Abstract
Antibiotic resistance has become the most significant threat to human health across the globe. Polymyxins are often used as the only available therapeutic option against Gram-negative ‘superbugs’, namely Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae. The limited pharmacological and clinical knowledge on the polymyxins in the old literature substantially limited optimizing their clinical use. The current chapter provides a general introduction to this first-ever polymyxin book which comprehensively reviews the significant progress over the last two decades in the chemistry, microbiology, pharmacology, clinical use and drug discovery of polymyxins. In particular, recent pharmacological results have led to the first scientifically-based dosing recommendations and facilitated the discovery of new-generation polymyxins. Future challenges in polymyxin research are highlighted, aiming at improving the clinical utility of this last-line defence.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
One of the most outstanding achievements of modern medicine was the development of antibiotics for treatment of bacterial infections that were widely fatal. Antibiotics are regarded as ‘miracle drugs’ and have significantly decreased mortality worldwide over the last century [1]. They have made many complicated surgical procedures and treatments possible; unfortunately, an increasing number of infections (e.g. pneumonia) are becoming more and more difficult to treat, as our current antibiotics are losing their efficacy. Over the last three decades resistance to these ‘magic bullets’ has presented the most significant threat to human health globally. If proactive solutions are not found to prevent the widespread antibiotic resistance , it is estimated that by 2050 approximately 10 million people per year will die of antimicrobial-resistant infections, which is more than the number of people dying from any other type of disease (Fig. 1.1) [2].
Predicted global deaths due to antimicrobial-resistant infections every year, compared to other major diseases [2]
Antibiotic resistance causes increased mortality, longer hospital stays and higher medical costs. Globally, the cost of antimicrobial resistance is enormous in terms of the economy and human health [3, 4]. It is predicted that a cumulative US$100 trillion of economic output by 2050 is at risk due to antimicrobial resistance [2]. Based upon the projections of the world economy in 2017–2050, The World Bank Group estimated that antibiotic resistance could cost the world economy $1 trillion every year by 2050 [5]. A recent study showed that the total economic cost of antibiotic resistance in Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii and Pseudomonas aeruginosa reached $2.8 billion per year in the US [6]. Furthermore, antibiotic resistance increases poverty worldwide and affects poorest countries the most [5].
Worryingly, many large pharmaceutical companies have left the antibiotic market, because the development of new antibiotics is scientifically challenging and not as profitable as for drugs used to treat chronic conditions and lifestyle issues [7,8,9]. A recent report reviewed the major pharmaceutical launches between 2014 and 2016 across a range of therapeutic areas [9]. It is evident that in anti-cancer drugs the risk of losing $450 million on a new molecular entity is easily offset by $8,200 million expected net present value. However, developing new antibiotics is astonishingly less attractive, as the expected net present value for the new antibiotics launched during 2014–2016 is −$100 million while with a financial risk of $500 million [9]. The World Health Organization (WHO) has urged all government sectors and society to act on antibiotic resistance . In 2017, WHO identified a list of priority pathogens which are resistant to the majority of currently available antibiotics and urgently require new therapeutic options (Fig. 1.2) [10].
A list of priority pathogens identified by WHO for research and development of new antibiotics [10]
As shown in the WHO Priority Pathogen List, carbapenem-resistant Gram-negative A. baumannii, P. aeruginosa and K. pneumoniae are particularly problematic, as efficacious therapeutic options are quickly diminishing against life-threatening infections caused by these pathogens [10]. All three ‘superbugs’ can develop resistance to almost all major classes of antibiotics via multiple mechanisms. A recent study investigated antibiotic resistance in A. baumannii infections with inpatients or outpatients from 54 studies (35 from the Organisation for Economic Co-operation and Development [OECD] countries with 57,188 bacterial isolates and 19 from non-OECD countries with 7,395 isolates) by searching Medline, Embase, Web of Science, and Cochrane databases [11]. Strikingly, a high prevalence of multidrug-resistance in A. baumannii infections is evident in both OECD and non-OECD countries, and a faster increase was clearly shown in OECD countries over the last decade (Fig. 1.3). In general, resistance to most commonly used antibiotics in A. baumannii is >70% in both OECD and non-OECD countries [11]. P. aeruginosa is intrinsically resistant to many antibiotics and is a major cause of healthcare-associated infections globally. The European Centre for Disease Prevention and Control (ECDC) has one of the most comprehensive antibiotic susceptibility surveillance programs in the world. According to its latest antibiotic surveillance report, the rate of resistance to three or more major classes of antipseudomonal antibiotics (including piperacillin/tazobactam, ceftazidime, carbapenems, fluoroquinolones and aminoglycosides) is disturbingly high, in particular in eastern and south-eastern European countries (Fig. 1.4) [12]. K. pneumoniae is another major pathogen which can become resistant to multiple classes of antibiotics and cause serious hospital-acquired infections , such as pneumonia, urinary tract infections and bloodstream infections. The resistance rate to fluoroquinolones, third-generation cephalosporins and aminoglycosides has reached >50% in Egypt [13] and a number of eastern European countries (Fig. 1.5) [12]. Sadly, few novel antibiotics will become available for these very problematic Gram-negative pathogens in the near future [2, 14, 15]. In many cases, polymyxins have to be used as the last resort for the treatment of life-threatening infections caused by A. baumannii, P. aeruginosa and K. pneumoniae [15,16,17,18,19,20].
Antibiotic resistance in A. baumannii. (a) Prevalence of multidrug-resistance to major antibiotics except colistin and tigecycline during 2000 and 2016 in the Organisation for Economic Co-operation and Development (OECD) and non-OECD countries. (b) Increasing antibiotic resistance in OECD and non-OECD countries between 2000 and 2016 [11]. http://creativecommons.org/licenses/by/4.0/
Antibiotic resistance in P. aeruginosa to three or more classes among piperacillin/tazobactam, ceftazidime, carbapenem, fluoroquinolones and aminoglycosides in Europe in 2017 [12]
Percentage (%) of K. pneumoniae isolates resistant to fluoroquinolones, third-generation cephalosporins and aminoglycosides in Europe in 2017 [12]
Polymyxins (i.e. colistin [also known as polymyxin E] and polymyxin B) entered the clinic in the late 1950s, but their use waned in the 1970s due to the potential nephrotoxicity and neurotoxicity [15, 16, 18, 21, 22]. Since the 2000s, however, clinicians have had to increasingly use colistin and polymyxin B as one of the very few therapeutic options for Gram-negative ‘superbugs’. This chapter serves as an introduction to this book and provides an overview of the microbiology, chemistry, pharmacology , clinical use , and drug discovery of polymyxins .
1.2 Polymyxins: A New ‘Old’ Class of Antibiotics
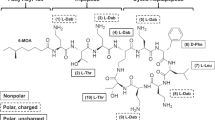
Colistin and polymyxin B (Fig. 1.6) were approved for clinical use in the late 1950s and were not subject to contemporary drug development evaluations and regulatory scrutiny. As polymyxins have been off patent for many years and were not widely used between the 1970s and 1990s, most pharmacological information on colistin and polymyxin B is from the studies conducted in academic research groups over the last two decades. Figure 1.7 clearly shows that polymyxins have attracted significant research and clinical interest since the early 2000s, due to increasing need to use them against multidrug-resistant Gram-negative pathogens. A number of major achievements have been made in the polymyxin field over the last two decades, including (1) a better understanding of the chemistry, structure-activity-toxicity relationships, and mechanisms of antibacterial activity, resistance and toxicity of polymyxins; (2) the first scientifically-based dosing recommendations for intravenous colistin based on the latest pre-clinical and clinical pharmacokinetic , pharmacodynamic and toxicodynamic information; and (3) the development of new-generation polymyxins informed by the newest chemical and pharmacological results. In this book, we invited international experts to provide comprehensive reviews on all of these major topics on polymyxins, with the aim of assembling the information needed to facilitate optimizing their clinical use and the development of novel, safer polymyxins.
This book starts with a comprehensive review on multidrug-resistance in Gram-negative ‘superbugs’ (Chap. 2) which highlights the urgent need to optimize the clinical use of both polymyxins and minimize any potential emergence of resistance. An in-depth introduction on the history, antibacterial spectrum and chemistry of polymyxins (Chap. 3) provides key information to understand how polymyxins kill bacterial cells (Chap. 4) and how bacteria develop resistance (Chap. 5). To optimize the dosage regimens of polymyxins, it is essential to develop sensitive and accurate analytical methods (Chap. 6), investigate the pharmacokinetics in animals (Chap. 7), characterize the pharmacodynamics using in vitro and animal models (Chap. 8), and determine antibacterial susceptibility breakpoints (Chap. 9). The two different conventions used to describe the dose of colistin, the complex composition of polymyxin products, and the different pharmacopoeial standards have caused considerable confusion in different parts of the world, and together with the outdated product information can affect the ability of clinicians to optimize the use of polymyxins in patients (Chap. 10). Chapters 11, 12, 13, 14, 15, and 16 review the latest achievements in improving the use of colistin, polymyxin B and potential synergistic combinations in the clinic, which is a major focus of this polymyxin book. As polymyxins have a narrow therapeutic window and nephrotoxicity is the major dose-limiting factor [17, 22, 23], understanding their toxicities (Chap. 17) and mechanisms (Chap. 18) are crucial to ensuring their optimum and safe use in the clinic. In addition, the anti-endotoxin effect of polymyxins has been extensively evaluated for the treatment of severe sepsis and septic shock (Chap. 19). Finally, Chap. 20 reviews the latest progress in developing new-generation polymyxins with better antibacterial activity and safety profiles, which is informed by the modern polymyxin pharmacology research.
A number of major challenges and gaps in knowledge have been identified in this book. There is an imperative to systematically evaluate the clinical efficacy of intravenous colistimethate (an inactive prodrug of colistin, see Chap. 3) and polymyxin B against different types of infections (e.g. blood and urinary tract infections) [24, 25]. A large clinical PK/PD/TD study on intravenous polymyxin B is being conducted in critically-ill patients funded by the National Institutes of Health (ClinicalTrials.gov Identifier: NCT02682355). Hopefully, scientifically-based dosing recommendations will be available in the near future for intravenous polymyxin B in different types of patients. Considering the narrow therapeutic window, prospective studies with therapeutic drug monitoring and adaptive feedback control are needed for optimizing the use of both polymyxins in patients. For the treatment of MDR Gram-negative respiratory tract infections, inhalation of polymyxins is very likely a better option than intravenous administration, because of the PK/PD considerations. However, the current dosage regimens of inhaled colistin and polymyxin B are empirical and not based on PK/PD/TD information. The literature on polymyxin combination therapy versus monotherapy is confusing. Most clinical studies evaluating the efficacy of polymyxin combinations in the literature have overlooked the significant PK/PD issues due to the limited polymyxin exposure in the lungs after intravenous administration. PK/PD/TD principles must be considered when optimizing polymyxin combination therapy , as it is not as simple as dosing multiple antibiotics together. To achieve synergistic killing in vivo , all drugs should achieve optimal exposure at the infection site at the right timing; otherwise, polymyxin ‘combination’ therapy is essentially monotherapy. As nephrotoxicity can occur in patients receiving intravenous polymyxins, innovative approaches are warranted to increase their therapeutic indices, thereby improving the efficacy. Development of new-generation polymyxins is challenging due to the narrow chemical space and the complex relations between the chemical structure , antibacterial activity, different resistance mechanisms, toxicity and PK (e.g. plasma protein binding). Taken together, collective efforts are essential to address these challenges in the coming years.
1.3 Summary
Almost 60 years after polymyxins were approved for clinical use , clinicians are now in a much better position to determine appropriate dosage regimens for intravenous polymyxins in patients, which is the result of extensive preclinical and clinical pharmacological investigations over the last two decades. Since 2013, three international conferences have been held with the contributions of distinguished speakers worldwide, most of whom are authors in this book, the first-ever on polymyxins. It is very encouraging that substantial progress has been made across all major areas of polymyxin research, and the list of high-priority issues and challenges identified at the international polymyxin conferences becomes shorter. In this ‘Bad Bugs, No Drugs’ era, polymyxins will continue to play an important role in the treatment of life-threatening infections caused by Gram-negative ‘superbugs’.
References
Armstrong GL, Conn LA, Pinner RW (1999) Trends in infectious disease mortality in the United States during the 20th century. JAMA 281:61–66
O’neill J (2015) Tackling a global health crisis: initial steps. Wellcome Trust, London. https://amr-review.org/sites/default/files/Report-52.15.pdf. Last accessed 12 April 2019
Hofer U (2019) The cost of antimicrobial resistance. Nat Rev Microbiol 17(1):3
Organisation for Economic Co-operation and Development (OECD) (2018) Stemming the superbug tide. http://www.oecd.org/els/health-systems/Stemming-the-Superbug-Tide-Policy-Brief-2018.pdf. Last accessed 12 April 2019
World Bank Group (2016) By 2050, drug-resistant infections could cause global economic damage on par with 2008 financial crisis. http://www.worldbank.org/en/news/press-release/2016/09/18/by-2050-drug-resistant-infections-could-cause-global-economic-damage-on-par-with-2008-financial-crisis. Last accessed 12 April 2019
Shrestha P, Cooper BS, Coast J et al (2018) Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob Resist Infect Control 7:98
Cooper MA, Shlaes D (2011) Fix the antibiotics pipeline. Nature 472:32
Infectious Diseases Society of America (IDSA) (2004) Bad bugs, no drugs. Infectious Diseases Society of America, Alexandria, VA. https://www.idsociety.org/globalassets/idsa/policy%2D%2Dadvocacy/current_topics_and_issues/antimicrobial_resistance/10x20/statements-manually-added/070104-as-antibiotic-discovery-stagnates-a-public-health-crisis-brews.pdf. Last accessed 12 April 2019
The Boston Consulting Group (BCG) for the German Federal Ministry of Health (2017) Breaking through the wall: a call for concerted action on antibiotics research and development. Follow-up report for the German GUARD initiative. https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Gesundheit/Berichte/GUARD_Follow_Up_Report_Full_Report_final.pdf. Last accessed 12 April 2019
World Health Organization (WHO) (2017) WHO priority pathogens list for R&D of new antibiotics. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. Last accessed 12 April 2019
Xie R, Zhang XD, Zhao Q et al (2018) Analysis of global prevalence of antibiotic resistance in Acinetobacter baumannii infections disclosed a faster increase in OECD countries. Emerg Microbes Infect 7:31
European Centre for Disease Prevention and Control (ECDC) (2018) Surveillance of antimicrobial resistance in Europe – annual report of the European antimicrobial resistance surveillance network (EARS-net) 2017. ECDC, Stockholm
World Health Organization (WHO) (2017) Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2016–2017. Geneva. ISBN: 978-92-4-151344-9. http://apps.who.int/iris/bitstream/handle/10665/259744/9789241513449-eng.pdf;jsessionid=9F6FFF4F4DA947B2346DFEAD91880B04?sequence=1. Last accessed 12 April 2019
Bush K, Courvalin P, Dantas G et al (2011) Tackling antibiotic resistance. Nat Rev Microbiol 9:894–896
Landman D, Georgescu C, Martin DA et al (2008) Polymyxins revisited. Clin Microbiol Rev 21:449–465
Li J, Nation RL, Turnidge JD et al (2006) Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis 6:589–601
Nation RL, Garonzik SM, Thamlikitkul V et al (2017) Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 64:565–571
Poirel L, Jayol A, Nordmann P (2017) Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev 30:557–596
Velkov T, Roberts KD, Nation RL et al (2013) Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol 8:711–724
Zavascki AP, Goldani LZ, Li J et al (2007) Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother 60:1206–1215
Li J, Nation RL, Milne RW et al (2005) Evaluation of colistin as an agent against multi-resistant gram-negative bacteria. Int J Antimicrob Agents 25:11–25
Nation RL, Li J, Cars O et al (2015) Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 15:225–234
Nation RL, Velkov T, Li J (2014) Colistin and polymyxin B: are they like peas in a pod or chalk and cheese? Clin Infect Dis 59:88–94
Cheah SE, Wang J, Nguyen VT et al (2015) New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 70:3291–3297
Landersdorfer CB, Wang J, Wirth V et al (2018) Pharmacokinetics/pharmacodynamics of systemically administered polymyxin B against Klebsiella pneumoniae in mouse thigh and lung infection models. J Antimicrob Chemother 73:462–468
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Li, J. (2019). Reviving Polymyxins: Achievements, Lessons and the Road Ahead. In: Li, J., Nation, R., Kaye, K. (eds) Polymyxin Antibiotics: From Laboratory Bench to Bedside. Advances in Experimental Medicine and Biology, vol 1145. Springer, Cham. https://doi.org/10.1007/978-3-030-16373-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-16373-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16371-6
Online ISBN: 978-3-030-16373-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)