). Embryos of the Milnesium cf. tardigradum exhibited higher sensitivity to radiation exposure during their early stage of development compared to the middle and late stages of development; egg mortality increased at radiation doses above 50 Gy (Beltrán-Pardo et al. 2013).
A recent study demonstrated the transgenerational effect of parental exposure to ionizing radiation on survival and fertility of directly exposed D. magna females and their first-generation progeny (Sarapultseva and Dubrova 2016). At the same time, F2 progeny of irradiated F0 Daphnia substantially recovered (Sarapultseva and Dubrova 2016). We also studied both maternal and transgenerational effects of exposure of resting eggs to ionizing radiation in population experiments (Zadereev et al. 2017). We performed experiments with populations of M. macrocopa that comprised at least four generations of females that were hatched from irradiated resting eggs. We found that population performance was affected by the irradiation of the resting stage of animals that initiated the population. However, the population response was detected at a higher level than individual responses. Only populations that were initiated from hatchlings from resting eggs exposed to 100 Gy were of smaller size and with fewer juvenile and parthenogenetic females compared to control populations (Zadereev et al. 2017).
Polikarpov (1998) proposed a conceptual model of biological responses to different dose rates of ionizing radiation: the dose rates that produce damage to populations and communities should be higher than the dose rates that produce detectable effects in individual organisms. Responses at the individual and population levels to the acute exposure of the resting eggs to ionizing radiation (Zadereev et al. 2017) were consistent with Polikarpov’s conceptual model (1998). The estimated ED50 for the R0 at the individual level was 50 Gy. However, populations initiated from resting eggs exposed to 50 Gy did not differ in size from the control population; populations initiated from resting eggs exposed to 80 Gy demonstrated reduced numbers and altered structure with fewer juvenile and parthenogenetic females in the initial stage of development, but later the population size recovered. Population responses were most probably masked by the individual variability and recovery of the subsequent progeny of F0 females irradiated in the resting stage.
We can conclude that resting eggs of Cladocera irradiated at different stages of development are resistant to relatively high doses, which corresponds to the accepted notion that invertebrates in aquatic habitats show low vulnerability to the radiation factor (Eisler 1994). Even though the survival of resting eggs is not affected by relatively high doses of radiation, the reproductive performance of hatchlings is suppressed. This effect is most probably not transgenerational, as we observed recovery of subsequent generations of females irradiated at the resting stage. At the same time, resting eggs demonstrated higher sensitivity when they were exposed to ionizing radiation during reactivation. Thus, contamination of bottom sediments by radioactive materials could affect zooplankton communities through either adverse chronic effects on resting eggs, which will be transmitted to hatchlings, or acute irradiation effects on resting eggs during reactivation.
4 Environmental Context
4.1 Heavy Metals and Organic Pollutants
To discuss the environmental significance of the data on the sensitivity of resting eggs to different pollutants, we need to estimate whether critical concentrations or doses observed in laboratory experiments are observed in natural habitats, too. We observed high resistance of resting eggs to heavy metals over a relatively long time (up to 8 months). Most probably, such concentrations are unattainable in natural habitats, even in the case of severe pollution. For example, copper concentrations in bottom sediments reached only 5 g/kg in contaminated swampy areas around the mines in China (Deng et al. 2004), 0.5 g/kg in the places where the city run-off enters the water body (Vesk and Allaway 1997) and 5 g/kg in the mining region of Mexico (Razo et al. 2004). While we demonstrated (Oskina et al. 2018) that the critical copper concentration that induced mortality of resting eggs after 8-month exposure was 30 g/kg, for cadmium, zinc or nickel, even concentrations of 50 g/kg had no effect on the survival of resting eggs.
The active concentrations of toxicants will be lower when we consider the long-term storage of dormant eggs in bottom sediments (e.g. for several years). For example, Rogalski (2015) observed that hatching rate of Daphnia hatched from diapausing eggs isolated from sediments from four lakes that experienced varying levels of metal contamination (Cu, Cd, Zn, Hg, Pb) was negatively influenced by metal contamination and sediment age. It means that negative effects of heavy metals in bottom sediments on resting eggs will be significant only for eggs that have been kept for a long time in the egg bank. However, long-term storage reduces the viability of resting eggs. In addition, the contribution to the population dynamics of eggs, buried deep in sediments, is low. The age of resting eggs that actively contribute to the zooplankton community in lakes varies between 10 and 20 years (Hairston 1996; Hairston Jr. et al. 2000). Resting eggs of zooplankton can survive longer (e.g. up to 300 years (Hairston Jr. et al. 1995)). However, it is highly improbable that eggs buried deep in sediments will contribute to the population dynamics. In addition, Rother et al. (2010) demonstrated that the contribution of hatched animals to the spring population dynamics can be negligible in situations with an overwintering population present and will only become relevant if densities are extremely low. Thus, ecological significance of such toxic effects will be low.
In addition, in the case of high concentrations of heavy metals in bottom sediments, there is a realistic assumption that elevated concentrations of toxicants will be also observed in water. Then, toxic effects of dissolved toxicants on the newborn juveniles hatched from resting eggs will be more valid. Sarabia et al. (2008) when they studied the effect of zinc on resting eggs of Artemia made a similar conclusion. Authors suggested that in natural habitats chronic toxicity of heavy metals dissolved in water to active animals will be more important than the effect of toxic bottom sediments on resting eggs. However, we should not underestimate evolutionary implications of the effect of heavy metals and other toxicants on complex interactions between active zooplankton community and the resting egg bank (e.g. Gutierrez et al. 2017; Turko et al. 2016; Aránguiz-Acuña and Pérez-Portilla 2017; Rogalski 2017). For example, metals in freshwater environments can modulate diapause adaptive efficacy and the selective process in egg banks (e.g. Aránguiz-Acuña and Pérez-Portilla 2017).
Toxic effects of pesticides or complex chemical compounds are observed at lower concentrations of toxicants than the effects of heavy metals. Organic pollutants not only directly affect survival of resting eggs but also modify life history parameters of hatchlings from exposed resting eggs. Thus, effects of such toxicants can be manifested at lower concentrations and will have higher environmental significance.
4.2 Ionizing Radiation
To estimate activities of radionuclides observed in natural and manmade lakes affected by various nuclear events, we can mention lake Inba affected by the Fukushima Daiichi Nuclear Power Plant accident (Cao et al. 2017) and Lake Maggiore (Italy, Switzerland), which received some 137Cs as a consequence of atmospheric nuclear fallouts (Putyrskaya et al. 2009). The maximal dose rate for the sediments of these habitats was estimated to be 6 mGy/h. Thus, it would take about 1900 years to accumulate the dose of 100 Gy, which would induce individual and population effects in hatchlings from resting eggs deposited in such sediments. For areas with a high level of radioactive contamination (Lake Karachai, lakes of the Southern Urals, the cooling pond of the Chernobyl NPP, and the marine cooling area of the Leningrad NPP), the estimated dose rates vary from 0.002 to 800 Gy per day (Kryshev and Sazykina 1998). The dose of 100 Gy in such sediments will be accumulated in 137 years (for a dose rate of 0.002 Gy per day) or in several hours (for a dose rate of 800 Gy per day).
We have already mentioned that the age of resting eggs that contribute to the zooplankton in lakes lies within the range of 10–20 years (Hairston 1996; Hairston Jr. et al. 2000). This time is long enough for resting eggs to accumulate critical doses in highly polluted lakes. We also observed that during reactivation, resting eggs were more sensitive to the effect of ionizing radiation than eggs in deep diapause. We can summarize that environmentally relevant unfavourable effects of ionizing radiation on the resting eggs in sediments can be manifested in areas with radioactive contamination that is high enough to produce either a cumulative dose or an acute dose during reactivation of resting eggs that will affect hatching of resting eggs and/or life cycle parameters of hatchlings (Fig. 7.1).
5 Conclusion
Our review demonstrated that in spite of the high significance of resting eggs of zooplankton for the ecology and evolution of natural communities, studies on the negative effects of sediments contaminated with anthropogenic pollutants on resting eggs are fragmentary. We observed a recent increase in the number of papers addressing sensitivity of resting eggs to various toxic compounds. This interest was mostly stimulated by the understanding of the role that resting stages play in natural habitats and by the advances in paleolimnological research. However, many of the studies focused on the effect of a specific toxicant on resting eggs lack dose-response curves or estimates of delayed responses.
We have covered a substantial gap in this field of research in our recent detailed study of the effects of several heavy metals and gamma radiation on dormant eggs of M. macrocopa. We determined critical concentrations of toxicants and the lethal dose of gamma radiation for resting eggs of M. macrocopa. We also studied the performance of hatchlings from resting eggs exposed to either toxic or radioactive effect of pollutants. However, this is just a small contribution to this complex research area.
It is highly important to investigate mechanisms that are responsible for the viability of resting eggs under a wide range of concentrations of various toxicants and different durations of direct contact of eggs with toxicants. Detailed studies of the structure of the ephippium and interactions of toxicants with these protective structures are needed. The exposure of resting eggs to contaminated bottom sediments can affect the performance of hatchlings. Individual and population level responses should be estimated both in laboratory experiments and in natural habitats. This information is necessary to predict the effect of contamination of natural habitats by different pollutants on the ecosystem structure and functioning.
References
Alekseev VR, de Stasio BT, Gilbert JJ (2007) Diapause in aquatic invertebrates: theory and human use. Springer, Netherlands, p 257
Alekseev V, Makrushin A, Hwang JS (2010) Does the survivorship of activated resting stages in toxic environments provide cues for ballast water treatment? Mar Pollut Bull 61:254–258
Alonzo F, Gilbin R, Zeman FA, Garnier-Laplace J (2008) Increased effects of internal alpha irradiation in Daphnia magna after chronic exposure over three successive generations. Aquat Toxicol 87:146–156
Alonzo F, Hertel-Aas T, Real A, Lance E, Garcia-Sanchez L, Bradshaw C, Batlle JV, Oughton DH, Garnier-Laplace J (2016) Population modelling to compare chronic external radiotoxicity between individual and population endpoints in four taxonomic groups. J Environ Radioact 152:46–59
Aránguiz-Acuña A, Pérez-Portilla P (2017) Metal stress in zooplankton diapause production: post-hatching response. Ecotoxicology 26:329–339
Avila-Perez P, Balcazar M, Zarazua-Ortega G, Barcelo-Quintal I, Dıaz-Delgado C (1999) Heavy metal concentrations in water and bottom sediments of a Mexican reservoir. Sci Total Environ 234:185–196
Beltrán-Pardo E, Jönsson KI, Wojcik A, Haghdoost S, Harms-Ringdahl M, Bermúdez-Cruz MR, Villegas JEB (2013) Effects of ionizing radiation on embryos of the tardigrade Milnesium cf. tardigradum at different Stages of development. PLoS ONE. https://doi.org/10.1371/journal.pone.0072098
Brendonck L, De Meester L (2003) Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491:65–84
Cao L, Ishii N, Zheng J, Kagami M, Pan S, Tagami K, Uchida S (2017) Vertical distributions of Pu and radiocesium isotopes in sediments from lake Inba after the Fukushima Daiichi nuclear power plant accident: source identification and accumulation. Appl Geochem 78:287–294
Dallas LJ, Keith-Roach M, Lyons BP, Jhaa AN (2012) Assessing the impact of ionizing radiation on aquatic invertebrates: a critical review. Radiat Res 2012(177):693–716
Deng H, Ye ZH, Wong MH (2004) Accumulation of lead, zinc, copper and cadmium by 12 wetland plant contamination sites in China. Environ Pollut 132:29–40
Donaldson LR, Foster RF (1957) Effects of radiation on aquatic organisms, National Academy of Sciences. The effects of atomic radiation on oceanography and fisheries. Publication 551. National Academy of Sciences-National Research Council, Washington, DC, pp 96–102
Eisler R (1994) Radiation hazards to fish, wildlife, and invertebrates: a synoptic review, Contaminant hazard reviews report. US National Biological Service. U.S. Deparment of the Interior, Washington, DC. №29. 147 p
Gilbin R, Alonzo F, Garnier-Laplace J (2008) Effects of chronic external gamma irradiation on growth and reproductive success of Daphnia magna. J Environ Radioact 99:134–145
Gladyshev E, Meselson M (2008) Extreme resistance of bdelloid rotifers to ionizing radiation. PNAS 105:5139–5144
Gutierrez MF, Battauz Y, Caisso B (2017) Disruption of the hatching dynamics of zooplankton egg banks due to glyphosate application. Chemosphere 171:644–653
Hairston NG (1996) Zooplankton egg banks as biotic reservoirs in changing environments. Limnol Oceanogr 41:1087–1092
Hairston NG Jr, Van Brunt RA, Kearns CM, Engstrom DR (1995) Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76:1706–1711
Hairston NG Jr, Hansen AM, Schaffner WR (2000) The effect of diapause emergence on the seasonal dynamics of a zooplankton assemblage. Freshw Biol 45:133–145
Iwasaki T (1964) Sensitivity of Artemia eggs to the gamma irradiation 1. Hatchability of encysted dry eggs. J Radiat Res 5:69–75
Jiang XD, Wang GZ, Li SJ, He JF (2007) Heavy metal exposure reduces hatching success of Acartia pacifica resting eggs in the sediment. J Environ Sci 19:733–737
Kansanen P, Jaakkola T, Kulmala S, Suutarinen R (1991) Sedimentation and distribution of gamma-emitting radionuclides in bottom sediments in southern Lake Päijänne Finland, after the Chernobyl accident. Hydrobiology 222:121–140
Kryshev II, Sazykina TG (1998) Radioecological effects on aquatic organisms in the areas with high levels of radioactive contamination: environmental protection criteria. Radiat Prot Dosim 75:187–191
Lampert W, Fleckner W, Rai H, Taylor BE (1986) Phytoplankton control by grazing zooplankton: a study on the spring clear-water phase. Limnol Oceanogr 31:478–490
Lopatina TS, Bobrovskaya NP, Oskina NA, Zadereev ES (2017) Comparative study of the toxic effect of cadmium and nickel on the active and resting stages of Cladoceran Moina macrocopa. J Siberian Federal Univ Biol 10:358–372
Lopatina TS, Zadereev ES, Oskina NA, Petrichenkov MV (2018) The sensitivity of resting eggs of the cladoceran Moina macrocopa to the effect of ionizing radiation during the reactivation. Dokl Biochem Biophys 480:169–172
Massarin S, Alonzo F, Garcia-Sanchez L, Gilbin R, Garnier-Laplace J, Poggiale JC (2010) Effects of chronic uranium exposure on life history and physiology of Daphnia magna over three successive generations. Aquat Toxicol 99:309–319
May RM, Maria M, Guimard J (1964) Action differentielle des rayons x et ultraviolets sur le tardigrade Macrobiotus areolatus, al’etat actif et desseche. Bull Biol Fr Belg 98:349–367
Most M, Chiaia-Hernandez AC, Frey MP, Hollender J, Spaak P (2015) A mixture of environmental organic contaminants in lake sediments affects hatching from Daphnia resting eggs. Environ Toxicol Chem 34:338–345
Navis S, Waterkeyn A, Voet T, De Meester L, Brendonck L (2013) Pesticide exposure impacts not only hatching of dormant eggs, but also hatchling survival and performance in the water flea Daphnia magna. Ecotoxicology 22:803–814
Navis S, Waterkeyn A, Putman A, De Meester L, Vanermen G, Brendonck L (2015) Timing matters: sensitivity of Daphnia magna dormant eggs to fenoxycarb exposure depends on embryonic developmental stage. Aquat Toxicol 83:159–176
Nielsen DL, Smith D, Petrie R (2012) Resting egg banks can facilitate recovery of zooplankton communities after extended exposure to saline conditions. Freshw Biol 57:1306–1314
Novikova N, Gusev O, Polikarpov N, Deshevaya E, Levinskikh M, Alekseev V, Okuda T, Sugimoto M, Sychev V (2011) Survival of dormant organisms after long-term exposure to the space environment. Acta Astronaut 68:1574–1580
Oskina N, Lopatina T, Zadereev E (2018) High resistance of resting eggs of cladoceran Moina macrocopa to the effect of heavy metals. Bull Environ Contam Toxicol. https://doi.org/10.1007/s00128-018-2473-7
Polikarpov GG (1998) Conceptual model of responses of organisms, populations and ecosystems to all possible dose rates of ionizing radiation in the environment. Radiat Prot Dosim 75:181–185
Putyrskaya V, Klemt E, Rollin S (2009) Migration of 137 Cs in tributaries, lake water and sediment of Lago Maggiore (Italy, Switzerland) - analysis and comparison with Lago di Lugano and other lakes. J Environ Radioact 100:35–48
Radzikowski J (2013) Resistance of dormant stages of planktonic invertebrates to adverse environmental conditions. J Plankton Res 35:707–723
Raikow DF, Reid DF, Maynard EE, Landrum PF (2006) Sensitivity of aquatic invertebrate resting eggs to Seakleen (menadione): a test of potential ballast tank treatment options. Environ Toxicol Chem 25(2):552–559
Raikow DF, Landrum PF, Reid DF (2007a) Aquatic invertebrate resting egg sensitivity to glutaraldehyde and sodium hypochlorite. Environ Toxicol Chem 26:1770–1773
Raikow DF, Reid DF, Blatchley ER, Jacobs G, Landrum PF (2007b) Effects of proposed physical ballast tank treatments on aquatic invertebrate resting eggs. Environ Toxicol Chem 26:717–725
Razo I, Carrizales L, Castro J, Diaz-Barriga F, Monroy M (2004) Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water Air Soil Pollut 152:129–152
Rogalski MA (2015) Tainted resurrection: metal pollution is shown in the Daphnia egg banks. Ecology 96:1166–1173
Rogalski MA (2017) Maladaptation to acute metal exposure in resurrected clones after decades of increasing contamination. Am Nat 189(4):443–452
Rother A, Pitsch M, Huelsmann S (2010) The importance of hatching from resting eggs for population dynamics and genetic composition of Daphnia in a deep reservoir. Freshw Biol 55:2319–2331
Sacmaci S, Yilmaz Y, Kartal S, Kaya M, Duman F (2014) Resting eggs as new biosorbent for preconcentration of trace elements in various samples prior to their determination by FAAS. Biol Trace Elem Res 159:254–262
Sarabia R, Del Ramo J, Varó I, Díaz-Mayans J, Torreblanca A (2008) Sublethal zinc exposure has a detrimental effect on reproductive performance but not on the cyst hatching success of Artemia parthenogenetica. Sci Total Environ 398:48–52
Sarapultseva EI, Dubrova YE (2016) The long-term effects of acute exposure to ionizing radiation on survival and fertility in Daphnia magna. Environ Res 150:138–143
Sarapultseva EI, Gorski AI (2013) Low-dose g-irradiation affects the survival of exposed Daphnia and their offspring. Dose-Response 11:460–468
Suyama I, Etoh H, Maruyama T, Kato Y, Ichikawa R (1981) Effects of ionizing radiation on the early development of Oryzias eggs. J Radiat Res 25:125–133
Turko P, Sigg L, Hollender J, Spaak P (2016) Rapid evolutionary loss of metal resistance revealed by hatching decades-old eggs. Evolution 70:398–407
United Nations Scientific Committee on the Effect of Atomic Radiation (2010) Sources and effect of ionizing radiation. United Nations, New York. Available online at: http://www.unscear.org/docs/reports/2008/09-86753_Report_2008_Annex_B.pdf
Van der Stricht S, Janssens A (2010) Radioactive effluents from nuclear power stations and nuclear fuel reprocessing sites in the European union, 2004e08. RP 164. Publications Office of the European Union, Luxembourg. http://dx.doi.org/10.2833/27366. Available online at: https://ec.europa.eu/energy/sites/ener/files/documents/164.pdf
Vesk PA, Allaway WG (1997) Spatial variation of copper and lead concentrations of water hyacinth plants in a wetland receiving urban run-off. Aquat Bot 59:33–44
Watanabe M (2006) Anhydrobiosis in invertebrates. Appl Entomol Zool 41:15–31
Wong CK, Wong PK (1990) Life table evaluation of the effects of cadmium exposure on the freshwater cladoceran, Moina macrocopa. Bull Environ Contam Toxicol 44:135–141
Wong CK, Wong PK, Tao H (1991) Toxicity of nickel and nickel electroplating water to the freshwater cladoceran Moina macrocopa. Bull Environ Contam Toxicol 47:448–454
Zadereev ES, Lopatina TS, Zotina TA, Dementyev DV, Oskina NA, Petrichenkov MV (2016) The effect of γ-radiation on resting eggs and life cycle of cladoceran Moina macrocopa. Dokl Biochem Biophys 466:61–65
Zadereev E, Lopatina T, Oskina N, Zotina T, Petrichenkov M, Dementyev D (2017) Gamma irradiation of resting eggs of Moina macrocopa affects individual and population performance of hatchlings. J Environ Radioact 175-176:126–134
Zampi M, Focardi S (1973) Effects of ionizing radiation on the eggs of Milax nigricans (Schultz) Mollusca Gastropoda Pulmonata. Bolletino di zoologia 40:291–303
Acknowledgements
The research was partially supported by the Russian Foundation for Basic Research (Project No. 15-04-05199) and the Krasnoyarsk Regional Science Foundation (Project No. 16-44-243041). We are grateful to Helen Krasova for linguistic improvements.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zadereev, E., Lopatina, T.S., Oskina, N. (2019). Resistance of Dormant Eggs of Cladocera to Anthropogenic Pollutants. In: Alekseev, V., Pinel-Alloul, B. (eds) Dormancy in Aquatic Organisms. Theory, Human Use and Modeling. Monographiae Biologicae, vol 92. Springer, Cham. https://doi.org/10.1007/978-3-030-21213-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-21213-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21212-4
Online ISBN: 978-3-030-21213-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)