Abstract
Scavenging is a key process in the ecosystems. Studying foraging movements of obligate scavengers such as vultures can contribute to a better understanding of the scavenging-related patterns and processes. Here we review methods that can be used to track foraging vultures in the field. Yet, in order to track, vultures need to be trapped and tagged in manner that would ensure their health and normal survival and behavior. GPS telemetry is currently the best tool to track vultures for foraging studies. In a review of recent studies, we highlight the predominance of studies of species from Europe, North America and Southern Africa, and we deplore the lack of knowledge of species from the Tropics. Home ranges vary tremendously between sites, season and species (from a few km2 to >300,000 km2) but also depending on the analysis method used. Daily distances travelled are more repeatable between species, with values ranging between 30 and 40 km. Yet the way that carrion distribution can affect scavenger distribution and foraging behavior is still poorly understood.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Studying carrion ecology and their importance in the ecosystem is difficult in the field, because carrions presence is usually unpredictable in time and space. Moreover, carrion is a pulsed resource that can be quite ephemeral in the ecosystems as they are usually rapidly eliminated by scavengers. Therefore, studying scavengers’ movement ecology can help to a better understanding of the scavenging-related patterns and processes. The role of vultures in the ecosystems are of prime importance because they are the most specialized terrestrial vertebrate scavengers (Ruxton and Houston 2004) and they are highly skilled movers able to transport scavenging services over large areas (DeVault et al. 2016). In this chapter we will focus on the methods to study vulture movement ecology, and review recent results about their foraging ecology and movements. Tracking vultures can help identifying mortality of them as well as of both wild and domesticated ungulates. Thus, tracking scavengers can be highly important in the context of global environmental change for detecting alterations in the ecosystems derived from changes in the carrion availability.
Trapping Vultures
Trapping vultures is generally not an easy task and must be performed professionally in order to minimize the possibility of injury or stress to birds. When planning trapping efforts, the time of day, season (breeding status of the birds), weather, number of birds to be captured, staff required, and the possibility of predation should be taken into account (Bird and Bildstein 2007).
It is possible to mark chicks at nest but climbing skills and gear are required. Furthermore, a detailed nest monitoring, from hatching date, is crucial because early arrival to the nest will prevent deployment of tagging equipment as it may affect body growth, whereas delayed arrival to the nest may entail premature fledging (and death).
After fledging, the most common methods are based on attracting vultures to a food source as bait to catch them on the ground (Fig. 1). There are several alternatives, most described in details by Bloom et al. (2007), and summarized in Table 1. According to the review detailed in Table 1, the most common method of capture was walk-in traps (10 studies), followed by leg-hold traps, projected-nets and nestling (4 studies each) and rehabilitated birds (2).
Four different systems of traps for capturing vultures: (a) Walk-in trap in an aviary (active because an observer is hidden nearby to trigger the sliding door, France; Photo credit O.Duriez); (b) Cage trap (passive because vultures fall into the cage through openings in the roof, Israel, Photo credit I. Shaked); (c) leg-hold trap (here more specifically Phai traps as noose surrounding a bait; the rope linking the nooses is hidden under the snow or ground; France, Photo credit O. Duriez); (d) Californian condor chick equipped at the nest with GPS tag embedded in patagial tag (photo courtesy of Joseph Brandt, USFWS California Condor Recovery Program)
Tagging and Tracking Vultures
The possibilities to tag vultures are diverse and so are the costs and the potential output for management and research (Fig. 2). Darvic rings (numbered plastic rings) are good for mass tagging and allow long distance observation, typically up to 400 m on large vultures (even 700 m for color-ring codes). However because they might be broken or lost (Mihoub et al. 2013), they must be complemented by metal rings that would allow a re-identification of bird if recaptured. In addition, leg rings are not suitable for Cathartidae due to their habit to defecate on their legs, presumably for thermoregulation (Houston 1994). Wing tags (also called “patagial tags”) enhance resighting of birds at long distance and in flight (Wallace et al. 1980; Reading et al. 2014). However, it must be reminded that the impact of wing tags on bird flight (by increasing drag) has never been properly investigated in large soaring raptors (Trefry et al. 2013). Radio Frequency Identification Tags (RFID) allow detection (presence-absence) by passive tags at focal sites (Bonter and Bridge 2011). RFID can be either passive and provide presence data at short distances (up to 0.5 m), or active (powered by batteries) and detected at longer distance (up to 100 m). If base stations are deployed in advance around carrions, data collected can provide information of arrival time, duration of stay and departure time.
Eurasian griffon vultures Gyps fulvus carrying a GPS tag, (top) attached by a back-pack harness in silicon threaded Teflon ribbon (photo by I. Shaked); and (bottom) attached by a leg-loop harness (photo by O. Duriez). The position of both tags is located close to the bird’s center of gravity. For long-distance visual identification, these birds are also marked with wing tags (top) and with a darvic coded ring (bottom)
Several techniques can be used to track vultures and we review here only the methods useful to study vulture foraging ecology. For foraging studies, we advise to use interval <10 min between consecutive positions because vultures can cover several km in a few minutes (see below) and sometimes can spend <15 min to land, feed and take off (Harel et al. 2016a; Monsarrat et al. 2013). VHF radio-tracking should not be advised nowadays for foraging studies because of its low precision and relative inefficiency (as flying vultures move much faster than land-based observers) despite intense fieldwork requirement (observers need to follow each bird and manually find its position by triangulation). Satellite telemetry using Argos transmitters provide geographic positions anywhere in the world but are not recommended for detailed foraging studies, due to low precision (typically >1 km) and time interval between positions often >1 h. GPS tracking is the most widely used system nowadays which allows precise tracking (typically <100 m error) at short time interval. Solar powered devices are preferred for multi-annual studies (the longest tracking duration in France and in Israel was 5 years; Duriez and Hatzofe, unpublished). GPS transmitters usually store data on-board but several companies have developed systems to remotely download data using a radio-link, GSM or satellite transmission.
Tracking devices should be placed near the center of gravity on the bird’s back. The common attachment method includes a Teflon ribbon harness which is robust and non-abrasive for skin. Some elasticity can be added by inserting a silicon tube inside the ribbon. Back-pack and body harness are efficient methods of attachment, but there is a risk of entanglement of the bird if one string is broken (Bögel et al. 2000). Leg-loop harness (Rappole and Tipton 1991) is fast and easy to fit, secure for the birds (as it prevents entanglement when broken), but power charging can sometimes be made more difficult because solar panels can be covered by wings when vultures are perched (Duriez unpublished). In condors telemetry devices have been mounted on wing-tag (Wallace et al. 1980), but this method is questionable because it adds weight on a sensitive place of the wing and can potentially change lift or drag forces.
Additional sensors coupled with the GPS transmitter can enhance the information collected on the behavior of individuals. Accelerometers are useful to estimate body posture and movements, and ultimately feeding behavior and thus determining the location of carrions found and eaten by vultures (Spiegel et al. 2013b; Nathan et al. 2012). Magnetometer data can provide additional data on body position and help in discriminating between different behaviors (Williams et al. 2017). In the near future, we believe that cameras can be deployed also on vultures’ back to collect data on social behavior around carrions, as done on seabirds (Tremblay et al. 2014), however this technique is currently limited by the technical difficulty to download large amount of image data remotely without recapturing the bird. Nowadays the use of cameras is limited to the ground, with camera traps that can record attendance of scavengers (obligate and facultative) around carrions and help population monitoring by reading rings (Mateo-Tomas et al. 2017; Wilson 2015; Moreno-Opo et al. 2015; Ogada et al. 2012).
How Carrion Affect Foraging Movement of Vultures?
A main research question concerning the study of vulture movements in space and time is “how vultures make use of unpredictable food like carrions, and how does this affect their foraging strategies depending on the environmental context?”
We reviewed 39 studies published between 2007 and 2018 about vulture foraging movements using telemetry (Table 2), excluding studies about migration, dispersal or demography. 34 studies (87%) used GPS tracking devices and the 5 others used combinations of Argos or VHF telemetry units. 31 studies (79%) concerned Old-World vultures (family Accipitridae) and 8 concerned New-World vultures (family Cathartidae). Studies on Old-World vultures concerned 9 species out of 16 described (56%) and studies on Cathartidae concerned 4 species out of 7 described (57%). The 10 species where no data on foraging movements has been published (thus absent from Table 2) are Gypohierax angolensis, Gyps indicus, G. ruppellii, G. tenuirostris, Necrosyrtes monachus, Sarcogyps calvus, Trigonoceps occipitalis, and Cathartes burrovianus, C. melambrotus, Sarcoramphus papa. From a biogeographical perspective, while all species from Palearctic and Nearctic have been studied (at least once), only half of the species from Afrotropical and Neotropical regions have been studied and only one species from the Indo-Malayan region (Fig. 3). A similar pattern can be found regarding the main habitat, where all species from temperate-mountainous habitat have been studied, half of species from arid-Savanna habitats have been studied and only one species from tropical forest (Fig. 3). There are studies from all IUCN Red-list groups, however only a third of the species listed as Critically Endangered have been studied, as well as 42% of Least-Concerned species (Fig. 3). Finally, most studies concerned species displaying social behaviors for nesting or feeding, and few studies concerned territorial species (the notable exceptions being the bearded, lappet-faced and Egyptian vultures).
Number of species in which detailed foraging studies have been performed (in blue) and species unstudied (in red), according to IUCN red list status (CR critically endangered, EN endangered, NT near threatened, LC least concerned), biogeographical region (distinguishing when species are found in different regions) and main habitat used (from studies detailed in Table 2). Credit: the chapter authors
Therefore, our review points a lack of studies of vultures from the tropics, where vulture communities are (or were) the most abundant and diverse. This sample is therefore biased taxonomically, geographically and behaviorally and our knowledge of vulture movements mostly represents the behavior of social species in temperate mountains or arid areas (savannas or deserts).
With the aim to compare foraging movement behaviors from various species and derive general patterns, we reported in Table 2 the most widely computed parameters: the radius of displacement from central place, the daily distance travelled and home range size.
The radius of displacement from central place (roost or nest) has been reported in 9 studies only, however it is probably the most repeatable metric because it is less dependent of technology and sampling rate. Yet, caution is needed in interpretation because some studies measured the daily maximal distance from central place while other measured the mean radius of feeding event from the central place (e.g. even possible to compute from pellet analyses in which ear-tags of livestock can be found; (Moreno-Opo et al. 2011). As expected, the foraging radius is shorter in territorial species than in social species, among which it varies between c. 30 km in European vultures and c. 50 km in African species (Table 2).
The daily distance travelled per day has been computed in 13 studies. It varies between 5 km day−1 in A. monachus juvenile birds in Caucasus before migration (Yamaç and Bilgin 2012) and 160 km day−1 in G. fulvus in Israel (Harel et al. 2016a). However the value reported is highly dependent on the interval between fixes, as illustrated in Israel in G. fulvus at the same time of year, with values of 90 km day−1 when recording at interval of 10 min and 160 km day−1 when recording at interval of 1 s (Harel et al. 2016a). Such difference is due to the high flight speed of vultures (mean “cross-country” speed ranging 20–40 km h−1, combining vertical soaring phases and horizontal gliding phases, but instantaneous ground speed >50 km h−1; (Pennycuick 1972)): at interval of 1 h between fixes, two fixes could be spaced of a few km while the bird could have travelled in reality several tens of km in one direction and be back on its sinuous path. At interval shorter than 5 s, the soaring circles become visible and further increase the distance covered (Harel et al. 2016a; Treep et al. 2016). When considering only studies with interval between fixes 1–10 min, the mean daily distances varied between 90 km (G. fulvus in France and Israel) and 120 km (G. africanus and T. tracheliotus in Namibia) (Harel et al. 2016a; Spiegel et al. 2013a). When considering only studies with interval between fixes around 1 h, the mean daily distances varied between 5 and 66 km, with an average at 34.9 ± 21.6 km. This range of values between 30 and 60 km day−1 are remarkably similar for C. aura in the Americas, G. africanus in Africa and G. fulvus in Europe.
The home range is defined as the area in which an animal lives and moves on a periodic basis. The simplest method uses Minimum Convex Polygon (MCP) to delineate all recorded positions of an animal, although it is well known to greatly overestimate home range size. More recent methods use kernel density methods to estimate Utilization Distributions (UD) as the probability that an animal has to be in each cell of its home range (Worton 1989). When positions are supposed to be independent (i.e. collected at large interval of time) it is possible to use fixed-kernel method, while movement-based kernels are advised when data are collected at short time intervals (Benhamou and Cornélis 2010). Home range methods have been the most popular methods to describe vultures’ space use since it was used in 46 sub-populations or seasons and in 31 studies. However inter specific and intra-specific comparisons are made difficult because of the heterogeneity of methods, and within a method, the researcher’s decision to use a probability threshold of 99%, 95% or 90% to define his home range (Table 2). When considering only studies using the most popular 95% kernel estimators, individual home range sizes of vultures varied tremendously between 11 km2 and 334,923 km2, with a mean of 42,680 ± 105,244 km2. The smallest home ranges (<100 km2) belonged to the two New-World species C. aura and C. atratus, while the largest home ranges, >10,000 km2, all belonged to species in Africa: G. africanus, N. percnopterus and G. barbatus. The season often played a role in home range size, but there was no clear general trend. In temperate and sedentary species, like G. fulvus, G. coprotheres or C. atratus, home ranges tended to be larger in summer than in winter, when flight is more constrained by adverse aerological conditions (Kane et al. 2016; Avery et al. 2011; Monsarrat et al. 2013). In migratory species like N. percnopterus, home ranges were smaller in breeding season in temperate summer than in tropical winter, where movements are not constrained by returning to a nest (Carrete et al. 2013; Lopez-Lopez et al. 2014). However the opposite pattern was found in the long-distance migrant C. aura (Dodge et al. 2014). The status or age of the birds also played a role, with breeding adults tending to have smaller home ranges than non-breeding adults or immature birds.
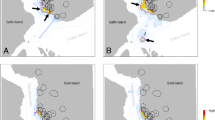
Foraging activity of avian scavengers may serve as an estimator for carrion distribution (Fig. 4). When searching for food, avian scavengers may follow specific land cover features, which are correlated with the presence of carrions (Houston 1974; Monsarrat et al. 2013) and may be limited by other factors, such as the roost site locations. However, within a suitable habitat, vultures may search in a random manner for food resources, or using their personal knowledge of the most likely places to find carrion (Kendall et al. 2014; Fluhr et al. 2017). To locate carrions, most vultures also use information retrieved from conspecifics (Houston 1974) or from other species (Kane et al. 2014; Spiegel et al. 2013a). Some gregarious species may use communal roosts as information centers to follow specifically the individuals that have been successful in foraging the previous day, probably using the state of the crop (extended) as proxy (Buckley 1996; Harel et al. 2017). Therefore, in order to establish the link between scavenger movements and the actual carrion distribution, the composition of foraging groups should be taken into account. Nevertheless, competition, diet preferences, human disturbance, topography and land cover may affect scavengers access to carrions and is expected to cause mismatches between the observed feeding behavior and the actual carrion distribution. Few modeling studies have compared the importance of self and social-information for vultures’ foraging efficiency and population persistence (Jackson et al. 2008; Deygout et al. 2009, 2010; Cortes-Avizanda et al. 2014). They suggested that acute reduction in population densities of avian scavengers may impair their foraging efficiency. Such models could be developed to predict the actual effect that avian scavengers can have on carrion distribution, but should incorporate individual characteristics, such as fasting period (Spiegel et al. 2013b), dominance hierarchies within species (Bosè et al. 2012) and between species (Houston 1975; Moreno-Opo et al. 2016; Sebastián-González et al. 2016), and different scenarios of population or community densities (Dupont et al. 2011).
Space-use patterns of griffon vultures Gyps fulvus in Israel as an estimator for carrion distribution patterns. (a) The spatial utilization distribution of foraging flights of GPS-tracked vultures may serve as a proxy for the area scanned by scavengers, while (b) feeding events (classified by accelerometry and movement data) represent the actual use of carrions available in the region. Colors in both panels represent the proportion of visits in a 2 × 2 km cell grid over the study period. Black dots in panel (b) represent active feeding stations. Credit: the chapter authors
Conclusions and Future Perspectives
In conclusion it is still premature to derive general conclusions about vulture foraging movements in space, partly due to the different methods used for tracking and estimating movement metrics, but also due to large differences in ecology and habitat of vulture species around the world. The only general conclusion possible to say so far is that vultures are able to cover large distances of tens of km per day, in search of carrions, over very large areas, and thus can extend scavenging services well beyond reach of their colonies. But we still lack data on many species, particularly from the tropics. However, the methods to catch vultures are plentiful and the development of GPS technology and remote download of data offers avenues for promising future research about the spatial use of carrions by specialized scavengers.
More practically, to improve intra-specific and inter-specific comparisons, we encourage researchers to standardize their methods. The most important would be to homogenize the statistical methods to describe space use, and systematically report for each individual or each group the daily maximal displacement (which is the less biased metric), daily travelled distance and 95% kernel UD. To study fine details about foraging behavior, vulture biologists must aim at collecting tracking data at intervals <5 min (Fluhr et al. 2017). Regarding the choice of loggers, the GPS system with automatic download with GSM network is by far the most accurate and cheaper. The most recent GPS devices, powered by solar panels over several years, allow recording GPS positions at intervals <5 min, and including burst of high resolutions recording 1 position per second, where it becomes easy to distinguish fine behavioral details (Harel et al. 2016a, b; Sherub et al. 2016). Two additional features can be very useful to study vulture feeding behavior if integrated into the device. The accelerometer can record body posture and movements associated with feeding events, and eventually can be used as a proxy of energy expenditure (Nathan et al. 2012; Spiegel et al. 2013b; Duriez et al. 2014). The possibility to define a “GPS fence” around areas of interest (like feeding stations, or wind farms) can allow to use special settings to be applied (like high resolution GPs recording), to better understand vulture behavior in these areas.
References
Alarcon PAE, Morales JM, Donazar JA, Sanchez-Zapata JA, Hiraldo F, Lambertucci SA (2017) Sexual-size dimorphism modulates the trade-off between exploiting food and wind resources in a large avian scavenger. Sci Rep 7:11461. https://doi.org/10.1038/s41598-017-11855-0
Arrondo E, Moleon M, Cortes-Avizanda A, Jimenez J, Beja P, Sanchez-Zapata JA, Donazar JA (2018) Invisible barriers: differential sanitary regulations constrain vulture movements across country borders. Biol Conserv 219:46–52. https://doi.org/10.1016/j.biocon.2017.12.039
Avery ML, Humphrey JS, Daughtery TS, Fischer JW, Milleson MP, Tillman EA, Bruce WE, Walter WD (2011) Vulture flight behavior and implications for aircraft safety. J Wildl Manag 75(7):1581–1587. https://doi.org/10.1002/jwmg.205
Bamford AJ, Diekmann M, Monadjem A, Mendelsohn J (2007) Ranging behaviour of cape vultures Gyps coprotheres from an endangered population in Namibia. Bird Conserv Int 17(04):331–339. https://doi.org/10.1017/S0959270907000846
Bamford AJ, Monadjem A, Diekmann M, Hardy ICW (2009) Development of non-explosive-based methods for mass capture of vultures. S Afr J Wildl Res 39(2):202–208. https://doi.org/10.3957/056.039.0201
Barber DR, Bildstein KL (2011) A lightweight portable, walk-in trap for catching vultures. Vulture News 60:22–25
Batbayar N, Reading R, Kenny D, Natsagdorj T, Kee PW (2008) Migration and movement patterns of cinereous vultures in Mongolia. Falco 32:5–7
Benhamou S, Cornélis D (2010) Incorporating movement behavior and barriers to improve Kernel home range space use estimates. J Wildl Manag 74(6):1353–1360. https://doi.org/10.2193/2009-441
Bird DM, Bildstein KL (2007) Raptor research and management techniques. Hancock House Publishers, Surrey
Bloom P, Clark W, Kidd J (2007) Capture techniques. In: Bird DM, Bildstein KL (eds) Raptor research and management techniques. Hancock House Publishers, Surrey, pp 242–248
Bögel R, Prinzinger R, Karl E, Walzer C (2000) A multisensor telemetry system for studying flight biology and energetics of free-flying griffon vultures - Gyps fulvus. A case study. In: Chancellor RD, Meyburg B-U (eds) Raptors at risk. WWGBP/Hancock House, Johannesburg, pp 879–883
Bonter DN, Bridge ES (2011) Applications of radio frequency identification (RFID) in ornithological research: a review. J Field Ornithol 82(1):1–10. https://doi.org/10.1111/j.1557-9263.2010.00302.x
Bosè M, Duriez O, Sarrazin F (2012) Intra-specific competition in foraging griffon vultures: 1. The dynamics of feeding in groups. Bird Study 59:182–192. https://doi.org/10.1080/00063657.2012.658639
Buckley NJ (1996) Food finding and the influence of information, local enhancement, and communal roosting on foraging success of North American vultures. Auk 113(2):473–488
Carrete M, Bortolotti GR, Sánchez-Zapata JA et al (2013) Stressful conditions experienced by endangered Egyptian vultures on African wintering areas. Anim Conserv 16(3):353–358. https://doi.org/10.1111/acv.12001
Castaño JP, Sanchez JF, Diaz-Portero MA, Robles M (2015) Dispersal and survival of juvenile black vultures Aegypius monachus in central Spain. Ardeola 62(2):351–361
Cortes-Avizanda A, Jovani R, Donazar JA, Grimm V (2014) Bird sky networks: how do avian scavengers use social information to find carrion? Ecology 95(7):1799–1808. https://doi.org/10.1890/13-0574.1
DeVault TL, Beasley JC, Olson ZH et al (2016) Ecosystem services provided by avian scavengers. In: Sekercioglu CH (ed) Why birds matter. University of Chicago Press, Chicago, p 36
Deygout C, Gault A, Sarrazin F, Bessa-Gomes C (2009) Modeling the impact of feeding stations on vulture scavenging service efficiency. Ecol Model 220(15):1826–1835
Deygout C, Gault A, Duriez O, Sarrazin F, Bessa-Gomes C (2010) Impact of food predictability on social facilitation by foraging scavengers. Behav Ecol 21(6):1131–1139. https://doi.org/10.1093/beheco/arq120
Dodge S, Bohrer G, Bildstein K, Davidson SC et al (2014) Environmental drivers of variability in the movement ecology of turkey vultures (Cathartes aura) in North and South America. Philos Trans R Soc London Ser B 369:1643. https://doi.org/10.1098/rstb.2013.0195
Dupont H, Mihoub JB, Becu N, Sarrazin F (2011) Modelling interactions between scavenger behaviour and farming practices: impacts on scavenger population and ecosystem service efficiency. Ecol Model 222(4):982–992
Duriez O, Kato A, Tromp C et al (2014) How cheap is soaring flight in raptors? A preliminary investigation in freely-flying vultures. PLoS One 9(1):e84887
Fluhr J, Benhamou S, Riotte-Lambert L, Duriez O (2017) Assessing the risk for an obligate scavenger to be dependent on predictable feeding sources. Biol Conserv 215:92–98
Garcia-Ripolles C, Lopez-Lopez P, Urios V (2011) Ranging behaviour of non-breeding Eurasian Griffon Vultures Gyps fulvus: a GPS-telemetry study. Acta Ornithol 46:127–134
Gavashelishvili A, McGrady M, Ghasabian M, Bildstein KL (2012) Movements and habitat use by immature cinereous vultures (Aegypius monachus) from the caucasus. Bird Study 59:449–462. https://doi.org/10.1080/00063657.2012.728194
Gil JA, Baguena G, Sanchez-Castilla E et al (2014) Home ranges and movements of non-breeding bearded vultures tracked by satellite telemetry in the Pyrenees. Ardeola 61:379–387
Gilbert M, Watson RT, Ahmed S et al (2007) Vulture restaurants and their role in reducing diclofenac exposure in Asian vultures. Bird Conserv Int 17(1):63–77. https://doi.org/10.1017/s0959270906000621
Harel R, Duriez O, Spiegel O et al (2016a) Decision-making by a soaring bird: time, energy and risk considerations at different spatio-temporal scales. Philos Trans R Soc London Ser B 371(1704):20150397. https://doi.org/10.1098/rstb.2015.0397
Harel R, Horvitz N, Nathan R (2016b) Adult vultures outperform juveniles in challenging thermal soaring conditions. Sci Rep 6:27865
Harel R, Spiegel O, Getz WM, Nathan R (2017) Social foraging and individual consistency in following behaviour: testing the information centre hypothesis in free-ranging vultures. Proc R Soc Lond B 284:1852. https://doi.org/10.1098/rspb.2016.2654
Holland AE, Byrne ME, Bryan AL, DeVault TL, Rhodes OE, Beasley JC (2017) Fine-scale assessment of home ranges and activity patterns for resident black vultures (Coragyps atratus) and turkey vultures (Cathartes aura). PLoS One 12(7):e0179819
Houston DC (1974) Food searching in griffon vultures. East Afr Wild J 12:63–77
Houston DC (1975) Ecological isolation of African scavenging birds. Ardea 63:55–64
Houston DC (1994) Family cathartidae (new world vultures). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world, New world vultures to guineafowls, vol 2. Lynx Edición, Barcelona, pp 24–41
Iezekiel S, Woodley B, Hatzofe O (2003) Cage traps for Gyps fulvus. Vulture News 49:14–16
Jackson AL, Ruxton GD, Houston DC (2008) The effect of social facilitation on foraging success in vultures: a modelling study. Biol Lett 4(3):311–313
Kane A, Jackson AL, Ogada DL, Monadjem A, McNally L (2014) Vultures acquire information on carcass location from scavenging eagles. Proc R Soc Lond B 281:1793. https://doi.org/10.1098/rspb.2014.1072
Kane A, Wolter K, Neser W, Kotze A, Naidoo V, Monadjem A (2016) Home range and habitat selection of cape vultures Gyps coprotheres in relation to supplementary feeding. Bird Study 63:387–394. https://doi.org/10.1080/00063657.2016.1214105
Kendall CJ, Virani MZ, Hopcraft JGC, Bildstein KL, Rubenstein DI (2014) African vultures don’t follow migratory herds: scavenger habitat use is not mediated by prey abundance. PLoS One 9(1):e83470
Krüger S, Amar A (2017) Insights into post-fledging dispersal of bearded vultures Gypaetus barbatus in Southern Africa from GPS satellite telemetry. Bird Study 64(2):125–131. https://doi.org/10.1080/00063657.2017.1295019
Krüger S, Reid T, Amar A (2014) Differential range use between age classes of Southern African bearded vultures Gypaetus barbatus. PLoS One 9(12):e114920
Lambertucci SA, Alarcon PAE, Hiraldo F, Sanchez-Zapata JA, Blanco G, Donazar JA (2014) Apex scavenger movements call for transboundary conservation policies. Biol Conserv 170:145–150. https://doi.org/10.1016/j.biocon.2013.12.041
Lopez-Lopez P, Garcia-Ripolles C, Urios V (2014) Food predictability determines space use of endangered vultures: implications for management of supplementary feeding. Ecol Appl 24(5):938–949. https://doi.org/10.1890/13-2000.1
Margalida A, Perez-Garcia JM, Moreno-Opo R (2017) European policies on livestock carcasses management did not modify the foraging behavior of a threatened vulture. Ecol Indic 80:66–73. https://doi.org/10.1016/j.ecolind.2017.04.048
Mateo-Tomas P, Olea PP, Moleon M, Selva N, Sanchez-Zapata JA (2017) Both rare and common species support ecosystem services in scavenger communities. Global Ecol Biogeo 26(12):1459–1470
Mihoub J-B, Prince K, Duriez O, Lécuyer P, Eliotout B, Sarrazin F (2013) Comparing release method effects on post-release survival of the European black vulture Aegypius monachus reintroduced population in France. Oryx 48:106–115
Monsarrat S, Benhamou S, Sarrazin F, Bessa-Gomes C, Bouten W, Duriez O (2013) How predictability of feeding patches affects home range and foraging habitat selection in avian social scavengers? PLoS One 8(1):e53077
Moreno-Opo R, Arredondo A, Guil F (2011) Foraging range and diet of Cinereous vulture Aegypius monachus using livestock resources in Central Spain. Ardeola 57:111–119
Moreno-Opo R, Trujillano A, Arredondo A, Gonzalez LM, Margalida A (2015) Manipulating size, amount and appearance of food inputs to optimize supplementary feeding programs for European vultures. Biol Conserv 181:27–35. https://doi.org/10.1016/j.biocon.2014.10.022
Moreno-Opo RN, Trujillano A, Margalida A (2016) Behavioral coexistence and feeding efficiency drive niche partitioning in European avian scavengers. Behav Ecol 27:1041–1052. https://doi.org/10.1093/beheco/arw010
Nathan R, Spiegel O, Fortmann-Roe S, Harel R, Wikelski M, Getz WM (2012) Using tri-axial acceleration data to identify behavioral modes of free-ranging animals: general concepts and tools illustrated for Griffon vultures. J Exp Biol 215:986–996
Ogada DL, Torchin ME, Kinnaird MF, Ezenwa VO (2012) Effects of vulture declines on facultative scavengers and potential implications for mammalian disease transmission. Conserv Biol 26(3):453–460. https://doi.org/10.1111/j.1523-1739.2012.01827.x
Oppel S, Dobrev V, Arkumarev V, Saravia V, Bounas A, Kret E, Velevski M, Stoychev S, Nikolov SC (2015) High juvenile mortality during migration in a declining population of a long-distance migratory raptor. Ibis 157:545–557. https://doi.org/10.1111/ibi.12258
Pennycuick CJ (1972) Soaring behaviour and performance of some East African birds observed from a motor glider. Ibis 114:178–218
Phipps WL, Willis SG, Wolter K, Naidoo V (2013a) Foraging ranges of immature African white-backed vultures (Gyps africanus) and their use of protected areas in Southern Africa. PLoS One 8(1):e52813
Phipps WL, Wolter K, Michael MD, MacTavish LM, Yarnell RW (2013b) Do power lines and protected areas present a catch-22 situation for cape vultures (Gyps coprotheres)? PLoS One 8(10):e76794
Rappole JH, Tipton AR (1991) New harness design for attachment of radio transmitters to small passerines. J Field Ornithol 62:335–337
Reading PP, Maude G, Hancock P, Kenny D, Garbett R (2014) Comparing different types of patagial tags for use on vultures. Vulture News 67:33–42
Reid T, Krüger S, Whitfield DP, Amar A (2015) Using spatial analyses of bearded vulture movements in Southern Africa to inform wind turbine placement. J Appl Ecol 52:881–892. https://doi.org/10.1111/1365-2664.12468
Rivers JW, Johnson JM, Haig SM, Schwarz CJ, Burnett LJ, Brandt J, George D, Grantham J (2014) An analysis of monthly home range size in the critically endangered California Condor Gymnogyps californianus. Bird Conserv Int 24:492–504. https://doi.org/10.1017/S0959270913000592
Ruxton GD, Houston DC (2004) Obligate vertebrate scavengers must be large soaring fliers. J Theor Biol 228(3):431–436
Sebastián-González E, Moleón M, Gibert JP, Botella F, Mateo-Tomás P, Olea PP, Guimarães PR, Sánchez-Zapata JA (2016) Nested species-rich networks of scavenging vertebrates support high levels of interspecific competition. Ecology 97(1):95–105. https://doi.org/10.1890/15-0212.1
Sherub S, Bohrer G, Wikelski M, Weinzierl R (2016) Behavioural adaptations to flight into thin air. Biol Lett 12:10. https://doi.org/10.1098/rsbl.2016.0432
Sherub S, Fiedler W, Duriez O, Wikelski M (2017) Bio-logging - new technologies to study conservation physiology on the move: a case study on annual survival of Himalayan vultures. J Comp Physiol A 203(6):531–542. https://doi.org/10.1007/s00359-017-1180-x
Spiegel O, Getz WM, Nathan R (2013a) Factors influencing search efficiency: why do scarce Lappet-faced vultures outperform ubiquitous white-backed vultures. Am Nat 181:5. https://doi.org/10.1086/670009
Spiegel O, Harel R, Getz W, Nathan R (2013b) Mixed strategies of griffon vultures’ (Gyps fulvus) response to food deprivation lead to a hump-shaped movement pattern. Mov Ecol 1(1):5
Treep J, Bohrer G, Shamoun-Baranes J, Duriez O, Prata de Moraes Frasson R, Bouten W (2016) Using high resolution GPS tracking data of bird flight for meteorological observations. Bull Am Meteorol Soc 97(6):951–961. https://doi.org/10.1175/bams-d-14-00234.1
Trefry S, Diamond A, Jesson L (2013) Wing marker woes: a case study and meta-analysis of the impacts of wing and patagial tags. J Ornithol 154(1):1–11. https://doi.org/10.1007/s10336-012-0862-y
Tremblay Y, Thiébault A, Mullers R, Pistorius P (2014) Bird-borne video-cameras show that seabird movement patterns relate to previously unrevealed proximate environment, not prey. PLoS One 9(2):e88424
Urios V, Lopez-Lopez P, Limiñana R, Godino A (2010) Ranging behaviour of a juvenile bearded vulture (Gypaetus barbatus meridionalis) in South Africa revealed by GPS satellite telemetry. Ornis Fenn 87:114–118
Vasilakis DP, Whitfield DP, Schindler S, Poirazidis KS, Kati V (2016) Reconciling endangered species conservation with wind farm development: cinereous vultures (Aegypius monachus) in South-Eastern Europe. Biol Conserv 196:10–17. https://doi.org/10.1016/j.biocon.2016.01.014
Wallace MP, Parker GP, Temple SA (1980) An evaluation of patagial markers for cathartid vultures. J Field Ornithol 51:309–314
Williams HJ, Holton MH, Shepard EL, Largey N, Norman JA, Ryan P, Duriez O, Scantlebury M, Quintana F, Magowan E, Wilson RP (2017) Identification of animal movement patterns using tri-axial magnetometry. Mov Ecol 5:6. https://doi.org/10.1186/s40462-017-0097-x
Wilson B (2015) An introduction to camera trapping of wing-tagged vultures in Southern Africa. Vulture News 69:3–22
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70(1):164–168
Xirouchakis SM, Andreou G (2009) Foraging behaviour and flight characteristics of Eurasian griffons Gyps fulvus in the island of Crete, Greece. Wildl Biol 15(1):37–52. https://doi.org/10.2981/07-090
Yamaç E, Bilgin CC (2012) Post-fledging movements of cinereous vultures Aegypius monachus in Turkey revealed by GPS telemetry. Ardea 100(2):149–156. https://doi.org/10.5253/078.100.0206
Acknowledgements
The authors want to thank all their colleagues who helped capturing and tagging vultures in the field, A. Camiña, I. Shaked and J. Brandt for providing photos of trapping and tagging techniques, and the editors of the book for giving the opportunity to write this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Duriez, O., Harel, R., Hatzofe, O. (2019). Studying Movement of Avian Scavengers to Understand Carrion Ecology. In: Olea, P., Mateo-Tomás, P., Sánchez-Zapata, J. (eds) Carrion Ecology and Management. Wildlife Research Monographs, vol 2. Springer, Cham. https://doi.org/10.1007/978-3-030-16501-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-16501-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16499-7
Online ISBN: 978-3-030-16501-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)