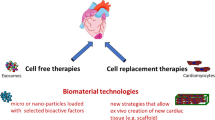
Abstract
Cardiovascular regenerative medicine is an interdisciplinary field that utilizes the principles of engineering and life sciences to restore the structure and/or function of damaged or diseased heart. While current cardiovascular tissue and organ transplantation therapies suffer from scarce donor supply and various immune system complications [1], regenerative medicine approaches have enabled bypassing some of these obstacles and heal or replace tissues damaged by acquired or congenital disease [2, 3]. A broad range of regenerative strategies are currently being investigated in preclinical and clinical stages [3]. These methods can be classified to (a) the use of exogenous materials (e.g., scaffolds or cardiac patch systems [4]) and/or cells [5] to replace or salvage the damaged tissue structure and function and (b) leveraging the body’s endogenous regenerative mechanisms [6, 7], although adult human heart possesses markedly restricted regenerative capacity. In many cases, a combination of these mechanisms is involved in healing the damaged tissue (e.g., paracrine effects or inducing innate therapeutic responses by implanted patch or cells) [8].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Regenerative medicine
- Tissue engineering
- Cardiovascular
- Translational medicine
- Additive manufacturing
- Biomaterials
Cardiovascular regenerative medicine is an interdisciplinary field that utilizes the principles of engineering and life sciences to restore the structure and/or function of damaged or diseased heart. While current cardiovascular tissue and organ transplantation therapies suffer from scarce donor supply and various immune system complications [1], regenerative medicine approaches have enabled bypassing some of these obstacles and heal or replace tissues damaged by acquired or congenital disease [2, 3]. A broad range of regenerative strategies are currently being investigated in preclinical and clinical stages [3]. These methods can be classified to (a) the use of exogenous materials (e.g., scaffolds or cardiac patch systems [4]) and/or cells [5] to replace or salvage the damaged tissue structure and function and (b) leveraging the body’s endogenous regenerative mechanisms [6, 7], although adult human heart possesses markedly restricted regenerative capacity. In many cases, a combination of these mechanisms is involved in healing the damaged tissue (e.g., paracrine effects or inducing innate therapeutic responses by implanted patch or cells) [8].
A variety of conventional and advanced tissue engineering methods are used to create cardiac patch systems including cell sheets [9], decellularized tissues [10], self- or wave-assembly techniques [11], and 3D bioprinting [12]. Although beneficial effects of engineered cardiac patch devices in a variety of in vitro and in vivo animal studies have been demonstrated, there still remain a number of challenges in their clinical applications. These challenges include (1) lack of sufficient vasculature in the patch, (2) precise control on the 3D scaffold structure, and (3) inadequate maturity/functionality of human cardiac muscle cells within engineered constructs [13, 14]. Additive manufacturing (i.e., 3D (bio)printing) technologies have emerged as powerful, versatile tools to manufacture 3D cardiac tissue constructs at remarkably greater precision, consistency, and reproducibility [14,15,16]. To date, a variety of bioprinted cardiovascular tissues/organs have been investigated including vasculature [17], cardiac patches [18, 19], coronary artery stents [20], and cardiac valves [21]. Despite the significant advances in cardiac tissue bioprinting, significant challenges remain, including but not limited to the need for large quantity of functional cardiac muscle cells and the necessity of incorporating functional vasculature in printed constructs [14]. Further work will be necessary to develop specialized bioink materials with biological and physiochemical properties that are optimized for cardiac tissue bioprinting [16].
While still in its infancy, the cardiovascular regenerative medicine field has made notable advances in recent years to enhance future applications to meaningfully regenerate damaged/diseased adult human heart [22, 23]. Efforts to understand the complexity of adult heart regeneration through basic sciences and translational studies in fields such as genetics, biomaterials and tissue engineering, nanotechnology, imaging, and cardiac cellular and molecular biology will pay dividends in the long run [23]. While the potential therapeutic benefits of many approaches to cardiac regenerative therapies have been examined thus far, their precise mechanisms of action are often unknown. For instance, while application of an epicardial patch, laden with the cardiogenic follistatin-like 1 protein , was recently shown to stimulate cardiomyocyte cell cycle re-entry and division, little is known about the cellular and molecular mechanisms underlying this regenerative effect [24, 25].
Looking ahead, the role for tissue engineering and regenerative therapy to treat patients with heart disease must contend and synergize with the large variety of device therapies that are currently being implemented in patients with cardiovascular diseases. For instance, while patients with sick sinus syndrome may benefit from implantation of biological pacemakers that are made from tissue engineered cells [26], the reliability and efficiency of these cells to maintain pacemaker function must be greater than that of an electronic pacemaker, which is the current standard of care [22]. It is expected that the future of regenerative therapies will focus on a selective number of cardiovascular diseases that are not served well by the current devices and treatments (e.g., genetic cardiomyopathies or acute myocardial infarction) [22]. Thus, economic, practical, and translational considerations must be more carefully taken into account in the future studies aiming at developing next-generation cardiovascular regenerative therapies.
References
Tonsho M, et al. Heart transplantation: challenges facing the field. Cold Spring Harb Perspect Med. 2014;4(5):a015636.
Mao AS, Mooney DJ. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A. 2015;112(47):14452–9.
Doppler SA, et al. Cardiac regeneration: current therapies-future concepts. J Thorac Dis. 2013;5(5):683–97.
Mahmoudi M, et al. Multiscale technologies for treatment of ischemic cardiomyopathy. Nat Nanotechnol. 2017;12(9):845–55.
Broughton KM, Sussman MA. Enhancement strategies for cardiac regenerative cell therapy: focus on adult stem cells. Circ Res. 2018;123(2):177–87.
Finan A, Richard S. Stimulating endogenous cardiac repair. Front Cell Dev Biol. 2015;3:57.
Xiang MS, Kikuchi K. Endogenous mechanisms of cardiac regeneration. Int Rev Cell Mol Biol. 2016;326:67–131.
Ye L, et al. Patching the heart: cardiac repair from within and outside. Circ Res. 2013;113(7):922–32.
Wang CC, et al. Direct intramyocardial injection of mesenchymal stem cell sheet fragments improves cardiac functions after infarction. Cardiovasc Res. 2008;77(3):515–24.
Wang B, et al. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J Biomed Mater Res A. 2010;94a(4):1100–10.
Serpooshan V, et al. Bioacoustic-enabled patterning of human iPSC-derived cardiomyocytes into 3D cardiac tissue. Biomaterials. 2017;131:47–57.
Pati F, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935.
Kolanowski TJ, Antos CL, Guan K. Making human cardiomyocytes up to date: derivation, maturation state and perspectives. Int J Cardiol. 2017;241:379–86.
Serpooshan V, et al. Bioengineering cardiac constructs using 3D printing. J 3D Printing Med. 2017;1(2):123–39.
Serpooshan V, et al. Chapter 8 – 4D printing of actuating cardiac tissue. In: Al’Aref SJ, et al., editors. 3D printing applications in cardiovascular medicine. Boston: Academic Press; 2018. p. 153–62.
Hu JB, et al. Cardiovascular tissue bioprinting: physical and chemical processes. Appl Phys Rev. 2018;5(4):041106.
Lim GB. Vascular disease: treatment of ischaemic vascular disease with 3D-printed vessels. Nat Rev Cardiol. 2017;14(8):442–3.
Hu JB, et al. Bioengineering of vascular myocardial tissue; a 3D bioprinting approach. Tissue Eng A. 2017;23:S158–9.
Ong CS, et al. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci Rep. 2017;7:4566.
Misra SK, et al. 3D-printed multidrug-eluting stent from graphene-nanoplatelet-doped biodegradable polymer composite. Adv Healthc Mater. 2017;6(11).
Jana S, Lerman A. Bioprinting a cardiac valve. Biotechnol Adv. 2015;33(8):1503–21.
Lee RT, Walsh K. The future of cardiovascular regenerative medicine. Circulation. 2016;133(25):2618–25.
Matsuda H. The current trends and future prospects of regenerative medicine in cardiovascular diseases. Asian Cardiovasc Thorac Ann. 2005;13(2):101–2.
Wei K, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525(7570):479–85.
van Rooij E. Cardiac repair after myocardial infarction. N Engl J Med. 2016;374(1):85–7.
Mandel Y, et al. Human embryonic and induced pluripotent stem cell–derived cardiomyocytes exhibit beat rate variability and power-law behavior. Circulation. 2012;125(7):883–93.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wu, S.M., Serpooshan, V. (2019). Cardiovascular Regenerative Medicine: Challenges, Perspectives, and Future Directions. In: Serpooshan, V., Wu, S. (eds) Cardiovascular Regenerative Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-20047-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-20047-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20046-6
Online ISBN: 978-3-030-20047-3
eBook Packages: MedicineMedicine (R0)