Abstract
The thyroid gland affects the development of a child at any age. A change in gland volume accompanies almost all thyroid pathologies in children. Inspection and palpation fail to assess the thyroid volume correctly in about 35% of cases. WHO proposed the international reference values for goiter screening presented as a function of age, sex, and body surface area. Congenital anomalies of the thyroid occur in <0.3–0.5% of the population. Thyroid size anomalies include aplasia (agenesis), hemiagenesis, and hypoplasia. Thyroid dystopia and ectopia are sonographically characterized by an absence of thyroid tissue at its typical location. Midline cysts of the neck are similar to dystopia in their pathogenesis. Failed obliteration of the thyroglossal duct during fetal thyroid migration results in the formation of an epithelial cavity with subsequent fluid accumulation. Diffuse thyroid diseases in children include pathologic processes characterized by hypertrophy and/or hyperplasia of glandular tissue with thyroid enlargement or by its atrophy with a decrease in thyroid size. The incidence of thyroid nodules in children does not exceed 0.5–2%. More than half of the nodules are detected with US screening, and they are more prevalent in elder children. Specific pitfall in diagnosis of thyroid nodules in children is aberrant thymic tissue within the thyroid gland that may be mistaken for thyroid nodule. Thyroid cancer is more aggressive in children than in adults. Papillary cancer dominates among all thyroid malignancies in children.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
The thyroid gland affects the development of a child at any age. Adequate thyroid function is necessary for the development of the brain and other organs and systems, including the immune system and sexual development. Much attention is paid to examining the thyroid gland in children, taking into account the influences of numerous negative factors [1]. The most significant factors are an iodine deficiency, an unfavorable ecological situation, an urbanization, and an intense rhythm of life with stressful influences. In this regard, regular screening has become a medical standard, especially in children suspected of having a thyroid pathology.
The technique of thyroid US in children actually does not differ from that in adults, except for special demands in relation to the accuracy of the volume calculation. Thyroid US in infants is preferably performed with an assistant due to certain difficulties in fixing the baby’s head. The assistant places the hand under the baby’s neck and upper back and slightly lifts it supporting the jaw with the thumb to prevent the baby from reflex flexing the head.
A change in gland volume accompanies almost all thyroid pathologies in children. Inspection and palpation fail to assess the thyroid volume correctly in about 35% of cases. In areas of mild to moderate iodine deficiency, the sensitivity and specificity of palpation are very poor. Measurement of thyroid size using ultrasound is preferable.
The thyroid volume is the sum of the volumes of both lobes. The volume of each lobe is calculated from the measurements of the depth (d), the width (w), and the length (l) by the standard formula: V (mL) = 0.479 × d × w × l (cm). The volume of the isthmus is not included.
The issue of correctly interpreting the US measurements obtained in children and teenagers is a pertinent one. Numerous attempts have been made to define normal values of thyroid volume in different age [2, 3].
WHO [4] proposed the international reference values for goiter screening presented as a function of age, sex, and body surface area (BSA) (Tables 3.1 and 3.2). These were based on the study of Zimmermann et al. [6] that conferred the data of 3529 children from areas of long-term iodine sufficiency in North and South America, Central Europe, the Eastern Mediterranean, Africa, and the Western Pacific. By using the ultrasonography criteria, a thyroid gland will be called goitrous when its values will be above the 97th percentile of the volume found in an iodine-replete population used as control. Typically, reference values for the 97th percentile for thyroid volume, as a function of both age and sex, are used (Table 3.1). BSA reference (Table 3.2) is potentially useful in countries with a high prevalence of child growth retardation due to malnutrition with both stunting (low height-for-age) and underweight (low weight-for-age). An advantage of the thyroid volume for BSA is that the age of the child is not required, which in some populations is not known with certainty.
The norms listed above for the thyroid volume with respect to age or BSA have been simplified and adapted for US screening. They do not take into account the physical development of a child and puberty in a teenager. Kasatkina et al. [7] suggested a method of defining thyroid hyperplasia and hypoplasia in children, which is linked to anthropometric parameters that depend on age and the presence of puberty. Thoracic circumference at maximal expiration is measured at the ages of 4–6 years, leg length (distance between the greater trochanter and the sole) at 7–9 years of age and in children over 10 years of age who are yet to enter puberty, and body weight in children undergoing puberty. Table 3.3 is used to interpret the obtained data.
The isthmus depth is usually neglected. The thickness of the isthmus can be taken into account indirectly if the thyroid volume appears close to the upper limit. The boundary volume of the thyroid gland is reported normal if the isthmus thickness is normal. Alternatively, if the isthmus is enlarged (thicker than 3 mm in children under 10 years of age and more than 5 mm in teenagers), the thyroid is reported enlarged.
The echodensity of thyroid tissue in children, as well as in adults, is compared to that of the salivary gland. Normal thyroid tissue shows homogeneous echostructure (Fig. 3.1). However, in rare cases, the homogeneity of the tissue does not exclude initial stages of sporadic or endemic diffuse euthyroid goiter.
CDI and PDI allow changes in the functional activity of both the thyroid gland and nodules to be gauged and several pathologic conditions to be differentiated based on the vascularization features (Fig. 3.2). However, quantitative assessment with PW Doppler is very subjective, especially for the vessels of the parenchyma, and is not used in everyday practice.
Congenital anomalies of the thyroid occur in <0.3–0.5% of the population. They appear at the stage of prenatal development. The embryonic primordium of the gland descends between weeks 3 and 5 of gestation as a median diverticulum from the floor of the pharynx, which makes its appearance at the level of the second pair of pharyngeal pouches. It evaginates, migrating caudally to the level of the III–IV pairs of pharyngeal pouches, and retains its connection with the pharynx only by a narrow thyroglossal duct at the root of the tongue. It is contributed to by the primordia, which arise laterally from the fourth pharyngeal pouches. The thyroglossal duct obliterates, and the germs of lateral lobes grow quickly and migrate caudally to the inferior part of the fetal neck. The first signs of independent function in the fetal thyroid are observed at week 8 of gestation. Thyroid function becomes apparent between weeks 12 and 14 of gestation.
At the stage of embryogenesis, the thyroid germ migrates from the level of the stomatopharynx to the inferior part of the neck. Various congenital anomalies of the thyroid gland can be formed in cases where the embryo experiences disturbances during histo- or organogenesis resulting in pathology of the thyroid primordium or the thyroid germ fails to successfully migrate. Thus, thyroid anomalies may be divided into size and position anomalies.
Thyroid size anomalies include the following:
-
Aplasia (agenesis)
-
Hemiagenesis
-
Hypoplasia
Thyroid aplasia is the complete absence of thyroid tissue. This is a widespread cause of congenital hypothyroidism, which has been recorded to occur in 1 in every 3000–5000 newborns. Here, the thyroid tissue cannot be sonographically visualized in its typical position or higher up.
Thyroid hemiagenesis is usually a purely sonographic finding and is not accompanied by thyroid disorders. Here, only one thyroid lobe can be detected at its typical site (Fig. 3.3). Its volume, as a rule, does not exceed the standard limits for the total volume of a normal thyroid gland. Echodensity and echostructure of the lobe in grayscale and Doppler modes are normal. The second lobe cannot be visualized.
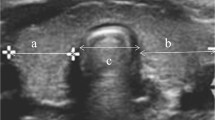
Congenital hypoplasia of the whole thyroid gland is the second cause of congenital hypothyroidism. Sonography reveals a low thyroid volume, which is more than 2–2.5 times below the lower limit. The gland parenchyma appears normal with normal blood flow. Alternatively, it may exhibit slightly increased echodensity with heterogeneous structure and irregular margins. Hypoplasia of only one thyroid lobe is much more common. Here, the volume of the affected lobe is 2–2.5 times smaller as compared with the contralateral one (Fig. 3.4).
Thyroid gland position anomalies are as follows:
-
Dystopia
-
Ectopia
Thyroid dystopia and ectopia are sonographically characterized by an absence of thyroid tissue at its typical location. Dystopia refers to the localization of thyroid tissue close to the typical site along the route of natural migration during embryogenesis (within the neck, along the thyroglossal duct). If thyroid tissue is found at an atypical site outside the path of the thyroglossal duct, it is known as thyroid ectopia (aberrant thyroid). An ectopic thyroid gland is at an increased risk of malignant transformation compared to either dystopic or normal thyroid glands.
Thyroid dystopia can take the form of the following variants, depending on the height of location:
-
Lingual (goiter of the tongue root)
-
Intralingual (lingual goiter)
-
Sublingual
-
Thyroglossal
-
Pre- and intratracheal
-
Intraesophageal
-
Intrathoracic (truly retrosternal, in cases with an entirely retrosternal location)
Ectopic thyroid can be found in the lateral neck (Fig. 3.5), in an ovary (struma ovarii), in a testicle (struma testis), in pericardium (struma pericardii), etc.
Midline cysts of the neck are similar to dystopia in their pathogenesis. Failed obliteration of the thyroglossal duct during fetal thyroid migration results in the formation of an epithelial cavity with subsequent fluid accumulation. US detects a cystic lesion cranial to the normal thyroid gland.
Thyroid pathology is widespread in children and teenagers. Girls suffer more often. Morbidity increases distinctly with age and reaches its peak in puberty.
Diffuse thyroid diseases in children include pathologic processes characterized by hypertrophy and/or hyperplasia of glandular tissue with thyroid enlargement or by its atrophy with a decrease in thyroid size. Different variants of diffuse euthyroid goiter dominate among diffuse thyroid diseases in children. Diffuse goiter is a universal pathologic sign of several diseases, such as the following:
-
Endemic goiter
-
Simple nontoxic (juvenile) goiter
-
Iodine-induced goiter
-
Idiopathic goiter
-
Autoimmune thyroid disease
-
Graves’ disease
-
Pendred syndrome
-
Congenital nontoxic goiter
Diffuse endemic goiter is the most common thyroid pathology in children and teenagers. It is associated with iodine deficiency and is identical to the same in adults. Children and adolescents contribute up to 40% of the cases in the total number of diffuse endemic goiter [7]. This disease is sonographically characterized by normal thyroid tissue. Thyroid echostructure remains homogeneous and isoechoic with unchanged tissue vascularity according to color-coded Doppler imaging (Fig. 3.6). The only sign of the disease is an increase in thyroid volume, which thus differentiates it from the norm.
Autoimmune thyroid disease (AITD) in children under 15 years of age reaches 20–25 cases per 100,000. It quite often results in hypothyroidism. The specific features of AITD in children are a consequence of short duration of the disease and minimal changes in the thyroid tissue. Hence, the disease is more difficult to diagnose than it is in adults. The US image is quite variable and is characterized by the heterogeneity of thyroid tissue due to hypoechoic foci (lymphocytic infiltration), which contrast with the normal or slightly hypoechoic surrounding tissue (Fig. 3.7). It may also show the heterogeneous decrease in echodensity of the entire thyroid gland. Increased vascularity of the parenchyma and irregular vascular pattern are registered less often than in adults. Fibrosis is sometimes possible to visualize as echogenic septa. Pseudonules in AITD are often difficult to differentiate with US. Reactive lymph nodes adjacent to lower poles of the lobes tending to upper mediastinum are typical. A specific feature of humoral immune response in children with early AITD is a high rate (up to 60%) of cases without any increase in both anti-thyroglobulin (anti-Tg) and antithyroid peroxidase (anti-TPO) antibodies [8]. Such cases of AITD are subject to puncture biopsy and cytology. Expressed autoimmune changes during the initial stages of AITD do not lead to a very big goiter, unlike in adults. The thyroid volume in the majority of children with AITD, as well as those with endemic goiters, is increased by <50–60% compared to the norm [7].
Graves’ disease in children and teenagers is a serious endocrine pathology. The annual morbidity is 2–4 cases per 100,000. The disease disproportionately affects girls 10–15 years of age. The clinical symptoms of Graves’ disease in children are variable, but they do not develop as quickly as in adults. Organ compression symptoms may arise in cases with a retrosternal thyroid location. However, the degree of thyroid enlargement does not define the severity of thyrotoxicosis. The results of treatment depend on the accuracy and the timeliness of diagnosis. Sonography usually reveals an enlarged thyroid gland with regular, well-defined margins and relatively homogeneous and significantly hypoechoic parenchyma. Blood flow supply in the parenchyma is substantially increased and demonstrates “thyroid inferno” in CDI and PDI. The blood flow velocities in the large arteries with PW Doppler are also significantly increased. Wide veins are often registered with possible arteriovenous shunts.
Thyroid nodules in children and teenagers are noted less often than in the adult population. The incidence of thyroid nodules in children does not exceed 0.5–2% [9]. More than half of the nodules (63.4%) are detected with US screening, and they are more prevalent in elder children. Solitary nodules are detected in about 88.6% of cases [10]. Nodule size, as a rule, does not correlate with age. Thyroid nodules in children do not show specific US features and are similar to such lesions in adults (Fig. 3.8). Specific pitfall in diagnosis of thyroid nodules in children is aberrant thymic tissue within the thyroid gland that may be mistaken for thyroid nodule. Intrathyroid ectopic thymic tissue is usually found incidentally in prepubertal pediatric population, rarely in adults due to age-related involution. It is detected with US in 1% of general pediatric population [11] and is presented by round, oval, or polygonal hypoechoic or hyperechoic lesion, with multiple granular and punctate echogenic foci that are typical for thymus (Fig. 3.9). It is predominantly soft with elastography making no difference as compared to the surrounding thyroid tissue.
Thyroid cancer is the most widespread tumor of the endocrine system in children. It accounts for up to 45.3% of all malignant neoplasms of endocrine glands at this age [12]. The incidence of pediatric thyroid cancer among all tumors of the head and neck is 8–22% [13, 14]. According to Kiyaev et al. [10], thyroid cancer in children is detected in 11.5% of suspicious thyroid nodules subject to puncture biopsy.
Papillary cancer dominates among all thyroid malignancies in children, as well as in adults. Children 8–14 years of age show the disease more often, with the peak occurring during puberty. The ratio of boys to girls with thyroid malignancy is about 1:1.6. US image of thyroid cancer in children is identical to the same in adults (Fig. 3.10).
The disease is more aggressive in children than in adults. Multicentric growth of thyroid cancer in children is observed in more than half of cases (up to 65%) [12]. Invasion of the tumor through the thyroid capsule is registered in 24–52% of patients. The incidence of lymph node metastases in children is 37–93% [15]. More than 30% of children have metastases in cervical lymph nodes before surgery, and up to 28% have distant metastases. Thyroid cancer recurrence occurs more often in children than in adults and constitutes 19–39%. Precise sonography with all accessible options in children with thyroid malignancy before and after surgery permits improved management of thyroid cancer.
References
Pykov MI, editor. Children’s ultrasound diagnosis. Textbook. Vol 5. Andrology, endocrinology, selected questions. Moscow: Vidar; 2016 (Book in Russian).
Delange F, Benker G, Caron P, et al. Thyroid volume and urinary iodine in European schoolchildren: standardization of values for assessment of iodine deficiency. Eur J Endocrinol. 1997;136:180–7.
Gutekunst R, Martin-Teichert H. Requirements for goiter surveys and the determination of thyroid size. In: Delange F, Dunn JT, Glinoer D, editors. Iodine deficiency in Europe: a continuing concern. New York: Plenum Press; 1993. p. 109–18.
World Health Organization, UNICEF, ICCIDD. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed. Geneva: WHO; 2007.
Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med Chic. 1916;17:863–71.
Zimmermann MB, Hess SY, Molinari L, et al. New reference values for thyroid volume by ultrasound in iodine-sufficient schoolchildren: a World Health Organization/Nutrition for Health and Development Iodine Deficiency Study Group report. Am J Clin Nutr. 2004;79(2):231–7.
Kasatkina EP, Shilin DE, Pykov MI. Ultrasound of the thyroid gland in children and adolescents. Moscow: Vidar; 1998 (Book in Russian).
Shilin DE, Pykov MI. Ultrasound examination of the thyroid gland. In: Pykov MI, Vatolin KV, editors. Clinical guidance on ultrasound in pediatrics. Moscow: Vidar; 2001 (Book in Russian).
Wang C, Crapo LM. The epidemiology of thyroid disease and implication for screening. Endocrinol Metab Clin N Am. 1997;26:189–218.
Kiyaev AV, Fechina LG, Shorikov EV, et al. Thyroid tumors in the structure of nodular goiter in children and the accuracy of fine-needle aspiration biopsy in their diagnosis. Pediatr Oncol. 2008;3:21–4 (Article in Russian).
Fukushima T, Suzuki S, Ohira T, et al. Prevalence of ectopic intrathyroidal thymus in Japan: the Fukushima health management survey. Thyroid. 2015;25(5):534–7.
Romanchyshen AF. Diagnostics, methods and results of surgical treatment of patients with advanced differentiated thyroid cancer. Vestnik RONTS im NN Blokhin RAMS. 2009;20(2):22 (Article in Russian).
Paches AI, Brzhezovsky VZ. Tumors of the thyroid gland. Tumors of the head and neck. Moscow: Practical Medicine; 2013 (Book in Russian).
Polyakov VG, Shishkov RV. Local prevalence and metastasis of the thyroid cancer in children and adolescents. Sib Oncol J. 2006;1:89–90 (Article in Russian).
Romanchyshen AF, Gostimsky AV. Diseases of the thyroid gland in children and adolescents. Surgical endocrinology. Sankt Petersburg; 2004 (Book in Russian).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sencha, A.N., Tukhbatullin, M.G. (2019). Congenital Thyroid Anomalies and Thyroid Diseases in Children. In: Sencha, A., Patrunov, Y. (eds) Thyroid Ultrasound. Springer, Cham. https://doi.org/10.1007/978-3-030-14451-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-14451-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14450-0
Online ISBN: 978-3-030-14451-7
eBook Packages: MedicineMedicine (R0)