Abstract
Drosophila suzukii, a fruit fly, also known as spotted wing drosophila, is a highly polyphagous invasive pest endemic to South East Asia, which has recently invaded western countries. Its serrated ovipositor allows this fruit fly to lay eggs on and damage unwounded ripening fruits, thus heavily threatening fruit production. D. suzukii is spreading rapidly and severe economic losses have been reported, making it a pest of great concern. Entomovector technology, which utilizes insects as vectors of biological control agents, for targeted precision biocontrol towards plant pests and diseases, could present an intriguing management option for the control of D. suzukii in an integrated pest management (IPM) system. The success of entomovectoring in the management of D. suzukii will be based on mutual and well suited interactions between the appropriate components of vector, control agent, formulation and dispenser, and needs to be safe for the environment and human health. This chapter highlights the threat of this invasive pest to fruit production. It describes the pest life history and its distribution across Europe. Finally, it presents the hypothetical potential of exploiting entomovectoring in the control of D. suzukii and also discusses the impact which the increased use of chemical pesticides to control D. suzukii has on the development of entomovectoring as a biocontrol method.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Drosophila suzukii Matsumura (Diptera: Drosophilidae), also commonly referred to as spotted wing drosophila (SWD) and native to Southeast Asia (Kanzawa 1939; Tan et al. 1949), is a polyphagous invasive pest in America and Europe (Lee et al. 2011; Kinjo et al. 2014; Deprá et al. 2014). From its early detection in 2008, in California (USA), Spain and Italy (Europe), D. suzukii has rapidly spread through these two continents with the aid of global trading and absence of niche competitors (Hauser 2011; Calabria et al. 2012; Cini et al. 2012; Rota-Stabelli et al. 2013; Cini et al. 2014; Wiman et al. 2014; Asplen et al. 2015). Contrary to other closely related Drosophila species that would preferentially infest over-ripened and damaged fruits, and thus are not considered serious pests (Lee et al. 2011), D. suzukii has the ability to bore holes into the skin of maturing and undamaged healthy fruits using its serrated ovipositor and oviposits into them. The oviposition wounds caused by D. suzukii flies very often provide access points to other insects and undesirable secondary infections by pathogens, including fungi, yeasts and bacteria, hence, causing additional losses (Hamby et al. 2012; Ioriatti et al. 2015). All these together make D. suzukii a pest of great concern to maturing and ripening fruits (Mitsui et al. 2006; Calabria et al. 2012). A wide range of different soft and stone fruits including strawberry, raspberry, plums, blueberry and grapes are potential targets under D. suzukii’s damage range (Dreves et al. 2009; Cini et al. 2012; Bellamy et al. 2013). The damage caused by D. suzukii has been reported to reach up to 80% crop loss (Dreves et al. 2009; Walsh et al. 2011; Goodhue et al. 2011). Furthermore, the management of D. suzukii is primarily challenging because the fly can continuously infest various fruits available throughout the year (Lee et al. 2011), it can survive in a wide range of different climatic conditions in which their natural predators can sometimes not keep up (Chabert et al. 2012) and it also has a very short generation time (Kanzawa 1939; Lee et al. 2011; Wiman et al. 2014). Limited knowledge on how to effectively control this pest and the zero tolerance attitude for infested fruit bound for the fresh market or various export markets, has motivated the priority for more research into possible control options for this pest.
Entomovector technology, which utilizes insects as vectors of biological control agents for targeted precision biocontrol towards plant pests and diseases (Hokkanen and Menzler-Hokkanen 2007; Mommaerts and Smagghe 2011; Menzler-Hokkanen et al. 2013), presents an intriguing management option for the control of D. suzukii in an integrated pest management (IPM) system. Multiple studies have reported on the success of exploiting both honey bees and bumblebees to vector different entomopathogenic control agents into flowers to control pest insects which either feed on, or inhabit, the flowers (Gross et al. 1994; Butt et al. 1998; Carreck et al. 2007; Albano et al. 2009). However, the success of entomovectoring in the management of D. suzukii will be based on mutual and suited interactions between the appropriate components of vector, control agent, formulation and dispenser, and it needs to be safe for the environment and human health.
This chapter presents the threat of the occurrence of D. suzukii in Europe, and places this in context to the possible effects that it might have on entomovectoring. Insights into the possibility of exploiting entomovectoring as a management option for the biocontrol of D. suzukii are also discussed.
2 Threat of Drosophila suzukii to Fruit Production
Contrary to most other Drosophilidae, with the exemption of D. subpulchrella, D. suzukii is able to lay eggs in healthy, unwounded fruit and not only on damaged or overripe fruits, thanks to the serrated female ovipositor (Fig. 1) (Sasaki and Sato 1995; Cini et al. 2012; Bellamy et al. 2013). Hence, ripening fruits are preferred over overripe ones (Mitsui et al. 2006).
Fly ovipositor. (A) Arrow indicates the serrated hook-like ovipositor of D. suzukii used in boring into unwounded ripening fruits on the fields (Photograph by Martin Hauser, California Department of Food and Agriculture). (B) Arrow indicates the shorter ovipositor of D. melanogaster used in boring into overripe and decaying fruits
Although most of the damage caused by D. suzukii is largely due to the larvae feeding on fruit flesh, the insertion of its prominent ovipositor into the skin of the fruit can cause physical damage to the fruit. This in turn provides access to secondary infections of pathogens such as, yeasts, filamentous fungi and bacteria, which may cause faster deterioration and further losses (Hamby et al. 2012; Ioriatti et al. 2015) (Fig. 2).
Indirect and direct damages caused by D. suzukii. (A) Arrow indicates an oviposition spot created by the serrated hook-like ovipositor of D. suzukii on a healthy cherry. (B) Arrow indicates larvae feeding inside a cherry. (C) Arrow indicates fungi growing around an oviposition spot (Photograph by Martin Hauser, California Department of Food and Agriculture). (D) Deterioration and softening of strawberries following infestation with D. suzukii
Additional costs associated with the field management of D. suzukii are mostly related to increased production costs (monitoring and chemical input costs, increased labour and fruit selection, reduction of the fruit shelf life, storage costs) and to the decrease of foreign market appeal for fruit production from contaminated areas (Goodhue et al. 2011). Nevertheless, the oviposition habit itself is not enough to explain the dramatic impact of D. suzukii on fruit production. In the next sections the main characteristics making D. suzukii a threat of high concern for the European fruit production sector are discussed.
2.1 High Fecundity in D. suzukii
Mating in D. suzukii optimally occurs from the first days of life and females start to lay eggs already from the second day from emergence. Females are known to typically lay 1–3 eggs per fruit in up to 7–16 fruits per day, depending on the temperature (Kinjo et al. 2014). Since they are capable of ovipositing for 10–59 days, they can lay up to a total of 600 eggs during their lifetime (around 400 eggs on average). The eggs hatch within 2–72 h after being laid inside the fruits, and larvae mature (inside the fruit) in 3–13 days. D. suzukii pupae reside for 3–15 days either inside or less frequently outside the fruit. Depending on the temperature, a minimum of 10 days is required from the time the egg is oviposited to adult emergence. This very short generation time exhibited by D. suzukii has a huge impact on fruit production. It implies that D. suzukii can complete several generations in a single cropping cycle and up to 7–15 generations in a year, depending on specific climatic conditions, thus allowing an explosive population growth [life-cycle details can be found in Kanzawa (1939); Mitsui et al. (2006); Walsh et al. (2011); Tochen et al. (2014); Wiman et al. (2014)].
2.2 D. suzukii is Tolerant to a Wide Range of Climatic Conditions
The ability to survive and reproduce in a wide range of different climatic conditions is obviously a relevant factor for pest insects. Limiting temperatures for D. suzukii reproduction have been reported to be between 10 and 32 °C for oviposition and up to 30 °C for male fertility (Sakai et al. 2005). Its development and peak activity is around 20–25 °C (Kanzawa 1939; Tochen et al. 2014). D. suzukii can also be described as being both heat tolerant (viable D. suzukii populations can resist hot summers in Spain) and cold tolerant (D. suzukii is present in cold areas, such as mountain regions in Japan and Alpine areas). Adult D. suzukii are particularly tolerant to cold compared to other drosophilids (Sasaki and Sato 1995; Mitsui et al. 2010) and mated females in reproductive diapause have been reported to be the D. suzukii stage that overwinters (Kanzawa 1939; Mitsui et al. 2010; Walsh et al. 2011). Whether the observed tolerance is physiological or mediated by behavioral adaptation is still unclear. However, several authors have suggested that D. suzukii survival under harsh conditions might be increased by acclimatization (Walsh et al. 2011), altitudinal migration (Mitsui et al. 2010), and/or overwintering in manmade habitats or other sheltered sites (Kimura 2004).
2.3 D. suzukii has a Broad Host Range
D. suzukii has a large host range, infesting both cultivated and wild soft-skinned fruits on host plants in both native and invaded areas, with berries being the preferred hosts (Table 1). Despite laboratory tests indicating that D. suzukii has a lower oviposition susceptibility and developmental rate on grapes compared to berries and cherry (Lee et al. 2011), reports from observations in vineyards in Northern Italy have clearly indicated that V. vinifera can become a field host (particularly with soft skinned varieties being more impacted) (Griffo et al. 2012). This could indicate that D. suzukii host preference is highly dependent upon the local abundance of hosts. D. suzukii can also be flexible with its host choice. This is demonstrated by its ability to develop on tomato under controlled laboratory conditions. However, tomato has not been so far recorded as its host in the field, even though D. suzukii adults have been trapped in France in tomato crop fields (EPPO website). In addition to cultivated fruits, many wild, ornamental, and uncultivated plants can serve as potentially important hosts (Lee et al. 2015; Klick et al. 2016).
Despite its relatively recent detection in Europe, D. suzukii has already caused severe yield losses in several small fruit crops grown across southern Europe, such as sweet cherries, strawberries, raspberries, blackberries, and blueberries. Extreme damage has been reported for locations in Northern Italy (Trentino) and in France, with up to 100% damage reported on cranberries, strawberries, and sweet cherries (Cini et al. 2012; Warlop et al. 2013). The first evaluation of the economic impact in Europe was presented by De Ros et al. (2013), although the study only focused on Trento Province, Italy. It was estimated in the study that 400-ha of soft fruit production areas faced losses of around 500,000 € in 2010, and three million € in 2011. Although the level of these economic impacts recorded in Trentino can be ascribed to high levels of blueberry production, this estimate is also somewhat conservative in that it did not consider the costs of control strategies and other societal consequences resulting from increased chemical inputs. In France, D. suzukii has also been reported on apples and peaches, although without economically significant damage (Warlop et al. 2013).
The wide host range of D. suzukii represents a pest management constraint in many affected regions. This is not only because D. suzukii can cause damage to many species, but also because populations can survive almost everywhere, alternating hosts with different ripening times through the year, both cultivated and wild. Crop plants usually cultivated in high density monoculture, allow rapid and impressive population growth, while wild hosts and ornamental plants may serve as refuges from management treatments, and provide later re-infestation sources and overwintering habitats observed (Klick et al. 2016). The ability to damage thick ripening fruits and the wide host range, gives to D. suzukii a wide but at the same time specialized ecological niche. Nevertheless, the overlap of niches and the possibility of competition with other drosophilids needs to be investigated.
2.4 D. suzukii has a High Potential for Dispersal
The rapid spread of D. suzukii in invaded countries and its presence on several continents, as well as remote islands [e.g. Hawaii; Kaneshiro (1983)], confirms its high dispersal potential (Hauser 2011; Calabria et al. 2012). Similar to many other invasive species (Westphal et al. 2008), passive diffusion due to global trade is most likely the main cause of the spread of D. suzukii. Before larval activity, the intact and healthy appearance of fruits infested with D. suzukii is likely to masked the damage caused to the fruit. This will lead to the risk of infestation remaining undetected and thus an increase in the risk of passive dissemination of D. suzukii (Calabria et al. 2012).
3 Rapid Worldwide Spread of D. suzukii
D. suzukii was initially described for the first time in 1916, in Japan, where it was reported to attack cherries, however, it is still uncertain whether it is native to this region or was introduced (Kanzawa 1939). The presence of D. suzukii has also been reported in the eastern part of China (Peng 1937), Taiwan (Lin et al. 1977), North and South Korea (Chung 1955, Kang and Moon 1968), Pakistan (ud Din et al. 2005), Myanmar (Toda 1991), Thailand (Okada 1976), the Russian Far East (Sidorenko 1992) and India (Kashmir region, (Parshad and Duggal 1965), where it was described as the D. suzukii subspecies indicus (Parshad and Paika 1964). D. suzukii is currently spreading in many areas, such as the USA (West and East coast), Canada, Brazil (Deprá et al. 2014), Mexico and Europe [a history of the introduction in North America is reviewed by Hauser (2011)]. A key feature of the rapid spread of D. suzukii was the initial lack of regulation over the spread of any Drosophila species.
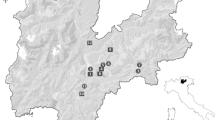
D. suzukii is rapidly spreading across Europe (Fig. 3). First reports of its presence in Europe were in autumn 2008 in Spain (Rasquera Province) (Calabria et al. 2012), although a later proposal suggested that southern France was the first propagation center (Cini et al. 2014). Moreover, malaise traps deployed in Tuscany (San Giuliano Terme, Pisa, Italy) in 2008 caught D. suzukii adults simultaneously with those deployed in Spain (Raspi et al. 2011). By 2009, in other regions of Spain, (Bellaterra, near Barcelona), France (Montpellier and Maritimes Alpes) and Italy (Trentino) (Grassi et al. 2009; Calabria et al. 2012), D. suzukii adults were trapped and recorded. In Trentino, first oviposition on wild hosts (Vaccinium, Fragaria and Rubus spp.) and economically important damage on several cultivated berries species were reported (Grassi et al. 2009; Sarto and Royo 2011). By 2010–2011, the range of D. suzukii was further enlarged. In Italy it was reported in several other regions: Piedmont, Aosta Valley, Lombardy, Veneto, Emilia Romagna, Liguria, Marche and Campania (Franchi and Barani 2011; Pansa et al. 2011; Süss and Costanzi 2010; Griffo et al. 2012; Baser et al. 2015; Mazzetto et al. 2015) and in France it was found from Corsica up to Ile de France. Then, many other European countries made their first record: Switzerland (Baroffio and Fischer 2011; Baroffio et al. 2014), Slovenia (Seljak 2011), Croatia (Milek et al. 2011), Portugal (Rota-Stabelli et al. 2013), Austria (Lethmayer 2011), Germany (Vogt et al. 2012; Vogt 2014; Briem et al. 2015), Belgium (Mortelmans et al. 2012; Belien et al. 2013), The Netherlands (Helsen et al. 2013), United Kingdom (EPPO 2012), Hungary (Kiss et al. 2014; Kiss et al. 2016), Poland (Łabanowska and Piotrowski 2015), Greece (Papachristos et al. 2013), Romania (Chireceanu et al. 2015), Bulgaria (EPPO 2015), Serbia (Toševski et al. 2014), Bosnia and Herzegovina (Zovko 2014) and Czech Republic (Brezıková et al. 2014). This reflects the distribution of D. suzukii in Europe.
Current worldwide D. suzukii distribution map (Asplen et al. 2015). It is worthwhile to note that the lack of reports from several areas is probably due to a lack of monitoring rather than to an actual absence of D. suzukii
D. suzikii seems to be spreading rapidly and all of continental Europe is at risk for invasion (Fig. 3). It is important to note that the lack of reports from several areas is probably due to a lack of monitoring rather than to an actual absence of D. suzukii. Thus, the history of reports might reflect differences in the sampling effort and/or problems of awareness rather than the true distribution of D. suzukii. Considering the reports together with the outputs of available degree-day phenological models (Damus 2009; Coop 2010) and analysis of the distribution of D. suzukii host plants (EPPO website), it is very likely that D. suzukii will spread all over Europe. Ecological simulations have indicated that the northern humid areas are more suitable ecosystems for D. suzukii compared to the Mediterranean drier environments, especially because desiccation seems to be a limiting factor for drosophilids (Walsh et al. 2011). Taking the current climate changes into account, even Scandinavian countries cannot be considered out of reach from the risk of D. suzukii invasion. On a wider geographic perspective, according to the biology of D. suzukii, global expansion in regions with climatic conditions spanning from subtropical to continental is highly likely to happen (Walsh et al. 2011). Furthermore, the occurrence of niche shifts, as was observed for other pests (e.g. Zaprionus indianus Gupta, Da Mata et al. 2010), should not be excluded (Calabria et al. 2012), suggesting that D. suzukii could become a global problem for fruit production.
4 Potential of Entomovectoring in the Management of D. suzukii
The success of entomovectoring in the management of D. suzukii will depend on mutual and suited interactions between the appropriate components of vector, control agent, formulation and dispenser, and it needs to be safe for the environment and human health. A typical scenario will be the delivery of the microbial control agent (MCA) to the flowers by the vector (e.g. honey bee or bumble bee), which will in turn lead to the protection of the resulting fruit against D. suzukii coming to feed on the ripening fruits (Fig. 4). In this scenario, the MCA has to be able to survive long enough in the flower to the maturation of the fruit and subsequently to the ripening of the fruit. The choice for an MCA which can survive on flower dwelling insect pest prior to fruit maturation could be a good option.
Illustration of the management of D. suzukii through entomovectoring. In this scenario, the vector delivers the MCA to the flower during pollination. The MCA then survives by feeding on other flower dwelling insects until fruit maturation and ripening. D. suzukii attacking the fruits are exposed to the MCA, which subsequently leads to mortality in D. suzukii
The potential MCA of choice to be used in the control of D. suzukii will need to fulfil the criteria as defined for agents against postharvest diseases by Droby et al. (2009) and Sharma et al. (2009): (a) effective at low concentrations, (b) not fastidious in its nutrient requirements, (c) genetically stable, (d) able to survive adverse environmental conditions, (e) non-pathogenic to the host, (f) resistant to pesticides, (g) preparable in a form that can effectively be stored and disseminated and (h) not detrimental to human health. In addition to these criteria three extra characteristics should be included for a suitable MCA, namely (i) safe for the vector and the crop, (j) effective against aerial and/or foliar plant insect pests, and (k) able to survive and grow under conditions present in the flower.
Metarhizium anisopliae, an entomopathogenic fungus has been observed to infect over 200 insect pest species (Cloyd 1999). M. anisopliae and its related species have been tested as biological insecticides against a number of pests such as termites, thrips, pollen beetle, cabbage seedpod weevil, sweet potato weevil and fruit flies (Butt et al. 1998; Carreck et al. 2007; Reddy et al. 2014; Quesada-Moraga et al. 2006; Yousef et al. 2015; Yousef et al. 2017). M. anisopliae could be exploited as a possible MCA for the management of D. suzukii. M. anisopliae does not infect humans or other animals and is therefore considered safe as an insecticide. Vectoring of M. anisopliae on oil seed rape and canola has been demonstrated to cause high mortality in some insect pests, including larvae/adults of Meligethes aeneus Fabricius (Coleoptera: Nitidulidae) and Ceutorhynchus assimilis Dejean (Coleoptera: Curculionidae) (Butt et al. 1998; Carreck et al. 2007). M. anisopliae typically causes the diseases known as ‘green muscardine disease’ (due to the green color of its spores) in insects. When the mitotic (asexual) spores (called conidia) of the fungus come into contact with the body of an insect host, they germinate and the hyphae that emerge penetrate the cuticle. Then, the fungus develops inside the insect body eventually killing it only after a few days. It is very likely that the lethal effect is aided by the production of insecticidal cyclic peptides (destruxins). Most insect species living close to the soil have evolved natural defenses against entomopathogenic fungi such as, M. anisopliae. To overcome the insect host defenses, this fungus is locked in an evolutionary battle, which has resulted to a large number of different isolates (or strains) that are adapted to certain groups of insects (Freimoser et al. 2003). This implies that screenings will need to be performed to select isolates with insecticidal activities against D. suzukii, prior to any field trials. In a recent study, Yousef et al. (2016) reported on the effectiveness of Metarhizium brunneum Petch (Hypocreales: Clavicipitaceae) and its crude extract in the control of D. suzukii. The study evaluated the use of two M. brunneum strains, EAMa 01/58-Su and EAMb 09/01-Su, and their extracts for the respective development of lure-and-infect and lure-and-kill devices for the control of D. suzukii (Fig. 5). The EAMa 01/58-Su strain designed for a lure-and-infect strategy, caused 62.2% mortality in adult D. suzukii (survival time of 3.6 days). Furthermore, the evaluation of horizontal transmission and sublethal reproductive effects of the fungal strain showed 48.0% mortality in untreated males after mating with fungus-treated females, whereas only 24.0% of untreated females were killed after mating with treated males, thereby revealing the horizontal transmission potential of the strain. These results show the high potential of using M. brunneum as an MCA in entomovectoring, contributing to an IPM program for the control of D. suzukii.
D. suzukii adult with M. brunneum EAMa01/58-Su strain fungal outgrowth (from Yousef et al. 2016)
Another MCA which could be used in the management of D. suzukii is the entomopathogenic fungus, Beauveria bassiana. It is known to attack a broad range of insects, acting as a parasite on various arthropod species (McNeiL Jr. 2005; Barbarin et al. 2012). Studies with honey bees vectoring B. bassiana GHA in canola showed 22–56% mortality in Lygus lineolaris (Palisot de Beauvois) (Hemiptera: Miridae) (Al Mazra’awi et al. 2006). B. bassiana causes white muscardine (due to the white color of its spores) disease in insects, using a similar mechanism as describe for M. anisopliae. When the microscopic spores of the fungus come into contact with an insect host body, they germinate, penetrate the cuticle, and grow inside, killing the insect within a matter of days. New spores are then produce from a white mold which emerges from the cadaver. Since various isolates of B. bassiana differ in their host range and the factors responsible for host susceptibility are unknown, further research will have to be done to select an appropriate isolate to be used in the management of D. suzukii. A preliminary screening of some isolates of B. bassiana showed up to 44% mortality in D. suzukii (Cuthbertson et al. 2014). Another example of a possible MCA is Isaria fumosorosea. Cuthbertson and Audsley (2016) demonstrated the efficacy of I. fumosorosea against D. suzukii by immersing blueberries in suspensions of these fungi pre- and post-infestation. I. fumosorosea caused >40% mortality in adult flies within 7 days of fly contact with the fungi.
Once appropriate MCAs against D. suzukii are identified and tested, the next crucial step will be the development of appropriate carriers in which the MCA will be transported by the vector. An appropriate carrier will need to fulfil three criteria (Kevan et al. 2008): (a) No effect on the life span of the MCA. A good example is reported by Hjeljord et al. (2000), where the germination of Trichoderma spp. and B. bassiana spores were significantly slower when formulated with talc; (b) Safe for the vector. A good example is reported by Israel and Boland (1993), where talc irritated honey bees causing them to groom, whereas with flours as carrier, grooming decreased by 50% (Kevan et al. 2008). Similarly, Pettis et al. (2004) reported that minerals such as talc adversely affected the honey bee brood; (c) Enhance the transport capacity of the vector. In this context, Al-Mazra’awi et al. (2007) showed that direct honey bee load increased with decreasing carrier particle size and moisture content. A start point to the carriers for the management of D. suzukii could be adaptations from existing carriers. So far, known carrier substances are corn flour (Shipp et al. 2006), corn meal (Peng et al. 1992), bentonite (Kevan et al. 2008) and polystyrene beads (Butt et al. 1998). Despite the high efficiency of the latter carrier, these beads are prohibitively expensive for commercial formulations, whereas flours and meals have the advantage to be easily available and inexpensive, safe and food grade qualified. These carrier options could be used as basis for the evaluation of identified MCAs against D. suzukii, while research continues for the identification of better carriers.
It is evident that success in dissemination and deposition of the MCA is crucial in an entomovector strategy. Therefore it is of paramount importance that the most efficient vector should be selected, and this selection depends on the species, the crop visitation rate by the vector, and the deposition capacity of the MCA by the vector to the target. Honey bees and solitary mason bees are used to vector MCAs onto crops under field conditions. Besides the carrier substance and selection of an appropriate MCA against D. suzukii, all of the other components of an entomovectoring system (such as, the selection of the vector, vector safety, transport of MCA, dispenser design and safety of the control agent to the environment and human health) will probably be the same as reported in other cases (Kevan et al. 2008; Mommaerts and Smagghe 2011). These indicate the feasibility for the development of an entomovectoring system, where bee-mediated dissemination of entomopathogenic MCAs could be exploited to target fruit pests, such as D. suzukii, within an IPM system that aims to enhance biological control and minimize insecticide use.
5 Effects of the Occurrence of D. suzukii on Entomovectoring
The control of D. suzukii populations in the field mainly relies on the use of chemical pesticides (Beers et al. 2011; Bruck et al. 2011; Whitener and Beers 2015; Andreazza et al. 2017), a practice with serious drawbacks such as indiscriminate killing of different insect species (including bees) and its use close to harvest which could lead to a risk of high residues left on fruits. The particular preference of D. suzukii for ripening fruit presents timing difficulties with respect to pollinator protection and pre-harvest intervals. This implies that the most effective time for applying chemical controls against D. suzukii is when the fruit is ripe or very nearly ripe, necessitating chemicals with a shorter pre-harvest interval. Therefore, growers of bee-pollinated crops may need to remove their bees slightly earlier than optimum to spray late-flowering fruit, before D. suzukii infestation, if bee kills are to be minimized.
The fast spread and establishment of D. suzukii in Europe will result to an increase in the use of chemical pesticides to manage this invasive pest. Certain pyrethrins and spinosad are among the authorized active materials for D. suzukii control (Diepenbrock et al. 2016). Increased pesticide usage to control D. suzukii will inevitably lead to an increase in bee mortality. Considering that bees are currently the only actively exploited vectors in the delivery of MCAs in entomovectoring, this will significantly impact efforts in promoting entomovectoring as an alternative to the use of chemical pesticides.
6 Conclusions
The rapid spread of D. suzukii poses a challenge to fruit production in Western countries. The biology of D. suzukii clearly indicates that an effective control effort requires an area wide IPM program. In order to accomplish this, research needs to address D. suzukii basic biology, the development of management tools, the transfer of knowledge and technology to users and, finally, the implementation of the IPM program also at a cultural and societal level. While short term solutions to limit the current dramatic damage are strongly needed, only long-term and environmentally friendly management approaches will allow a sustainable control of this pest. To this aim, research into entomovectoring as a possible biocontrol option, should be carried out to shed light on many knowledge gaps that are still present.
References
Al Mazra’awi M, Shipp J, Broadbent A, Kevan P (2006) Dissemination of Beauveria bassiana by honey bees (Hymenoptera: Apidae) for control of tarnished plant bug (Hemiptera: Miridae) on canola. Environ Entomol 35:1569–1577
Albano S, Chagnon M, De Oliveira D, Houle E, Thibodeau P, Mexia A (2009) Effectiveness of Apis mellifera and Bombus impatiens as dispersers of the Rootshield® biofungicide (Trichoderma harzianum, strain T-22) in a strawberry crop. Hell Plant Protect J 2:57–66
Al-Mazra’awi MS, Kevan PG, Shipp L (2007) Development of Beauveria bassiana dry formulation for vectoring by honey bees Apis mellifera (Hymenoptera: Apidae) to the flowers of crops for pest control. Biocontrol Sci Tech 17:733–741
Andreazza F, Bernardi D, Baronio CA, Pasinato J, Nava DE, Botton M (2017) Toxicities and effects of insecticidal toxic baits to control Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae). Pest Manag Sci 73(1):146–152
Asplen MK, Anfora G, Biondi A, Choi D-S, Chu D, Daane KM, Gibert P, Gutierrez AP, Hoelmer KA, Hutchison WD (2015) Invasion biology of spotted wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci 88:469–494
Barbarin AM, Jenkins NE, Rajotte EG, Thomas MB (2012) A preliminary evaluation of the potential of Beauveria bassiana for bed bug control. J Invertebr Pathol 111:82–85
Baroffio C, Fischer S (2011) Neue bedrohung für obstplantagen und beerenpflanzen: die kirschessigfliege. UFA-Revue 11:46–47
Baroffio CA, Richoz P, Fischer S, Kuske S, Linder C, Kehrli P (2014) Monitoring Drosophila suzukii in Switzerland in 2012. J Berry Res 4:47–52
Baser N, Broutou O, Lamaj F, Verrastro V, Porcelli F (2015) First finding of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in Apulia, Italy, and its population dynamics throughout the year. Fruits 70:225–230
Beers EH, Van Steenwyk RA, Shearer PW, Coates WW, Grant JA (2011) Developing Drosophila suzukii management programs for sweet cherry in the western United States. Pest Manag Sci 67:1386–1395
Belien T, Thys T, Fassotte C, Walrant C, Tomme M, Bolen M, Bylemans D (2013) Population dynamics of Drosophila suzukii (Diptera: Drosophilidae) in Belgium in 2013. Commun Agric Appl Biol Sci 79:169–175
Bellamy DE, Sisterson MS, Walse SS (2013) Quantifying host potentials: indexing postharvest fresh fruits for spotted wing drosophila, Drosophila suzukii. PLoS One 8:e61227
Bolda MP, Goodhue RE, Zalom FG (2010) Spotted wing drosophila: potential economic impact of a newly established pest. Agric Resour Econ Update 13(3):5–8
Brezıková M, Dvorák L, Máca J (2014) Faunistic records from the Czech Republic—367. Diptera: Drosophilidae. Klapalekiana 50:247–248
Briem F, Breuer M, Köppler Vogt H (2015) Phenology and occurrence of spotted wing Drosophila in Germany and case studies for its control in berry crops. IOBC-WPRS Bull 109:233–237
Bruck DJ, Bolda M, Tanigoshi L, Klick J, Kleiber J, Defrancesco J, Gerdeman B, Spitler H (2011) Laboratory and field comparisons of insecticides to reduce infestation of Drosophila suzukii in berry crops. Pest Manag Sci 67:1375–1385
Butt T, Carreck N, Ibrahim L, Williams I (1998) Honey-bee-mediated infection of pollen beetle (Meligethes aeneus fab.) by the insect-pathogenic fungus, Metarhizium anisopliae. Biocontrol Sci Tech 8:533–538
Calabria G, Máca J, Bächli G, Serra L, Pascual M (2012) First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J Appl Entomol 136:139–147
Carreck NL, Butt TM, Clark SJ, Ibrahim L, Isger EA, Pell JK, Williams IH (2007) Honey bees can disseminate a microbial control agent to more than one inflorescence pest of oilseed rape. Biocontrol Sci Tech 17:179–191
Chabert S, Allemand R, Poyet M, Eslin P, Gibert P (2012) Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol Control 63:40–47
Chireceanu C, Chiriloaie A, Teodoru A (2015) First record of the spotted wing Drosophila, Drosophila suzukii (Diptera: Drosophilidae) in Romania. Rom J Plant Protect 8:86–95
Chung Y (1955) Collection of wild Drosophila on Quelpart Island, Korea. Drosophila Inf Serv 29:111
Cini A, Ioriatti C, Anfora G (2012) A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull Insectol 65:149–160
Cini A, Anfora G, Escudero-Colomar L, Grassi A, Santosuosso U, Seljak G, Papini A (2014) Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J Pest Sci 87:559–566
Cloyd RA (1999) The entomopathogenic fungus Metarhizium anisopliae. Midwest Biol Control News 6
Coop L (2010) Online phenology and degree-day model for agricultural and decision-making in the US. Integrated Plant Protection Center, Botany & Plant Pathology Dep. Oregon State University, Corvallis, Oregon. http://uspest.org/risk/models
Cuthbertson AG, Audsley N (2016) Further screening of Entomopathogenic Fungi and nematodes as control agents for Drosophila suzukii. Insects 7:24
Cuthbertson AG, Collins DA, Blackburn LF, Audsley N, Bell HA (2014) Preliminary screening of potential control products against Drosophila suzukii. Insects 5:488–498
Da Mata RA, Tidon R, Côrtes LG, De Marco P Jr, Diniz-Filho JA (2010) Invasive and flexible: niche shift in the drosophilid Zaprionus indianus (Insecta, Diptera). Biol Invasions 12:1231–1241
Damus M (2009) Some preliminary results from CLIMEX and MAXENT distribution modelling of Drosophila suzukii. Version 2. CFIA Plant Health Risk Assessment, Ottawa. http://swd.hort.oregonstate.edu/files/files/DrosophilaSuzukiiInfestationModel.pdf
De Ros G, Anfora G, Grassi A, Ioriatti C (2013) The potential economic impact of Drosophila suzukii on small fruits production in Trentino (Italy). IOBC-WPRS Bull 91:317–321
Deprá M, Poppe JL, Schmitz HJ, De Toni DC, Valente VL (2014) The first records of the invasive pest Drosophila suzukii in the South American continent. J Pest Sci 87:379–383
Diepenbrock LM, Rosensteel DO, Hardin JA, Sial AA, Burrack HJ (2016) Season-long programs for control of Drosophila suzukii in southeastern US blueberries. Crop Prot 81:76–84
Dreves AJ, Walton VM, Fisher GC (2009) A new pest attacking healthy ripening fruit in Oregon: spotted wing Drosophila: Drosophila suzukii (Matsumura). Extension Service, Oregon State University, Corvallis
Droby S, Wisniewski M, Macarisin D, Wilson C (2009) Twenty years of postharvest biocontrol research: is it time for a new paradigm? Postharvest Biol Technol 52:137–145
EPPO (2012) First report of Drosophila suzukii in the United Kingdom
EPPO (2015) Reporting service no. 01-2015 number article: 2015/006
Franchi A, Barani A (2011) Un nuovo agente di danno per frutta e vite in Emilia. Notiziario Fitopatologico 2:14
Freimoser FM, Screen S, Bagga S, Hu G, St Leger RJ (2003) Expressed sequence tag (EST) analysis of two subspecies of Metarhizium anisopliae reveals a plethora of secreted proteins with potential activity in insect hosts. Microbiology 149:239–247
Goodhue RE, Bolda M, Farnsworth D, Williams JC, Zalom FG (2011) Spotted wing Drosophila infestation of California strawberries and raspberries: economic analysis of potential revenue losses and control costs. Pest Manag Sci 67:1396–1402
Grassi A, Palmieri L and Giongo L (2009) Nuovo fitofago per i piccoli frutti in Trentino. Terra trentina
Grassi A, Giongo L, Palmieri L, Linder C, Vétek G (2011) Drosophila (Sophophora) suzukii (Matsumura), new pest of soft fruits in Trentino (North-Italy) and in Europe. IOBC/wprs Bull 70:121–128
Griffo R, Frontuto A, Cesaroni C, Desantis M (2012) L’insetto Drosophila suzukii sempre più presente in Italia. L’Informatore Agrario 68:56–60
Gross HR, Hamm JJ, Carpenter JE (1994) Design and application of a hive-mounted device that uses honey bees (Hymenoptera: Apidae) to disseminate Heliothis nuclear polyhedrosis virus. Environ Entomol 23:492–501
Hamby KA, Hernández A, Boundy-Mills K, Zalom FG (2012) Associations of yeasts with spotted-wing Drosophila (Drosophila suzukii; Diptera: Drosophilidae) in cherries and raspberries. Appl Environ Microbiol 78:4869–4873
Hampton E, Koski C, Barsoian O, Faubert H, Cowles RS, Alm SR (2014) Use of early ripening cultivars to avoid infestation and mass trapping to manage Drosophila suzukii (Diptera: Drosophilidae) in Vaccinium corymbosum (Ericales: Ericaceae). J Econ Entomol 107(5):1849–1857
Hauser M (2011) A historic account of the invasion of Drosophila suzukii (Matsumura)(Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag Sci 67:1352–1357
Helsen H, Van Bruchem J, Potting R (2013) De suzuki-fruitvlieg Drosophila suzukii, ennieuweplaagopfruit in Nederland. Gewasbescherming 44:72–76
Hjeljord LG, Stensvand A, Tronsmo A (2000) Effect of temperature and nutrient stress on the capacity of commercial Trichoderma products to control Botrytis cinerea and Mucor piriformis in greenhouse strawberries. Biol Control 19:149–160
Hokkanen H, Menzler-Hokkanen I (2007) Use of honeybees in the biological control of plant diseases. Entomol Res 37:A62–A63
Ioriatti C, Walton V, Dalton D, Anfora G, Grassi A, Maistri S, Mazzoni V (2015) Drosophila suzukii (Diptera: Drosophilidae) and its potential impact to wine grapes during harvest in two cool climate wine grape production regions. J Econ Entomol 108(3):1148–1155
Israel M, Boland G (1993) Influence of formulation on efficacy of honey bees to transmit biological controls for management of Sclerotinia stem rot of canola. Can J Plant Pathol 14:244
Kaneshiro K (1983) Drosophila (Sophophora) suzukii (Matsumura). Proc Hawaiian Entomol Soc 24:179
Kang Y-S, Moon K-W (1968) Drosophilid fauna of six regions near the demilitarized zone in Korea. Korean J Zool 11:65–68
Kanzawa T (1939) Studies on Drosophila suzukii Mats. Kofu. Rev Appl Entomol 29:622
Kenis M, Tonina L, Eschen R, van der Sluis B, Sancassani M, Mori N et al (2016) Non-crop plants used as hosts by Drosophila suzukii in Europe. J Pest Sci 89(3):735–748
Kevan PG, Kapongo J-P, Ai-Mazra’awi M, Shipp L (2008) Honey bees, bumble bees, and biocontrol. Bee pollination in agriculture ecosystems. Oxford University Press, New York
Kimura MT (2004) Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia 140:442–449
Kinjo H, Kunimi Y, Nakai M (2014) Effects of temperature on the reproduction and development of Drosophila suzukii (Diptera: Drosophilidae). Appl Entomol Zool 49:297–304
Kinjo H, Kunimi Y, Ban T, Nakai M (2013) Oviposition efficacy of Drosophila suzukii (Diptera: Drosophilidae) on different cultivars of blueberry. J Econ Entomol 106(4):1767–1771
Kiss B, Lupták R, Kis A, Szita É (2014) A pettyesszárnyú muslica tömeges megjelenése Magyarországon 2014-ben. Integrált termesztés a kertészeti és szántóföldi kultúrákban 31:34–35
Kiss B, Kis A, Kákai Á (2016) The rapid invasion of spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), in Hungary. Phytoparasitica 44:429–433
Klick J, Yang W, Walton V, Dalton D, Hagler J, Dreves A, Lee J, Bruck D (2016) Distribution and activity of Drosophila suzukii in cultivated raspberry and surrounding vegetation. J Appl Entomol 140:37–46
Łabanowska B, Piotrowski W (2015) Drosophila suzukii stwierdzona w Polsce. Truskawka, malina, jagody 1:16
Lee JC, Bruck DJ, Curry H, Edwards D, Haviland DR, Van Steenwyk RA, Yorgey BM (2011) The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag Sci 67:1358–1367
Lee JC, Dreves AJ, Cave AM, Kawai S, Isaacs R, Miller JC, Van Timmeren S, Bruck DJ (2015) Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann Entomol Soc Am 108(2):117–129
Lethmayer C (2011) Gefhrliche Fliegen für Äpfel & Co. Bessers Obst 12:4–5
Lin F, Tseng H, Li W (1977) Catalogue of the family Drosophilidae in Taiwan (Diptera). Q J Taiwan Mus 30(3/4):345–372
Mann RS, Stelinski LL (2011) Spotted wing Drosophila Drosophila suzukii (Matsumura) (Insecta: Diptera: Drosophilidae). Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida
Mazzetto F, Pansa MG, Ingegno BL, Tavella L, Alma A (2015) Monitoring of the exotic fly Drosophila suzukii in stone, pome and soft fruit orchards in NW Italy. J Asia Pac Entomol 18:321–329
Mazzi D, Bravin E, Meraner M, Finger R, Kuske S (2017) Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. Insects 8(1):18
Mcneil Jr DG (2005) Fungus fatal to mosquito may aid global war on malaria. The New York Times, 10
Menzler-Hokkanen I, Hokkanen H, Maccagnani B, Lahdenperä M-L, Mommaerts V, Smagghe G, Karise R, Muljar R, Mänd M (2013) Entomovectored biocontrol of strawberry grey mould shows promise Europe-wide. Proceedings of the 65th International Symposium on Crop Protection. p. 40
Milek T, Seljak G, Šimala M, Bjeliš M (2011) First record of Drosophila suzukii (Matsumara, 1931) (Diptera: Drosophilidae) in Croatia. Glasilo Biljne Zaštite 11:377–382
Mitsui H, Takahashi KH, Kimura MT (2006) Spatial distributions and clutch sizes of Drosophila species ovipositing on cherry fruits of different stages. Popul Ecol 48:233–237
Mitsui H, Beppu K, Kimura MT (2010) Seasonal life cycles and resource uses of flower-and fruit-feeding drosophilid flies (Diptera: Drosophilidae) in Central Japan. Entomol Sci 13:60–67
Mommaerts V, Smagghe G (2011) Entomovectoring in plant protection. Arthropod Plant Interact 5:81–95
Mortelmans J, Casteels H, Beliën T (2012) Drosophila suzukii (Diptera: Drosophilidae): a pest species new to Belgium. Belg J Zool 142(2):143–146
Okada T (1976) New distribution records of the drosophilids in the oriental region. Acta Dipterologica 8:1–8
Pansa M, Frati S, Baudino M, Tavella L, Alma A (2011) First record of Drosophila suzukii in Piedmont. Protezione delle Colture 2:108
Papachristos D, Matakoulis C, Papadopoulos N, Lagouranis A, Zarpas K, Milonas P (2013) First report of the presence of Drosophila suzukii (Diptera: Drosophilidae) in Greece. Abstracts of the 15th entomology meeting of the Hellenic Entomological Society
Parshad R, Duggal K (1965) Drosophilidae of Kashmir, India. Drosophila Inf Serv 40:44
Parshad R, Paika I (1964) Drosophilid survey of India. II. Taxonomy and cytology of the subgenus Sophophora (Drosophila). Res Bull Punjab Univ 15:225–252
Pelton E, Gratton C, Isaacs R, Van Timmeren S, Blanton A, Guédot C (2016) Earlier activity of Drosophila suzukii in high woodland landscapes but relative abundance is unaffected. J Pest Sci 89(3):725–733
Peng FT (1937) On some species of Drosophila from China. Annotationes Zool Japonenses 16:20–27
Peng G, Sutton J, Kevan P (1992) Effectiveness of honey bees for applying the biocontrol agent Gliocladium roseum to strawberry flowers to suppress Botrytis cinerea. Can J Plant Pathol 14:117–129
Pettis JS, Kochansky J, Feldlaufer MF (2004) Larval Apis mellifera L. (Hymenoptera: Apidae) mortality after topical application of antibiotics and dusts. J Econ Entomol 97:171–176
Quesada-Moraga E, Ruiz-García A, Santiago-Alvarez C (2006) Laboratory evaluation of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against puparia and adults of Ceratitis capitata (Diptera: Tephritidae). J Econ Entomol 99:1955–1966
Raspi A, Canale A, Canovai R, Conti B, Loni A, Strumia F (2011) Insetti delle aree protette del comune di San Giuliano Terme. Felici Editore, San Giuliano Terme, Pisa, pp 1–192
Reddy GV, Zhao Z, Humber RA (2014) Laboratory and field efficacy of entomopathogenic fungi for the management of the sweetpotato weevil, Cylas formicarius (Coleoptera: Brentidae). J Invertebr Pathol 122:10–15
Rota-Stabelli O, Blaxter M, Anfora G (2013) Drosophila suzukii. Curr Biol 23:R8–R9
Sarto V, Royo RS (2011) Drosophila suzukii (Matsumura, 1931), nueva amenaza para las producciones agrícolas. La revista profesional de sanidad vegetal, Phytoma España, pp 54–59
Sakai M (ed) (2005) Cherry Drosophila suzukii, in primary color pest and disease encyclopedia. Rural Culture Association Japan, Tokyo, Japan
Sasaki M, Sato R (1995) Bionomics of the cherry drosophila, Drosophila suzukii Matsumura (Diptera: Drosophilidae) in Fukushima prefecture (Japan). Annu Rep Soc Plant Protect N Jpn 46:164–172
Seljak G (2011) Spotted wing Drosophila, Drosophila suzukii (Matsumura), a new pest of berry-fruit in Slovenia. Sad Publ 22:3–5
Sharma R, Singh D, Singh R (2009) Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol Control 50:205–221
Shipp L, Broadbent B, Kevan P (2006) Biological control of Lygus lineolaris (Hemiptera: Miridae) and Frankliniella occidentalis (Thysanoptera: Thripidae) by Bombus impatiens (Hymenoptera: Apidae) vectored Beauveria bassiana in greenhouse sweet pepper. Biol Control 37:89–97
Sidorenko V (1992) New and unrecorded species of Drosophilidae from soviet Far East (Diptera, Brachycera). Spixiana 15:93–95
Süss L, Costanzi M (2010) Presence of Drosophila suzukii (Matsumura, 1931)(Diptera Drosophilidae) in Liguria (Italy). J Entomol Acarol Res 42:185–188
Tan C, Hsu T, Sheng T (1949) Known Drosophila species in China with descriptions of twelve new species. University of Texas Publications, 4920, 194
Tochen S, Dalton DT, Wiman N, Hamm C, Shearer PW, Walton VM (2014) Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ Entomol 43:501–510
Toda M (1991) Drosophilidae (Diptera) in Myanmar (Burma) VII. The Drosophila melanogaster species-group, excepting the D. montium species-subgroup. Orient Insects 25:69–94
Toševski I, Milenković S, Krstić O, Kosovac A, Jakovljević M, Mitrović M, Cvrković T, Jović J (2014) Drosophila suzukii (Matsumura, 1931) (Siptera: Srosophilidae): a new invasive pest in Serbia. Zaštita Bilja 65:99–104
Ud Din MA, Mazhar K, Haque S, Ahmed M (2005) A preliminary report on Drosophila fauna of Islamabad (Capital, Pakistan). Drosophila Inf Serv 88:6–7
Van Timmeren S, Isaacs R (2013) Control of spotted wing drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop Prot 54:126–133
Vogt H (2014) A new pest, the spotted wing Drosophila, Drosophila suzukii (Matsumura 1931), is threatening fruit-growing and viticulture. Mitteilungen der Deutschen Gesellschaft für allgemeine und angewandte Entomologie 19:211–221
Vogt H, Baufeld P, Gross J, Kopler K, Hoffmann C (2012) Drosophila suzukii: eine neue bedrohung für den Europäischen obst-und weinbau—bericht über eine internationale tagung in trient, 2, Dezember 2011. J Kult 64:68–72
Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, Bruck DJ, Walton VM, O’neal SD, Zalom FG (2011) Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J Integr Pest Manag 2:G1–G7
Warlop F, Mandrin J-F, Weydert C, Filleron E, Turquet M, Gallia V (2013) Un nouveau ravageur menaçant pour les cultures fruitières biologiques: Drosophila suzukii
Westphal MI, Browne M, Mackinnon K, Noble I (2008) The link between international trade and the global distribution of invasive alien species. Biol Invasions 10:391–398
Whitener A, Beers EH (2015) Baseline mortality of spotted wing Drosophila after exposure to insecticides. J Integr Pest Manag 2(1):1–7
Wiman NG, Walton VM, Dalton DT, Anfora G, Burrack HJ, Chiu JC, Daane KM, Grassi A, Miller B, Tochen S (2014) Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. PLoS One 9:e106909
Yousef M, Quesada-Moraga E, Garrido-Jurado I (2015) Compatibility of herbicides used in olive orchards with a Metarhizium brunneum strain used for the control of preimaginal stages of tephritids in the soil. J Pest Sci 88:605–612
Yousef M, Aranda-Valera E, Quesada-Moraga E (2016) Lure-and-infect and lure-and-kill devices with Metarhizium brunneum and its extract for spotted wing Drosophila control. J Pest Sci 91(1):227–235
Yousef M, Garrido-Jurado I, Ruíz-Torres M, Quesada-Moraga E (2017) Reduction of adult olive fruit fly populations by targeting preimaginals in the soil with the entomopathogenic fungus Metarhizium brunneum. J Pest Sci 90(1):345–354
Zovko M (2014) First record of spotted wing Drosophila suzukii (Matsumura, 1931) in Bosnia and Herzegovina. Radovi Poljoprivrednog Fakulteta Univerziteta u Sarajevu (Works of the Faculty of Agriculture University of Sarajevo), 59, 127–133
Acknowledgements
The authors acknowledge support by the EU via Core-Organic II (Bicopoll, Targeted precision biocontrol and pollination enhancement in organic cropping systems), the Special Research Fund of the Ghent University, the Flemish agency for Innovation by Science and Technology (IWT-Flanders, Brussels), and the Research Foundation-Flanders (FWO-Vlaanderen, Brussels).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Taning, C.N.T., Smagghe, G. (2020). Threat of Drosophila suzukii as an Invasive Species and the Potential of Entomovectoring. In: Smagghe, G., Boecking, O., Maccagnani, B., Mänd, M., Kevan, P. (eds) Entomovectoring for Precision Biocontrol and Enhanced Pollination of Crops. Springer, Cham. https://doi.org/10.1007/978-3-030-18917-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-18917-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-18916-7
Online ISBN: 978-3-030-18917-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)