Abstract
Since their early invention, pacemakers have reached a considerable level of technological progress and a myriad of clinical indications. However, they have a reported complication rate of 1–6%. This obligates every emergency room physician to know and understand the basis of pacing and to be familiarized with the most common complications. Moreover, a fast and precise diagnosis has a considerable impact on mortality and morbidity. Hence, this chapter focuses on the most common pacemaker complications that could present to the emergency room. These emergencies can be divided into mechanical and electrical and range from mild to fatal. By including the most recent information from guidelines, reviews, and case studies, this chapter will give the emergency room physician a quick review of the complications, clinical diagnostic keys, high-clinical suspicion signs, and possible treatments. Additionally, a brief section about the basis of pacing is included. Other cardiac devices, such as defibrillators and cardiac resynchronization therapy, are out of the scope of this chapter and will just be briefly commented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pacemaker emergencies
- Pacemaker complications
- Pacemaker malfunctions
- Pacemaker abnormalities
- Pacemaker in the emergency room
- Pacemaker late complications
- Physical basis
- Magnetic resonance imaging
16.1 The Scope of the Problem
Pacemakers have won a preponderant role in today’s cardiology and nowadays are used to treat a huge variety of conditions. For instance, 425 new pacemakers are implanted per 100,000 people every year in America [1]. In addition, in 2009, 737,840 pacemakers were implanted, and 264,824 were replaced worldwide. Specifically, most of them (225,567) were implanted in the United States, whereas demographically speaking, Germany had the greatest quantity of newly implanted pacemakers per million population (927). Additionally, the most common indications for pacemaker implantation are high-degree atrioventricular block and sick sinus syndrome. The most common pacing mode is VVI/VVIR, especially in developing countries [2, 3]. Furthermore, the majority of leads are transvenous and bipolar and have an active fixation [2]. All of this obligates every emergency room (ER) physician to know how to appropriately and efficiently treat a pacemaker emergency.
16.2 Prevalence
Overall issues associated with pacemakers have a prevalence ranging from <1% to 6% [4] or 3% to 7.5% [5]. Complications can be classified according to the time elapsed after the implantation in immediate (related to the procedure), intermediate, late, and in mechanical or electrical (Table 16.1).
Moreover, rates of up to 19.5% of right ventricular (RV) pacing-induced cardiomyopathy (≥10% decrease in LVEF with LVEF <50%) were related with frequent RV pacing in patients with preserved ejection fraction. Other risk factors for pacing-induced cardiomyopathy are male sex, wide native QRS duration, and frequent RV pacing (>20%) [4, 13].
16.3 Pacemaker Functionality Aspects
Cardiac pacing has advanced a great deal since Elmqvist’s and Senning’s first totally implantable pacemaker in 1958 [14]. Basically, a pacemaker consists of a pulse generator and a lead or various leads implanted in the heart’s chambers. Nowadays pacemakers are more complex, and a five-letter code, proposed by the North American Society of Pacing and Electrophysiology and the British Pacing and Electrophysiology Group and reaffirmed by the Heart Rhythm Society in 2018, is used to describe their function (Table 16.2) [15, 16].
The first letter makes allusion to the chamber paced (V for ventricle, A for atrium, and D for dual/both), the second letter refers to the chamber sensed (V for ventricle, A for atrium, D for dual/both, and O for none), the third letter indicates how the device responds to sensed stimuli (I for inhibit, T for trigger, D for dual/both, or O for nothing), the fourth letter indicates if rate response is on (R), and the fifth letter identifies if multisite pacing is used (none O, in atrium A, in ventricle V, or in both atrium and ventricle D) [4]. The most common use of the fifth letter is for biventricular pacing used for heart failure treatment [3].
Some common pacing modes are AAI/AAIR, VVI/VVIR, VDD, DDD, DDDR, and VOO/DOO [4, 17], which are hereby presented:
-
AAI/AAIR : in this mode, pacing occurs in the atrium and is inhibited by a detected P wave (atrial event). It is used when the sinus node is dysfunctional, but the AV node conduction is conserved. The main advantage of this mode (when used with a single-chamber pacemaker) is that it avoids ventricular pacing and crossing the tricuspid valve. Rate response (AAIR) is added for patients with chronotropic incompetence.
-
VVI/VVIR : this mode was devised to pace the ventricle in the absence of an intrinsic ventricular event or to inhibit in the presence of one (inhibition by the QRS complex). Moreover, this mode is employed in cases of chronic atrial fibrillation, infrequent pauses, or bradycardias [4]. This is explained by the fact that VVI/VVIR is unable to sense stimuli from the atrium. Rate response (VVIR) is used in patients with chronotropic incompetence. This pacing mode can be delivered by a single-chamber pacemaker with a lead in the ventricle.
-
VDD : pacing can be delivered by a single lead that senses the atrium and the ventricle but only paces the ventricle. If an atrial event is detected, after a certain time interval, the ventricle is paced. On the other hand, if the intrinsic atrial impulse travels through the AV node normally or if there is an ectopic spontaneous ventricular complex resulting in a sensed ventricular event, the pacemaker is inhibited.
-
DDD/DDDR : when the sinus node is functional, but the AV conduction is abnormal, a dual-chamber pacemaker may be the option. This pacing mode is able of pacing the atrium in case the frequency drops below a set value and is also capable of pacing the ventricle if the AV conduction is dysfunctional. Additionally, by sensing the atrium, the pacemaker turns the sinus node into a biosensor for increasing the heart rate when needed [17]. Moreover, rate response (DDDR) is used as an additional indicator of physical activity for increasing the heart rate.
-
VOO/DOO : although only used temporarily, this mode is of great utility in certain situations. Specifically, asynchronous stimulation is employed when there is a risk of oversensing, which means that certain electromagnetic interfering signals (MRI or electrocautery, etc.) can be taken as intrinsic cardiac events. For instance, if one of these signals is detected in the atrium, the impulse could be carried to the ventricles, which may exceed the upper limit. Also, it is possible that the interfering signal is sensed in the ventricle as a native ventricular event and hence pacing would stop, leading to bradycardia or asystole in a pacemaker-dependent patient.
16.4 Most Common Indications to Implant a Pacemaker
The most common indications to implant a pacemaker, ICD, and CRT are summarized in the following table (Table 16.3).
16.5 Main Pacemaker Malfunctions/Abnormalities
Pacemaker malfunctions/abnormalities can be divided into mechanical or electrical complications:
-
Mechanical complications
-
Lead damage
-
Infections
-
Thrombosis
-
Lead perforation
-
-
Electrical complications
-
Failure to capture
-
Failure to pace
-
Failure to sense
-
Pacemaker-induced tachycardia
-
Runaway pacemaker syndrome
-
Battery depletion
-
Left ventricular dyssynchrony
-
Pacemaker syndrome
-
-
Mechanical and electrical complications
-
Tricuspid regurgitation
-
16.6 Mechanical Complications
16.6.1 Lead Damage
Leads may experience fracture or twisting. In a few severe cases such as Twiddler’s syndrome, Reel syndrome, or Ratchet mechanism, lead dislodgement may occur due to manipulation of the generator, causing it to twist inside its pocket [8, 18]. Additionally, lead’s resistance is a variable factor dependent on body position or edema (to name a few), but a resistance change of >30% might imply a lead defect/damage [4]. Moreover, it is crucial to understand that the term “impedance” (measured in ohms Ω) refers to all the forces that oppose to the current flux in an electric circuit or pacemaker [19]. The normal impedance value of a lead typically ranges from 250 to 1200 Ω, with an output of 5 V [19]. In the one hand, an impedance value lower than 250 Ω suggests that the lead’s insulation may be damaged (fewer forces opposing to the current flux). On the other hand, a high impedance along with a high myocardial depolarization threshold suggests a broken lead (stronger forces opposing the current flux) [19].
16.6.2 Infections
Infections are severe complications of cardiac implantable electronic devices (CIED) . For instance, device-related endocarditis has an incidence of 10–23%, while infection of a pacemaker following implantation goes from 0.13% to 19.9%. Additionally, the incidence of ICD infection ranges from 0.7% to 1.2% [9]. Cardiac device infective endocarditis has a high mortality rate of 24.5–29% (with up to a year follow-up periods) and an 80–100% explantation rate [20]. Moreover, 68–93% of infections are caused by Staphylococci and Gram-positive bacteria, whereas less than 18% of infections are due to Gram-negative bacteria. The fact that 15% of implantable cardiac device bacteria are culture negative must be considered [20].
Most of the infections related to pacemakers occur in the implantation pocket [9]. Device infection may present a few weeks later (a most common scenario) or up to 1 year after the procedure [4]. As a result of infected leads, vegetations can appear through all the lead path, which includes the tricuspid valve, the endocardium of the right atrium, and less frequently the right ventricle [9]. Echocardiography is effective in visualizing and measuring vegetations along with evaluating the hemodynamic state of the heart. Transesophageal echocardiography must be performed in pacemaker bearers with suspected infective endocarditis [21].
Clinical presentation of systemic infections and endocarditis of the leads or valves commonly are fever, chills, positive blood cultures, and intracardiac vegetation. Pocket infection signs are swelling, redness, erosion, purulent discharge, chronic pocket pain, and alterations in the scar. Pocket fluid collection (visible with ultrasonography) and soft swelling may also present [22]. In this case, recommendations are to take a blood culture, to perform sensitivity testing (if possible), and to initiate broad-spectrum antibiotics with focus on cutaneous flora (most commonly Staphylococcus aureus or Staphylococcus epidermidis) such as vancomycin [1, 22, 23]. Needle aspiration or incision of the pocket should be avoided, and the patient must be referred to a center experienced in treating infected devices to program removal and/or antibiotic therapy [4].
In case empirical treatment needs to be initiated, a list of possible antibiotics is provided according to the “Guidelines for the diagnosis, prevention, and management of implantable cardiac electronic device infection” published on behalf of the British Society for Antimicrobial Chemotherapy (BSAC) as host organization [20]: (iv, intravenous; q, every)
-
Generator pocket infection without further complications
-
Vancomycin (1 g BID iv) or
-
Daptomycin (4 mg/kg OD iv) or
-
Teicoplanin (6 mg/kg to a maximum of 1 g given at 0, 12, and 24 h and then OD)
-
-
Lead-associated infective endocarditis or lead infection or complicated generator pocket infection with pending blood cultures, like in the scenario of severe sepsis
-
Vancomycin (1 g bid iv) AND meropenem (1 g tid iv) or
-
Daptomycin (8–10 mg/kg od iv) AND meropenem (1 g tid iv)
-
-
Lead-associated infective endocarditis or lead infection or complicated generator pocket infection with negative blood cultures
-
Vancomycin (1 g bid iv) AND gentamicin (1 mg/kg bid iv) or
-
Daptomycin (8–10 mg/kg od iv) AND gentamicin (1 mg/kg od iv)
-
It is important to consider that doses need to be adjusted to the renal state of the patient. Moreover, daptomycin may be used to replace vancomycin in glycopeptide-intolerant patients or if nephrotoxicity is an issue. When selecting gentamicin, pre-dose levels must be <1 mg/L and post-dose levels 3–5 mg/L. Additionally, gentamicin may be replaced by meropenem.
16.6.3 Thrombosis
Venous thrombosis and stenosis are severe complications of pacemakers with an incidence of 1–3% [4]. Right atrial thrombosis is an uncommon pathology that can present asymptomatically or with signs of right-sided heart failure, obstruction, or pulmonary embolism [9]. Moreover, in 2 out of 53 autopsies performed in pacemaker bearers, a large right atrial thrombus was found. Both patients were older women and presented the thrombotic event approximately 1 month after device implantation and had signs of congestive heart failure and superior vena cava syndrome [9, 24, 25].
Echocardiography is an insightful tool for determining if the thrombus is recent or longstanding. According to Almomani et al., long-standing thrombi may contain calcium and most of the times are stationary . On the other hand, recent thrombi have a lower echo density and are highly mobile [9]. General signs for thrombosis are a pain, swelling, vein distention, and shortness of breath. As for standard venous thromboembolism, anticoagulants are the core of the treatment [1]. Finally, deciding whether to remove or change a lead or not is the responsibility of the implantation team, and it is not an emergency [1].
16.6.4 Lead Perforation
Perforation by a lead of a cardiac implantable device is an uncommon complication with an incidence of less than 1%. Moreover, perforation rates for pacemakers go from 0.1% to 0.8% and for implantable cardioverter defibrillators from 0.6% to 5.2%. This type of complication can be further divided into acute perforation , commonly resulting from the procedure, and subacute or delayed perforation, which takes place past the 1 month of implantation [9]. According to Hirschl et al., atrial perforation is more common than ventricular perforation , and ventricular perforation is more frequently caused by an implantable cardioverter defibrillator than by a pacemaker [26].
Apart from cardiac perforation , pleural perforation is also an acute complication of pacemaker implantation . Figure 16.1 depicts an anteroposterior chest X-ray of a pneumothorax case with subcutaneous emphysema after pacemaker implantation.
Almomani et al. conducted a review of 35 cases of delayed lead perforation reported in the literature in which his group concluded that the risk for cardiac tamponade and death is low [9]. Furthermore, Refaat et al. found that the symptoms accompanying a delayed perforation are variable, but some examples are syncope, chest pain, stimulation of extracardiac muscles such as the diaphragm, shortness of breath (possibly related to pneumothorax, hemothorax, hemopneumothorax, pneumomediastinum , and/or tamponade), chest discomfort (due to delayed pericarditis or mammary hematoma near the device pocket), hiccups caused by the stimulation of the phrenic nerve, swelling of the device pocket, and repetitive shocks due to a malfunctioning device. Moreover , patients may present unspecific symptoms such as dizziness or fatigue or be completely asymptomatic [27].
If the lead perforation is suspected, the following diagnostic sequence can be followed: device interrogation, chest radiography, echocardiography, and fluoroscopy [28]. Chest CT aids when other methods do not provide a clear diagnosis [9]. As such, myocardial perforation can sometimes be seen with a chest X-ray, and in much of the cases, it will show the lead’s displacement to a different position from the one it was originally implanted (Fig. 16.2). Hence, when possible, it is important to compare the chest X-ray taken in the ER with a control one ideally taken within 24 h after the pacemaker implantation [29].
Two-dimensional transthoracic echocardiography is also of help to diagnose lead perforation or dislodgement, along with some accompanying pathologies such as pericardial effusion and tamponade. Since transthoracic echocardiography beam may not pass through the wire’s path at first, it is important to keep in mind that multiple tomographic images should be taken to achieve a complete diagnosis [9]. Real-time 3D transthoracic echocardiography complements the 2D modality and is better and quicker to visualize the intracardiac part of the device’s lead [9]. Thus, if available, real-time 3D transthoracic echocardiography should be used when lead perforation is suspected.
16.7 Electrical Complications
16.7.1 Failure to Capture
In this complication, the pacing spike is delivered, but the cardiac muscle does not depolarize. On the ECG this can be identified as pacing spikes with no atrial or ventricular complexes following [1]. Figure 16.3 depicts an example of a failure to capture on the ECG. Some common causes for this complication are lead dislodgement or malposition, inflammation of the electrode-myocardium interphase, and electrolyte imbalances. Imaging techniques, ranging from a chest X-ray or echocardiography to chest CT, are useful to determine the position of the lead. Symptoms of the disease by which a pacemaker was initially indicated can appear. Standard ACLS management is suggested, and a transcutaneous pacemaker should be considered on pacemaker-dependent patients [1].
ECG of a patient with a dislodged atrial lead (same case of Fig. 16.2) that depicts a failure to capture and to pace. On DII, pacing spikes 2, 4, 5, 7, and 9 fail to elicit a ventricular contraction. Additionally, pacing spikes 3, 6, and 8 are incorrectly delivered due to a failure to sense. ECG parameters: heart rate = 40 bpm, QRS complex = 94 ms, QT/QTc = 510/449 ms, average RR = 1485 ms, QTcB = 425 ms, QTcF = 454 ms, speed = 25 mm/s, voltage = 10 mm/mV, filter = 0.05–300 Hz W
16.7.2 Failure to Pace
Here, the pacemaker is sensing correctly but not delivering pacing spikes when needed. On the ECG, there will be no pacing spikes, and thus, the native rhythm of the patient will be observed. The most common causes are lead fracture, battery depletion, failure of the generator, and oversensing [1]. Oversensing refers to the event when the pacemaker is affected by electrical interference (muscular potentials or electrical noise) and incorrectly senses it as coming from the heart. This inhibits the delivery of stimuli.
Another important cause of oversensing is called pacemaker crosstalk . This phenomenon happens with dual-chamber devices when the lead in one chamber delivers a pacing spike which is sensed by the lead on the second chamber as an intrinsic depolarization, therefore inhibiting the delivery of pacing spikes in the second chamber [30]. For example, the ventricular lead could sense an atrial depolarization spike as being ventricular in nature and inhibit ventricular pacing.
Causes of generator damage potentially leading to failure to pace are an internal malfunction, blunt trauma, MRI, radiation therapy, and use of electrocautery. Symptoms of pacing failure are frequently the same as those of the native pathology, such as bradycardia or high-degree atrioventricular block. Treatment consists of ACLS bradycardia management and interrogation and reprogramming of the pacemaker [1]. In the case of oversensing, switching the device into an asynchronous pacing mode (a constant frequency of 80–100 bpm) by placing a magnet over the pulse generator may help to avoid oversensing of the device (and therefore to avoid pacemaker inhibition). Extreme caution is advised in pacemaker-dependent patients [1].
16.7.3 Failure to Sense
In this malfunction, the pacemaker is not detecting the intrinsic chamber activity, and thus, regardless of the intrinsic beats, the device sends electrical impulses. Some frequent causes are lead dislodgement, lead fracture, scar tissue between the lead and myocardium interface, battery depletion, or low-amplitude cardiac signal [1]. The ECG will show inappropriately delivered pacing spikes (Fig. 16.3). Signs and symptoms of failure to sense will be those of congestive heart failure. Pacemaker under sensing must be considered when there is no obvious explanation for an exacerbation of congestive heart failure [1]. Interrogation of the device to obtain key functionality parameters is suggested alongside with pacemaker reprogramming.
16.7.4 Pacemaker-Induced Tachycardia
This complication occurs most commonly in old dual-chamber devices and is caused by atypical conduction through the heart [1]. Specifically, a retrograde P wave may initiate a reentry circuit by falling just after the preprogrammed refractory period. This will make the device deliver rapid ventricular stimuli as a result of the continuously sensed atrial impulses [1]. First-line intervention for pacemaker-induced tachycardia /runaway pacemaker syndrome is to apply a magnet since it could break the anomalous rhythm. When the above method fails in an unstable patient, possible management options are reprogramming the device or external pacing [1].
16.7.5 Runaway Pacemaker Syndrome
The present malfunction is intrinsic to the device and represents an infrequent but serious pacemaker complication with an estimated incidence of 2–4% with 30–40% mortality [7]. This malfunction also occurs with implantable defibrillators. Additionally, runaway pacemaker events have been reported to present in a wide time range, from 2 days to 9 years after implantation [7]. Nonetheless, runaway pacemaker events can occur throughout the entire lifetime of the device. They can also present intermittently and remain undetected [7].
Although some authors [1] treat runaway pacemaker syndrome and pacemaker-induced tachycardia as synonyms, they have certain specific differences and distinct treatment methods. Runaway pacemaker syndrome can present in two forms : pacemaker-induced ventricular tachycardia and extreme bradycardia as a result of ventricular capture failure (due to “rapid, low-amplitude sub-threshold pulses”) [7]. In both forms mortality rates are high. Runaway pacemaker syndrome must be considered when pacing frequency exceeds the established upper limit, thus excluding pacemaker-mediated tachycardia [31].
The precise cause of runaway pacemaker is unknown, but it is associated with:
-
Primary circuit failure
-
Generator hermetic seal defects
-
Circuit damage due to an electric scalpel or radio-frequency ablation
-
Generator sterilization with heat
-
Electromagnetic interference during radiotherapy [32]
-
Low battery voltage [33]
It is important to mention that this syndrome is refractory to defibrillation therapy and antiarrhythmic agents since the problem is limited to the device. Poor results have been achieved by reprogramming the device or by overstimulation with a temporal electrode. Moreover, since magnet placement just disables the sensing feature of the device, this approach may be inefficient. Last treatment option is to disconnect the leads from the generator [7, 23].
16.7.6 Battery Depletion
Battery life is a variable parameter but a very important one for pacemaker-dependent patients. Hence, it is valuable to know some common clinical manifestations of a dying battery:
-
Pacing mode change into an asynchronous one (VOO or AOO)
-
Change on the width of the pacing spike
-
Battery voltage or impedance change [19]
Two important terms to have in mind are ERI and BOL, which mean elective replacement indicator and beginning of life, respectively, and inform on the power left on the device’s battery.
16.7.7 Left Ventricular Dyssynchrony
Right ventricular apical pacing is a risk factor for left ventricular dyssynchrony , which can lead to systolic and diastolic dysfunction, and ventricular remodeling. All of this is reflected clinically by worsening of heart failure. Furthermore, tissue Doppler and speckle tracking echocardiography are helpful to evaluate left ventricular dyssynchrony [9].
16.7.8 Pacemaker Syndrome
This pathology does not imply a malfunctioning pacemaker but rather a patient presenting unfavorable hemodynamics, namely, atrioventricular dissociation. This is common to see with VVI pacemakers since the synchrony between auricular and ventricular depolarization is lost. According to the Mode Selection Trial (MOST), 18.3% of the patients with sinus node dysfunction assigned to a VVIR pacing mode developed pacemaker syndrome [34]. Some of the most common symptoms presented are neurological of low cardiac output and of congestive heart failure such as general discomfort, fatigability, dyspnea, orthopnea, cough, dizziness, atypical chest discomfort, throat fullness sensation , and, less frequently, presyncope or syncope [3, 35]. Furthermore, patients may present hypotension, rales, jugular vein distention accompanied with cannon A waves, peripheral edema, and tricuspid or mitral (or both) regurgitation murmurs [3]. Lastly, when patients with a VVI pacemaker present pacemaker syndrome , a change to a dual-chamber device, such as DDD/DDDR, could be considered in some cases [3].
16.8 Mechanical and Electrical Complications
16.8.1 Tricuspid Regurgitation
Severe tricuspid regurgitation due to valve interference with an intracardiac device lead is an infrequent cause of progressive right-sided cardiac insufficiency and represented 2.8% of all the tricuspid valve surgeries [9]. Higher rates of tricuspid regurgitation were reported when more than 1 RV lead is implanted and with ICD leads because of their thickness and stiffness [12]. Tricuspid regurgitation can be functional or structural. When tricuspid regurgitation is associated with a pacemaker, the most common cause is functional (87%). On the other hand, when the regurgitation is directly induced by a pacemaker, the structural causes are divided as follows: restricted leaflet mobility (41%), adherent leaflet to the leads (37%), leaflet perforation (12%), scarring of leaflets (8%), and chordal entrapment (7%). The most commonly affected leaflet was the septal one (73%) [36].
Tricuspid valve regurgitation due to a pacemaker must be suspected in every patient with progressive right-sided cardiac insufficiency with early or late onset, without an apparent cause, and in cases that are refractory to habitual diuretic treatment. Echocardiography is central to the diagnosis of tricuspid regurgitation, and both 2D and 3D modalities may be used. However, 3D echocardiography has better efficacy to evaluate the route of the lead through the tricuspid valve [9].
16.9 High-Clinical Suspicion in the ER
A pacemaker emergency must be suspected when a patient arrives at the ER with low- cardiac output symptoms (hypotension, syncope, lipothymia, dyspnea, fatigability, etc.). Additionally, lead perforation should be highly suspected in thin elderly females and in patients taking steroids or anticoagulants [27]. Moreover, device infection needs to be considered in light of Staphylococcus aureus bacteremia, since it is the most common infectious agent related to lead endocarditis and device pocket infection [9]. Furthermore, in a patient with an embolic event (especially pulmonary embolism) and a cardiac device, a right-sided origin of the thrombus must be suspected [21]. Pacemaker undersensing (failure to sense) must be suspected when there is no obvious explanation for congestive heart failure exacerbation [1].
16.10 Risk Factors
Although establishing clear risk factors is complicated, Refaat et al. [27] reported that patients with a lower body mass and elderly female patients were specifically vulnerable to lead perforation [9, 27]. Additionally, patients with a thin myocardial wall, possibly due to dilated myocardiopathy or a previous infarction, are also vulnerable to lead perforation. However, patients with a normal myocardium or a hypertrophic one are not considered to be at lower risk [27, 29]. Twiddler’s syndrome is more common in female, elder, obese, and psychiatric patients [8]. Risk factors for pacemaker infection (pocket, endovascular leads, and valves) are diabetes, heart failure, renal failure, corticosteroid use, postoperative hematoma, lack of antibiotic prophylaxis, oral anticoagulation , previous cardiac device infection, generator change, and use of temporary pacemaker [4]. Finally, passive fixation leads and coronary sinus pacing leads (LV) have a higher risk of dislodgement [4].
16.11 Clinical Presentation
A typical patient with a malfunctioning pacemaker presents with bradycardia and/or hemodynamic instability due to abnormal stimulation. Additionally, the baseline rhythm of the patient (his indication for pacing) may manifest due to the malfunctioning device. Patients may also present tachycardia due to oversensing (pacemaker-mediated tachycardia). In either case, low cardiac output symptoms are common. On the other hand, patients with pocket infections more commonly present local signs of erythema or edema. Finally, hemodynamic instability could also be due to severe cases of lead infection or thrombosis.
16.12 Main Clinical Characteristics
-
Low cardiac output symptoms
-
Hypotension, dizziness, syncope, dyspnea, lipothymia, and fatigability
-
-
Return to baseline rhythm before pacemaker implantation (bradycardia or advanced degree AV block)
-
High pacing frequencies
-
Shock or hemodynamic instability
-
Suggestive signs of pocket infection such as erythema, edema, or tenderness to palpation
16.12.1 Physical Examination
Physical examination and device interrogation are the cornerstone to identify a pacemaker complication. When myocardial perforation is suspected, mammary hematoma, pericardial/pleural effusion, and chest wall bruising are key signs that may support the diagnosis [27]. Moreover, setting the device to a maximal stimulation output and hence the stimulation of the right or left hemidiaphragm or the chest wall indicate most of the times that a lead has perforated the atrial or ventricular wall. Additionally, interrogation of the device may show change in impedance, change in pacing parameters, loss of capture, elevated capture threshold, undersensing, and a noisy electrogram [27]. Nevertheless, normal parameters do not exclude lead perforation, and in case some of the above signs are found, image confirmation must be undertaken.
16.12.2 Clinical Stability
Some patients with a pacemaker complication may be asymptomatic, as in the case of lead perforations or right atrial thrombus discovered incidentally by chest CT or echocardiography, respectively [9, 26].
16.12.3 Clinical Instability
Since pacemakers are essentially antibradycardia devices, bradycardia or asystole in pacemaker-dependent patients may occur . Although some of the patients with right atrial thrombosis are asymptomatic, they can also present with symptoms of right-sided heart failure, obstruction, or embolization of the pulmonary artery [9]. Patients may present with septic shock in less than 10% of the cardiac device infection cases [20].
16.12.4 Chest X-ray
Chest X-ray is helpful in identifying twisted, fractured, or dislodged pacemaker cables (Fig. 16.3). It is also valuable to diagnose myocardial perforation by a pacemaker lead, since the migrated lead may be appreciated outside the heart. Furthermore, lead perforation must be suspected when the separation between the electrode tip and the epicardial fat is less than 3 mm [9]. In addition to posteroanterior chest radiography, a lateral projection is also of help to assess for the correct position of the device’s leads [29].
16.12.5 Electrocardiogram
The electrocardiogram is an important part of the clinical assessment of a pacemaker. A functional pacemaker produces a spike or artifact on the surface ECG . Commonly, these spikes will anticipate atrial or ventricular depolarization [1]. These spikes are often difficult to appreciate, but setting the ECG filter to 150 or 300 Hz should make them more visible.
Most of the atrial leads are placed in the right atrial appendage, and thus P waves are normally positive on the inferior wall, DI, and AVL. An apical pacing lead will be seen as a left bundle block (QS or rS morphology in V1–V2 and wide QRS) since the depolarization stimulus travels from the RV to the LV. Moreover, the QRS complex will be discordant from the T wave [1]. On the other hand, a right bundle branch block suggests that the lead is in the left ventricle, which can result in thromboembolism or in ventricular arrhythmias. If this is discovered during the implantation procedure, leads must be repositioned. If this is detected after the implant, anticoagulation must be initiated, and a repositioning procedure must be planned [4].
Monophasic pacemakers (older devices) produce a clearly noticeable artifact on the ECG, while biphasic pacemakers (modern devices) produce a mostly indiscernible spike [1]. In the case of biphasic pacemakers, sometimes it is useful to increase the amplitude of the ECG to make the pacing spike noticeable [1]. Biphasic pacemakers (which can also act as monophasic) reduce the risk of over detecting muscular potentials, far-field detection, and stimulating the skeletal muscle [19]. In case of lead perforation suspicion, right bundle branch morphology might be seen in V1, while the right ventricle is paced [27].
As it was previously stated, pacing leads are normally placed on the apex of the RV. Other implantation sites higher up in the septum are also possible, but the left bundle branch block morphology will persist . However, inferior ECG leads can have a variable axis [37]. The following table summarizes the most common electrocardiographic features found according to the lead implantation site as reported in [38] (Table 16.4).
Finally, a recently published algorithm called TBC helps to quickly assess for complications in the electrocardiograms of patients with pacemakers [39]. This method is easy to remember since each of its letters represents a sign of alarm:
-
Tachycardia with spikes (T): spikes (pacing artifacts) stimulating at a frequency of 120 bpm or more (2.5 big squares [500 ms] or less after the previous QRS complex)
-
Bradycardia without spikes (B): no QRS complex during a 1500 ms time period (7.5 big squares) after the previous QRS, which translates in a frequency of 40 bpm
-
Chaos (C): spikes with no relation to the QRS complex (pacing artifacts within the QRS-T complex or not followed by a QRS and at different distances from the following QRS complex)
If the T criterion is found (most commonly produced by pacemaker-mediated tachycardia), elective referral to a specialist is recommended. On the other hand, both B and C require urgent pacemaker evaluation by a specialist and are indicative of severe malfunctions.
The sensitivity and specificity of this quick test are high, with 86.3% and 94.2%, respectively. Moreover, it has a positive predictive value of 88% and a negative predictive value of 93.3%, which means that if none of the above criteria are meet, the chances of finding an abnormal device are very low. Additionally, the algorithm improved the diagnostic and referral ability of non-cardiologist (including ER physicians) when dealing with patients with pacemakers. Unfortunately, atrial lead dysfunction, VOO programming , and advanced pacemaker functions are part of the limitations of this method [39].
16.12.6 Transthoracic, Transesophageal, and 3D Echocardiography
Echocardiography is a convenient diagnostic tool for detecting and, thus, properly treating pacemaker-related complications. Transthoracic echocardiography is useful to locate the path of pacemaker leads within the heart cavities (Figs. 16.8 and 16.9) and identify lead dislodgement, cavity perforation by lead, hemopericardium, or images suggesting a thrombus, but the diagnosis must be confirmed by other means such as transesophageal echocardiography, which is more sensible. Moreover, transesophageal echocardiography can be used to inspect for vegetations or masses with a sensibility of 92–96%, compared to a 22–30% of the transthoracic echocardiography [9]. Specifically, transesophageal echocardiography may be used when a thrombus on a pacemaker lead is suspected [40]. Real-time three-dimensional echocardiography , along with 2-dimensional echocardiography , is also helpful in the diagnosis of pacemaker complications, especially lead issues [9]. It is important to keep in mind that due to right ventricular pacing, patients may normally present paradoxical septal motion as a cause of the anticipated electrical activation of the right ventricle [9].
CT scan of the patient described in Fig. 16.8 mitral prosthetic mechanical valve, total hip replacement, and a VVI pacemaker can be appreciated
Transthoracic echocardiography may be limited as a result of a poor acoustic window and because of the presence of lead reverberation artifacts. Additionally, sometimes it is difficult to distinguish between the lead tip, abnormal masses, or the tricuspid valve with a transthoracic echocardiogram due to poor echogenicity, limited window, or artifacts. On the contrary, transesophageal echocardiography is better to view the entire lead passage through the heart cavities. Furthermore, real-time transthoracic 3D echocardiography offers multiple views from a single acquisition and is helpful in the assessment of masses adhered to the leads [9].
Echocardiography is the preferred imaging technique to inspect masses on cardiac device leads since MRI is contraindicated in some types of pacemakers and CT is generally affected by metal artifacts. Vegetation usually looks as an oscillating intracardiac mass located on the pacemaker leads, valve leaflets, or endocardium [9]. Nevertheless, distinguishing between thrombus or vegetation as the origin of the mass is complicated. Hence, echocardiography must always be complemented with clinical and laboratory evidence [9]. Finally, echocardiography is an operator-dependent study, and thus, having an echocardiography expert perform the studies in pacemaker patients could be an important factor to achieve a correct diagnosis.
16.12.7 Chest Cardiac Tomography (CT)
Chest CT is an important diagnostic tool for pacemaker complications. It is of special utility when lead perforation is suspected, and other diagnostic modalities were inconclusive. For instance, 15 of 100 completely asymptomatic patients with a cardiac device were incidentally diagnosed with subacute lead perforation when they underwent a CT whose primary clinical indication was other than lead perforation [26]. Leads create a star artifact when imaged with a CT, a common artifact caused by metal implants (Fig. 16.10). Commonly, the lead tip may be defined as the center of the star artifact [26].
ECG synchronized chest CT can be used in the diastole phase to assess for myocardial lead perforation [29]. CT 3D reconstruction could also be performed and offers good visualization of the lead. Finally, chest CT is safe to use with cardiac device bearers with no serious or permanent complications reported [28].
16.13 Laboratory Evaluation
Laboratory test is of special utility since a failure to capture or undersensing may be due to electrolyte imbalances [30]. Moreover, blood and lead tip cultures may help to identify a pacemaker infection. Especially, Staphylococcus aureus bacteremia could be related to lead endocarditis or device pocket infection [9].
16.14 Multimodal Diagnosis Approach
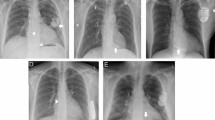
Some pacemaker complications may be asymptomatic, but others generally present as palpitations, anxiety, lightheadedness, or as full cardiac arrest (Fig. 16.11). If a pacemaker abnormality is suspected , the patient must be connected to a cardiac monitor. Next, a 12-lead ECG (to evaluate cardiac rhythm and to look for electrical malfunctions) and a chest X-ray should be taken (to assess for mechanical problems such as a lead fracture or dislodgement). General laboratory tests are also suggested since the myocardial depolarization threshold could increase (leading to failure to capture) with electrolyte imbalances or ischemia [1].
16.15 Differential Diagnosis
Pacemaker complications are subject to be confused with a wide range of pathologies. For instance, paradoxical septal motion, which is a normal echocardiographic finding in some patients with right ventricular pacing, can also be observed in patients with RV volume/pressure overload or that have undergone cardiac surgery [9]. Additionally, observing noninfected strands adhered to the cardiac device leads is frequent. Those strands typically measure between 1 and 2 mm in width and 3 and 5 mm in length and are commonly localized in the right atrium [9]. Nevertheless, 6% of patients with an infection presented abnormal long filaments of more than 3 mm in width which were infected [9, 41]. Hence, clinical correlation is central to adequate differentiation between fibrin deposits, vegetations, or thrombi. Thus, it is vital to apply a multimodal diagnosis approach in which data from the image studies, the electrocardiogram, and the echocardiogram are fully integrated with the clinical history.
16.16 Treatment
In case of a failure to capture, ACLS management is suggested in addition to a transcutaneous pacemaker , or a temporal venous pacemaker if available, in case of a pacemaker-dependent patient [1]. Moreover, in case of a failure to pace, ACLS bradycardia management is recommended. Then, the device must be interrogated and reprogrammed [1].
Transesophageal echocardiography is of great help when establishing treatment for device-related infections. Indeed, if the patient presents myocardial abscess or lead vegetation bigger than 5 cm, surgery may be preferred over percutaneous extraction [9]. Apart from device removal, antibiotic therapy must be started [9].
When lead perforation is confirmed, there is a vast set of possible treatments according to the characteristics of the perforation and the device. If the electrode tip is inside the mediastinum and no bleeding events are registered, then a second cable may be implanted without the retraction of the perforating lead [27]. Nevertheless, maintaining an inoperative lead must be weighed against the risk for further migration of the perforating lead . In the presence of a cardiac tamponade possibly caused by lead perforation, drainage of the pericardial effusion and conservative management are recommended [27]. Extraction must be performed in a patient with uncontrolled bleeding or evolving hematoma and lead migration outside of the pericardium with the risk of vascular, pulmonary, or adjacent structures injury [27]. When micro-perforation is suspected, indications for repositioning a lead are refractory pericarditis pain, persistent effusion, or pacemaker malfunction (pacing or sensing abnormalities) [4].
The method of choice to extract a perforating lead depends on the fixation system. If the lead has an active fixation system, transvenous extraction can be performed with a low complication risk according to some electrophysiologists [27]. The above procedure must be executed under TEE vigilance , general anesthesia, and if possible with excimer laser sheath [27]. Moreover, the procedure can be done both in the electrophysiology laboratory or in the operating room, but the cardiac surgery service must be present in case of an emergency [27]. On the other hand, if the electrode has a passive fixation system, two-stage cardiac surgery is preferred since this type of electrodes is thicker and has a higher chance of injuring tissue if retracted. Finally, the risk of bleeding or injuring nearby tissues during extraction is diminished by cutting the lead tip first [27].
16.17 Response to Magnet
The following table applies to most of the devices. For a specific list, please consult the references listed (Table 16.5).
In most of the devices, when the magnet is removed, the device will return to normal programmed function. However, reprogramming might be needed by some ICD models after being exposed to a magnet [1]. It is important to remember, that no matter the manufacturer, pacing behavior at or below EOL is unpredictable. Finally, physicians will be in warning when applying a magnet, and to ponder its usage against reprogramming the device instead, since asystole complication have been reported with it use. Consider the limited availability of technicians with the skills to reprogram the device and the time this process could take reprogramming the device (time is taken to reprogram and availability of technicians with such skills), since asystole cases have been reported [47, 48].
16.18 Electrosurgery
Electrosurgery alludes to the usage of electric scalpels during a surgical procedure. Its main risk with pacemakers is the production of electromagnetic interference potentially leading to pacing inhibition, rapid delivery of stimuli, tissue damage, or sudden change in pacing parameter (power-on reset). There are two modalities of electrosurgery monopolar and bipolar. In monopolar electrosurgery, the active electrode is included in the cautery pen, but a dispersive electrode needs to be placed on the patient. In the case of bipolar electrosurgery , both electrodes are built into the cautery pen, making the electric current to be localized. For this reason, in patients with pacemakers, bipolar electrosurgery should be used when possible. If the monopolar modality is selected, the current pathway between the active and return electrodes should avoid the generator (at least 6 inches away from the device) [49]. Additionally, cautery burst duration should be limited to 5 seconds with 5 seconds or more gap between bursts [4].
When the surgical site is below the umbilicus, and the dispersive path is placed on the lower limbs, there is no need to reprogram the device, except when several inhibition events are observed [4, 49]. In case the operation site is over the umbilicus, and especially for pacemaker-dependent patients, the device must be interrogated before the procedure; pacing mode should be changed to asynchronous (DOO, AOO, VOO), either by reprogramming or by using a magnet; and at the end of the procedure, the device must be reprogrammed to its original parameters [4]. Finally, always have magnet ready to use during the procedure, especially if no device reprogramming is decided [49].
16.19 Magnetic Resonance Imaging (MRI): Yes or No?
MRI is a powerful diagnostic tool in clinical practice. It is estimated that half of the patients with a cardiac implantable electronic device will need an MRI scan once in their life [50]. MRI conditional systems include both a generator and leads that were specifically tested in combination. Thus, an MRI conditional generator with non-MRI conditional leads is not considered to be an MRI conditional system. Abandoned or fractured leads, epicardial leads, or components from multiple vendors make an MRI nonconditional system [50]. Especially, patients with epicardial leads should not be scanned with MRI.
The most frequent effect of MRI on pacemakers is an increase in the pacing capture threshold. Battery level could also result affected, and power-on reset events may occur [50, 51]. Apart from MRI , radiotherapy can also interfere with pacemakers, while high-dose radiation may create electrical currents in the semiconductor circuit of the device (Table 16.6) [28].
It is important to know that MRI conditional generators have an MRI programming pathway that must be turned on before the scan and off after the scan. Scanning should be performed with the prerequisites specified for the device (I A HRS recommendation).
MRI conditional devices have an exempt period in which the conditionality does not apply (commonly 3 months after implantation). Despite the later, it is reasonable to perform an MRI scan during this period with a profound risk-benefit analysis (IIa C-EO HRS recommendation).
In the case of MRI nonconditional devices, risk-benefit must be thoroughly pondered. MRI scans are reasonable for patients with cardiac implantable electronic devices if the following criteria are meet, no fractured, epicardial, or abandoned leads, and MRI is superior to other testing modalities (IIa B-NR HRS recommendation). In such cases, pacemakers should be programmed to an asynchronous pacing mode, and tachyarrhythmia detection should be disabled on implantable cardioverter defibrillators (I B-NR HRS recommendation).
16.20 A Brief Comment on the Physical Bases of Pacemakers and MRI Compatibility
MRI has its theoretical basis on nuclear magnetic resonance (NMR) spectroscopy . This essentially consists of analyzing the radio-frequency energy absorbed and emitted by certain atomic nuclei placed in an artificial magnetic field. Hydrogen is the most commonly used atom for clinical purposes. Moreover, MRI is especially useful when imaging regions with a high quantity of water and fat since hydrogen atoms are densely present in those tissue components [50].
It is important to have in mind that MRI scan procedures require the use of the following fields: static magnetic, gradient magnetic, and radio frequency. All these fields might interfere negatively with susceptible electronic devices, including cardiac electronic implantable devices. For instance, the static magnetic field strength used by MRI scanners ranges from 0.2 to 9 Tesla, which could lead to mechanical injuries by moving objects if the appropriate security standards are not followed [50].
Apart from Tesla, gauss is an alternative unit for measuring the strength of magnetic fields [52]. To convert these units, the following formula is used:
The clinical importance of this formula resides in the fact that the “safe” magnetic field strength area is 5 gauss [50].
A final comment is to be made on the meaning of SAR, a concept commonly used when talking about the energy absorbed by a tissue due to exposure to a radio-frequency field on MRI. Specifically, SAR is used to limit the energy delivered to a tissue to avoid thermic damage. The following formula is used to calculate SAR:
where E represents the peak electric field strength, σ the local tissue conductivity, and ρ the local tissue mass density [53]. Thus, the clinical significance of the formula is that SAR depends on both scanner parameters (electric field) and tissue factors (conductivity and mass density). As such, the effect of MRI scanning on patients with pacemakers is determined by the device, patient’s tissue condition, and the pulse sequence used for the study.
16.21 Guideline Recommendations
A selection of guideline recommendations in relation to pacemaker emergencies is given in Table 16.7.
16.22 Additional Clinical Practice Takeaways
-
It is important to remember that pacemakers are essentially antibradycardia devices. Hence, patients with a malfunctioning pacemaker may present to the ER with bradycardia or low cardiac output symptoms.
-
Not all pacemaker complications imply an abnormally functioning device. For instance, pacemaker syndrome is caused by the adverse hemodynamics created by atrioventricular dissociation.
-
The decision to remove or to implant a new lead without removing the previous one must be accompanied by the clinical data, a multimodality image approach (chest X-ray, echocardiography, fluoroscopy, and tomography), and device interrogation.
-
If the decision has been taken to extract an electrode in the case of a subacute (late) lead perforation, the cardiac surgery service must be called even if the lead is going to be transvenous extracted or repositioned.
-
In case electrosurgery is needed, try to direct the electrical current pathway at least 6 inches away from the device, and always have a pacemaker magnet ready to use during the procedure.
References
Cabrera D, Decker WW. Management of emergencies related to implanted cardiac devices. Emergency Medicine. 2nd ed. Saunders; 2012. p. 11. https://www.elsevier.com/books/emergency-medicine/adams/978-1-4377-3548-2.
Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009-a World Society of Arrhythmia’s project: 2009 SURVEY CARDIAC PACEMAKERS AND ICDS. Pacing Clin Electrophysiol. 2011;34:1013–27.
Hayes DL. Modes of cardiac pacing: nomenclature and selection. Link MS, Downey BC, editors. UpToDate; 2018. https://www.uptodate.com/contents/modes-of-cardiac-pacing-nomenclature-and-selection. Accessed 25 Sept 2018.
Mulpuru SK, Madhavan M, McLeod CJ, Cha Y-M, Friedman PA. Cardiac pacemakers: function, troubleshooting, and management. J Am Coll Cardiol. 2017;69:189–210.
Vanezis AP, Prasad R, Andrews R. Pacemaker leads and cardiac perforation. JRSM Open. 2017;8:1–3.
Aggarwal RK, Connelly DT, Ray SG, Ball J, Charles RG. Early complications of permanent pacemaker implantation: no difference between dual and single chamber systems. Br Heart J. 1995;73:571–5.
Pindado J, Cabrera JA, Farréa J. The runaway phenomenon: an unexplained pacemaker dysfunction. Rev Esp Cardiol. 2005;58:1130–1.
Mansur S, Kassab I, Sarsam N, Hansalia R. Twiddler’s syndrome: a rare but serious complication of pacemaker implantation. J Am Coll Cardiol. 2018;71:A2586.
Almomani A, Siddiqui K, Ahmad M. Echocardiography in patients with complications related to pacemakers and cardiac defibrillators. Echocardiography. 2014;31:388–99.
Sato D, Kitajima H, Mani H, Park C-H, Chun Y-H. Pacemaker lead fracture without an increase in lead impedance caused by cardiac fibroma. J Arrhythmia. 2013;29:357–9.
Vurgun VK, Baskovski E, Goksuluk H, Ozyuncu N, Tan TS, Altin AT, et al. Evaluation of right ventricular pacing parameters in patients with proliferative scar. J Interv Card Electrophysiol. 2018;53:249–54.
Chang JD, Manning WJ, Ebrille E, Zimetbaum PJ. Tricuspid valve dysfunction following pacemaker or cardioverter-defibrillator implantation. J Am Coll Cardiol. 2017;69:2331–41.
Khurshid S, Epstein AE, Verdino RJ, Lin D, Goldberg LR, Marchlinski FE, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014;11:1619–25.
Aquilina O. A brief history of cardiac pacing. Images Paediatr Cardiol. 2006;8:17–81.
Bernstein AD, Daubert J-C, Fletcher RD, Hayes DL, Luderitz B, Reynolds DW, et al. The revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. Pacing Clin Electrophysiol. 2002;25:260–4.
Heart Rhythm Society. 2002 NASPE position statement: the revised NASPE/BPEG generic code for antibradycardia, adaptive-rate, and multisite pacing. 2018. https://tinyurl.com/ycwwnyqt. Accessed 23 July 2018.
Castellano C, Pérez de Juan MA, Attie F. Electrocardiografía Clínica. 2nd ed. Barcelona: Elsevier; 2004.
Díaz JC, Mejía-Zuluaga M, Aristizábal JM, Marín JE, Velásquez JE, Uribe W, et al. A lost cable: «reel» syndrome. Rev Mex Cardiol. 2018;29:41–4.
Andersen H, Nielsen J. Marcapasos Cardíaco. Diagnóstico y tratamiento en cardiología. 2nd ed. México: Manual Moderno; 2003.
Sandoe JAT, Barlow G, Chambers JB, Gammage M, Guleri A, Howard P, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint working party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother. 2015;70:325–59.
Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, et al. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2010;11:461–76.
Aktuerk D, Lutz M, Luckraz H. An unusual swelling at the pacemaker pocket site. Ann Emerg Med. 2014;63:391–403.
Allison MG, Mallemat HA. Emergency care of patients with pacemakers and defibrillators. Emerg Med Clin North Am. 2015;33:653–67.
Nicolosi GL, Charmet PA, Zanuttini D. Large right atrial thrombosis. Rare complication during permanent transvenous endocardial pacing. Br Heart J. 1980;43:199–201.
Pech-Alonso B, Fermín-Hernández C, Saavedra-de Rosas SI, Cicero-Sabido RJ. Superior vena cava syndrome: clinical considerations. Rev Med Hosp Gen Méx. 2018;81:59–65.
Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD Lead perforation on CT. Pacing Clin Electrophysiol. 2007;30:28–32.
Refaat MM, Hashash JG, Shalaby AA. Late perforation by cardiac implantable electronic device leads: clinical presentation, diagnostic clues, and management. Clin Cardiol. 2010;33:466–75.
Mak GS, Truong QA. Cardiac CT: imaging of and through cardiac devices. Curr Cardiovasc Imaging Rep. 2012;5:328–36.
Awamleh García P, Talavera Calle P. Perforación ventricular por cable de marcapasos: diagnóstico con tomografía computarizada. Radiologia. 2014;56:472–4.
Safavi-Naeini P, Saeed M. Pacemaker troubleshooting: common clinical scenarios. Tex Heart Inst J. 2016;43:415–8.
Makaryus AN, Patrick C, Maccaro P. A rare case of “runaway” pacemaker in a modern CPU-controlled pacemaker. Pacing Clin Electrophysiol. 2005;28:993–6.
Zweng A, Schuster R, Hawlicek R, Weber HS. Life-threatening pacemaker dysfunction associated with therapeutic radiation: a case report. Angiology. 2009;60:509–12.
Ortega D, Sammartino M, Pellegrino G, Barja L, Albina G, Segura E, et al. Runaway pacemaker: a forgotten phenomenon? Europace. 2005;7:592–7.
Link MS, Hellkamp AS, Estes NAM, Orav EJ, Ellenbogen KA, Ibrahim B, et al. High incidence of pacemaker syndrome in patients with sinus node dysfunction treated with ventricular-based pacing in the Mode Selection Trial (MOST). J Am Coll Cardiol. 2004;43:2066–71.
Ausubel K, Furman S. The pacemaker syndrome. Ann Intern Med. 1985;103:420–9.
Saran N, Said SM, Schaff HV, Maltais S, Stulak JM, Greason KL, et al. Outcome of tricuspid valve surgery in the presence of permanent pacemaker. J Thorac Cardiovasc Surg. 2018;155:1498–1508.e3.
Olaya Sanchez A, Trujillo GJ. Hallazgos electrocardiográficos en pacientes con marcapasos definitivos: revisión de la literatura. Repert Med Cir. 2017;26:67–77.
Chavarriaga A, Duque M, Díaz JC, Duque L. Electrocardiograma de superficie en pacientes con dispositivos de estimulación cardíaca. RCC. 2014;21:308–17.
Higueras J, Olmos C, Palacios-Rubio J, Gómez-Polo JC, Martínez-Losas P, Ruiz-Pizarro V, et al. TBC: a simple algorithm to rule out abnormalities in electrocardiograms of patients with pacemakers. Cardiol J. 2018. https://doi.org/10.5603/CJ.a2018.0079.
Raut MS, Maheshwari A, Dubey S. Thrombus on pacemaker lead. Indian Heart J. 2015;67:S120–1.
Dumont E, Camus C, Victor F, De Place C, Pavin D, Alonso C, et al. Suspected pacemaker or defibrillator transvenous lead infection prospective assessment of a TEE-guided therapeutic strategy. Eur Heart J. 2003;24:1779–87.
Boston Scientific Corporation. Expected magnet response of Boston scientific pacemakers and defibrillators. Boston Scientific Corporation; 2016. https://www.bostonscientific.com/content/dam/bostonscientific/quality/education-resources/english-a4/EN_ACL_Magnet_Response_20160330.pdf. Accessed 03 Aug 2018.
Medtronic. MAGNET OPERATION CRHF technical services standard letter medtronic; 2016. https://wwwp.medtronic.com/crs-upload/letters/102/102_CQES-StandardLetter-MagnetInstructions-Combined-IPG-and-ICD-FINALv2-2016-Sep02.pdf. Accessed 25 Sept 2018.
St. Jude Medical. Magnet use for SJM implanted cardioverter-defibrillators. St. Jude Medical; 2016. https://www.sjm.com/professionals/resources-and-reimbursement/technical-resources/emi-mri-and-other-interference/medical-and-dental/magnet-use-icds?halert=show&clset=af584191-45c9-4201-8740-5409f4cf8bdd%3ab20716c1-c2a6-4e4c-844b-d0dd6899eb3a. Accessed 25 Sept 2018.
St. Jude Medical. Magnet use for SJM pacemakers. St. Jude Medical; 2015. https://www.sjmglobal.com/professionals/resources-and-reimbursement/technical-resources/emi-mri-and-other-interference/household/magnet-use-pacemakers?halert=show&clset=92f57278-460e-4300-b7fe-89e52a04194f%3acadddb93-fcc4-47f2-8ceb-fd88f01ca17f. Accessed 25 Sept 2018.
Jacob S, Panaich SS, Maheshwari R, Haddad JW, Padanilam BJ, John SK. Clinical applications of magnets on cardiac rhythm management devices. Europace. 2011;13:1222–30.
Ip JE, Liu TJ, Chen CL, Lerman BB. Asystole during pacemaker magnet application. Pacing Clin Electrophysiol. 2017;40:1176–9.
Schulman PM, Rozner MA. Use caution when applying magnets to pacemakers or defibrillators for surgery. Anesth Analg. 2013;117:422–7.
Crossley GH, Poole JE, Rozner MA, Asirvatham SJ, Cheng A, Chung MK, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) expert consensus statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Heart Rhythm. 2011;8:1114–54.
Indik JH, Gimbel JR, Abe H, Alkmim-Teixeira R, Birgersdotter-Green U, Clarke GD, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm. 2017;14:e97–153.
Higgins JV, Sheldon SH, Watson RE, Dalzell C, Acker N, Cha Y-M, et al. “Power-on resets” in cardiac implantable electronic devices during magnetic resonance imaging. Heart Rhythm. 2015;12:540–4.
Grover VPB, Tognarelli JM, Crossey MME, Cox IJ, Taylor-Robinson SD, McPhail MJW. Magnetic resonance imaging: principles and techniques: lessons for clinicians. J Clin Exp Hepatol. 2015;5:246–55.
Wang Z, Collins CM. Effect of RF pulse sequence on temperature elevation for a given time-average SAR. Concepts Magn Reson Part B Magn Reson Eng. 2010;37B:215–9.
Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–51.
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt O-A, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281–329.
Acknowledgments
Images are courtesy of Ana Lilia Rayas Gómez, MD, Hospital San Jose, Queretaro, Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jerjes-Sánchez, C., Gonzalez-Rayas, J.M. (2019). Pacemaker Emergencies in the ER. In: Cardiology in the ER. Springer, Cham. https://doi.org/10.1007/978-3-030-13679-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-13679-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-13678-9
Online ISBN: 978-3-030-13679-6
eBook Packages: MedicineMedicine (R0)