Abstract
Cardiovascular diseases remain a leading cause of death worldwide with ischemic heart disease being the most common cause. Due to the poor proliferation capability of adult cardiomyocytes, an injured heart is incapable of replacing its lost myocardium. Instead, the heart heals through formation of fibrotic scar tissue which, unlike cardiomyocytes, lacks contractile capability. This gradually weakens the heart and in the long term may result in heart failure. Efforts to aid the heart in regenerating its lost cardiomyocytes have been concentrated on using stem cells as a source of new cells. In this chapter, we focus on the recent developments, challenges, and improvements that researchers around the world have made in the field of cell-based regenerative therapy, such as pluripotent stem cell- and adult stem cell-derived cardiomyocytes. Although there are still problems that need to be addressed, studies up to date have shown promising results in recovering the lost cardiomyocytes and ultimately improving heart function after injury.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cardiovascular disease is one of the leading causes of death in the world. According to the report published by the American Heart Association, out of an estimated 54 million deaths throughout the world in 2013, cardiovascular diseases contributed to 17.3 million (31.5%) deaths, with ischemic heart disease being the most common cause [1]. As such, there is an urgent need to address this problem.
The left ventricle of an adult human heart contains around two to four billions cardiomyocytes (heart muscle cells). When a person suffers from cardiovascular diseases such as myocardial infarction (MI), as much as one billion of his/her cardiomyocytes die within a few hours [2]. Adult cardiomyocytes are known to have poor proliferation capability, meaning that they possess poor ability to generate new cells to replace the lost cardiomyocytes. Hence, subsequent healing of the heart post-MI mainly takes place through scar formation rather than muscle regeneration. This eventually results in the loss of heart muscle strength and over time, when more and more cardiomyocytes are lost without new ones being regenerated, the patient will eventually develop heart failure.
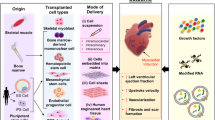
In some cases, cardiac transplantation is performed as a last resort; however patients who are in need of transplantation far outnumber the available donors. Furthermore, there is always a risk of organ rejection posttransplantation by the recipient’s immune system. With this in mind, researchers around the world have focused on alternative therapeutic methods that are capable of preventing the progression of cardiovascular diseases. This can be achieved either by preventing the death of cardiomyocytes or by regenerating the lost cells. This chapter focuses on the latter, specifically on myocardial regeneration by means of differentiation of stem cells and other modified cell types as shown in Fig. 9.1.
Pluripotent Stem Cells
Pluripotent Stem Cells for Cardiomyocyte Regeneration
Stem cells are defined by their capacity to maintain their undifferentiated state while proliferating , but are also capable of differentiating into various cell types when necessary. Pluripotent stem cells (PSCs) are able to differentiate into different cell lineages, including cardiac cell lineage, and are one of the most promising candidates among various stem cell sources for cardiac regeneration [3]. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are two main PSCs that have been extensively investigated for cardiac regeneration.
ESCs are derived from the inner cell mass of a blastocyst. These pluripotent cells can be directly differentiated into the desired cell types from any lineages. Following the isolation of the inner cell mass, a blastocyst is destroyed and is therefore not able to develop into an embryo (human or murine). This has led to controversies concerning the ethical issues of using human embryo for stem cell research.
Unlike ESCs, iPSCs are derived from somatic cells, such as fibroblasts or peripheral blood. These terminally differentiated cells can regain their pluripotency by undergoing a reprogramming process , through the introduction of several transcription factors that are important for pluripotency [4,5,6]. In their seminal work published in 2006, Yamanaka and associates successfully reprogrammed mouse fibroblast to iPSCs by using a cocktail of four transcription factors, Oct3/4, Sox2, c-Myc, and Klf4 [5]. In the years that followed, many other groups also reported successful reprogramming of human somatic cells such as dermal fibroblasts [6,7,8], peripheral blood cells [9,10,11,12], and keratinocytes [13, 14] to iPSCs by using various combinations of transcription factor cocktails. The resulting reprogrammed pluripotent cells are highly similar to ESCs [15] and can subsequently be differentiated into the desired cell types from any lineages. One obvious advantage of iPSCs compared to ESCs is that they do not involve isolation of cells from an embryo and therefore circumvent the aforementioned ethical issues.
Aside from circumventing the ethical issue posed by using ESCs , another advantage of using iPSCs as an alternative is the clinical advantage. It is well known that patients who undergo organ transplantation are at risk of rejection from their own immune system. Similarly, there is a high risk that the transplanted ESC-derived cells are rejected by the patient’s immune system [16]. Since iPSCs can be derived from patient’s own somatic cells, patient-specific iPSCs can be generated. Together with the aid of immunosuppressant drugs, the risk of rejection by the patient’s immune system should be reduced when transplanting autologous iPSC-derived cells [16, 17].
PSCs are able to differentiate into many types of cells including cardiomyocytes. However, poor differentiation and purification of desired cells in culture may result in PSCs differentiating into unintended cell types. If undifferentiated PSCs are transplanted along with differentiated cells, then they may lead to teratoma formation. Efficient differentiation and purification of target cells from unwanted ones are crucial in avoiding the potential formation of teratomas or other types of cancer.
Differentiation of PSCs to Cardiomyocytes and Their Maturation
Both ESCs and iPSCs are capable of differentiation into many types of cells from various lineages, including cardiac lineage. In order to achieve this, there are several important stages that PSCs have to undergo and several important growth factors that have to be administered during the differentiation. There are two most commonly utilized methods for differentiating cardiomyocytes from human PSCs (hPSCs) , namely, embryonic body (EB) formation and monolayer culture during the initial stages of PSC differentiation. Either of these strategies lead to the induction of mesoderm which can then be stimulated to cardiac specification and cardiac maturation.
In order for the cells to achieve each of these stages, specific conditions and growth factors are needed, and these have been reported by various groups [18,19,20,21,22,23,24]. EB can be formed by culturing hPSCs in StemPro34 culture medium [19,20,21]. On the other hand, monolayer culture is achieved by culturing them in mTeSR1 or MEF-conditioned (mouse embryonic fibroblast conditioned medium, CM) culture medium which is then replaced with Roswell Park Memorial Institute (RPMI) medium containing B27 supplement [22, 23]. Either EB or monolayer PSC culture then undergoes mesoderm induction through treatment with WNT activator/GSK3-beta inhibitor such as CHIR99021 or by combination Activin A/BMP4 [19, 20, 22, 23]. The culture media is largely not changed to allow for the accumulation of secreted growth factors. After mesodermal differentiation has been induced, inhibitors of WNT signaling such as IWR-1 or IWP-4 are then added to direct specification toward cardiac differentiation [21, 23]. Finally, factors that promote cardiac differentiation such as VEGF, FGF2, or small molecule triiodothyronine [19,20,21, 24] are added to enhance the yield of cardiomyocytes.
One of the limitations in using PSC-derived cardiomyocytes (PSC-CMs) for cardiac repair is their immaturity. These cells tend to exhibit the phenotype of fetal cells rather than adult cells. This is to be expected, as adult cardiomyocytes in the human body have undergone years of maturation. In contrast, newly differentiated PSC-CMs have only undergone a few weeks of maturation [25]. Some of the characteristics, among others, that can be used to differentiate immature and mature cells are size, shape, alignment, resting membrane potential, and metabolic substrate [25, 26].
Immature PSC-CMs are small and round-shaped, have disorganized sarcomeres, and possess a resting membrane potential of about −60 mV [25]. They rely on glucose and glycolysis as their main metabolic substrate and metabolism pathway, respectively [26]. When cardiomyocytes mature, they become larger and rod-shaped, with highly organized sarcomeres and a resting membrane potential of about −80 to −90 mV [25]. Their oxidative ability increases as they mature, and therefore, mature cardiomyocytes tend to use fatty acid as their main metabolic substrate and hence the β-oxidation as their metabolism pathway [26].
Transplantation of immature PSC-CMs may result in arrhythmia (irregular heartbeat) and less efficient contraction [24], and therefore, maturation should be achieved either prior or shortly after transplantation to ensure patient’s safety. There are several ways to promote the maturation of PSC-CMs, such as treating with small molecules (triiodothyronine) [24], co-culturing cardiomyocytes along with non-cardiomyocytes such as endothelial cells, or electrical and mechanical stimulation [25, 27, 28]. These methods work synergistically to enhance the electrophysiological and mechanical maturation of cardiomyocytes [24, 28].
Alignment of cardiomyocytes is also an important issue, as immature PSC-CMs tend to be disorganized. One study proved the importance of alignment by culturing cardiomyocytes and endothelial cells aligned and nonaligned nanofibrous electrospun patches [29]. The resulting aligned and nonaligned patches were transplanted on rats with MI hearts. It was found that the cardiac function of the hearts treated with aligned patches was improved. On the contrary, hearts treated with nonaligned patches deteriorated after the implantation [29].
PSC-CMs in Regenerative Cardiac Therapy
One of the most common methods to deliver PSC-CMs to the heart is through direct intramyocardial injection. This is usually performed using cells enclosed within a biomaterial scaffold, such as Matrigel® or collagen , to help retaining the cells in the heart. Over time, the cells secrete extracellular matrices, promoting the formation of cell-cell connection, while the biomaterial scaffold slowly degrades. In the end, biomaterial-free cells are engrafted and transplanted to the heart. Although the scaffold helps in ensuring the retention, it does not provide the support for the cells to form connections with the host cardiomyocytes. Therefore, this may result in poor engraftment as the individual cells will have to form the connections with host cardiomyocytes.
In order to optimize the engraftment of stem cells onto the heart, investigators have embedded cells onto a sheet of polymer scaffold, and the resulting construct is then transplanted [3, 30]. This transplantable scaffold technology enables the retention of cells without relying on enzymatic digestion [30] and ensures long-term engraftment of cells on the heart. Studies have been performed whereby murine ESC cardiomyocyte (mESC-CM) cell sheets were transplanted in rats with MI [30] and human iPSC cardiomyocyte (hiPSC-CM) cell sheets were transplanted in pigs with ischemic cardiomyopathy [31, 32]. In all cases, the utilization of cell sheets improved the engraftment of the cells in the heart while at the same time also improves the heart function [3, 30,31,32]. A clinical study has been performed using human ESC (hESC) cardiac progenitors embedded on a fibrin patch [33]. The result of this study showed that there was no arrhythmia or tumor formation, given that no cells were found surviving after patch engraftment. However, the issue of long-term efficacy and safety will need to be addressed [33].
Despite all the issues, in vivo transplantation of PSC-CMs has been studied using small and large animal models [17, 22, 34,35,36,37,38,39,40,41]. Human ESC-derived cardiomyocytes (hESC-CMs) have been transplanted on the infarct heart of rodents such as mice [34], rats [22, 35], and guinea pigs [36]. Mouse iPSC-derived cardiomyocytes have also been transplanted in MI mice which resulted in improved left ventricular function [37]. These studies have shown that in all cases, PSC-CMs improved heart function postinfarction in small animals [22, 34,35,36,37].
In large animals, sheep were used in the study that exploited cardiac-committed murine ESCs (mESCs) . In addition pigs were used in studies using human iPSC (hiPSC)-derived cells. Furthermore, nonhuman primates were used in the transplantation study using hESC-CMs and monkey iPSC-derived cardiomyocytes (monkey iPSC-CMs) [17, 38,39,40,41]. In sheep with MI, transplantation of mESCs successfully improved the function of the left ventricle [39]. Improvement of the heart function was also observed in pigs with acute MI transplanted with hiPSC-CMs, hiPSC-derived endothelial cells, and hiPSC-derived smooth muscle cells [38]. For the study using nonhuman primates, macaque monkeys (pigtail macaque Macaca nemestrina [40, 41] and cynomolgus monkey Macaca fascicularis [17]) were selected. Transplantation of hESC-CMs in pigtail macaque monkeys with MI resulted in successful short-term (<90 days) engraftment of cardiomyocytes [40, 41], while monkey iPSC-CMs transplanted in cynomolgus monkeys improved the cardiac contractile function [17]. It should be noted, however, that transplantation of PSC-CMs in large animals may give different result from that in small animals. Transplantation of PSC-CMs in macaque monkeys resulted in arrhythmia that had not previously been observed in rodents [17, 40, 41].
Adult Stem Cells
Bone Marrow-Derived Adult Stem Cells for Angiogenesis and Cardiac Regeneration
Aside from pluripotent stem cells, adult stem cells show relatively limited but useful potential for heart regeneration. The well-established isolation protocol and autologous transplantation have led adult stem cell-based therapy to undergo preclinical or clinical trials to test the therapeutic efficiency for heart disease in a clinical setting [42].
Bone marrow (BM)-derived mesenchymal and adult stem cells including unselected or selected mononuclear cells were reported to support angiogenesis and secrete a number of paracrine factors to protect cardiomyocytes from death [43]. They do this by inducing several cytokines and chemokines such as platelet-derived growth vector (PDGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF-1) for angiogenesis and arteriogenesis, resulting in improved heart function after injury [44, 45]. After transplantation, these BM-derived adult stem cells seldomly differentiate into cardiomyocytes or endothelial cells in the damaged region of the heart, therefore, aiding heart regeneration [46, 47]. Other BM-derived adult stem cells, such as CD34-positive hematopoietic stem cell, would migrate and infiltrate into the infarct area, increasing microvascularity and decreasing fibrosis, ultimately resulting in improved heart function after MI [48].
Cardiac Stem Cells for Heart Regeneration
Endogenous cardiac stem cells (CSCs) were found to express embryonic cardiac progenitor markers and show great efficiency to differentiate into cardiac lineage cells. CSCs, initially reported as c-Kit positive cells, reside in the atrium and ventricular apex of adult hearts [49, 50]. Over time, several other markers were gradually discovered to define CSCs such as Sca-1 or MDR-1 [51], indicating potential ability for multipotency, self-renewal, and limited differentiation into cardiac cells including cardiomyocytes, endothelial cells, and smooth muscle cells [52]. Cardiac stem cells were reported to participate in cardiomyocyte and vasculature formation, and to protect existing cardiomyocytes from death [53]. Nevertheless, using genetic fate-mapping, recent studies suggested that the cardiogenic capability of c-Kit or Sca-1 cells might be quite low [54, 55].
Cardiosphere-Derived Cells for Heart Regeneration
Cardiosphere-derived cells have been isolated and cultured from heart biopsy to form a 3D sphere with 20–150 μm in diameter [56, 57]. The cardiosphere was packed with CSCs inside and coated with cardiac-differentiated cells outside. The outer layer protected the internal CSCs, maintaining their activity and function. CSCs would proliferate and improve heart function after the cardiosphere had been attached to the fibronectin on the infarct site after injury [58, 59]. The cardiosphere-derived cell therapy is currently undergoing clinical trial and the preliminary results of the trial so far have been encouraging, where significant decrease in scar formation in the infarct area of the heart after injury was observed [59].
Proliferative Cardiomyocytes
Gene Modification for Cardiomyocyte Proliferation and Heart Regeneration
The loss of proliferative ability of adult cardiomyocytes is one of the reasons why heart disease remains a leading cause of death. Although delivery of mature cardiomyocytes may decrease potential problem with synchronization between host and transplanted cells, their limited proliferative ability is still a concern for therapy. Several cell cycle inducers such as cyclin D1 or cyclin A2 or repressor Rb1 were manipulated in cardiomyocytes to regain their proliferative ability; however, the efficacy of the proliferative ability was still low [60,61,62,63]. Therefore, upstream cell cycle regulators were taken into consideration to obtain more powerful cardiomyocyte proliferation advancement. Transcription factor E2F4 was found to co-localize with kinetochore in cardiomyocytes, and together, they support mitosis [64]. Knockdown of E2F4 by siRNA significantly affected cardiomyocyte proliferation due to reduction in mitosis. RE1 silencing transcription factor (REST) supports cardiomyocyte proliferation through inhibition of the cell cycle inhibitor p21 [65]. Under REST deletion, p21 knockout rescued cell cycle arrest in cardiomyocytes, indicating the importance of REST-p21 signaling in the proliferative ability of cardiomyocytes. Transcription factor with opposite function reported as Meis1 was found to be capable of inhibiting cyclin-dependent kinase inhibitor and therefore increasing cardiomyocyte proliferation ability [66]. Through careful examination of cardiomyocyte reprogramming process, combined gene modification (FoxM1, Id1, Jnk3 inhibitor) was determined to efficiently increase cardiomyocyte proliferation by inducing each step of cell cycle for heart regeneration after injury [4].
Small RNAs and Signalings Induce Cardiomyocyte Proliferation for Heart Regeneration
Small RNAs also play a role in producing cardiomyocyte progenitors for heart regeneration. Through high throughput screening, several microRNAs such as miR-590 and miR-199a have been shown as candidates that could increase neonatal cardiomyocyte proliferation [67].
Some signaling pathways were reported to be essential for cardiomyocyte proliferation. Hippo signaling induces Yap phosphorylation, which was reported to be present in low level in neonatal mice with high proliferative ability but present in high level in adult mice [68, 69]. Furthermore, Yap activation stimulates cardiomyocyte proliferation after heart injury, and Hippo/Yap pathway has been shown to possess excellent potential for heart regeneration without causing cardiac hypertrophy [70]. Neuregulin (Nrg1)-ErbB2 signaling is another regulator of cardiomyocyte cell cycle. Nrg1 induction led to increased proliferative ability of cardiomyocytes through its receptor ErbB2 signaling [71, 72]. Induction of ErbB2 led to enlarged size and enhanced proliferation in cardiomyocytes, showing therapeutic potential for improving heart function after MI [73].
Transdifferentiation from Cardiac Fibroblasts to Cardiomyocytes
Aside from manipulating cardiomyocytes in order to regain their proliferative ability as abundant cell source for heart therapy, cardiac fibroblasts have also been taken into consideration for their potential cardiomyocyte transformation. Cardiac fibroblasts and cardiomyocytes are both differentiated from cardiac-committed mesoderm, and the cardiomyocyte progenitor markers (Gata4, Mef2C, and Tbx5, GMT) have been reported as the main factors for cardiomyocyte differentiation. With this in mind, a new technique capable of performing GMT expression in cardiac fibroblasts or dermal fibroblasts was developed. This technique is called transdifferentiation, and it has been used for producing induced cardiomyocyte-like cells (iCMs) with excellent contractility [74]. This technique has been tested successfully in vitro and in vivo [75]; however, the efficacy of the transdifferentiation should still be improved for better practical clinical application [76].
A different strategy has also been reported where fibroblasts were reprogrammed directly to cardiac progenitor cells using a combination of five genes, Mesp1, Gata4, Tbx5, Nkx2–5, and Baf60c [77]. Transplantation of the resulting cells into mice hearts post-MI showed improved survival. This strategy bypassed the pluripotent stage and therefore could potentially reduce the risk of teratoma formation [77].
Conclusion
Heart disease remains a leading cause of death worldwide and the main reason is the inability of the heart itself to regenerate the lost cardiomyocytes. Cell-based therapy has shown great potential in replacing or complementing the lost cells, and overall in improving heart function after injury. In this chapter, we integrate various cell types including stem cells and modified cell types to demonstrate their potential as cell sources for heart disease. Even though large-scale production of candidate cells is no longer a problem for cell-based therapy, safety, cell retention, survival, engraftment, and maturation after transplantation are the main issues that should be addressed for better and more efficient therapeutic and clinical applications. Although at this point the prospect for iPS cell-based therapy to reach clinical use remains uncertain, ongoing efforts by numerous groups around the world should ensure improvement in efficacy and safety of this therapeutic approach. We believe that all of the studies so far have set the stage for cardiac cell-based regenerative therapy to succeed and that in the near future, we will be able to demonstrate the ability for cell transplantation to save the lives of people suffering from heart failure.
References
Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–603.
Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–35.
Masumoto H, Ikuno T, Takeda M, et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep. 2015;4:6716.
Cheng Y-Y, Yan Y-T, Lundy DJ, Lo AH, Wang Y-P, Ruan S-C, Lin P-J, Hsieh PCH. Reprogramming-derived gene cocktail increases cardiomyocyte proliferation for heart regeneration. EMBO Mol Med. 2017;9:251–64.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Park I-H, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6.
Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20.
Ye Z, Zhan H, Mali P, Dowey S, Williams DM, Jang Y-Y, Dang CV, Spivak JL, Moliterno AR, Cheng L. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114:5473–80.
Seki T, Yuasa S, Oda M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–4.
Loh Y-H, Hartung O, Li H, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–9.
Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, Mostoslavsky G, Jaenisch R. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–4.
Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–84.
Carey BW, Markoulaki S, Hanna J, Saha K, Gao Q, Mitalipova M, Jaenisch R. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci. 2009;106:157–62.
Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678–84.
Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49.
Shiba Y, Gomibuchi T, Seto T, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–91.
Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–60.
Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8.
Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–40.
Willems E, Spiering S, Davidovics H, Lanier M, Xia Z, Dawson M, Cashman J, Mercola M. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res. 2011;109:360–4.
Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24.
Hudson J, Titmarsh D, Hidalgo A, Wolvetang E, Cooper-White J. Primitive cardiac cells from human embryonic stem cells. Stem Cells Dev. 2012;21:1513–23.
Lee Y-K, Ng K-M, Chan Y-C, Lai W-H, Au K-W, Ho C-YJ, Wong L-Y, Lau C-P, Tse H-F, Siu C-W. Triiodothyronine promotes cardiac differentiation and maturation of embryonic stem cells via the classical genomic pathway. Mol Endocrinol. 2010;24:1728–36.
Lee DS, Chen JH, Lundy DJ, et al. Defined MicroRNAs induce aspects of maturation in mouse and human embryonic-stem-cell-derived cardiomyocytes. Cell Rep. 2015;12:1960–7.
Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114:511–23.
Hsieh PCH, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66.
Kim C, Majdi M, Xia P, Wei KA, Talantova M, Spiering S, Nelson B, Mercola M, Chen HV. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev. 2010;19:783–95.
Lin YD, Ko MC, Wu ST, et al. A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomater Sci. 2014;2:567–80.
Masumoto H, Matsuo T, Yamamizu K, et al. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196–205.
Kawamura M, Miyagawa S, Miki K, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–37.
Kawamura M, Miyagawa S, Fukushima S, et al. Enhanced therapeutic effects of human iPS cell derived-cardiomyocyte by combined cell-sheets with omental flap technique in porcine ischemic cardiomyopathy model. Sci Rep. 2017;7:8824.
Menasché P, Vanneaux V, Hagège A, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–7.
van Laake LW, Passier R, Monshouwer-Kloots J, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24.
Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–93.
Shiba Y, Fernandes S, Zhu WZ, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–5.
Rojas SV, Kensah G, Rotaermel A, Baraki H, Kutschka I, Zweigerdt R, Martin U, Haverich A, Gruh I, Martens A. Transplantation of purified iPSC-derived cardiomyocytes in myocardial infarction. PLoS One. 2017;12:e0173222.
Ye L, Chang YH, Xiong Q, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–61.
Ménard C, Hagège AA, Agbulut O, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005;366:1005–12.
Chong JJH, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–7.
Liu YW, Chen B, Yang X, et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018;36:597–605.
Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–48.
Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42.
Erbs S, Linke A, Adams V, et al. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion: first randomized and placebo-controlled study. Circ Res. 2005;97:756–62.
Yao K, Huang R, Qian J, et al. Administration of intracoronary bone marrow mononuclear cells on chronic myocardial infarction improves diastolic function. Heart. 2008;94:1147–53.
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow–derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6.
Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5.
Nygren JM, Jovinge S, Breitbach M, Säwén P, Röll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SEW. Bone marrow–derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501.
Ellison GM, Vicinanza C, Smith AJ, et al. Adult c-kitpos cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–42.
Smith AJ, Lewis FC, Aquila I, Waring CD, Nocera A, Agosti V, Nadal-Ginard B, Torella D, Ellison GM. Isolation and characterization of resident endogenous c-kit+ cardiac stem cells from the adult mouse and rat heart. Nat Protoc. 2014;9:1662–81.
Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21.
Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76.
Ellison GM, Torella D, Dellegrottaglie S, et al. Endogenous cardiac stem cell activation by insulin-like growth Factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58:977–86.
van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin S-CJ, Middleton RC, Marbán E, Molkentin JD. C-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–41.
Vagnozzi RJ, Sargent MA, Lin S-CJ, Palpant NJ, Murry CE, Molkentin JD. Genetic lineage tracing of Sca-1+ cells reveals endothelial but not myogenic contribution to the murine heart. Circulation. 2018. https://doi.org/10.1161/CIRCULATIONAHA.118.035210.
Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of Cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908.
Davis DR, Zhang Y, Smith RR, Cheng K, Terrovitis J, Malliaras K, Li T-S, White A, Makkar R, Marbán E. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195.
Johnston PV, Sasano T, Mills K, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–83.
Chimenti I, Smith RR, Li T-S, Gerstenblith G, Messina E, Giacomello A, Marbán E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–80.
Soonpaa MH, Koh GY, Pajak L, Jing S, Wang H, Franklin MT, Kim KK, Field LJ. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest. 1997;99:2644–54.
Shapiro SD, Ranjan AK, Kawase Y, Cheng RK, Kara RJ, Bhattacharya R, Guzman-Martinez G, Sanz J, Garcia MJ, Chaudhry HW. Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci Transl Med. 2014;6:224ra27.
Foglia MJ, Poss KD. Building and re-building the heart by cardiomyocyte proliferation. Development. 2016;143:729–40.
Mohamed TMA, Ang Y-S, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell. 2018;173:104–116.e12.
van Amerongen MJ, Diehl F, Novoyatleva T, Patra C, Engel FB. E2F4 is required for cardiomyocyte proliferation. Cardiovasc Res. 2010;86:92–102.
Zhang D, Wang Y, Lu P, et al. REST regulates the cell cycle for cardiac development and regeneration. Nat Commun. 2017;8:1979.
Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–53.
Eulalio A, Mano M, Ferro MD, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–81.
Diez-Cuñado M, Wei K, Bushway PJ, Maurya MR, Perera R, Subramaniam S, Ruiz-Lozano P, Mercola M. miRNAs that induce human Cardiomyocyte proliferation converge on the hippo pathway. Cell Rep. 2018;23:2168–74.
Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61.
von Gise A, Lin Z, Schlegelmilch K, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci. 2012;109:2394–9.
Gemberling M, Karra R, Dickson AL, Poss KD. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. elife. 2015. https://doi.org/10.7554/eLife.05871.
Wadugu B, Kühn B. The role of neuregulin/ErbB2/ErbB4 signaling in the heart with special focus on effects on cardiomyocyte proliferation. Am J Physiol Heart Circ Physiol. 2012;302:H2139–47.
D’Uva G, Aharonov A, Lauriola M, et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17:627–38.
Ieda M, Fu J-D, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–86.
Fu J-D, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct reprogramming of human fibroblasts toward a Cardiomyocyte-like state. Stem Cell Reports. 2013;1:235–47.
Fu J-D, Srivastava D. Direct reprogramming of fibroblasts into cardiomyocytes for cardiac regenerative medicine. Circ J. 2015;79:245–54.
Lalit PA, Salick MR, Nelson DO, et al. Lineage reprogramming of fibroblasts into proliferative induced cardiac progenitor cells by defined factors. Cell Stem Cell. 2016;18:354–67.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Prajnamitra, R.P., Cheng, YY., Chen, LL., Hsieh, P.C.H. (2019). Cell-Based Cardiovascular Regenerative Therapies. In: Serpooshan, V., Wu, S. (eds) Cardiovascular Regenerative Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-20047-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-20047-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20046-6
Online ISBN: 978-3-030-20047-3
eBook Packages: MedicineMedicine (R0)