Abstract
The study aims to present advances made by the academia in terms of multidisciplinary work among groups formed by industrial designers, industrial engineers, physiotherapists, and physicians, related to a University Hospital in a local environment in order to consolidate a collaborative strategy that allows the development of specific medical devices. Methodology A product portfolio consolidated by surgical devices and lower limb prostheses was the outcome of undergraduate projects, master and medical-surgical specialization projects working together. The baseline of surgical devices contains virtual pre-planning, biomodels, surgical guides, and implants according to requirements from different anatomical areas, predominantly skull and knee treatments. The baseline of lower limb prostheses presents cases developed and tested with users who had transtibial or transfemoral unilateral amputation. Results As the number of actors who shared data and limited resources increased, a gradual implementation of PLM strategy was established by building collaborative databases based on an established conceptual framework proposed by previous tool selection, so that the roles for project execution were defined in terms of access according to the role. To achieve comprehension among participants, a visualization model was adapted to involve workflows, roles, capabilities, and resources. Several data were collected from study cases to be stored and retrieved for further development according to stage development, understanding time and resources implemented to respond to a short period request when schedule uncertainties demand those requirements. Regardless of those results, the further project needs biocompatible materials as well as machines capable of transforming this raw material in order to achieve high-quality standards.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Technological changing promoted by the digital era has merged with the real world, integrating physical and biological systems. This situation has created opportunities to alter the shape and reality of the environment around us. Our reality has been built by new materials studies that apply to personalized issues and bioprinting, redefining the way of conceiving processes, products, and value creation according to the World Economic Forum [1].
Specific cases are related to the development of orthopedic surgical medical devices. Instead of the traditional approach of mass standard device production, some products have emerged from new manufacturing concepts such as flexible factories [2] and Direct Digital Manufacturing (DDM) [3]. These advances have granted process flexibility for developing Medical Devices (MD) obtained via 3D printing such as Patient-Specific Implants (PSI) [4], surgical guides for cutting, guiding or drilling, 3D printing from skin cells for tissue replacement and even printing organs. Those are pieces of evidence about the way how technological change has impacted on the new medical devices conception [5]. It was by far demonstrated that Medical Devices have been effective for patient functional recovery and also improvement in health professional performance [6].
The development of these Medical Devices requires the integration of a large amount of data that must be kept updated and traced through the process. Those systems are heterogeneous and allow the exchange of data between different roles, processes, communication tools and digital visualization apps [7]. Healthcare organizations face new challenges that are derived from a greater focus on controlling health system costs regarding increased expectations on treatment efficiency and personalized medicine [8]. It must be oriented to create and improve value throughout collaborative strategies for the organization and patients [9]. A correct strategy must integrate the patient’s profitability with successful care. Therefore, financial success would be a desirable consequence instead of the most relevant strategy in healthcare treatment [10].
In the light of this approach, it was identified that implementing product lifecycle management strategy PLM grants value creation [9]. The studies on literature review showed positive results to PLM implementation by improving processes acceleration [11] that allows reduction of information access time, number of errors, improvement of communication between actors and reduction of design time and product costs [12].
Just a few studies carried out on the PLM approach in the medical sector have been able to identify three intervention topics: implants, biomedical imaging and product portfolio in medical device companies. Likewise, it was possible to identify development issues from Medical Devices, mainly focused on information exchange and the relationship among roles [13]. Another difficulty reported has been obtaining relevant data for raw biomedical images [14], as well as handling the transmission of patients’ data between collaborators [15]. Finally, technology integration deals with different processes. From reverse engineering RE to computer-aided design CAD, computer-aided engineering CAE, rapid prototyping RP, computer-aided manufacturing CAM and other data files that must be integrated in order to ensure interoperability [16].
On the other hand, despite the fact that PLM strategy is known and implemented by automotive and aeronautics in countries with emerging economies since the 90s [17], the PLM strategy applied to the orthopedic medical sector is still uncertain and needs to be explored in more detail. To our knowledge, a few studies on PLM in Latin America in the health sector have been oriented to osteosynthesis implants [18,19,20], and no studies on socket development for lower limb disabilities have been found. However, in order to execute a management strategy, it was found that some researches approximate to the data control on early design stage in sockets for inferior members [21,22,23,24] and customized implants [13, 16].
Different authors recognize the importance of health technologies for value creation in surgical innovation to improve patients’ life quality within industries 4.0 framework, to configure a System of Systems based on CAx. Those technologies spin on design and manufacturing labor, supporting patient-centered activities, applying the principles of flexible manufacturing by using a PDM system integrating virtual technologies and low-cost 3D printing. Those isolated systems were selected by technology assessment. Based on these criteria, authors designed the PLM strategy in order to enhance the development of medical devices, articulating process areas, tasks, and roles with the technologies mentioned above.
The following sections describe the background, the methodology implemented to define the strategy and the procedure in which the PLM reference framework was established for surgical and prosthetic medical devices development. Finally, the results, discussion, and conclusions are presented.
1.1 Background
Almost 1.5 million different medical devices, a vast variety of artifacts, integrate Medical Devices [25]. Since 2002, the global market was calculated at US$14 billion [6], but four years later this value increased around US$260 billion [26]. In 2014, the orthopedic medical devices world market was estimated at US$375.2 million [27] from standard devices produced by countries such as Australia, Canada, The United States, The European Union, and Japan, which belong to The Global Harmonization Task Force GHTF. Comparatively, Europe was just the third worldwide market, which was led by the USA with the 50%, although emerging countries such as China and India have also risen [28]. In Latin America, Mexico and Brazil were the largest manufacturers of Medical Devices in the region [29].
Due to the fact that medical devices have been so profitable, they have had two drawbacks during exportation. First, medical devices in developed countries are not suitable for contextual, anthropometric and epidemiological needs for the Third World population [26]. Second, technological dependence and weak regulations in developing countries had already opened the door to adulterated and even degraded products [30].
In contrast to this trend, personalization in product development [31] is a correct path to generate solutions that correspond to patients’ real needs, applying technologies to value creation in clinical practice [32]. The technologies involved in medical devices development come from the integration of architecture systems defined by the inclusion of Reverse Engineering RE [33] or CAD systems [34]. These systems implement segmentation techniques such as tomography to obtain soft tissue or hard tissue virtual models as bone geometries; or by point cloud techniques to generate the use of a light scanner or contact reconstruction. These systems integrate the application of virtual CAD technologies for modeling, CAE for evaluation by simulation, 3DP printing [35] or CAM for the fabrication of final devices [36].
Recent studies on new technologic products for specific patients apply RE based on image segmentation technique to create implants adapted to bone geometry for reduction or restitution of complex fractures in: skull [37], jaw [38], spine [39], knee [40], ankle [41], elbow [42] and shoulder [43]. Apart from implants, other related technologies have also been developed as temporary guides to assist surgeries [44], virtual pre-planning [45] and use of physical Biomodels [46]. At the same time, the application of RE by point cloud has allowed the generation of reference models from residual limbs that serve as a reference for the design of customized prostheses for lower limb amputation [47] and upper limb, [48] using virtual technologies and additive manufacturing for socket generation.
Despite the successful evidenced applications, there were identified three drawbacks to achieve technology implementation for medical devices. The first one is related to equipment and specialized software cost. Although different tools have been proposed, communication difficulties between reverse engineering activity and CAD/CAE software remain [22]. These difficulties arise due to the investment limitation on commercial PDM licensed from robust use, as well protection and migration of data [49]. The second drawback refers to the complexity represented by the development of these devices, corresponding to compliance with regulatory requirements, clinical requirements, established monitoring processes and treatment [50]. Finally, insufficient knowledge management causes the third problem, since specialized processes depend on tacit knowledge that only few people have.
Based on the previous approach, the health sector requires solutions focused on creating robust and reliable design methods, which involve flexible manufacturing articulated with low-cost systems. That purpose could be attained by means to defining workflows that allow a reliable implementation of virtual technologies [51], oriented towards compliance by quality, safety and efficacy criteria. Not less important is to guarantee that knowledge is transferable and becomes explicit by routines and practices from organizational knowledge, to guide and supervise each role performance during the lifecycle phases of the process development [23].
2 Methodology
A conceptual framework was established based on the PLM model visualization, and Martinez’s definition of process areas involved in each phase [52]. Strategy construction methodology was designed as established by Stark [53], since the design of the strategy aims to meet the user’s expectations quickly and sustainably. The implementation objective was defined on the basis of the need to organize the basic data related to the product portfolio, as aimed by Schuh et al. [54]. Thus, goals were defined accordingly as follows. On the first stage, two objectives that brought together the key activities to carry out PLM strategy implementation were defined. Namely, the aims were:
-
To define a System of System (SoS) or an integration model related to flexible manufacturing technologies based on low-cost health techniques to obtain surgical Medical Devices (MD) and orthopedic prosthetics MD for specific patients.
-
To define a reference framework that integrates the stages and process areas with technologies, establishing practices and diagnostic tools, process protocols and quality verification for results generated in each case in order to contribute with the fulfillment of the quality criteria in the processes associated to the stages of ideation, definition and implementation, by means of case studies.
The fulfillment of first objective was executed by means of two stages. The first one was concerned about the strategy configuration, for which was to be required to define the software architecture to be able for developing medical devices. In accordance with the SoS theory, RE-CAx-3DP systems were defined, selecting potential technologies to support health software as well as low-cost hardware. From this study, the inputs and outputs of each system were defined as measurable milestones throughout the entire workflow [55]. The measurement of performance and interoperability between technologies were identified. To do this, user requirements were defined on software and hardware capabilities such as file weight, virtual volume, final part weight, virtual and physical processing times, affordability and compatibility of CAx file formats [56, 57]. The definition of the types of technologies was conducted while projects were oriented and addressed to medical devices design, which allowed defining activities by stages and alternatives to support RE-CAx-3DP architecture.
In the second stage, requirements were established to configure data management and government system. Like the SoS from low-cost RE-CAx-3DP, this system was made up with PDM low-cost product data management platforms. Three sorts of data were set up to frame the PDM. First, the file storage of RE-CAx-3DP that is a primary line to support the product development process. Second, for documents concerned to process management that allows traceability to be carried out in terms of progress, quality and compliance with product requirements in accordance with the operational line advance. Third, supporting materials for training and research such as catalogs, tutorial videos and design guides. These three types of data were the ones required to administer in any case study.
According to the second objective and based on the model established by Martinez [52], the SoS was articulated in a conceptual framework of the PLM strategy, delimiting the development process to the first three stages of the product life cycle: ideation, definition and implementation. The process areas derived from conceptual framework guidelines are fuzzily involved in different lifecycle stage. We proceeded to define a reference framework to adjust a PLM strategy to medical devices that was supported by the development of research projects for undergraduate, masters and doctoral studies that contribute to do more research and configure both SoS, for RE-CAx-3DP and for cloud storage and documentation. On this strategy, workflows, roles, activities, and tools were integrated within the strategy.
Finally, a strategy was defined for two kinds of cases, surgical medical device and a prosthetic medical device. Those involve flexible manufacturing technologies and SoS, RE-CAx-3DP and Storage documentation by low-cost resources. The strategy incorporated workflows, stages, process areas implemented, actors involved and sort of information generated through product development.
3 Results
This section describes the main results related to the guidelines for the PDM platform, the selection of technologies for the SoS RE CAx 3DP definition, the conceptual framework of the PLM strategy, the description of case studies and the PLM strategy of health technologies for the development of orthopedic medical devices.
3.1 Guidelines to Build a PDM Framework
The four areas proposed by Schuh et al. [54] were considered for the configuration of the strategy: data management, basic product data management, project data management, business administration and system integration. The first area is product data management, which involves tools for the storage of source files, planning, hierarchy, information coding, document creation and editing, file management and change and configuration management. The selection of the platform for information management required the identification of the capabilities of the system. It was established that the platform should first allow the administration of the technical and operational information associated with the device development process, as opposed to the storage of DICOM formats and the generation of CAx files: RE CAD CAE RP [58]. Second, the platform had to include office tools for the creation and edition of documents for administrative processes and quality management. Third, the platform should allow the storage and administration of audiovisual material, created for learning processes [59].
The second one is the project management area, where formats were organized through planning tools, quality assessment and maintenance support documentation. The third area, business management, is related to the management of multiple projects, tracking and document backup, change control, process performance indicators (time, cost, quality), access levels and workflow management. The fourth area, collaboration and integration, concatenating each system into a single storage and data exchange interface. Since commercial PDMs of a robust type require a considerable investment [60], it was decided to evaluate isolated technologies that in an integrated version make up a SoS with the capacity to support the basic features described above [61].
3.2 Technology Selection
A matrix of capabilities of software tools was generated in relation to the type of software and possible actions to be carried out. This matrix was built for the preliminary selection of software tools, to be used in the configuration of the software architecture in order to develop medical devices and PDM platform setting oriented to storage, configuration and changes of files derived from process development, data management and knowledge transfer.
Regarding the actions required to perform in these software, criteria such as file editing, CAD volume visualization, storage, office documents edition and data management creation of CAx files for product development processes are described in Fig. 1. Based on this matrix, it was identified software with greater capacity to respond to the requirements. We identified public software that allows us to perform 5 actions regarding the 6 requirements; however, information is uploaded to a cloud service. This is followed by educational software that allows performing 4 types of actions in respect of the file size, the learning cave, its cost, its accuracy, availability of supporting material and data security; these are the six requirements proposed. This procedure can be carried out once the software typology, the selected systems and typology of low-cost integrated software tools have been identified.
3.3 Strategy Conceptual Framework
The main requirement for the development of these devices is to respond to the need to generate personalized products, tailored to the patient. This feature has implications such as order of production by customer demand and generation of production requirements based on the clinical case typology. Consequently, the development of each product generates a geometry adjusted to a specific patient and product.
The way these products should be developed requires the definition of a flexible manufacturing model, appropriating the use of technologies that can be implemented in the health area. In accordance with the above, since the conception of 4.0 industries in the integration of the virtual world materialized physically by 3D printing manufacturing techniques, a conceptual framework for the construction of the PLM strategy could be configured. The visualization model proposed by Martínez [52] was taken as reference due to its contribution in the definition of process areas involved in the development of new CAD-CAM products and the correspondence of this visualization model with regard to the development process to obtain a variety of products through SoS RE-CAx-3DP.
Figure 2 describes the conceptual framework of product development processes according to the process areas and the general workflow involved. In addition, it entails the definition of two parallel SoS that respond to the capabilities requested by user requirements, which are the platform related to document editing and storage, and the RE-CAx-3Dp system for the project definition and implementation according to the needs of the multidisciplinary team.
According to Fig. 2, the process areas and the stages of the product lifecycle generate a matrix in which SoS are included. Based on this structure, the workflow of the process is configured. It starts in Product Marketing area, where the service request is made. By approval, it is then taken by Product Requirement, where the product specifications of the portfolio to be developed are defined.
Then, in the Product Design, Product Production and Product Testing areas, the software tools defined in the SoS RE-CAx-3DP are distributed to get the product requested. Meanwhile, the work plan is defined from the Project Management area and consists of assigning roles, tasks and quality formats to meet requirements such as delivery times and availability of resources. In the Configuration and Change area, storage, management, and change management are formalized according to the client’s request or the assigned roles. Finally, the Marketing area receives the result generated in the Production Product area in order to deliver it to the client, the one who provides lifecycle feedback.
3.4 Case Studies
3.4.1 Technologies for Specific Patients
The development of different projects was made according to the relevant area of knowledge for the development of medical devices. Different multidisciplinary teams were formed between Industrial Designers, Physiotherapists and Surgeons. This synergy allowed the exchange of technical knowledge and facilitated access to clinical information, as well as the opportunity to generate solutions on real cases and situations, based on clinical cases of patients with pathologies of congenital, trauma or oncological origin.
Ethical principles practices were defined and carried out, as well as precautions such as the codification of the information to maintain the patient’s data anonymously were taken, as established by the ethics committees. Once the level of information security was guaranteed and the interaction roles between the actors involved in the device development processes were defined, the data were shared through public platforms, editing and 3D visualization. The working groups were divided into those responsible for specific medical devices projects and those in charge of lower limb prosthetic devices.
Figure 3 describes the main workflow with digital manufacturing defined for the development of Patient-Specific Implant PSI. The process was carried out through data acquisition via Computer Tomography CT established as input data. A reverse engineering process was performed to obtain a virtual reference model. Subsequently, once the specifications of the clinical case by the specialist surgeon are defined, the list of requirements is stablished and the PSI is modeled in a CAD model. Virtual simulation tests are performed in CAE by Finite Element Analysis FEM and finally, the biomodels and PSI are taken to 3D printing.
A pilot study was carried out and structured in two stages. In the first stage, diagnostic and planning cases were developed. In this phase, clinical cases are addressed for diagnosis of tibial plateau fractures, pre-surgical planning for craniosynostosis, orbital-malar region trauma, PSI design process for cranioplasty, reduction of type B hemipelvis fracture or replacement of mandibular edentulous areas. In the second stage, cases were submitted on verification processes by geometry matching between device and tissue, namely a surgical approach of LeFort 1 type to reduce cleft lip LPH sequelae and maxillary retrognathia, segmental mandibulectomy for reduction of sequelae of mandibular fracture, multiple reductions in pseudoarthrosis and severe facial trauma.
There were 10 cases of skull and face trauma, 2 of jaw, 1 of hip damage, 22 of knee and 1 case for dental PSI. Derived from these cases, according to the service requirement definition, four kinds of products were generated. Virtual biomodels that were used as a reference for diagnosis in order to define surgical pre-planning processes. In complex cases, the decision-making process was also supported by pre-surgical or post-surgical physical biomodels, the design of PSI to replace blemished zones and the design of template guides to assist activities such as cutting, drilling, and repositioning a bone tissue during surgical activity. All the obtained 3DP virtual products were developed on a natural scale, and the scope of the results allowed them to be implemented in surgery.
3.4.2 Lower Limb Prosthesis
Another type of tailored medical devices is the lower limb prostheses. This device works as a support structure to replace the amputated anatomical region, allowing the rehabilitation process to recover its ability to walk [62]. These devices are made up of the socket, the cane, the ankle and the foot [63]. The socket is the main and most important component of the prosthesis since it is the interface between the stump and the prosthesis and therefore, it must be adjusted to the stump anatomy [64]. Problems in the development and manufacture of the sockets obtained by the traditional technique have been identified in the literature; these problems are associated with factors such as development time [65,66,67], information management [24, 68,69,70] and product quality [24, 66, 70].
A process based on digital manufacturing, is a SoS RE CAx 3DP, was proposed in the framework of the PLM strategy. Figure 4 describes the process flow defined for the development of lower limb sockets. This process starts with reverse engineering obtaining input data by means of a 3D scanner for the generation of the virtual reference model of the stump.
A process based on digital manufacturing was proposed, that is a SoS RE CAx 3DP, within the PLM strategy framework. Figure 4 describes the workflow defined for lower limb sockets development. This process starts with reverse engineering, obtaining input data through the 3D scanner as a virtual stump reference model. Subsequently, the list of requirements are defined based on prosthetic technician’s specifications and a traditional mold definition technique was performed by emulation. Thus, the socket was modeled in CAD software based on this reference model. Finally, the sockets are taken to 3D printing. To carry out the verification of the socket, adjustment and walking testing on the patient must be done.
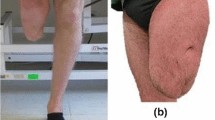
There were 4 cases of sockets for lower limb, patients with amputations due to accidents and victims of war. It was supported by the treatment of a case of a female patient that required a transfemoral socket of quadrilateral type. The remaining 3 cases presented in adult patients requested transtibial sockets of type PTB and KBM. These devices were assembled with standard mechanical components according to the designation of an orthopedist and the concept of the prosthetist. The sockets were prototyped in 3D printing. In the framework of the pilot study, the lace tests and the walking tests were performed, obtaining satisfactory results from the first iteration.
During the pilot study, it was possible to verify the applicability of the conceptual framework for the PLM strategy proposed in Fig. 2. According to this framework, each case was developed from specific requirements in order to achieve a suitable product. Different formats were created for the evaluation and verification of the outputs of each activity, since in socket design it is necessary to verify patient measures. This procedure was implemented in order to manage all the information generated and control each one of the activities.
3.5 The Proposed Strategy
The strategy reference frame was defined relying on the experiences in the case studies described in the previous section. The development of this research was based on Martinez’s visualization model [52] and the general process detailed by Ngo et al. [13] represented in the framework conceptual of Fig. 2. The strategy was concentrated in the first three stages of the product life cycle, establishing workflow for the different specific medical devices, organizing the type and level of interaction of the different roles according to the active process areas in the cycle development stage. The structure flexibility of the strategy facilitated the development of the two types of cases: lower limb sockets and specific medical devices [71].
Figure 5 shows that the different types of products (PSI and sockets) are guided by a common general workflow. However, there are differences that lie in the inputs and outputs that the products generate in the topological data acquisition activities and in the intervention activity with the specific medical device. According to each activity, a responsible role must interact with other roles to obtain or verify the information needed to achieve each task. Those roles can be internal and external roles to the organization and intervene in different stages of the life cycle of the product. Patient data were derived from interactions. These data should be collected and safeguarded as they are shared among the different areas of the process. In this way, the pieces of information are centralized in a database that acts as a repository and a visualizer.
All the information generated and shared in each activity is managed, stored and shared through the use of a web platform that permits different actors to be able to edit or visualize the data according to their role. The technologies implemented and integrated in the SoS RE-CAx-3DP are some tools of public access, free software, or licensed for educational and demonstrative purposes, which has allowed the consolidation of an integration model that evolves as open type tools are developed and adopted. This is evidenced in Figs. 1 and 2.
Although a general PLM strategy was proposed, different factors influenced the generation of operational differences to obtain the products and the respective results. Table 1 shows comparisons between two case types according to the process stages in which the SoS RE-CAx-3DP are involved. The differences and similarities were identified through four main components: actors involved, technologies, generated information and verification formats in each stage.
The reconstruction stage contains differences between the 4 components. It was observed that in terms of sockets, the patient and the prosthetist technician are present in order to obtain data by means of 3D scanner technology. On the other hand, the designer obtains patient’s data from computer tomographies or DICOM images in specific medical devices. Verification by simulation could be another example. In the case of implants, it is carried out by Finite Element Methods, but in the case of sockets, the evaluation protocol has not yet been implemented. The similarities presented in the two cases were found in the stage of modeling in CAD and 3D printing, in which the role component does not exist since it is carried out by an industrial designer in both cases.
It was possible to verify the applicability of the conceptual PLM strategy framework proposed in Fig. 2 throughout the pilot study. In accordance with this framework, each project was developed from case requirements to obtain a product.
For that purpose, different requests were received: definition of diagnostic protocols using virtual biomodels for pre-planning and complexity analysis for surgical procedure, the design of PSI to evaluate biomechanical behavior by simulation. Furthermore, there were personalized surgical guides for cutting, drilling, or positioning in order to verify the capability to generate precise devices and therefore evaluate the fit in the bone tissue during the surgical procedure. However, 3D virtual reference biomodels were obtained regardless of the type of request in all cases and virtual surgical pre-planning was carried out according to the surgical technique selected for treatment implementation.
4 Discussion and Conclusions
In this chapter of the book, it was evidenced how it was possible to build a PLM strategy integrating a digital manufacturing SoS for production in flexible factory contexts. In fact, the development of the devices through digital manufacturing was selected regarding the advantages presented by Jones in relation to: the decrease in the cost of the additive manufacturing machinery, going from the industrial sector to the desktop, the release of designs and open source programs RepRap for manufacturing by using fused deposition modelling, and the connectivity that makes the design, modification and exchange of virtual information possible [72]. Thereby, the final products can be manufactured anywhere, according to the user’s requirements, obtaining a specific final product [36].
Hence, in the development of a complex product such as custom medical devices, the process involves the exchange of ideas from multiple sources, digital data volumes and decisions that require administration [73]. In addition to collaboration between distanced actors, different organizations try to configure their value proposition to be more efficient when solving problems, using fewer resources, implementing new technologies for product development and administration [74]. That is why industries 4.0 seek to digitize the supply chain that provides personalized treatments to the patient in the health sector [75], taking advantage of additive manufacturing such as reduction in distances, production times, material consumption, energy consumption and part complexity [76].
In the present study, the evaluation of technologies allowed the identification and selection of technological tools, following the vision proposed by Schuh et al. [54], on the contribution of technologies to the PLM implementation objective, considering the processes, roles, availability and centralization of product information [77] and the activities required to be performed, the control of engineering and quality requirements [78], the complexity and technological maturity of the prototype or final product. In accordance with the above, when considering the importance of implementation costs, the low-cost strategy is highly relevant for countries with emerging economies, as well as the reduction of development times and costs to guarantee applicability, especially in the initial stages of development, where the organization has more control [79].
The authors’ strategy is based on a close communication with the specialists generating interactions and solutions in co-creation, involving the health expert, as an actor who is part of the solution. This strategy is related to the model proposed in this book chapter, unlike Zdravković et al. [16] who articulated a network of providers between the clinical system, the common or pre-planning system, the design system and the manufacturing system specialized by request.
The decisions made regarding the definition of process areas, workflow, technologies, roles and defined tools to guarantee the control of the results and obtain precise products, show that low cost PLM strategy built for the development of these devices in specific patients and prostheses, meet the expectations. However, as Allanic et al. [14] and Pham et al. [15] proposed, when given the volume of cases developed up to now as shown in Table 1, it is necessary to continue working to increase the PDM capacity in order to generate greater control in the interoperability between roles and the data generated. So that with the proposed base strategy, it is possible to achieve higher levels of maturity and control strategy processes in relation to the performance of the roles involved, amount of information, information custody and data traceability generated during the process.
The implementation of the PLM as a strategy based on the visualization model proposed by Martínez [52], facilitated the definition and detailed organization of the operational guidelines, both, process and information flow. This action involves functional departments, activities, inputs and outputs. This is how the activities were distributed [20] within 7 of the 8 visualization model processing areas. However, four of these process areas are: Product Requirements, where the guidelines for the device design were established; Product Design, where these parameters were converted into virtual or physical objects; Production Area, where the products were obtained in 3DP; and Testing Area, in which the fulfillment of requirements was verified by means of different tests. These were the areas of higher level of development in the current strategy defined by the authors to guarantee the development of the precise medical devices.
Nonetheless, other process areas were also intervened. From Marketing, a proposal is prepared to delimit the scopes, delivery times and products requested, also monitoring product satisfaction from a consumer. The Management Area is responsible for synchronizing activities among the execution of different projects, while the Configuration and Changes Area coordinate many change requests during the product development from different sources. These previous departments functioned as Cross-Cutting Areas and assumed a key role in the management of compliance with requests at an appropriate development time. Mainly in the area of configuration and change processes, from the defined low-cost SoS, the storage, management, evolution, and traceability of the project data were guaranteed in a safe manner to provide information integrity and personal data anonymization from medical records.
The digitization of information and the use of 3D printing tools tie in with the concept of Industry 4.0, with PLM defined as the strategy to orderly consolidate the required procedures according to the product profile. The way in which the cases were supported by the resources shared between the design, engineering and medicine teams within the framework of the PLM strategy, allowed obtaining specific medical devices and precise sockets according to clinical requirements. The previous statement is supported by the results obtained in the second stage of pilot studies. The tests implemented in surgery were the evidence of satisfactory results. These tests verified if the implants and the guides fitted in during the surgical procedure. On the other hand, there were socket and walking tests for the case of the amputations, which meant good results as well.
Further projects require to strengthen manufacturing capabilities to comply regulatory procedures that permit to obtain end-user medical devices. It is necessary to continue exploring low-cost software alternatives for obtaining or programming a centralized PDM, in accordance with the needs of surgeons, orthopedists, prosthetists, designers, and engineers. In this way, the process performance in the product development could be controlled, obtaining key performance indicators while different case studies are carried out. Similarly, it is important to address the observations of Ahmed, et al. [80] on collaborative work and linking in hospital centers through PDM platforms and PLM strategies. This could improve articulation and coordination to access and monitor data from patient treatment, in a way that it facilitates decision making in real time with respect to device design requirements.
References
WEF (2018) Key issues of the global agenda. Design for manufacturing by addition. World Economic Forum, 2017. Available: https://toplink.weforum.org/knowledge/insight/a1Gb0000001k6I5EAI/explore/dimension/a1Gb0000004481zEAA/summary. Accessed 18 Apr 2018
Theorin A et al (2017) An event-driven manufacturing information system architecture for Industry 4.0. Int J Prod Res 55(5):1297–1311
Chen D, Heyer S, Ibbotson S, Salonitis K, Steingrímsson JG, Thiede S (2015) Direct digital manufacturing: Definition, evolution, and sustainability implications. J Clean Prod 107:615–625
Rotaru H et al (2012) Cranioplasty with custom-made implants: analyzing the cases of 10 patients. J Oral Maxillofac Surg 70(2):e169–e176
Vyas D, Udyawar D (2018) A review on current state of art of bioprinting. In: 3D printing and additive manufacturing technologies. Springer, Singapore, pp 195–201
Long PH (2008) Medical devices in orthopedic applications. Toxicol Pathol 36(1):85–91
Lantada AD, Morgado PL (2013) Introduction to modern product development. In: Handbook on advanced design and manufacturing technologies for biomedical devices. Springer International, pp 1–17
Nilsson S, Ritzén S (2014) Exploring the use of innovation performance measurement to build innovation capability in a medical device company. Creat Innov Manag 23(2):183–198
Walton ALJ, Grieves MW, Sandall DL, Breault ML (2016) Product lifecycle management for digital transformation of industries. In: IFIP international conference on product lifecycle management, vol 492, pp 569–578
Porter ME, Teisberg EO (2004) Redefining competition in health care. Harv Bus Rev, p 14
Brandao R, Wynn M (2008) Product lifecycle management systems and business process improvement. A report on case product lifecycle management systems and business process improvement—a report on case study research. In: Third international multi-conference on computing in the global information technology ICCGI
Martínez-caro E, Campuzano-bolarín F, Villaescusa-chocano JA (2011) Improving product development from a knowledge management based approach. The case of Navantia. Dyna 86(6):699–707
Ngo T, Belkadi F, Bernard A (2017) Applying PLM approach for supporting collaborations in medical sector: case of prosthesis implantation. Adv Mech Des Eng Manuf 871–878
Allanic M, Hervé P-Y, Durupt A, Joliot M, Boutinaud P, Eynard B (2015) PLM as a strategy for the management of heterogeneous information in bio-medical imaging field. Int J Inf Technol Manag 1:5–30 (in press)
Pham CC et al (2016) How to share complex data and knowledge: application in bio-imaging. IFAC-PapersOnLine 49(12):1098–1103
Zdravković MM, Stojković MS, Mišić DT, Trajanović MD (2012) Towards semantic interoperability framework for custom orthopaedic implants manufacturing. IFAC Proc Vol 14(Part 1):1327–1332
Mora-Orozco J, Guarín-Grisales Á, Sauza-Bedolla J, D’Antonio G, Chiabert P (2016) PLM in a didactic environment: the path to smart factory, pp 640–648
Garro F (2016) Proposed implementation of the product lifecycle management system in the development of medical devices. Universidad Nacional de Córdoba
Murillo AB, López CG, Gómez JM (2017) Product lifecycle management and the industry of the future. IFIP Int Fed Inf Process 517:231–240
Ardila CC, López CI, Martínez JM, Meléndez GL, Navarro DC, Galeano CF (2018) Study for development of a patient-specific 3D printed craniofacial medical device: design based on 3D virtual biomodels/CAD/RP. Procedia CIRP 70:235–240
Hsu LH, Huang GF, Lu CT, Hong DY, Liu SH (2010) The development of a rapid prototyping prosthetic socket coated with a resin layer for transtibial amputees. Prosthet Orthot Int 34(1):37–45
Colombo G, Filippi S, Rizzi C, Rotini F (2010) A new design paradigm for the development of custom-fit soft sockets for lower limb prostheses. Comput Ind 61(6):513–523
Gabbiadirii S, Colombo G, Facoetti G, Rizzi C (2009) Knowledge management and customised 3D modelling to improve prosthesis design. In: ASME International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, DETC2009, vol 2, no. PART A, pp 625–633
Herbert N, Simpson D, Spence WD, Ion W (2005) A preliminary investigation into the development of 3-D printing of prosthetic sockets. J Rehabil Res Dev 42(2):141
WHO (2010) Medical devices: managing the mismatch. An outcome to the priority medical devices project, Geneva
WHO (2003) Medical device regulation. Global overview and guiding principles, vol E2440.001, Geneva
Evaluate (2015) Evaluate MedTech. World Preview 2015, Outlook to 2020
Maresova P, Penhaker M, Selamat A, Kuca K (2015) The potential of medical device industry in technological and economical context. Ther Clin Risk Manag 11:1505–1514
Torres D, López D, Sandria JC, Hermosillo GC, Rubio R, Zurita MA (2012) Medical products cluster from Baja California, Nov 2016. Universidad Privada en Puebla de Zaragoza, p 1.21
Glass BBD (2014) Counterfeit drugs and medical devices in developing countries. Res Rep Trop Med 2014(5):11–22
Watson JK, Taminger KMB (2018) A decision-support model for selecting additive manufacturing versus subtractive manufacturing based on energy consumption. J Clean Prod 176:1316–1322
Frow P, McColl-Kennedy JR, Payne A (2016) Co-creation practices: their role in shaping a health care ecosystem. Ind Mark Manag 56:24–39
Bruns J, Habermann CR, Rüther W, Delling D (2010) The use of CT derived solid modelling of the pelvis in planning cancer resections. Eur J Surg Oncol 36(6):594–598
Senalp AZ, Kayabasi O, Kurtaran H (2007) Static, dynamic and fatigue behavior of newly designed stem shapes for hip prosthesis using finite element analysis. Mater Des 28(5):1577–1583
ASTM (2015) Standard terminology for additive manufacturing—general principles—terminology, vol i. ASTM, pp 1–9
Gibson I, Rosen D, Stucker B (2015) Additive manufacturing technologies, 2nd edn. Springer, New York
Moiduddin K, Darwish S, Al-Ahmari A, ElWatidy S, Mohammad A, Ameen W (2017) Structural and mechanical characterization of custom design cranial implant created using additive manufacturing. Electron J Biotechnol 29:22–31
Thieringer FM, Sharma N, Mootien A (2018) Patient specific implants from a 3D printer—an innovative manufacturing process for custom PEEK implants in cranio-maxillofacial surgery. In: Industrializing additive manufacturing—proceedings of additive manufacturing in products and applications—AMPA2017, vol 2
Provaggi E, Leong JJH, Kalaskar DM (2017) Applications of 3D printing in the management of severe spinal conditions. Proc Inst Mech Eng Part H J Eng Med 231(6):471–486
Zarychta P (2017) A new approach to knee joint arthroplasty. Comput Med Imaging Graph
Weigelt L, Fürnstahl P, Hirsiger S, Vlachopoulos L, Espinosa N, Wirth SH (2017) Three-dimensional correction of complex ankle deformities with computer-assisted planning and patient-specific surgical guides. J Foot Ankle Surg 56(6):1158–1164
Zheng W et al (2017) Application of 3D printing technology in the treatment of humeral intercondylar fractures. Orthop Traumatol Surg Res
Deore VT, Griffiths E, Monga P (2017) Shoulder arthroplasty—past, present and future. J Arthrosc Jt Surg 1–6
Numajiri T, Nakamura H, Sowa Y, Nishino K (2016) Low-cost design and manufacturing of surgical guides for mandibular reconstruction using a fibula. Plast Reconstr Surg Glob Open 4(7):e805
Gandedkar NH, Chieh K, Kiat C (2017) Recent advances in orthognathic surgery diagnosis and management: 3D image acquisition, virtual surgical planning, rapid prototyping, and seamless surgical navigation. J Contemp Orthod 1(2):12–19
Ripley B et al (2017) 3D printing from MRI data: harnessing strengths and minimizing weaknesses. J Magn Reson Imaging 45(3):635–645
Paterno L, Ibrahimi M, Gruppioni E, Menciassi A, Ricotti L (2018) Sockets for limb prostheses: a review of existing technologies and open challenges. IEEE Trans Biomed Eng 0018
Mounika MP, Phanisankar SS, Manoj M (2017) Design & analysis of prosthetic hand with EMG technology in 3-D printing machine. Int J Curr Eng Technol 77(11):2277–4106
Bedolla JS, Ricci F, Gomez JM, Chiabert P (2013) A tool to support PLM teaching in universities. In: IFIP international conference on product lifecycle management, pp 510–519
Wassyng A et al (2015) Can product-specific assurance case templates be used as medical device standards? IEEE Des Test 32(5):45–55
Comotti C, Regazzoni D, Rizzi C, Vitali A (2017) Additive manufacturing to advance functional design: an application in the medical field. J Comput Inf Sci Eng 17(3):031006
Martínez J (2013) Visualization model for product lifecycle management: processes, activities, roles and items involved in product lifecycle. Politecnico di Torino
Stark J (2016) Product lifecycle management, vol 2, 3rd edn. Springer International, Geneva
Schuh G, Rozenfeld H, Assmus D, Zancul E (2008) Process oriented framework to support PLM implementation. Comput Ind 59(2–3):210–218
Meilich A (2006) System of systems (SoS) engineering & architecture challenges in a net centric environment. In: IEEE/SMC international conference on system of systems engineering, Apr 2006, pp 1–5
Castro GP (2014) Evaluation of a software tools integration model aimed at the biomedical-orthopedic sector. Universidad Industrial de Santander
Bermudez J (2017) Reference practices for the process of ideation and development of new custom orthopedic products. A case study of craniofacial trauma. Universidad Industrial de Santander
Wu D, Terpenny J, Schaefer D (2017) Digital design and manufacturing on the cloud: a review of software and services. Artif Intell Eng Des Anal Manuf 31(01):104–118
Akter M, Gani A, Rahman MO, Hassan MM, Almogren A, Ahmad S (2018) Performance analysis of personal cloud storage services for mobile multimedia health record management. IEEE Access 6:1
Enríquez JG, Sánchez-Begines JM, Domínguez-Mayo FJ, García-García JA, Escalona MJ (2019) An approach to characterize and evaluate the quality of product lifecycle management software systems. Comput Stand Interfaces 61:77–88
Chiabert P, Lombardi F, Martinez Gomez J, Sauza Bedolla J (2012) Visualization model for product lifecycle management. In: Proceedings of international scientific conference management of technology step to sustainable production—MOTSP 2012, pp 109–117
Shuxian Z, Wanhua Z, Bingheng L (2005) 3D reconstruction of the structure of a residual limb for customising the design of a prosthetic socket. Med Eng Phys 27(1):67–74
Laing S, Lee PV, Goh JC (2011) Engineering a trans-tibial prosthetic socket for the lower limb amputee. Ann Acad Med Singapore 40(5):252–259
Buzzi M, Colombo G, Facoetti G, Gabbiadini S, Rizzi C (2012) 3D modelling and knowledge: tools to automate prosthesis development process. Int J Interact Des Manuf 6(1):41–53
Vitali A, Rizzi C, Regazzoni D (2017) Design and additive manufacturing of lower limb prosthetic socket. In: Proceedings of ASME 2017 International Mechanical Engineering Congress and Exposition, pp 1–7
Doubrovski EL, Tsai EY, Dikovsky D, Geraedts JMP, Herr H, Oxman N (2015) Voxel-based fabrication through material property mapping: a design method for bitmap printing. CAD Comput Aided Des 60:3–13
Dickinson AS, Steer JW, Woods CJ, Worsley PR (2016) Registering a methodology for imaging and analysis of residual-limb shape after transtibial amputation. J Rehabil Res Dev 53(2):207–218
Sengeh DM, Herr H (2013) A variable-impedance prosthetic socket for a transtibial amputee designed from magnetic resonance imaging data. J Prosthet Orthot 25(3):129–137
Colombo G, Facoetti G, Rizzi C (2013) A digital patient for computer-aided prosthesis design. Interface Focus 3(2):20120082
Sanders JE, Severance MR, Allyn KJ (2012) Computer-socket manufacturing error: how much before it is clinically apparent? J Rehabil Res Dev 49(4):567
FDA (2018) Medical application on 3D printing. Available: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/3DPrintingofMedicalDevices/ucm500539.htm. Accessed 05 May 2018
Jones R et al (2011) Reprap—the replicating rapid prototyper. Robotica (Special issue) 29(1):177–191
Thompson M et al (2016) Design for additive manufacturing: trends, opportunities, considerations, and constraints. CIRP Ann Manuf Technol 65:737–760
Radziwon A, Bilberg A, Bogers M, Madsen ES (2014) The smart factory: exploring adaptive and flexible manufacturing solutions. Procedia Eng 69:1184–1190
Branke J, Farid SS, Shah N (2016) Industry 4.0: a vision for personalized medicine supply chains? Cell Gene Ther. Insights 2(2):263–270
Ford S, Despeisse M (2016) Additive manufacturing and sustainability: an exploratory study of the advantages and challenges. J Clean Prod 137:1573–1587
Grieves MW, Tanniru M (2008) PLM, process, practice and provenance: knowledge provenance in support of business practices in product lifecycle management. Int J Prod Lifecycle Manag 3(1):37
Saaksvuori A, Immonen A (2008) Product lifecycle management, 3rd edn. Helsinki, Finland
Labbi O, Ouzizi L, Douimi M, Aoura Y (2016) A model to design a product and its extended supply chain integrating PLM (product lifecycle management) solution. Int J Sci Eng Res 7(10):1190–1205
Ahmed Z, Dandekar T, Majeed S (2012) ADAM: potential of PDM into clinical patient data management. Int J Emerg Sci 2:280–299
Acknowledgements
The authors wish to express their gratitude to the work team from research groups at Universidad Industrial de Santander: INTERFAZ at Industrial Design School, GRICES at Medical School, INNOTEC at Industrial and Business Studies School and the program of engineering doctorate at Electrical, Electronic and Telecommunications Engineering School for support on inner research projects that contributed to results generation. In addition, to the cooperation between COLCIENCIAS (Department of Science, Technology and Innovation) and the Government of Santander, who provided great support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Martínez Gómez, J.M., López Gualdrón, C.I., Murillo Bohórquez, A.P., Garnica Bohórquez, I. (2019). PLM Strategy for Developing Specific Medical Devices and Lower Limb Prosthesis at Healthcare Sector: Case Reports from the Academia. In: Stark, J. (eds) Product Lifecycle Management (Volume 4): The Case Studies. Decision Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-16134-7_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-16134-7_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16133-0
Online ISBN: 978-3-030-16134-7
eBook Packages: EngineeringEngineering (R0)