Abstract
Interventions directed at the sphenopalatine ganglion (SPG, also called the pterygopalatine ganglion) with the intention of treating headache dates back over 100 years to work done by Dr. Sluder [1]. In the time since, a number of treatments targeting the SPG have been applied in headaches resistant to standard therapy. These include complete ganglionectomy [2], radiofrequency lesioning [3], injections using alcohol, corticosteroids, lidocaine, cocaine, or botulinum toxin injections to the SPG [4–8]. While some of these interventions provided relief, this was often fleeting, requiring multiple procedures, or they were accompanied by unacceptable side effects. A subset of cluster headache (CH) patients are treatment refractory [9], and especially in the chronic subform (cCH), with a high headache burden, this combination presents a considerable clinical challenge. Due to abortive medications also including oxygen inhalation or injections with sumatriptan, motivation to develop new treatment strategies is high. Especially preventive medications including verapamil, corticosteroids, methysergide, divalproex sodium (valproate), or lithium carbonate show very significant side effects in many cases and not all patients have relief. These side effects can be very incisive, including bradycardia, AV block, myocardial infarction, nausea, and fatigue to hypotension [10]. A new treatment option for refractory CH is the sphenopalatine ganglion stimulation via an inserted miniaturized microstimulator (Pulsante™ Microstimulator System, previously referred to as the ATI Neurostimulation System) has been made available as both an acute and preventive therapeutic option [10]. After a proof-of-concept case study was published in 2007 [11], in two small series of CH and migraine patients, electrical stimulation was initially tested in an experimental setting [12, 13]. A few years later, the first randomized controlled study of the Pulsante™ system, developed by Autonomic Technologies Inc., was published [14]. Today, we have long-term data available for CH and guidelines on patient selection and technical aspects available [10]. The majority of the evidence is for SPG stimulation (SPGS) in CH patients (episodic (eCH) and chronic), and this will be reflected below. This chapter gives an overview of the surgical and technical aspects as well as the evidence available for SPG stimulation.
Presentation of Technical Aspects—Presentation of Evidence Base Medicine for Use in Headache Field
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Interventions directed at the sphenopalatine ganglion (SPG, also called the pterygopalatine ganglion) with the intention of treating headache dates back over 100 years to work done by Dr. Sluder [1]. In the time since, a number of treatments targeting the SPG have been applied in headaches resistant to standard therapy. These include complete ganglionectomy [2], radiofrequency lesioning [3], injections using alcohol, corticosteroids, lidocaine, cocaine, or botulinum toxin injections to the SPG [4,5,6,7,8]. While some of these interventions provided relief, this was often fleeting, requiring multiple procedures, or they were accompanied by unacceptable side effects. A subset of cluster headache (CH) patients are treatment refractory [9], and especially in the chronic subform (cCH), with a high headache burden, this combination presents a considerable clinical challenge. Due to abortive medications also including oxygen inhalation or injections with sumatriptan, motivation to develop new treatment strategies is high. Especially preventive medications including verapamil, corticosteroids, methysergide, divalproex sodium (valproate), or lithium carbonate show very significant side effects in many cases and not all patients have relief. These side effects can be very incisive, including bradycardia, AV block, myocardial infarction, nausea, and fatigue to hypotension [10]. A new treatment option for refractory CH is the sphenopalatine ganglion stimulation via an inserted miniaturized microstimulator (Pulsante™ Microstimulator System, previously referred to as the ATI Neurostimulation System) has been made available as both an acute and preventive therapeutic option [10]. After a proof-of-concept case study was published in 2007 [11], in two small series of CH and migraine patients, electrical stimulation was initially tested in an experimental setting [12, 13]. A few years later, the first randomized controlled study of the Pulsante™ system, developed by Autonomic Technologies Inc., was published [14]. Today, we have long-term data available for CH and guidelines on patient selection and technical aspects available [10]. The majority of the evidence is for SPG stimulation (SPGS) in CH patients (episodic (eCH) and chronic), and this will be reflected below. This chapter gives an overview of the surgical and technical aspects as well as the evidence available for SPG stimulation.
2 Anatomy and Neurophysiology
The SPG is a bilateral, parasympathetic ganglion situated in the sphenopalatine (pterygopalatine) fossa, which is a defined anatomical bilateral space, bounded anteriorly by the maxillary bone, and posteriorly by the medial plate of the pterygoid process and the greater wing of the sphenoid process. Medially the PPF is anatomically restricted by the palatine bone and superiorly by the body of the sphenoid process. The pterygomaxillary fissure (PMF) is marking the lateral border, which results into the infratemporal fossa [10, 15]. The PPF communicates with the infratemporal fossa via the PMF and the nasal cavity via the sphenopalatine foramen. Superiorly in the fossa, as if suspended by two sensory branches from the maxillary nerve, lies the SPG. Pain attacks experienced by the patients during a cluster headache seem to be the result of an activation of the trigeminal-autonomic reflex. Cluster headache is a neurovascular disorder in which neural elements cause vessel dilatation and/or an activation of trigeminal nociceptive fibers, which is perceived as referred pain [16]. The anatomy of the SPG can be quite variant, in up to a third partitioned into smaller morphological structures [17]. This individual variation is proposed as a possible reason for failure of some ablation procedures.
The relevance of the SPG in the pathophysiology of migraine and CH stems from its effector role in the trigeminal-autonomic reflex [16]. The afferent branch of this reflex is composed of the ophthalmic and maxillary nerves. These trigeminal afferents project in part to the superior salivatory nucleus (SSN) in the brain stem and from here, via the facial nerve, to the SPG where efferents from the SSN synapse. Postganglionic SPG fibers release transmitter substances which directly or indirectly activate the previously mentioned trigeminal afferents causing increased sensory, afferent signaling again increasing the parasympathetic outflow from the SSN in a positive feedback manner. Parasympathetic outflow is also the cause of the observable autonomic symptoms including conjunctival injection, rhinorrhea, and lacrimation. Only parasympathetic neurons synapse in the SPG, but also motor and trigeminal sensory fibers transit the structure, the latter innervating the nasal and pharyngeal mucosa.
3 Technical Aspects
Aspects pertaining to the technical success of SPG stimulation include planning of the surgery, whereby anatomical compatibility is ensured, correct surgical placement, and finally programming of the microstimulator. At the time of writing, the only system available for on-demand stimulation of the SPG is the Pulsante™ Microstimulator System (Fig. 5.1) by ATI (Autonomic Technologies Inc., Mountain View, CA 94043, USA) which is CE marked in Europe, labeled for the acute and preventive treatment of CH and in some patients was associated with a reduction in the number of cluster headaches [10]. The implanted microstimulator consists of an integral lead containing six electrodes and a device body housing electronics [10, 14]. The device is inductively powered by the remote control (RC) held up against the cheek when stimulation is desired (Fig. 5.2).
3.1 Planning
Preoperative planning of the surgical implantation is important, due to the various differences in the facial anatomy and especially in the PPF. This affects not only between different patients, but also within one patient, as the left side may be different from the right [18], [10].
In all patients, the presence of dental pathology should be excluded prior to implantation as this may result in postsurgical infection of the implant site. To address these issues, prior to surgery, a panoramic view, dental cone beam computed tomography (CBCT), or computed tomography scans (CT) of the oral cavity an the maxillary sinus should be performed [19]. Interpretation and description of the radiological investigations should focus on signs of osteopathology including infection, pericoronitis, and impacted wisdom teeth as well as the morphology of the pterygomaxillary fissure. The latter bears importance as the lead diameter of the microstimulator is 1 mm. A minimum width of the fissure of 1.2 mm is preferable.
A unique challenge in CH is constituted by patients who have been treated for many years with pulse therapy of corticosteroids as this may lead to osteoporosis, increasing the risk of perioperative posterior maxillary wall fracture [10]. These, as well as the described radiological findings, weigh in on the final confirmation of patient eligibility at the surgeon’s discretion. Thus far, in the published studies, no patients have been rejected on the basis of surgical ineligibility but some have had preoperative dental procedures. On the establishment of surgical eligibility, the manufacturer performs 3D modeling and makes a recommendation for stimulator size—short (3.6 cm), medium (4.4 cm), long (5.2 cm), and extra-long (6.0 cm). As power and control is provided by the RC, these recommendations also figure the resulting depth of the microstimulator housing as this is essential for a functioning connection.
3.2 Surgery
All insertion procedures have to be done under general anesthesia. The risk of dislocation of the stimulator during local anesthesia is quit to high, as well as pain attacks during implantation caused by manipulation of nerval structures inside the PPF during the insertion procedure. As the procedure is sterile contaminate, it should be performed under antibiotic cover and with prior oral decontamination per local guidelines. Local anesthetic with adrenaline may be applied to reduce bleeding and postoperative swelling and pain. The microstimulator is inserted transorally. Depending on the patients’ dentition, gingival buccal approach, a crestal incision, or a marginal incision can be performed. After subperiosteal tissue dissection from the lateral and posterior maxilla, the microstimulator can be placed with its stimulating electrodes into the PPF of the affected side. The microstimulator is then fixated with its osteosynthesis plate to the anterior wall of the maxillary sinus by using three osteosynthesis screws [19] (Fig. 5.3).
The procedure is aided by the ATI surgical introducers, starting with the SI-100 (Fig. 5.4a), which is a curved subperiosteal elevator; the posterior lateral maxilla is prepared by subperiosteal dissection into the PMF. When reaching the expected area inside the PPF, a.p. and lateral fluoroscopy is used to verify the position of the SI-100 (Fig. 5.5). The SPG is expected in the lower third of the distance in between the two anatomical structures “foramen rotundum” and “Vidian canal” [10]. If position is good, the SI-110 (Fig. 5.4b) is used and also inserted into the same position inside the PPF (Fig. 5.6). Both instruments, SI-100 and SI-110 allow for blunt atraumatic subperiosteal dissection while maintaining close contact to the posterior wall of the sinus to avoid trauma to the surrounding tissues [10]. The surgeon can optimize the contour of the instruments by adapting the curvature of the instruments to the shape of the posterior maxillary sinus curvature and may avoid soft tissue destruction and inside the infratemporal fossa and minimize intraoperative bleeding. When the SI-110 is placed at the entrance of the PPF, the lead blank (LB-100) (Fig. 5.6) is used to create an implant path within the PPF [10].
In some anatomical variations, the SI-120 may not fit into the PPF due to a small entrance and may be precluded. In those cases, placement of the microstimulator may be achieved by inserting the microstimulator along the insertion groove and split tip of the SI-110 (Fig. 5.7). Especially in those cases, periodic images are necessary to verify correct positioning of the microstimulator inside the PPF at the expected target point.
If anatomy allows, SI-120 can be used to insert the microstimulator into the PPF. The microstimulator is then placed into the SI-120, which is inserted using the existing surgical plane created by the SI-100 or SI-110. Here too, repeated fluoroscopy is recommended to visualize the position of the SI-120 and to maintain the trajectory toward the cranial and medial aspect of the PPF [10] (Fig. 5.8).
If target placement is achieved and the positioning of the microstimulator is checked against the DRR images, it can be pushed slightly forward and be removed from the SI-120 anchoring hub. While pushing the body against the lateral wall of the sinus, the SI-120 is retracted as the sheath opens around the lead. Finally, three 4–6 mm osteosynthesis screws are used to fixate the microstimulator on the anterior wall of the maxillary sinus and final anterior–posterior and lateral fluoroscopy is performed to check the final position (Figs. 5.9 and 5.10).
Microstimulator function is checked prior to insertion and closing suturing. After surgery, correct placement of the device is assured by intraoperative 3D-CT if possible, or a postoperative 3D-CT. If necessary, placement may be revised. The procedure is considered minimally invasive—comparable in extent to that associated with wisdom tooth extractions or dental implants procedures. The average duration of the surgery for the first 99 patients was 80 min (range 25–175) [19].
3.3 Surgical Intraoperative Navigation
When possible, intraoperative navigation can also be used for this procedure. Navigation technique allows, especially in the initial phase of this procedure, precise placement of the surgical introducers without using radiation [20]. In preparation for navigation CBCT or CT scans of each patient are required. Those images allow, next to a detailed analyzation of the PPF, whether the microstimulator implantation procedure was suitable and to estimate the necessary length of the SPG microstimulator. Overwork of all scans and files are performed for each patient separately and are reconstructed as DRR for intraoperative comparison (Figs. 5.11 and 5.12). All preoperative planning is performed using special software solutions. Those are available by different companies. All 3D-CBCT data and the virtual treatment plan are then uploaded into the used navigation system (Fig. 5.13).
Surgical implantation using navigation is also performed under general anaesthesia. Before starting implantation, marker spheres of the used system have to be attached to the patients’ calvarian bone, called skull reference array (SRA) (Fig. 5.14). Further marker spheres are attached to the surgical instruments (SI-100 and SI-110) (Fig. 5.15), which are used as described before. This procedure enables the surgeon to visualize the exact position of the instruments inside the patients’ anatomy during surgical insertion without the use of radiation. The system displays the actual position during insertion of the instruments inside the PPF (Fig. 5.16). When the expected position inside the PPF is reached, insertion of the microstimulator can be done using the SI-120 [20].
Insertion is performed using the SI-100 or SI-110 as a guided tool. After implantation of the SPG microstimulator, real-time verification of correct placement can be done by conventional anterior–posterior and lateral fluoroscopy. After fixation of the SPG microstimulator on the zygomatic process with two to three osteosynthesis screws, final confirmation of the exact position can be done using intraoperative 3D-CBCT/CT scans. The electrode tip has to be positioned in close vicinity to the previously defined target points (Vidian canal and foramen rotundum) [10, 20]. The final scans can be matched with the preoperative 3D-CBCT or CT scans using built-in image alignment software integrated to most navigation systems. Image fusion allows direct comparison of the expected SPG microstimulator position and the real-time result (Fig. 5.17) [20]. Due to direct verification of the position or misplacement, immediate revision and reinsertion are possible [20].
3.4 Postsurgical Aspects
It is relatively common that the patient wake up with a CH attack immediately after the general anesthesia, which the patient of course should be informed in advance. Postoperatively, patients should pay special attention to avoiding pressure on the cheek on the affected side. Diet should be limited to soft and cool food on the first postoperative day. Smoking should be avoided in the first couple of days. Oral decontamination, for example, Chlorhexidine 0.12% should be continued twice daily for 1 week. Peroral antibiotics on the first three postoperative days are recommended. One to two weeks after surgery, the wound should be inspected and sutures removed if resorbable suture is not used. Infections may be treated at this point using debridement or intravenous antibiotics. The patient can subsequently receive normal dental treatment, filling and crown therapy, etc., but the dentist should avoid injection of local anesthetic behind the tuber maxillae on the operated side to prevent damage to the electrode or inappropriate relocation of the lead.
3.5 Programming
First programming and stimulation can be attempted 2 weeks following insertion. Earlier attempts are generally avoided as healing is still ongoing. The adjustable parameters for the Pulsante™ system include pulse frequency, width, and amplitude. Furthermore, the anode/cathode state of the six electrodes on the lead can be changed. The overall guiding principle of programming is to obtain a sensation of parasthesia in the posterior nasopharynx. The reasoning behind this is that this is the innervation area for the sensory components of the SPG. Thus, if parasthesia is obtained here, by inference, the SPG must be in the stimulation field. The programming is done by a technician in cooperation with the patient. Several attempts, weeks apart, may be necessary before a suitable setting is found.
3.6 Side Effects
The microstimulator is small and, barring surgical side effects, does not give rise to cosmetic issues. However, a majority of patients experience postoperative sequalae. These include pain and swelling (47%) and sensory disruptions (67%) [14, 19, 21]. Resolution of these surgical side effects is usually swift, within 2–3 months, and would typically be classified as mild–moderate. Some patients experience a transient increase in attacks in the days and weeks following surgery. No late side effects (24 months) have been reported. Most importantly there has been no need for repeated surgery due to lead breaks or migrations [21]. Special consideration has been given to the possibility of contralateral attacks or even side shifts. However, it does not appear that that SPGS can be causally related to observed contralateral attacks, which do occur with some regularity in CH in general [22].
4 Clinical Evidence
4.1 Patient Selection
Neurostimulation remains a developing therapeutic pathway and its use is still restricted to highly specialized tertiary centers. Thus far, we only have clinical studies of SPGS available for CH. In CH, 10–20% of patients may develop drug-resistant headaches [23, 24]. Further, in some patients, verapamil, the first-choice preventive strategy, cannot be used as it may induce atrioventricular block [25]. Triptans are contraindicated in peripheral artery disease, severe hypertension, and ischemic heart disease and may be associated with increased risk of cerebrovascular events [26, 27]. For this reason, the daily dose must not exceed 12 mg. Additionally, there are logistical disadvantages to oxygen use which may also cause rebound headaches in some [28]. With this in mind, the vast majority of both migraine and CH patients who have been selected for SPGS have been patients with a high headache burden who were dissatisfied with available treatment options. How this is defined has been the subject of some debate and a preliminary expert consensus on patient selection and standards of care has been set forth [29]:
-
(a)
A documented history of refractory cluster headache for at least 2 years before the implantation.
-
(b)
Headache meets the current ICHD criteria for chronic cluster headache.
-
(c)
Detailed headache diary (on a daily basis) for at least 1 month: number of attacks, severity and mean duration of attacks, consumption of rescue medication, circadian (and circannual) rhythmicity, and response to preventatives including dosage, duration of intake, and response.
-
(d)
Attacks are side-locked or occur predominantly (>90% of the time) on one side.
-
(e)
There is significant disability and socio-professional impairment as measured by established questionnaires.
-
(f)
The patient is able to comprehend and comply with the instructions on how and when to use the device and will present to the headache specialist for regular follow-up visits.
Additionally, medication overuse headache should be ruled out as this may lead to a higher attack frequency and may result in lower efficacy of initiated regimens [30]. Lastly, it should be noted that spontaneous remissions may occur at any time in CH which was observed in a previous study where some patients, while on the waiting list for deep brain stimulation, went into spontaneous remission [31].
4.2 Available Studies
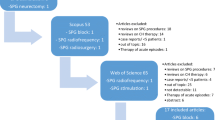
SPG stimulation has been tested in CH and a trial is ongoing in migraine. In CH, evidence is available from two studies: the randomized, controlled pathway CH-1 trial [29] and the long-term 24-month follow-up study [21, 32]. A large registry study has recently been published [33].
The first evidence of SPGS efficacy is from a 2007 case report describing a young CH patient treated with electrical stimulation of the SPG by a device, not specifically designed to accomplish this [11]. Nevertheless, the results were convincing and the patient remained asymptomatic for almost a year, attacks reoccurring as technical difficulties interrupted stimulation. In 2010, Ansarinia et al. [12] published results from the first pioneering study of transcutaneous stimulation of the SPG in a series of six patients with refractory cCH [12]. Patients were subjected to stimulation with varying pulse widths, frequencies, and amplitudes during spontaneous and provoked attacks. In total, 18 attacks were studied and SPG stimulation resulted in complete pain freedom in 11 of these.
The first blinded, randomized, controlled trial investigating SPGS using the Pulsante™ microstimulator in CH was published in 2013 [14]. Twenty-eight patients completed the experimental period where attacks were treated with either sham, sub-perception, or full stimulation. Results were convincing—67% of attacks treated with full stimulation resulted in pain relief compared to 7.4% and 7.3% for sham and sub-perception, respectively. These results encompass the expected effect—acute relief of ongoing attacks. However, an unexpected effect was noticed—the frequency of attacks fell by 50% or more in around one-third of the patients. Thus, at the time, SPGS became the first neurostimulation for headache to elicit both an acute and a preventive effect.
The preventive effect was better characterized in a follow-up publication of a slightly expanded cohort [21]. In these 33 patients, the ability to treat attacks (n = 5956) acutely was maintained through 24 months following implant with 45% of patients being acute responders. Acute response being defined as the ability to treat ≥50% of attacks. A reduction in the number of attacks ≥50% was observed in 33% of patients. Thus, 61% of patients were acute responders, frequency responders, or both at 24 months. The clinical response was maintained in the majority of patients, a few loosing effect after some time, others gaining it. The major finding of the study was that the clinical response is stable in the long run. In some patients of this cohort, complete remission of attacks were seen, and using the ICHD definition of remission in CH [34], these were characterized in a separate publication [32]. Around one-third of patients experienced at least one such remission period, the periods on average lasting 5 months, in some almost a year. The ability to treat attacks was sustained after the remission periods.
Results from the first large, open-label registry study has recently been published [33]. Of 85 patients enrolled in the study, 68% were responders (≥50% frequency drop, able to treat ≥50% attacks acutely). For the first time, seven episodic CH patients were included. These all had a high headache burden and were dissatisfied with conventional treatments. Effect in these patients was comparable to cCH patients.
The initial cost of neurostimulation is high, but over time this cost may be offset by a reduced use of other treatments. CH is associated with a considerable headache burden [35], but it also represents a considerable healthcare expense. A German study found that the average yearly direct and indirect cost per cCH patient averages ca. €21,000 [36]. A subsequent study analyzing the cost-effectiveness of SPGS found that it was either cost-effective or cost-saving across all tested scenarios [37].
Any trial investigation a potential frequency response in CH must heed the fact that CH is a cyclical disorder. Both circannual and circadian rhythmicity can be observed, also in cCH [38]. Thus, due caution and conservatism in the interpretation of results must be exercised as any drop, or indeed increase, in frequency could be due to spontaneous fluctuations in activity and not necessarily administered interventions. Another problem with regard to the interpretation of results and the designing of studies is represented by the different effects which can be observed in SPGS and possibly also other forms of neurostimulation in CH. Deep brain stimulation and occipital nerve stimulation both elicit a preventive response exclusively. No attempts at stimulation during attacks have resulted in pain freedom or reduction [39]. In the previously mentioned trials, based on the knowledge of the trigeminal-autonomic reflex, the preventive effect was simply not anticipated and consequently the trials were not designed to capture the preventive effect. Thus, these results rested on post hoc analyses.
The field of neurostimulation in headache is dynamic and still evolving; experiences from previous trials are applied moving forward which was demonstrated in the subsequent registry study of SPGS in which the frequency of attacks was a prespecified outcome. Please see the chapter on Methodological Difficulties in Clinical Trials Investigating Neurostimulation for further considerations on this matter.
4.3 Mechanism of Action
The exact mechanism of effect of SPG stimulation remains unknown, especially so because two types of effect are observed—preventive and acute. The acute effect could be attributed to induction of unphysiological, rapid firing of parasympathetic neurons in the ganglion leading to rapid depletion of neurotransmitter and thus a cessation of firing [40]. The mechanism behind the preventive effect is more elusive as there is an incomplete understanding of the interaction between central and peripheral mechanisms in headache. A possible explanation could be long-term modulation of central nociceptive processing. This is also seen in deep brain stimulation and occipital nerve stimulation and has been theorized to be the reason behind the weeks to months interval between initiation of stimulation and manifestation of the preventive effect. A possible direct effect on maxillary division sensory fibers of the trigeminal nerve which converge on second-order neurons in the TNC has not been excluded.
The Pulsante™ system has provided clinical researches with a new tool for studying the pathogenesis of attacks. Whereas high frequency has been shown to have a therapeutic effect, animal studies have shown that low-frequency stimulation induces ipsilateral dilatation of cerebral, pial, and carotid arteries accompanied by an increase in cortical blood flow [40,41,43]. In one study, Schytz and colleagues used low-frequency (5 Hz) stimulation to provoke CH attacks and study autonomic changes. A subsequent study with a larger sample size found that low-frequency stimulation (20 Hz) induced cranial autonomic symptoms, but not reliably attacks or increases in plasma PACAP and VIP [44]. Interestingly though, changes were detected in cardiac autonomic regulation [45].
5 Conclusion
In the group of CH patients where there is a combination of refractoriness to conventional treatments and a high headache burden, the advent of clinically feasible neurostimulation has expanded the clinician toolbox. The choice of which form of neurostimulation is offered to these patients has thus far been decided by availability at the headache clinic. Following the positive clinical studies, in a number of European countries, full reimbursement is now provided for SPGS. Since SPGS provides both an acute and preventive effect, it may be particularly useful in patients in whose acute treatments are poorly tolerated, ineffective, or contraindicated. The effect is sustained in the long term and the treatment is generally well tolerated, with an attractive surgical side effect profile. As a follow-up to the multicenter CH-1 pathway study, which initially tested SPGS in Europe, the CH-2 study has been undertaken in the United States and results are eagerly awaited.
References
Sluder G. The role of the sphenopalatine (or Meckel’s) ganglion in nasal headaches. N YMed J. 1908;87:989–90.
Meyer JS, Binns PM, Ericsson AD, Vulpe M. Sphenopalatine gangionectomy for cluster headache. Arch Otolaryngol (Chicago, Ill 1960). 1970;92(5):475–84.
Narouze S, Kapural L, Casanova J, Mekhail N. Sphenopalatine ganglion radiofrequency ablation for the management of chronic cluster headache. Headache. 2009;49(4):571–7.
Kittrelle JP, Grouse DS, Seybold ME. Cluster headache. Local anesthetic abortive agents. Arch Neurol. 1985;42(5):496–8.
Felisati G, Arnone F, Lozza P, Leone M, Curone M, Bussone G. Sphenopalatine endoscopic ganglion block: a revision of a traditional technique for cluster headache. Laryngoscope. 2006;116(8):1447–50.
Yang l Y, Oraee S. A novel approach to transnasal sphenopalatine ganglion injection. Pain Physician. 2006;9(2):131–4.
Devoghel JC. Cluster headache and sphenopalatine block. Acta Anaesthesiol Belg. 1981;32(1):101–7.
Sanders M, Zuurmond WW. Efficacy of sphenopalatine ganglion blockade in 66 patients suffering from cluster headache: a 12- to 70-month follow-up evaluation. J Neurosurg. 1997;87(6):876–80.
Mitsikostas DD, et al. Refractory chronic cluster headache: a consensus statement on clinical definition from the European Headache Federation. J Headache Pain. 2014;15:79.
Assaf AT, Klatt JC, Blessmann M, Kohlmeier C, Friedrich RE, Pohlenz P, May A, Heiland M, Jürgens TP. Value of intra- and post-operative cone beam computed tomography (CBCT) for positioning control of a sphenopalatine ganglion neurostimulator in patients with chronic cluster headache. J Craniomaxillofac Surg. 2015;43(3):408–13.
Ibarra E, El Dolor B, Long CW, Rico P. Neuromodulación del Ganglio Esfenopalatino para Aliviar los Síntomas de la Cefalea en Racimos. Reporte de un Caso. Boletín El Dolor. 2007;46(16):12–8.
Ansarinia M, et al. Electrical stimulation of sphenopalatine ganglion for acute treatment of cluster headaches. Headache. 2010;50:1164–74.
Tepper SJ, Rezai A, Narouze S, Steiner C, Mohajer P, Ansarinia M. Acute treatment of intractable migraine with sphenopalatine ganglion electrical stimulation. Headache J Head Face Pain. 2009;49(7):983–9.
Schoenen J, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 2013;33(10):816–30.
Daniels DL, et al. Osseous anatomy of the pterygopalatine fossa. AJNR Am J Neuroradiol. 1998;19(8):1423–32.
Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002;1(4):251–7.
Rusu MC, Pop F, Curcă GC, Podoleanu L, Voinea LM. The pterygopalatine ganglion in humans: a morphological study. Ann Anat Anat Anzeiger. 2009;191(2):196–202.
Lang J, Keller H. The posterior opening of the pterygopalatine fossa and the position of the pterygopalatine ganglion. Gegenbaurs Morphol Jahrb. 1978;124(2):207–14. German
Assaf AT, et al. Technical and surgical aspects of the sphenopalatine ganglion (SPG) microstimulator insertion procedure. Int J Oral Maxillofac Surg. 2016;45(2):245–54.
Kohlmeier C, et al. Improved surgical procedure using intraoperative navigation for the implantation of the SPG microstimulator in patients with chronic cluster headache. Int J Comput Assist Radiol Surg. 2017;12(12):2119–28. https://doi.org/10.1007/s11548-016-1512-2.
Jürgens TP, et al. Long-term effectiveness of sphenopalatine ganglion stimulation for cluster headache. Cephalalgia. 2017;37(5):423–34.
Meyer EL, et al. Lateralization in cluster headache: a Nordic multicenter study. J Headache Pain. 2009;10(4):259–63.
May A. Cluster headache: pathogenesis, diagnosis, and management. Lancet. 2005;366(9488):843–55.
Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick D. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168–70.
Lanteri-Minet M, Silhol F, Piano V, Donnet A. Cardiac safety in cluster headache patients using the very high dose of verapamil (≥720 mg/day). J Headache Pain. 2011;12(2):173–6.
Roberto G, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia. 2015;35(2):118–31.
Roberto G, Piccinni C, D’Alessandro R, Poluzzi E. Triptans and serious adverse vascular events: data mining of the FDA Adverse Event Reporting System database. Cephalalgia. 2014;34(1):5–13.
Geerlings RP, Haane DY, Koehler PJ. Rebound following oxygen therapy in cluster headache. Cephalalgia. 2011;31(10):1145–9.
Jürgens TP, et al. Stimulation of the sphenopalatine ganglion in intractable cluster headache: expert consensus on patient selection and standards of care. Cephalalgia. 2014;34(13):1100–10.
Paemeleire K, Evers S, Goadsby PJ. Medication-overuse headache in patients with cluster headache. Curr Pain Headache Rep. 2008;12(2):122–7.
Schoenen J, et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128(Pt 4):940–7.
Barloese M, et al. Cluster headache attack remission with sphenopalatine ganglion stimulation: experiences in chronic cluster headache patients through 24 months. J Headache Pain. 2016;17(1):67.
Barloese M, Petersen A, Stude P, Jürgens T, Jensen RH, May A. Sphenopalatine ganglion stimulation for cluster headache, results from a large, open-label European registry. J Headache Pain. 2018;19(1):6.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808.
Jensen RM, Lyngberg A, Jensen RH. Burden of cluster headache. Cephalalgia. 2007;27(6):535–41.
Gaul C, et al. Treatment costs and indirect costs of cluster headache: a health economics analysis. Cephalalgia. 2011;31(16):1664–72.
Pietzsch JB, Garner A, Gaul C, May A. Cost-effectiveness of stimulation of the sphenopalatine ganglion (SPG) for the treatment of chronic cluster headache: a model-based analysis based on the pathway CH-1 study. J Headache Pain. 2015;16:530.
Barloese M, Lund N, Petersen A, Rasmussen M, Jennum P, Jensen R. Sleep and chronobiology in cluster headache. Cephalalgia. 2015;35(11):969–78.
Pedersen JL, Barloese M, Jensen RH. Neurostimulation in cluster headache: a review of current progress. Cephalalgia. 2013;33(14):1179–93.
Ekstrom J, Brodin E, Ekman R, Hakanson R, Mansson B, Tobin G. Depletion of neuropeptides in rat parotid glands and declining atropine-resistant salivary secretion upon continuous parasympathetic nerve stimulation. Regul Pept. 1985;11(4):353–9.
Takahashi M, Zhang Z-D, Macdonald RL. Sphenopalatine ganglion stimulation for vasospasm after experimental subarachnoid hemorrhage. J Neurosurg. 2011;114(4):1104–9.
Suzuki N, Hardebo JE, Kahrstrom J, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab. 1990;10(3):383–91.
Suzuki N, Gotoh F, Gotoh J, Koto A. Evidence for in vivo cerebrovascular neurogenic vasodilatation in the rat. Clin Auton Res. 1991;1(1):23–6.
Guo S, et al. Cranial parasympathetic activation induces autonomic symptoms but no cluster headache attacks. Cephalalgia. 2018;38(8):1418–28.
Barloese M, Petersen AS, Guo S, Ashina M, Mehlsen J, Jensen RH. Sphenopalatine ganglion stimulation induces changes in cardiac autonomic regulation in cluster headache. Clin Physiol Funct Imaging. 2018;38(5):808–15.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Assaf, A.T., Barloese, M.C.J., Rostgaard, J. (2020). Sphanopalatine Ganglion Stimulation. In: Lambru, G., Lanteri-Minet, M. (eds) Neuromodulation in Headache and Facial Pain Management. Headache. Springer, Cham. https://doi.org/10.1007/978-3-030-14121-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-14121-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14120-2
Online ISBN: 978-3-030-14121-9
eBook Packages: MedicineMedicine (R0)