Abstract
Cluster headache (CH) is a trigeminal autonomic cephalalgias (TAC) characterized by strictly unilateral, excruciating painful headaches that occur in association with ipsilateral cranial autonomic features. Neuromodulation for the treatment of CH can involve both peripheral and central targets. Peripheral targets include stimulation of the sphenopalatine ganglion, vagus nerve and the greater occipital nerve. Central targeting includes deep brain stimulation of the posterior hypothalamus/ventral tegmental area as well as high cervical spinal cord stimulation. This chapter explores the mechanisms, operative techniques and clinical evidence supporting each method.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Trigeminal autonomic cephalalgia
- Cluster headache
- Deep brain stimulation
- Spinal cord stimulation
- Occipital nerve stimulation
- Sphenopalatine ganglion stimulation
- Vagal nerve stimulation
1 Introduction
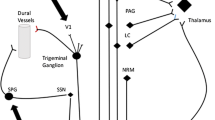
Trigeminal autonomic cephalalgias (TAC) refers to a group of strictly unilateral primary headache syndromes with cranial autonomic features, and includes cluster headache (CH), short-lasting unilateral neuralgiform headache attacks (SUNHA), paroxysmal hemicrania (PH) and hemicrania continua (HC) [1]. These syndromes are thought to be caused by dysfunction in the pain matrix involving the hypothalamic region and trigeminocervical complex as well as the trigemino-parasympathetic reflex [2].
Cluster headache (CH) is characterized by attacks of severe, strictly unilateral pain which is orbital, supraorbital, temporal, or in any combination of these sites, lasting 15–180 min and occurring from once every other day to eight times a day. The pain is associated with ipsilateral conjunctival injection, lacrimation, nasal congestion, rhinorrhoea, forehead and facial sweating, miosis, ptosis and/or eyelid oedema, and/or with restlessness or agitation [1]. CH has a prevalence of 0.1–0.2% and chronic cluster headache (CCH) occurs in 10–15% of sufferers whose attacks occur for more than 1 year without remission, or with remissions lasting less than 3 month [3, 4].
Neuromodulation for the treatment of CH can involve both peripheral and central targets. Peripheral targets include stimulation of the sphenopalatine ganglion, vagus nerve and the greater occipital nerve. Central targeting includes deep brain stimulation of the posterior hypothalamus/ventral tegmental area as well as high cervical spinal cord stimulation. This chapter explores the mechanisms, operative techniques and clinical evidence supporting each method.
2 Peripheral Neuromodulation Techniques
2.1 Sphenopalatine Ganglion Stimulation
2.1.1 Mechanism and Use of SPG Stimulation
The sphenopalatine ganglion (SPG) lies in the pterygopalatine fossa and receives trigeminal sensory inputs as well as cranial parasympathetic outflow from the superior salivary nucleus. Meningeal vessels and facial structures are innervated by postganglionic SPG fibres, and neurotransmitters released by these fibres activate trigeminal nociceptors and thus the trigeminal system. This positively feeds back on the parasympathetic outflow and forms the trigemino-parasympathetic reflex [5]. Stimulation of the SPG is thought to work by disrupting this trigemino-parasympathetic reflex. Acute attacks are terminated by a direct effect on the trigeminal inflow and/or parasympathetic output and attack prevention may be mediated by changes in neurotransmitter production over time [6].
Sphenopalatine ganglion stimulation involves implanting a small neurostimulator device into the pterygopalatine fossa via a small transoral incision through the gum above the upper premolar teeth, overlying the maxilla (Pulsante ATI®). The stimulator delivers an electrical current by induction from a remote held over the cheek by the patient in both abortive and preventive contexts. After the procedure, the patient is initially evaluated every 1–2 weeks to ensure that optimal stimulation settings result in comfortable soft palate paraesthesia. At the initiation of an attack, the patient activates the device by placing the remote on the cheek over the implant and stimulates for a least 15 min. If the attack persists, stimulation should be turned off and rescue medication used. The device can also be used prophylactically by stimulating for 15 min one to two times daily. Ongoing studies are currently assessing the optimal regimen for both abortive and preventive control [6].
2.1.2 Evidence for SPG Stimulation and Lesioning
A multicentre trial of 28 CCH patients treated with SPG stimulation demonstrated a significant difference in number of attacks reported as showing pain relief at 15 min between stimulation and sham groups (67.1% vs. 7.3%, p < 0.0001) as well as number of attacks reported as demonstrating pain freedom at 15 min between the stimulation vs. sham groups (34.1% vs. 1.6%, p < 0.0001). After 2 months of therapy, acute rescue medications were only being used in 31% of cluster attacks in the stimulation group vs. 77.4% of CH attacks in the sham group (p < 0.0001). Complications encountered included infection (6%), lead misplacement or migration (15%), and transient sensory deficits in the maxillary nerve distribution (81%) [7].
In a series of 33 CCH patients treated with SPG stimulation, Barloese et al. reported ten patients (30%) who experienced at least one remission period lasting at least 1 month, with an average remission period of 134 ± 86 days. All ten patients were taking triptans pre-operatively, and at 24 months post-operatively, 60% were not using triptans and 30% were not using any acute medications [8].
Expert consensus published in 2014 recommended SPG stimulation for patients with unilateral chronic cluster headache who have failed all medical therapies. The device may be especially effective in patients with a high number of daily attacks and those who are non-responsive to or cannot tolerate triptans [9]. Given its minimally invasive nature and potential to serve as both a preventive and abortive treatment, SPG stimulation may be considered as a possible first-line option for medically refractory CH patients. The device does, however, require patient cooperation to turn it on and off during acute attacks and this must be emphasized since clinical improvement may only occur after weeks or months of stimulation.
2.2 Occipital Nerve Stimulation (ONS)
2.2.1 Mechanism and Clinical Use of ONS
Occipital nerve stimulation (ONS) has been used to treat medically refractory CCH and involves implanting one or two electrodes over the occiput to stimulate the greater occipital nerves [10,11,12,13]. Electrodes are connected to an internal pulse generator, typically in the subclavicular area. After implantation, the neurostimulator is programmed to achieve tolerable levels of paraesthesia in the greater occipital nerve distribution and used as a preventive therapy for TACs. Implantation of bilateral leads is recommended given the reports of conversion from unilateral to bilateral symptoms after initiating unilateral stimulation. Symptom improvement may not be seen for up to 3 months post-implantation; however, there is unlikely to be clinical benefit after 1 year of clinical unresponsiveness [6].
While the exact mechanism of ONS for TACs is unclear, it likely involves non-specific modulatory effects on descending pain-control systems. Although the paraesthesia induced by stimulation follows the occipital nerve distribution, the therapeutic goal is to mimic the ‘extra-occipital’ effects that were initially seen in glucocorticoid injection studies for primary headache prevention [14,15,16]. Early animal studies demonstrated anatomical convergence of somatic, cervical and trigeminovascular afferents on trigeminocervical complex nociceptors [17, 18], which serve as an important relay for head and facial pain to higher centres of pain processing in the thalamus, hypothalamus, and brainstem. These animal studies were later supported by flurodeoxyglucose positron emission tomography (FDG-PET) imaging in drug-resistant cluster headache patients who were treated with occipital nerve stimulation. Hypermetabolism in several pain areas normalized after 3–6 months of stimulation, whereas hypermetabolism in the untreated ipsilateral hypothalamus remained unchanged [19].
2.2.2 Evidence for ONS
To date, outcomes on 200 patients undergoing ONS for chronic cluster headache have been published in ten major studies, with reported efficacies ranging from 36% to 100% [10,11,12,13, 19,20,21,22,23,24]. In these studies, a positive therapeutic response was defined as patients who have achieved ≥50% improvement in headache attack frequency and/or severity compared to baseline. Three recent studies with larger cohorts report that 53–60% of patients were responder. Fontaine et al. reported a 60% responder rate in 44 CCH patients being treated with ONS at 12 months [22]; Miller et al. demonstrated a response rate of 53% in 51 CCH patients with mean follow-up of 39.2 months (range 2–81) [12], and Leone’s group reported 57% response rate in 35 CCH patients with a median follow-up of 6.1 years (range 1.6–10.7) [13]. Overall, 62.5% of patients were responders (see Table 13.1). Adverse events encountered can include electrode migration, hardware malfunction, hardware erosion through the skin and infection (2%) (see Table 13.1).
2.3 Vagal Nerve Stimulation
2.3.1 Mechanism and Use of Vagal Nerve Stimulation
The vagus nerve has connections to several brain centres important in pain regulation including the spinal trigeminal nucleus and the nucleus tractus solitarius. Studies in rats have identified reduction in pain and allodynia in the trigeminal distribution through stimulation of the vagus nerve, most likely secondary to ascending antinociceptive effect of that nerve on the second order neurons of the spinoreticular and spinothalamic tract [26, 27]. Early studies suggested direct inhibition of the afferents to the caudal trigeminal nucleus with acute stimulation of the vagus nerve [28]. Recent neuroimaging studies have shown inhibition of the activation of several structures involved in the pain matric of headaches, including the thalamus, limbic system and nucleus tractus solitarius, with chronic vagus nerve stimulation [29]. Studies have also suggested that stimulation of the vagus nerve may reduce glutamate concentration in the trigeminal nucleus caudalis, in turn possibly reversing central sensitization in chronic headache [30]. Therefore, activation of parasympathetic systems during attacks supports the use of non-invasive vagus nerve stimulator (nVNS) through the inhibition of afferent networks and neurotransmitters [26, 28, 30].
The gammaCore device is a non-invasive handheld transcutaneous vagal nerve stimulator applied to the neck (Electrocore©).
2.3.2 Evidence Base for Vagal Nerve Stimulation
The current evidence for the use in prevention of cluster headache attacks is limited to a manufacturer-sponsored trial involving 97 subjects. This trial of standard of care plus vagal nerve stimulation versus standard of care alone was conducted on the preventative and acute treatment of CCH using the gammaCore device Regular use of gammaCore for 4 weeks was associated with a significant reduction in attack frequency in the active compared to standard of care group (5.9 vs. 2.1 less attacks per week; p = 0.02) [31]. Similarly, the rate of subjects reporting more than 50% reduction in weekly attacks was higher in the active group (40% vs. 8.3%; p < 0.001). For comparison, the responder rate for verapamil 360 mg daily was 80% versus 0% for placebo in the single small randomized controlled trial available [32]. There are no well-controlled trial data on the use of VNS as a preventive treatment in ECH.
There are two randomized sham-controlled trials of vagus nerve stimulation in acute treatment of cluster headache [33, 34]. These studies were performed in episodic and chronic cluster headaches. Both studies failed to meet the primary end points of the trials. However, a post-hoc analysis showed superiority of nVNS in ECH. In ACT1, gammaCore resulted in a higher response rate (RR) (RR, 3.2; P = 0.014), higher pain-free rate for >50% of attacks (RR, 2.3; P = 0.045), and shorter duration of attacks (mean difference [MD], −30 min; P < 0.01) compared with the sham group [33]. In ACT2, gammaCore resulted in higher odds of achieving pain-free attacks in 15 min (OR, 9.8; P = 0.01), lower pain intensity in 15 min (MD, −1.1; P < 0.01) and higher rate of achieving responder status at 15 min for ≥50% of treated attacks (RR, 2.8; P = 0.058) compared with the sham group [34]. These data suggest that nVNS stimulation may be beneficial as an acute treatment in ECH but not CCH.
Reported side effects of nVNS are mild and include transient hoarseness, voice change, skin irritation, muscle ache and uncomfortable paraesthesia.
From the current evidence, the nVNS can be considered for the preventative treatment of chronic cluster headache as well as acute treatment of ECH but not CCH.
3 Central Neuromodulation Techniques
3.1 Cervical Spinal Cord Stimulation (SCS)
3.1.1 Mechanism and Use of SCS
Application of high cervical spinal cord stimulation (SCS) to treat TACs is based on clinical data from studies using SCS to treat other chronic pain conditions, in particular chronic back pain [35, 36]. In animal spinal cord models, afferent nociceptive inputs have been found to be inhibited by modulating a wide range of neuronal activity. For example, in chronic pain states, wide dynamic range (WDR) neurons are frequently hyperactive. Preclinical models have demonstrated that stimulation of these neurons at high frequency results in desensitization and decreased neuronal output, subsequently restoring them closer to their preinjury condition [35].
3.1.2 Operative Technique
SCS implantation for CH is similar to the techniques used for chronic back pain. Patients initially undergo a test stimulation phase for 7–14 days, where either one or two octad leads are placed in the epidural space. Fluoroscopy is used to determine the appropriate entry point on the skin, based on accessing the upper thoracic spine (usually the T2–3 interspace). After local anaesthetic is injected, a small incision is made under conscious sedation. Using fluoroscopic guidance, a 14-gauge Touhy needle is inserted into the T2–3 interspace and advanced cranially into the dorsal epidural space. Epidural placement is confirmed using a saline probe with loss of resistance technique.
Electrode(s) are advanced cranially in the dorsal epidural space until the distal lead tip reaches the area between the occiput and the C2 vertebral body. For normal frequency stimulation, intraoperative test stimulation is performed to confirm the presence of ipsilateral paraesthesia over the neck, occipital, parietal and frontal scalp areas, as well as the facial areas encompassing the C2 root sensory supply and V1–V2 trigeminal division. Test stimulation is not performed for high-frequency, paraesthesia-free stimulation systems.
Leads are anchored by suturing them to the supraspinal ligament and temporary extensions are connected and tunnelled under the skin surface. The extensions are then connected to an external stimulator during the trial period. High-frequency stimulation targets the dorsal columns at the C2–3 level, with parameters performed at 10 kHz frequency, 30 μs pulse width and 1.4–4 mA. If test stimulation is successful, permanent extensions and an internal pulse generator are implanted, typically in the gluteal region [37, 38].
3.1.3 Evidence for SCS
One small series evaluated SCS for treatment of CH both involve high-frequency, paraesthesia-free stimulation at 10 kHz and low-frequency stimulation with induced paraesthesia. Wolter et al. treated seven medication-resistant CCH patients with low-frequency SCS and followed them for a mean of 23 months (range 3–78). Continuous stimulation was used in all cases but one, where intermittent stimulation was used. Stimulation settings were as follows: frequency: 40–110 Hz, pulse width: 100–500 μs and amplitude: 2.0–25.5 mA. Six patients (85.7%) achieved at least 50% or more reduction in attack frequency and/or intensity and one patient achieved pain freedom. Baseline mean frequency of attacks decreased from 6 to 1.4 attacks/day. Five patients (71.4%) were able to discontinue triptan use, and the remaining two were able to reduce triptan dosages. Four patients were completely medication-free. All seven patients stated that they would recommend the treatment to other patients and six of seven would undergo the procedure again if given the option. Adverse events included one lead fracture requiring revision and two lead migrations requiring revision [37]. In addition to the case series described above, Lambru et al. treated one chronic cluster headache patient with high-frequency SCS who reported 50% improvement in attacks at 9 months [38]. See Table 13.2 for summary.
3.2 Deep Brain Stimulation (DBS)
3.2.1 Mechanism and Use of DBS
CH shows a striking periodicity. This consists of a circadian periodicity of single attacks often occurring at given hours of night and day, as well as a circannual periodicity of the cluster headache periods (the bouts) that typically follow a seasonal pattern. This periodicity strongly suggests that the biological clock mainly located in the hypothalamus has a role in the pathophysiology of cluster headache. A number of neuroendocrinological studies showed abnormalities confirming a derangement in some hypothalamic functions as the regulation of circadian hormone secretion, melatonin, cortisol and others.
The role of the hypothalamus in cluster headache was confirmed in a positron emission tomography (PET) study that showed activation in the ipsilateral inferior hypothalamic grey matter during a cluster headache attack. However, a comparison of the PET and fMRI studies reveals that the diencephalic/mesencephalic activation is more posteroinferior in the PET studies, straddling the hypothalamus and midbrain tegmentum, whereas the activation is centred on the hypothalamus in the higher spatial resolution fMRI studies [39]. A voxel-based morphometry study demonstrated increased neuronal density in the same area, but the study design was poor and another larger better designed study failed to reproduce this finding [40, 41].
These observations led to hypothesize that high-frequency hypothalamic stimulation could inhibit activation seen in cluster headache [7]. This was the case when a completely refractory chronic cluster headache patient received high-frequency hypothalamic stimulation with complete remission of attacks [7]; when stimulation was interrupted, attacks recurred, confirming that placebo effect was not behind the efficacy [8].
Potential DBS candidates should be evaluated at a specialized DBS centre by a multidisciplinary team consisting of neurologists, neurosurgeons and a neuropsychologist. In large-volume DBS centres, overall risks of the procedure can be lower than 1% for intracranial haemorrhage [42,43,44] and 2% for hardware infection [43, 45]. Other potential complications include seizure, hardware discomfort and hardware failure. Seizures are rare and typically transient, occurring only in the immediate post-operative period. Transient side effects associated with stimulation in the hypothalamic and ventral tegmental area may include vertical diplopia, dizziness, vertigo and emotional disturbances (i.e. panic, anxiety) [46, 47].
3.2.2 Operative Technique
DBS leads can be implanted with myriad stereotactic techniques and utilizing magnetic resonance imaging (MRI)-guided techniques or MRI-computerized tomography (CT) fusion. Most studies for TACs use frame-based (Leksell) stereotaxy with intraoperative microelectrode recording and test stimulation. Many centres target the posterior hypothalamus using atlas coordinates based on the midcommisural point (MCP). Target location varied between 2 and 6 mm posterior to the MCP, 0–2 mm lateral to the MCP and 1–3 mm below the mid-commissural plane. The procedure is performed under conscious sedation, and the electrode is introduced in a rigid cannula, 10 mm to target. Intraoperative test stimulation is performed typically at 60 μs, 180 or 185 Hz. Side effects seen with higher voltage macrostimulation of the posterior hypothalamus include diplopia, subjective mood changes (i.e. feelings of anxiety, fear and/or panic), vertigo and changes in blood pressure or pulse rate [48,49,50,51,52,53,54,55,56,57].
The institute of one of the authors, The National Hospital for Neurology and Neurosurgery, Queen Square, UK, has adopted an MRI-guided, MRI-verified approach, without microelectrode recording, utilizing frame-based stereotaxy (Leksell frame model G) under general anaesthesia. This technique has been previously published for other DBS targets used in movement disorders [58, 59] and was used in our recent reports of chronic cluster headache and SUNA patients treated with ventral tegmental area (VTA) DBS. The most distal contact on the Medtronic 3389 lead is placed in the ventral tegmental area, which is visualized on a 1.5 T T2-weighted axial MRI sequence at a level immediately superior to the mammillary bodies, anteromedial to the red nucleus, and posterolateral to the mammillothalamic tract. An immediate post-implantation stereotactic MRI is obtained for patients without ONS implants to confirm lead positioning, and a stereotactic computerized tomography scan (CT) is obtained for patients with existing ONS hardware. Internal pulse generators are implanted in the infraclavicular area either in the same procedure or within a week after surgery [46].
After implantation, stimulators are programmed at 60 μs, 180–185 Hz, and the voltage is titrated based on clinical benefit and side effect profiles. The stimulation is delivered chronically, and patients are not typically given adjustable parameters, as is sometimes done during therapy for movement disorders such as Parkinson’s disease or essential tremor. Patients are usually evaluated more frequently in the initial 2–3 months. Similar to occipital nerve stimulation, if there has been no improvement after 6–12 months of stimulation, it is unlikely that stimulation will provide any clinical benefit [6].
3.2.3 Evidence for DBS
Initial functional neuroimaging studies in CCH patients reporting activation of the ipsilateral posterior hypothalamic area during acute CH attacks led to the first successful DBS electrode implantation in a CCH patient in 2001, with lead placement reported to be in the ipsilateral posterior inferior hypothalamic area. The patient experienced complete resolution of symptoms within 48 h of initiating stimulation and remained pain free at 13-month follow-up [48]. Since then, there have been over 100 patients implanted with DBS (Table 13.3) [46, 49,50,51,52,53,54,55,56,57, 63, 65]. The target used in DBS for CH was initially called ‘the posterior hypothalamus’; however, the area between mammillothalamic tract and red nucleus is more accurately referred to as the ventral tegmental area [39].
Overall improvement has been reported in more than 60% of the patients [23]. Continuous stimulation takes weeks to months to exert its preventive effect [9,10,11,12,13,14,15,16,17,18,19,20,21,22] and this latency clearly suggests that inhibition of stimulated neurons is a too simplistic hypothesis to explain DBS effect, pointing to a more complex mechanism of action [66]. In accordance with this is the observation that acute hypothalamic stimulation does not improve ongoing CH attacks [51].
The only sham-controlled trial for DBS was a multicentre study led by Fontaine and colleagues, randomizing 11 chronic cluster headache patients to receive active versus sham stimulation over a 1-month period. There were no differences in primary and secondary outcomes measures during the blinded sham versus active stimulation phase. However, this may have been related to the relatively short duration of the randomized phase, given that it is now established that 3–6 months may be needed to develop a response to DBS. After an additional 10 months of open-label stimulation in all patients, 54.5% (n = 6) achieved >50% improvement in frequency of attacks and three of these patients were pain-free [56].
Due to its invasiveness, DBS has to be considered as the last-line preventive treatment for the most severe chronic CH patients [67, 68]. An experienced multidisciplinary team combining headache and functional neurosurgery expertise is essential for the care of these cohorts of patients. Severe selection criteria have been published and should be strictly followed [60, 68]. It is of interest that endoventricular stimulation of the hypothalamus using a floating DBS electrode on the floor of the third ventricle has been effective in relieving chronic drug-resistant cluster headache [65].
3.3 Putative Mechanisms of Action of DBS
The trigemino-hypothalamic tract carries sensory information from trigeminal territories to posterior hypothalamus [29]. It is now clear that posterior hypothalamus modulates neuronal activity inside the trigeminal nucleus caudalis (TNC). Injection of orexins A and B into the posterior hypothalamus change neuronal pattern discharge in the TNC [30] and based on these observations, it has been hypothesized that hypothalamic orexinergic system has a role in CH pathophysiology [31]. The role in analgesia exerted by the orexinergic system involves also other hypothalamic areas as the lateral hypothalamus through activation of orexin-1 in the periaqueductal grey matter (PAG) [32]. The reduction of orexin-1 in the CSF of both episodic and chronic CH patients supports involvement of orexinergic system [33] even if this reduction could be due to both a hypofunctioning hypothalamic descending antinociceptive pathway or be a consequence of pain. From historical point of view, it is of interest that involvement of posterior hypothalamus in pain control in humans was first demonstrated by Sano et al. who lesioned the posteromedial hypothalamus to treat intractable facial pain [34]. Genetic studies also lend some support to the involvement of the orexinergic system in CH even with conflicting results [35,36,37].
GABAA receptors in the posterior hypothalamus can also be involved. Bicuculline is a GABAA receptor antagonist; when is injected into the posterior hypothalamus, it affects neuronal discharge in the TNC [30]. Verapamil and topiramate are effective in CH prevention [38] and are both able to inhibit GABAA receptors in the CNS [69].
When hypothalamus is stimulated in CH patients, it produces activation at the site of the stimulator tip (the ipsilateral posterior hypothalamic area) and at the same time, activation of the ipsilateral trigeminal system [70]. It is of note that the activation was not associated with headache attacks [70]. Together with the observations that acute hypothalamic stimulation is not effective to abort CH attacks [26] and the latency needed by DBS to improve CH [71] suggest that the cluster generator is not located in the posterior hypothalamic area. A modulatory activity of the posterior hypothalamic area can be hypothesized instead [72], terminating CH attacks by regulating attack duration [70]. Accordingly, activation of posterior hypothalamic area occurs also in other TACs form [39, 40] and could be the key structure in the CNS responsible of the attack duration in the various forms of TACs.
CH patients undergoing chronic hypothalamic stimulation an increased ipsilateral cold pain threshold in V1 territories has been found, suggesting that hypothalamic stimulation improves CH by modulating the antinociceptive system [41]. In addition to orexinergic and GABAergic systems, dopamine and calcitonin gene-related peptide (CGRP) cells could be involved in the mechanism of action of hypothalamic DBS: in the periventricular posterior hypothalamic region, very close to that of placement of hypothalamic electrode tip in human hypothalamic stimulation [16, 48], lies the A11 nucleus containing dopamine cells, CGRP cells and dopamine cells co-localized with CGRP [49]. A11 nucleus projections to the spinal cord inhibit sensory and pain responses in TNC [49]. The increased V1 cold pain threshold observed in chronically stimulated DBS CH patients [4] could be mediated by enhanced A11 nucleus inhibitory activity on TNC cells.
In a positron emission tomography (PET) study, hypothalamic stimulation enhanced cerebral blood flow in areas of the so-called pain matrix: thalamus, somatosensory cortex and anterior cingulate cortex [70]. At the same time, deactivation was observed in the middle temporal gyrus, posterior cingulate cortex and insula [70]. In CH patients, altered metabolism in brain areas of the pain matrix, including posterior cingulate cortex, prefrontal cortex, insula, thalamus and temporal cortex, was reported in an 18F-fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET) [50], suggesting that hypothalamic stimulation could restore metabolism, in turn improving the top-down regulation on TNC. Areas involved in imaging studies in CH as anterior cingulate, insula and frontal lobe seem to play a role in the chronification of pain [51], probably through long-term potentiation [52]. Since blood flow is altered in these brain areas during hypothalamic stimulation, it can be argued that the stimulation acts on mechanisms behind pain chronification [53]. Reversal of chronic CH into episodic CH has been reported in patients undergoing long-term hypothalamic stimulation [71].
As an alternative, hypothalamic stimulation could improve CH by modulating parasympathetic activity in the superior salivatory nucleus [54].
4 Summary
Neuromodulation for CH includes stimulation of both peripheral and central targets. The non-invasive neurostimulation technique available is VNS, while the invasive neurostimulation techniques include SPG stimulation, ONS, high-cervical SCS and DBS. Non-invasive VNS may be used as an acute treatment in ECH and preventive treatment in CCH. Invasive neuromodulation should be considered in patients who have failed all conservative therapies and non-invasive neuromodulation. We recommend SPG stimulation or ONS as initial therapeutic options in compliant patients. Given that SPG stimulation is a minimally invasive implantation technique and can be used in both an abortive and preventive therapy, it is an attractive first-line therapy in CCH patients. Though used only as a preventive therapy, ONS can also be considered given its low risk of adverse events and well-established efficacy. Should peripheral neuromodulation strategies fail or be contraindicated, central neuromodulation methods can be considered. The response rates of DBS thus far appear comparable to ONS, though the therapy is associated with slightly different risks, albeit low, given the intracranial nature of the procedure. DBS can be considered as an alternative therapy for those who have failed SPG and/or ONS or those in whom peripheral modulation is contraindicated. High cervical SCS has recently emerged as an alternate central modulation technique, although current evidence is limited to small case series, and larger cohort and randomized placebo-controlled trials will be needed. Thorough patient evaluation by a multidisciplinary team at a specialist centre is necessary to determine the most appropriate treatment modality for the unique symptoms and clinical needs of each individual patient.
References
Headache Classification Subcommittee of The International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211.
Leone M, Proietti Cecchini A. Advances in the understanding of cluster headache. Expert Rev Neurother. 2017;17(2):165–72.
Russell MB. Epidemiology and genetics of cluster headache. Lancet Neurol. 2004;3(5):279–83.
Gaul C, Diener HC, Muller OM. Cluster headache: clinical features and therapeutic options. Dtsch Arztebl Int. 2011;108(33):543–9.
May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab. 1999;19(2):115–27.
Miller S, Sinclair AJ, Davies B, Matharu M. Neurostimulation in the treatment of primary headaches. Pract Neurol. 2016;16(5):362–75.
Schoenen J, Jensen RH, Lanteri-Minet M, Lainez MJ, Gaul C, Goodman AM, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 2013;33(10):816–30.
Barloese MC, Jurgens TP, May A, Lainez JM, Schoenen J, Gaul C, et al. Cluster headache attack remission with sphenopalatine ganglion stimulation: experiences in chronic cluster headache patients through 24 months. J Headache Pain. 2016;17(1):67.
Jurgens TP, Schoenen J, Rostgaard J, Hillerup S, Lainez MJ, Assaf AT, et al. Stimulation of the sphenopalatine ganglion in intractable cluster headache: expert consensus on patient selection and standards of care. Cephalalgia. 2014;34(13):1100–10.
Burns B, Watkins L, Goadsby PJ. Treatment of medically intractable cluster headache by occipital nerve stimulation: long-term follow-up of eight patients. Lancet. 2007;369(9567):1099–106.
Magis D, Allena M, Bolla M, De Pasqua V, Remacle JM, Schoenen J. Occipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot study. Lancet Neurol. 2007;6(4):314–21.
Miller S, Watkins L, Matharu M. Treatment of intractable chronic cluster headache by occipital nerve stimulation: a cohort of 51 patients. Eur J Neurol. 2017;24(2):381–90.
Leone M, Proietti Cecchini A, Messina G, Franzini A. Long-term occipital nerve stimulation for drug-resistant chronic cluster headache. Cephalalgia. 2017;37(8):756–63.
Afridi SK, Shields KG, Bhola R, Goadsby PJ. Greater occipital nerve injection in primary headache syndromes—prolonged effects from a single injection. Pain. 2006;122(1–2):126–9.
Ambrosini A, Vandenheede M, Rossi P, Aloj F, Sauli E, Pierelli F, et al. Suboccipital injection with a mixture of rapid- and long-acting steroids in cluster headache: a double-blind placebo-controlled study. Pain. 2005;118(1–2):92–6.
Anthony M. Headache and the greater occipital nerve. Clin Neurol Neurosurg. 1992;94(4):297–301.
Bartsch T, Goadsby PJ. Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain. 2002;125(Pt 7):1496–509.
Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain. 2003;126(Pt 8):1801–13.
Magis D, Bruno MA, Fumal A, Gerardy PY, Hustinx R, Laureys S, et al. Central modulation in cluster headache patients treated with occipital nerve stimulation: an FDG-PET study. BMC Neurol. 2011;11:25.
de Quintana-Schmidt C, Casajuana-Garreta E, Molet-Teixido J, Garcia-Bach M, Roig C, Clavel-Laria P, et al. Stimulation of the occipital nerve in the treatment of drug-resistant cluster headache. Rev Neurol. 2010;51(1):19–26.
Fontaine D, Christophe Sol J, Raoul S, Fabre N, Geraud G, Magne C, et al. Treatment of refractory chronic cluster headache by chronic occipital nerve stimulation. Cephalalgia. 2011;31(10):1101–5.
Fontaine D, Blond S, Lucas C, Regis J, Donnet A, Derrey S, et al. Occipital nerve stimulation improves the quality of life in medically-intractable chronic cluster headache: results of an observational prospective study. Cephalalgia. 2017;37(12):1173–9.
Mueller O, Diener HC, Dammann P, Rabe K, Hagel V, Sure U, et al. Occipital nerve stimulation for intractable chronic cluster headache or migraine: a critical analysis of direct treatment costs and complications. Cephalalgia. 2013;33(16):1283–91.
Burns B, Watkins L, Goadsby PJ. Treatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patients. Neurology. 2009;72(4):341–5.
Magis D, Gerardy PY, Remacle JM, Schoenen J. Sustained effectiveness of occipital nerve stimulation in drug-resistant chronic cluster headache. Headache. 2011;51(8):1191–201.
Bossut DF, Maixner W. Effects of cardiac vagal afferent electrostimulation on the responses of trigeminal and trigeminothalamic neurons to noxious orofacial stimulation. Pain. 1996;65(1):101–9.
Miller S, Matharu M. Non-invasive neuromodulation in primary headaches. Curr Pain Headache Rep. 2017;21(3):14.
Oshinsky ML, Murphy AL, Hekierski H Jr, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155(5):1037–42.
Kraus T, Kiess O, Hosl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal—a pilot study. Brain Stimul. 2013;6(5):798–804.
Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010;27(2):130–8.
Gaul C, Diener HC, Silver N, Magis D, Reuter U, Andersson A, et al. Non-invasive vagus nerve stimulation for PREVention and acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia. 2016;36(6):534–46.
Leone M, D’Amico D, Frediani F, Moschiano F, Grazzi L, Attanasio A, et al. Verapamil in the prophylaxis of episodic cluster headache: a double-blind study versus placebo. Neurology. 2000;54(6):1382–5.
Silberstein SD, Mechtler LL, Kudrow DB, Calhoun AH, McClure C, Saper JR, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache. 2016;56(8):1317–32.
Goadsby PJ, de Coo IF, Silver N, Tyagi A, Ahmed F, Gaul C, et al. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: a randomized, double-blind, sham-controlled ACT2 study. Cephalalgia. 2018;38(5):959–69.
Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation. 2013;16(1):59–65.
Al-Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15(3):347–54.
Wolter T, Kiemen A, Kaube H. High cervical spinal cord stimulation for chronic cluster headache. Cephalalgia. 2011;31(11):1170–80.
Lambru G, Trimboli M, Palmisani S, Smith T, Al-Kaisy A. Safety and efficacy of cervical 10 kHz spinal cord stimulation in chronic refractory primary headaches: a retrospective case series. J Headache Pain. 2016;17(1):66.
Matharu MS, Zrinzo L. Deep brain stimulation in cluster headache: hypothalamus or midbrain tegmentum? Curr Pain Headache Rep. 2010;14(2):151–9.
May A, Ashburner J, Buchel C, McGonigle DJ, Friston KJ, Frackowiak RS, et al. Correlation between structural and functional changes in brain in an idiopathic headache syndrome [see comments]. Nat Med. 1999;5(7):836–8.
Matharu MS. Functional and structural neuroimaging in primary headache disorders. PhD Thesis. Institute of Neurology, University of London, London. 2006.
Zrinzo L, Foltynie T, Limousin P, Hariz MI. Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review. J Neurosurg. 2012;116(1):84–94.
Chen T, Mirzadeh Z, Chapple K, Lambert M, Ponce FA. Complication rates, lengths of stay, and readmission rates in “awake” and “asleep” deep brain simulation. J Neurosurg. 2017;127(2):360–9.
Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120(1):132–9.
Patel DM, Walker HC, Brooks R, Omar N, Ditty B, Guthrie BL. Adverse events associated with deep brain stimulation for movement disorders: analysis of 510 consecutive cases. Neurosurgery. 2015;11(Suppl 2):190–9.
Akram H, Miller S, Lagrata S, Hyam J, Jahanshahi M, Hariz M, et al. Ventral tegmental area deep brain stimulation for refractory chronic cluster headache. Neurology. 2016;86(18):1676–82.
Leone M, Franzini A, Cecchini AP, Bussone G. Efficacy of hypothalamic stimulation for chronic drug-resistant cluster headache. Cephalalgia. 2012;32(4):267–8.
Leone M, Franzini A, Bussone G. Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med. 2001;345(19):1428–9.
Franzini A, Ferroli P, Leone M, Broggi G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery. 2003;52(5):1095–9.
Schoenen J, Di Clemente L, Vandenheede M, Fumal A, De Pasqua V, Mouchamps M, et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128(Pt 4):940–7.
Leone M, Franzini A, Broggi G, Bussone G. Hypothalamic stimulation for intractable cluster headache: long-term experience. Neurology. 2006;67(1):150–2.
Starr PA, Barbaro NM, Raskin NH, Ostrem JL. Chronic stimulation of the posterior hypothalamic region for cluster headache: technique and 1-year results in four patients. J Neurosurg. 2007;106(6):999–1005.
Owen SL, Green AL, Davies P, Stein JF, Aziz TZ, Behrens T, et al. Connectivity of an effective hypothalamic surgical target for cluster headache. J Clin Neurosci. 2007;14(10):955–60.
Bartsch T, Pinsker MO, Rasche D, Kinfe T, Hertel F, Diener HC, et al. Hypothalamic deep brain stimulation for cluster headache: experience from a new multicase series. Cephalalgia. 2008;28(3):285–95.
Bartsch T, Paemeleire K, Goadsby PJ. Neurostimulation approaches to primary headache disorders. Curr Opin Neurol. 2009;22(3):262–8.
Fontaine D, Lazorthes Y, Mertens P, Blond S, Geraud G, Fabre N, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 2010;11(1):23–31.
Seijo F, Saiz A, Lozano B, Santamarta E, Alvarez-Vega M, Seijo E, et al. Neuromodulation of the posterolateral hypothalamus for the treatment of chronic refractory cluster headache: experience in five patients with a modified anatomical target. Cephalalgia. 2011;31(16):1634–41.
Holl EM, Petersen EA, Foltynie T, Martinez-Torres I, Limousin P, Hariz MI, et al. Improving targeting in image-guided frame-based deep brain stimulation. Neurosurgery. 2010;67(2 Suppl Operative):437–47.
Foltynie T, Zrinzo L, Martinez-Torres I, Tripoliti E, Petersen E, Holl E, et al. MRI-guided STN DBS in Parkinson’s disease without microelectrode recording: efficacy and safety. J Neurol Neurosurg Psychiatry. 2011;82(4):358–63.
Leone M, Franzini A, Proietti Cecchini A, Bussone G. Success, failure, and putative mechanisms in hypothalamic stimulation for drug-resistant chronic cluster headache. Pain. 2013;154(1):89–94.
Leone M, Franzini A, Broggi G, May A, Bussone G. Long-term follow-up of bilateral hypothalamic stimulation for intractable cluster headache. Brain. 2004;127(Pt 10):2259–64.
D’Andrea G, Nordera GP, Piacentino M. Effectiveness of hypothalamic stimulation in two patients affected by intractable chronic cluster headache. Neurology. 2006;5(Suppl 2):140.
Hidding U, May A. Mere surgery will not cure cluster headache—implications for neurostimulation. Cephalalgia. 2011;31(1):112–5.
Seijo-Fernandez F, Saiz A, Santamarta E, Nader L, Alvarez-Vega MA, Lozano B, et al. Long-term results of deep brain stimulation of the mamillotegmental fasciculus in chronic cluster headache. Stereotact Funct Neurosurg. 2018;96:1–8.
Chabardes S, Carron R, Seigneuret E, Torres N, Goetz L, Krainik A, et al. Endoventricular deep brain stimulation of the third ventricle: proof of concept and application to cluster headache. Neurosurgery. 2016;79(6):806–15.
Leone M, Bussone G. Pathophysiology of trigeminal autonomic cephalalgias. Lancet Neurol. 2009;8(8):755–64.
Leone M, May A, Franzini A, Broggi G, Dodick D, Rapoport A, et al. Deep brain stimulation for intractable chronic cluster headache: proposals for patient selection. Cephalalgia. 2004;24(11):934–7.
Martelletti P, Jensen RH, Antal A, Arcioni R, Brighina F, de Tommaso M, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86.
Kudrow L. Cluster headache: mechanisms and management. Oxford: Oxford University Press; 1980.
Leone M, Bussone G. A review of hormonal findings in cluster headache. Evidence for hypothalamic involvement. Cephalalgia. 1993;13(5):309–17.
May A, Bahra A, Buchel C, Frackowiak RS, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352(9124):275–8.
Morelli N, Pesaresi I, Cafforio G, Maluccio MR, Gori S, Di Salle F, et al. Functional magnetic resonance imaging in episodic cluster headache. J Headache Pain. 2009;10(1):11–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
D’Antona, L., Cecchini, A.P., Leone, M., Matharu, M. (2020). Neuromodulation in Cluster Headache. In: Lambru, G., Lanteri-Minet, M. (eds) Neuromodulation in Headache and Facial Pain Management. Headache. Springer, Cham. https://doi.org/10.1007/978-3-030-14121-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-14121-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14120-2
Online ISBN: 978-3-030-14121-9
eBook Packages: MedicineMedicine (R0)