Abstract
Simple (unicameral) bone cyst (SBC) and aneurysmal bone cyst (ABC) are common benign bone tumors in children and adolescents. Although non-cancerous, they can cause substantive physical and psychosocial problems. Particularly for ABC, they mimic malignant tumors causing understandable anxiety and fear. As such, early and accurate diagnosis is essential. Primarily lytic lesions, they weaken the involved bones which predisposes to pathologic fractures, even with minimal trauma. Children are often restricted from sport and some daily living activities because of the fear of these fractures. Bone cysts involving the hip in children have special importance. Being a weight bearing joint, fractures in this area are a genuine risk, with concomitant occurrence of avascular necrosis being a concern. There are surgical challenges related to the pathoanatomy of these lesions, with only few surgical implants that can reliably stabilise the proximal femur without causing injury to the growth plate. This chapter provides an overview of both SBC and ABC, emphasizing currently accepted treatments and reported outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Part I: Simple (Unicameral) Bone Cyst
Introduction
A simple bone cyst (SBC) is a common non-neoplastic bone lesion in children. It is a fluid-filled cavity which consists of a single chamber (unicameral). Septations within the cyst are common, however, dividing the lesion into two or more chambers. As such, the term simple bone cyst is a more appropriate name [1]. The earliest description of SBC in the literature was attributed to Virchow in 1870, ([2]) and was subsequently identified in the remains of a child who lived in the early Middle Ages [3]. Early reports confused simple and aneurysmal bone cysts (ABC), often using the two names interchangeably.
The causes of SBC are not fully understood. Several theories have been proposed to explain why SBC is formed. The vascular theory is one of the earliest proposed, attributing cyst formation as being due to a localized blockage of interstitial fluid drainage from cancellous bone [4]. This was supported by two discoveries: a relative increase in pressure within the SBC compared to the contralateral normal bone marrow, and the low oxygen tension within the cyst fluid as compared to venous and/or arterial samples taken simultaneously [5]. Proponents theorized that venous obstruction within the bone could cause the formation of a bone cyst with these characteristics [5].
The presence of synovial cells within a SBC suggests that this entity may actually represent entrapped intraosseous synovium (secreting synovial fluid) within the bone. This was further supported by the striking similarities between synovial and SBC fluids [6, 7]. More recently, genetic abnormalities have been described in children with SBC. Chromosomal translocation between chromosome 16 and 20 has been identified as a sole abnormality in SBC [8]. Cytogenetic analysis of an 11-year-old boy with surgically resected SBC, revealed a highly complex clonal structural rearrangement involving chromosomes 4, 6, 8, 12, 16, and 21. These findings reinforce the need for further research to understand the possible genetic causes of SBC [9].
“The causes of simple bone cyst are not yet fully understood but may be genetically determined”.
Pathophysiology
SBC usually starts as a small endosteal lesion which gradually increases in size to occupy most of the metaphysis [10, 11]. SBC is typically asymptomatic unless there is an acute or impending fracture. As the cyst grows in size it causes cortical thinning via endosteal erosion, reducing its structural integrity and predisposing to pathologic fracture. When an SBC is juxta-physeal (within 0.5 cm), it is described as being “active” which is believed to have a worse prognosis. SBC may cross the growth plate into the epiphysis and if so may cause growth disturbance and deformity. Furthermore, treatments directed at cyst healing may have increased risk of growth arrest if cyst crosses into epiphysis. When an SBC migrates away from the physis, it is called a “latent cyst” which is believed to have a more benign course [1, 12].
Histologically, SBC appears as a single cyst that is lined a thin layer of epithelium. The walls of the cyst are thin and usually contains reactive bone formation, scattered giant cells and occasionally cholesterol clefts are seen on the cyst wall. There are usually amorphous cement-like fibrin depositions which are considered important clues for the diagnosis (Fig. 33.1) [13].
Simple bone cyst histology. Simple bone cyst showing a thin-walled cyst filled with serous fluid. One-layer mesothelium-like epithelium lining the thin cyst wall. There is cholesterol deposition on the cyst wall and amorphous pink fibrinous substance. (H&E; ×100). (Courtesy Edmund Cheesman, Royal Manchester University Hospital)
In a series of 143 patients with various bone cysts, Doganavsargil and colleagues showed that 8.4% of patients exhibiting typical SBC features also had ABC-consistent areas either in the same biopsy sample (n = 5) or in the repeat biopsy (n = 7).
It is reported that SBC can mimic ABC if there is a fracture and the fluid is replaced with blood. History of fracture, trauma or radiological images could aid the diagnosis.
The content of SBC has been studied. The cyst fluid is rich with prostaglandin E2, lysosomal enzymes, IL-1 and oxygen free radicals [14, 15]. These are often implicated with respect to the erosive changes of bone during cyst expansion. Since steroids are known to inhibit prostaglandin synthesis [16, 17], this may help explain the beneficial effect of intralesional steroid injection for the treatment of SBC [18,19,20].
All SBCs are thought to eventually resolve but the bony changes can remain for a long time, making the definition of cyst healing more difficult [21]. Radiographic evidence of cyst healing have been suggested as follows: [22].
-
1.
Not healed:
-
(a)
Grade 1: clearly visible cyst
-
(b)
Grade 2: cyst that is visible but multilocular and opaque.
-
(a)
-
2.
Healed
-
(a)
Grade 3: sclerosis around or within a partially visible cyst
-
(b)
Grade 4: obliteration of the cyst (complete healing).
-
(a)
Natural History
Our understanding of the natural history of SBC has advanced over the last decade, with more studies identifying aspects of its natural history and prognosis. The relative rarity of SBC in adults supports the hypothesis that some proceed to spontaneous resolution but not all cysts resolve after skeletal maturity and prognostic factors have not yet been determined.
Contrary to the common belief that SBC heals after a pathological fracture, this phenomenon is inconsistent and likely coincidental. The healing fracture callus initially fills the cyst with bone—either fully or partially—but this usually resorbs after six months; with a low likelihood of persistent cyst healing (Fig. 33.2). The reported healing rates of SBC following a fracture ranged between only 5–15% [23,24,25,26]. Neer reported 70% of the cysts had a repeat fracture within two years of the initial one [21].
Pathological fracture through a simple bone cyst. This young boy was treated with a hip spica for a pathological fracture secondary to a simple bone cyst. Although the cyst initially healed with bone filling the lesion; this bone eventually resorbed and the cyst was larger than pre-fracture at 3 year follow-up
“The reported healing rates of simple bone cyst following a fracture have been reported to be between 5–15%, and the majority do not heal”
Although SBC is considered a benign bone lesion—in the sense that it does not risk life or limbs—the potential physical, psychological and social problems that it can cause are serious. Kaelin studied the natural history of 58 SBCs in 57 patients. They developed 64 fractures in total; which were the initial presenting findings in 94% of the humeral cyst SBC but for only 47% of patients with femoral SBC [27]. Interestingly, there were more fractures in humeral cysts (50 fractures in 31 patients) than femoral cysts (14 fractures in 21 patients) but patients with femoral cyst underwent more operations (20 operations in 12 patients) than those with humeral fractures (11 operations in 9 patients). The authors also observed the natural history of SBC in 11 untreated humeral cysts that were followed for more than one year. Spontaneous healing occurred in five cases only (<50%), who had sustained a total of nine fractures. Other studies reached same conclusion [1, 28].
Several characteristics have been studied to identify features that could predict pathologic fractures associated with SBC. Kaelin and colleagues [27] introduced the concept of a “cyst index” (Fig. 33.3). The index is based on the proportion of the radiographic area of the cyst and the size of the involved bone. A low cyst index indicates a small cystic area in relation to the bone; conversely, a high index indicates a large cyst. The smallest cyst in their series was measured as a cyst index of 0.1, the largest 12.78. They reported that there were no fractures in 40 patients who had a cyst index lower than 3.5, even when they did not curtail their activities. They also showed that in 57 patients, no further increase in the cyst index occurred after its spontaneous decrease, which means that all cysts which begin to heal spontaneously will ultimately heal completely. Cysts with at least two consecutive decreased indices of less than 3 and a cortical wall thicker than 2 mm were considered to be healed; as fractures were found to never occur in these patients.
The value of the cyst index has been contested as a good predictor for future fracture or healing. A study involving 32 SBCs (femoral and humeral) showed that the sensitivity, specificity, positive and negative predictive values were small; particularly for the femoral cysts (50%, 33%, 33% and 50% respectively). The 1.4-year short follow-up of the study was a major weakness, however [29].
In a series of 75 children with SBC, Ahn and colleagues found that the width of the lesion occupied more than 85% in both AP and lateral radiographs for every case of associated pathological fracture [23] . Leong and colleagues introduced the use of a new model to assess bone structural integrity associated with SBC based on quantitative computed tomography [30]. In their study, the resistance of the affected bone to compressive, bending, and torsional loads was calculated via a rigidity analysis, performed using serial trans-axial quantitative computed tomography data. They reported that no patient sustained a fracture when a favourable rigidity analysis resulted.
SBC has been shown to cause bone deformity even without fracture. This happens when the bone cysts involve or are close to the physis [31,32,33,34,35,36].
Epidemiology
SBC is one of the most common benign bone tumours in children; along with osteochondroma and non-ossifying fibroma. SBC occurs most frequently in children between 5–15 years old. It is rare in adults. Any bone can be affected but the proximal humerus and proximal femur are the most common sites for SBC; accounting for nearly 90% of all cases [37,38,39]. Neer reported the demographic distribution of 175 patients with SBC with the proximal femur being involved in 18% of cases Table 33.1 [21]. The acetabulum was not an identified site for SBC in their series.
Clinical Presentation
There is a spectrum of how patients with SBC may present. Some patients may be asymptomatic with the cyst being found incidentally during investigation after an injury or for unrelated reasons. Some patients may present with pain in the involved bone that has been gradually getting worse. When pain is associated, it is typically activity related and relieved with rest. Pain associated with a proximal femoral SBC may initially present as knee pain referred from the hip, leading to a delay in diagnosis and unnecessary investigations involving the knee (Fig. 33.4). Groin and thigh pain are also common presentations.
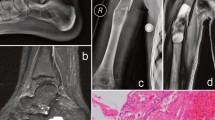
10 year-old male with simple bone cyst (SBC) of the proximal femur and right knee pain. (a) Anteroposterior (AP) and lateral X-rays of the knee showing no abnormality. (b) After many months, AP pelvis and lateral hip X-rays are performed, identifying the presence of a bone cyst in the proximal femur. (c) T2-weighted MRI shows the lesion to be fluid-filled (homogenous high signal), consistent with the diagnosis of a SBC. (Courtesy Jason J. Howard)
A pathological fracture was reported to be the initial presentation in just under 50% of patients with femoral SBC [27]. These fractures may occur even after a trivial trauma mechanism.
Physical examination of the patient with a bone cyst should include a general assessment to look for features associated with a more aggressive lesion (decreased weight percentile, cachexia) that may suggest a neoplastic cause. The presence of fever and sweating may suggest an infectious cause. Associated pallor and jaundice may suggest a haematopoetic cause.
A complete examination of the hip is required. For lesions associated with an impending pathologic fracture, an antalgic, and/or Trendelenburg gait may be apparent. Otherwise, the hip examination is typically normal. The examination should also include the axial skeleton and the uninvolved limbs. Any swelling, tenderness or deformities should prompt radiologic examination of any affected areas.
Palpation of major lymph node areas (e.g., the axillary and inguinal areas) is also appropriate as infection and malignancy are part of the differential diagnosis.
Essential Clinical Tests
There is no specific clinical test for simple bone cyst. However, several tests should be performed to exclude other conditions:
-
General (fever, sweating, weight loss, pallor and jaundice are NOT features of simple bone cyst)
-
Involved limb (unless there is a fracture, a limb with an associated SBC looks and feel normal in contrast to aneurysmal bone cyst or other cystic conditions)
-
The child with an impending pathologic fracture of the proximal femur may have a gait disturbance: either antalgic or Trendelenburg.
-
Pain associated with a proximal femoral UBC may initially present as knee pain referred from the hip. Groin and thigh pain are also common presentations.
-
The rest of uninvolved part (simple bone cyst is a single bone disease. It is extremely rare to have more than one lesion present)
Imaging
Plain Radiograph
The plain radiograph appearance of SBC is virtually diagnostic. SBC is a well-defined lucent lesion with a narrow zone of transition. It is rare in skeletally mature patients. The lesion is often centrally located with well-defined sclerotic margin in the majority of cases with no periosteal reaction or soft tissue component. The cyst can expand the bone with thinning of the endosteum without breaching the cortex unless there is a pathologic fracture (Fig. 33.5) [40].
Simple bone cyst involving the proximal femur. Simple bone cyst in a 5-year-old boy. The cyst is a well-defined lucent lesion with a narrow zone of transition. The lesion is often centrally located with well-defined sclerotic margins in the majority of cases, with no periosteal reaction or soft tissue component. The cyst can expand the bone with thinning of the endosteum without breaching the cortex unless there is a pathologic fracture associated
The “fallen fragment” (a.k.a. “fallen leaf”) radiographic sign was first described by Reynolds as pathognomonic in the radiographic diagnosis of a SBC (Fig. 33.6) [41]. The fallen fragment sign refers to the presence of a bone fracture fragment within the bone cyst. Although this sign is widely considered to be pathognomonic for SBC, it has occasionally been reported with other cystic lesions as well (e.g. eosinophilic granuloma) [42].
“The fallen fragment radiographic sign is not pathognomonic for simple bone cyst given its presence in other cystic lesions as well”
Cross-Sectional Imaging
CT and MRI are not routinely required and often add little to the diagnosis, however, they can be helpful in eliminating other conditions that mimic SBC, such as ABC. They are also helpful if a SBC is in close proximity to a growth plate where growth disturbance can potentially occur, particularly if the lesion is planned for curettage and bone grafting [43,44,45].
SBC can produce a wide variety of appearances on MRI. The characteristic MRI signal for an uncomplicated lesion are low signals on T1 and high signal on T2 (Fig. 33.7). For SBC, there are usually no fluid-fluid levels present on MR (indicative of blood within the cyst; a common finding associated with ABC) unless an associated fracture has occurred.
Essential Imaging Tests
-
Plain radiographs
-
Fallen leaf (i.e. fragment sign)
-
Cyst index
-
For knee pain with normal radiographs, consider imaging the pelvis
-
-
MRI scan
-
Low signal on T1 and high signal on T2
-
No soft tissue swelling
-
No fluid-fluid levels unless there is an associated fracture
-
Non-Operative Management
There is a role for non-operative treatment in selected cases with SBC. A small cyst with a good cortical thickness in the upper limb which is completely asymptomatic can be observed and treated non-operatively. However, this is not usually the case in SBC involving the hip. In general, the following factors should be considered when deciding on non-operative treatment of SBC:
-
1.
The site of the cyst: upper limbs are general under lower stress than lower limbs. A cyst in the neck of femur are at more risk of a fracture and complications than a cyst in the femoral shaft or the iliac wings.
-
2.
The age of the child: younger children are more amenable to cast immobilization and life style restriction than older adolescents.
-
3.
The general health of the child and any associated co-morbidities.
-
4.
The available expertise and equipment.
-
5.
The child and the family willingness to accept risk of fractures/surgery.
Simple bone cyst involving the proximal femur are not ideal for non-operative treatments even when there is a fracture which is amenable to non-operative treatment. (Fig. 33.2) The reliability of a fracture to heal a bone is low. The risk of having a fracture in a weight bearing bone is high. The time for cyst healing is often uncertain. This often leads to prolonged and arguably unnecessary restriction of children from living a healthy and active life. Therefore, we recommend to have a low threshold for operative intervention. This has also the advantage of providing histological confirmation of the type of cyst.
Essential Non-operative Management Methods
-
1.
Observation.
-
2.
Restriction of risky sports.
Non-operative Pitfalls
-
1.
The persistence with non-operative treatments when it is clearly not working.
-
2.
The long term restriction of sport and social activities to prevent “re-fracture.”
-
3.
Mistaking atypical malignant tumours for being simple bone cyst.
Operative Management
Several operative interventions have been used to treat SBC with varying degrees of reported success. New treatments are still being developed to achieve better results. The operative interventions can be categorised into the followings [1, 46]:
-
1.
Intra-lesional injection
-
2.
Mechanical disruption of cyst lining (curettage) with or without graft
-
3.
Trepanation and decompression
-
4.
Excision and subtotal resection with or without grafting
-
5.
Combination of the methods above
Intra-Lesional Injection
This is the simplest interventional treatment of SBC. Some authors consider it as a non-operative treatment. Steroid and bone marrow injection are the most common injections that are used for treating simple bone cyst.
Injecting a SBC is performed under C-Arm fluoroscopic localisation followed by the introduction of a sharp needle percutaneously through the thinnest area of the cyst wall. Some surgeons use a radio-opaque dye to ensure correct needle position and to determine the presence of septations which may require multiple injection points to distribute the steroid evenly throughout the cyst (Fig. 33.8).
Steroid injection to treat SBC was first introduced by Scaglietti, who noted that the fluid aspirated from these lesions have similar characteristics to the synovial fluid from patients with rheumatoid arthritis who often improve after intra-articular steroid injection [47]. He treated 72 cysts with up to 6 injections of methylprednisolone acetate per case over 18 months, reporting that 60% healed completely, 36% healed partially, and 4% did not heal in that period. A few years later, he published a larger series (163 cysts) and reported that 55% of cysts healed completely and 45% had partial healing with steroid treatment alone [48]. Capanna reported similar findings using steroid injection with a low recurrence rate of 7% [49,50,51,52]. He advocated the use of radiopaque contrast examination to identify non-contiguous septated areas to ensure the steroid is distributed through the whole cyst [53].
Various authors have reported comparable results and suggested refinements of percutaneous steroid techniques using different types of steroids, different steroid doses, and combining with bone graft augmentation [54,55,56,57,58].
Lokiec [38] published a preliminary case series of 10 patients with SBC who were treated with bone marrow injection. The rationale of using bone marrow derived from the availability of stem cells and undifferentiated cells that have the potential to become osteogenic. All patients in the series became pain free after a mean of two weeks post-injection and resumed full activities by six weeks. All cysts consolidated radiologically and demonstrated remodeling within four months. A longer term review at 12 to 48 months post-injection showed satisfactory healing without any complications.
Lokiec findings have not been replicated by others [59,60,61]. Yandow [61] reported an 83% (10/12) success rate with bone marrow injection. Delloye and colleagues [62] reported good results in approximately 88% (7/8) of their patients. Kose and colleagues [60] reported complete healing in only 16% (2/12), partial healing in 75% (9/12), and no healing in 8% (1/12). They also reported a 59% recurrence rate.
Chang and co-workers did not find bone marrow superior to steroid for SBC injection in a comparative study of 79 patients [63]. The injection technique and demographics were standardised between the two groups so that the only difference was the substance injected into the cysts. Repeated injections were required in 57% of patients after bone marrow and in 49% after steroid. No complications were noted in either group.
Wright and colleagues conducted a randomized controlled trial comparing bone marrow injection with steroid (methylprednisolone acetate) injection in 90 patients. They reported that sixteen (42%) of the 38 cysts treated with methylprednisolone acetate healed, and 9 (23%) of the 39 cysts treated with bone marrow healed (p = 0.01). There was no significant difference between the groups with respect to function, pain, number of injections, additional fractures, or complications [64].
Kadhim and co-workers conducted a systematic review and meta-analysis of 62 studies (3211 patients with 3217 SBC) [65]. The pooled estimate of steroid injection showed a healing rate of (77.4%), comparable to bone marrow injection (77.9%). A higher healing rate was observed with steroid injection when inner wall disruption was performed.
Mechanical Disruption of Cyst Lining (Curettage) with or Without Bone Graft
The rationale of this modality of treatment is based on the theory that the SBC is caused by entrapped synovial tissue within the bone. This synovial tissue secretes the fluid within the cyst. The combination of intracystic pressure and the erosive constituents of the fluid leads to a gradual increase in cyst size. Therefore, removing the ectopic tissue in theory should heal SBC. This has been shown to be somewhat true.
The relative success rate of steroid in healing SBC spread enthusiasm to experiment with various types of cyst fillers to find a superior material that induce a consistent and reliable bone healing. Kadhim and co-workers investigated the published evidence of various type of fillers [65]. Their findings are summarised in Table 33.2. They demonstrated a success rate of 39% with conservative treatment (observational management only), 51% with curettage only, and consistently higher success rates when the curated cysts were filled with bone graft or a bone substitute (over 75%).
Trepanation and Cyst Decompression
A less invasive technique was proposed by Komiya and colleagues named “trepanation” [10, 11]. It involves creating multiple drill holes through the SBC, draining of cyst fluid, and lavage of the cystic cavity with saline. They reported a 91% (10/11) success rate with one patient having a pathological fracture and two others with partial recurrence. In a series of 23 patients who underwent he “trepanation” technique, recurrence of the cyst occurred in 15 cases after the initial operation [66]. Twelve of these were treated with re-operations. At 5-year follow-up, 15 cases had healed and the 8 that did not required no further treatment.
The increased intracystic pressure combined with corrosive nature of cystic fluid have been implicated with the pathogenesis of SBC. Therefore, it is logical to think that continuous drainage would lead to cyst healing or at least slow its progression [14, 15]. This is the basis for decompression and continuous drainage treatment. Several implants have been proposed to achieve decompression and continuous drainage including K-wires, elastic or solid intramedullary nails and cannulated screws (Fig. 33.9). In addition, K-wires and intramedullary nails provide stability and early return to daily activities. Using cannulated screws for continuous decompression has a reported success rate of 77% [49, 67,68,69,70].
Excision and Subtotal Resection with and Without Grafting
Historically, a more aggressive surgical approach was used to minimize the reported recurrence rates. Kadhim and co-workers pooled data of 83 patients from 6 studies that performed subtotal excision with a reported success rate of 91.5%. This approach came with higher complications rates, however. McKay and Nason reported on 21 patients who underwent subtotal resection but without bone grafting. Seven of these patients developed fractures at the time of surgery (6, humerus; 1, femur) [71]. More solid internal fixation techniques have emerged to overcome iatrogenic fractures [72, 73].
Combinations of the Methods Above
Many surgeons have adopted a multimodal treatment including opening of the cyst wall, curettage, filling the void with a bone substitute and stabilisation using appropriate fixation (Fig. 33.10) [74]. The search for the most optimal treatment of SBC is ongoing.
“The available evidence is insufficient to support a single approach for treating simple bone cyst. However, the trends indicate that success rates were lowest with conservative treatment, higher with curettage alone, and the highest with curettage and grafting”
Multimodal treatment of simple bone cyst. This 5-year-old boy presented with a simple bone cyst of the femoral neck (image 1). He was treated with curettage, artificial bone substitute (using calcium sulfate pellets), continuous decompression using a cannulated screw (second short screw from the top), and stabilisation using locking femoral proximal plate (image 2). The cyst has almost disappeared 5 months after treatment (image 3)
Essential Surgical Techniques
Percutaneous Curettage and grafting
-
1.
Preoperative planning is essential. The approach is dictated by the location of the bone cyst and the adjacent structures that can be at risk. We will describe the techniques using the following example of a proximal femoral SBC.
-
2.
Patient is positioned supine on radiolucent table. X-ray check to ensure adequate visualisation of the lesion.
-
3.
Plan the type of graft to fill the bone defect (if you plan to graft it) and ensure that adequate supply is available. Some of commercially available pellets are mixed with antibiotic (although antibiotic is not required in SBC). Caution should be taken not to exceed the maximum dose of the antibiotic.
-
4.
Prepare for the worst case scenario should an intraoperative fracture occur. An adequate stabilisation system should be available.
-
5.
Under x-ray guidance, introduce a 3.2 mm guide wire percutaneously toward the cyst. Utilise the X-ray fluoroscopy for accurate positioning (Fig. 33.11)
-
6.
A small stab wound is made along the track of the guide wire to create a space for the drill sleeve. Use a cannulated 6.5 mm drill to create a hole in the lateral wall of the cyst. (Fig. 33.12)
-
7.
Send cyst fluid for cytology (and microbiology, if infection is suspected). Then perform a thorough and systematic curettage using angled curettes and suction. Open the cyst toward the medullary canal (Fig. 33.13). Send tissue for histology. Gentle curettage is required close to the growth plate. X-ray screening is helpful. Some surgeons recommended using endoscopy to aid this step [75,76,77,78].
-
8.
After adequate curettage and washout, the cyst cavity can be packed with either bone graft or artificial bone substitutes. Our preference is to use calcium sulfate pellets. We adopted a useful technique to introduce the pellets into the cavity by using a Yankauer sucker cut in half. This is introduced over a guide wire into the cavity then the pellets are pushed through it (Fig. 33.14).
-
9.
Ensure the bone graft fills the cyst completely (Fig. 33.15). Based on compliance, we usually put the child in a hip spica.
-
10.
Using a device(s) to promote continuous drainage and provide stability (such as flexible nailing, cannulated screws or plates) has become popular but this approach has yet to show superior sucess rates.
Operative Pitfalls
-
1.
Poor informed consent. It is striking how often we see patients and parents who have been poorly counselled about the condition, the treatments options and outcomes.
-
2.
Persisting with non-operative treatment when it is clearly not working.
-
3.
Failure to take adequate sample for histology.
-
4.
The reluctance to perform a second curettage when the first one has not been optimally successful.
-
5.
Underestimating the growth effect on the metal ware position and effect (Fig. 33.16)
Distal migration of metal ware of proximal femur. This is the same 5-year-old boy presented in Fig. 33.10. The 4 X-rays demonstrate how the effect of the proximal femoral physeal growth on the metal ware position over 3 years; from September 2015 until September 2018. As the bone grows the metal ware migrate distally with the bone. Patient underwent revision of the proximal screws only in July 2016.
Part II: Aneurysmal Bone Cyst
Introduction
Aneurysmal bone cyst (ABC) is different to the simple bone cyst (SBC). Although, it is often described as a benign bone condition, it is locally aggressive with much higher recurrence rates than SBC. ABC typically affects people in the second decade of life. On plain X-ray, ABC appears as a solitary, expansile, radiolucent lesion in the metaphyseal region of the bone [12]. Patients usually present with pain, swelling, a pathologic fracture, or a combination of these features.
Early reports confused SBC and ABC. In 1940, Cope described a 19-year-old patient presented with pain in the right hip. X-ray showed a large cystic space. Intraoperatively, he found thin walled loculi containing blood-stained fluid (Fig. 17). Histological examination showed amorphous debris and organized blood clot. Cope regarded the cyst as a simple bone cyst. Two years later, Jaffe and Lichtenstein published on “unicameral bone cysts with peculiar blood-containing cysts of large size”. These cysts were expansile and showed evidence of erosion of the surrounding bone with encroachment of the surrounding tissues. Intraoperatively, the cysts had thin, bony walls and contained bloody fluid [79]. It is mandatory to distinguish ABC from SBC, as their prognosis and treatment are different [80].
“It is mandatory to distinguish ABC from SBC, as their prognosis and treatment are different”
Pathophysiology
Several aspects of the aetiology and pathophysiology of ABC remain unknown; despite advancements in basic and molecular research. Several theories have been proposed to explain the nature of ABC but none has been widely accepted. Most investigators believe that ABC is a vascular malformation within the bone. This can be a primary malformation (when no cause can be found) or secondary to other pathologies. Histologically, ABC is characterized by cystic spaces filled with blood and separated by fibrous septa containing osteoclast-like giant cells. However, these cystic spaces lined by fibroblasts, myofibroblasts and histiocytes but not endothelium (Fig. 33.18).
Aneurysmal bone cyst histology. (1) Cystic spaces filled with blood and separated by fibrous septa (H&E; ×25). (2) Fibrous septa containing giant cells, hemosiderin-laden macrophages and osteoid (H&E; ×50). (3) Cysts and septa lined by fibroblasts, myofibroblasts and histiocytes but not endothelium (H&E; ×100). (Courtesy Edmund Cheesman, Royal Manchester University Hospital)
In around 60% of ABC cases, a characteristic genetic abnormality has been demonstrated in which there is a clonal translocation between chromosome 16 and 17. This abnormality was not found in secondary ABC [81,82,83,84,85]. The mechanism on how this translocation leads to ABC is still unknown.
ABC can occur secondary to other bony abnormalities, including bony trauma [86]. It has been reported that over 20% of ABC was associated with other bony abnormalities such as giant cell tumours, fibrous dysplasia, osteoblastoma, chondromyxoid fibroma, nonossifying fibroma, chondroblastoma, osteosarcoma, chondrosarcoma, unicameral or solitary bone cyst, hemangioendothelioma, osteosarcoma and metastatic carcinoma [87]. Although secondary, the ABC features may predominate, masking the real lesion and this poses a diagnostic challenge [88].
Natural History
Benign bone tumours have been classified into 3 groups or stages [89]:
Stage 1: Inactive (latent). This refers to lesions which are not causing pain and show no evidence of active growth. Stage 1 lesions are generally treated with observation only.
Stage 2: Active. This refers to lesions which are causing pain or some form of disability. If a lesion has weakened the structure of the bone such that fracture may occur, the lesion would also be considered a Stage 2 lesion.
Stage 3: Aggressive. This refers to lesions which are large, have broken into the soft tissues, or have caused a pathologic fracture. These lesions are usually prone to local recurrence and have the potential of causing a major problem for the patient.
ABC, although benign, can present in any of the three stages above: latent, active and aggressive. On one hand, the lesion can be asymptomatic; resolving spontaneously or after a simple biopsy. On another hand, it can be very aggressive, expanding at a fast rate and destroying one end of the bone [80]. Contrary to SBC, malignant transformation of ABC has been well documented; particularly when treated with radiotherapy, further complicating its diagnosis and treatment [49, 90, 91].
Malignant transformation has been reported in ABC that was treated several years before. Some linked this to previous radiotherapy [49, 90, 91].
Epidemiology
Several epidemiologic aspects of ABC are similar to SBC. ABC is more common in long bones (67% of cases) with the femur being the most frequently involved bone; followed by the tibia, humerus and fibula. Virtually any bone can be involved. The pelvis has been involved in only 9% of the cases [90]. Some reported that there is a predilection for the distal femur metaphysis but our personal experience is that ABC is more common in the proximal femoral metaphysis [80, 90]. Pelvic lesions involve the obturator foramen more often and are typically peripheral to the triradiate cartilage (Fig. 33.19) [90].
The incidence of ABC is 0.14 to 0.32 per 100,000 per year, with 75 to 90% of cases occurring before the age 20 years. ABC is rare after the age 30 years and exceptional after the age 50 [88, 92].
Clinical Presentation
Unlike SBC, patients with ABC are often symptomatic. Associated symptoms and signs may include pain, a palpable mass or swelling, pathologic fracture, or a combination of these. As ABCs usually expand slowly, the symptoms are usually insidious; often present for several weeks to months before the diagnosis is made. Pathologic fracture is not a common presentation of ABC and accounts for 8% of the cases.
Children with proximal femoral ABC, like symptomatic SBC, may present with an antalgic or Trendelenberg gait. They may also be tender over the proximal femoral metaphysis. Pain with weightbearing suggests an impending pathologic fracture which should be treated with prophalactic surgery.
Essential Clinical Tests
There are no specific clinical tests for aneurysmal bone cyst; however, the following signs are helpful
-
1.
Deformity is more common than with simple bone cyst
-
2.
Decreased range of motion, weakness, or stiffness
-
3.
Bruit over the affected area
-
4.
Warmth over the affected area
Imaging
Plain Radiographs
ABC classically appears as an eccentric, radiolucent cystic lesion circumscribed by a thin layer of the cortical bone (Fig. 33.20). Septations within the cyst gives it a multi-locular appearance, which has been colloquially described as a “soap bubble appearance.” (Fig. 33.20).
Cross-Sectional Imaging
Computed tomography (CT) scan is helpful in assessing cortical breach and soft tissue extension into soft tissues. It can also help determine whether the ABC is secondary to another primary lesion (Fig. 33.21). Magnetic resonance imaging (MRI), however, is the gold standard to image ABC. It can show the characteristic fluid-fluid levels beautifully (Fig. 33.22) but, more importantly, it can identify the presence of a solid component; suggesting that the aneurysmal bone cyst is secondary. Although fluid-fluid levels are characteristic MRI features associated with ABC, they have also been reported in other conditions such as SBC, giant cell tumours, chondroblastoma, and telangiectatic osteosarcomas, and as such are not pathognomonic.
On MRI scan, ABC gives a variable signal reflecting its blood contents which gives different signals depending on the aging of the blood and clot. This is usually surrounded by a rim of low T1 and T2 signal (Fig. 33.22) [87].
The close resemblance between ABC and telangiectatic osteosarcoma (TOS) deserves emphasis. Both lesions have an osteolytic expansile appearance on plain radiographs with fluid–fluid levels within cystic cavities identified on MRI. Even histologically, they overlap considerably. Three radiological signs favour the diagnosis of TOS over ABC [93]:
-
1.
Thick, nodular, and contrast enhancing tissue surrounding the cyst-like spaces on cross-sectional imaging, as opposed to the thin non-nodular borders of ABC
-
2.
The presence of matrix mineralization reflecting an underlying osteoid-producing tumour. Any solid area that is 1 cm or larger should raise the suspicion that this is not primary ABC [12].
-
3.
Cortical destruction indicative of the more aggressive lesion with associated soft-tissue mass as opposed to the typically well-defined encapsulated margins of ABC and lack of soft-tissue mass.
Essential Imaging Tests
-
MRI scan
-
Fluid-fluid levels
-
Variable signals surrounded by a rim of low T1 and T2 signal
-
-
FOG MACHINES differential diagnosis list for ABC:
-
F: fibrous dysplasia (FD) or fibrous cortical defect (FCD)
-
O: osteoblastoma
-
G: giant cell tumour (GCT)
-
M: metastasis(es)/myeloma
-
A: aneurysmal bone cyst (ABC)
-
C: chondroblastoma or chondromyxoid fibroma
-
H: hyperparathyroidism (brown tumour)
-
I: infection (osteomyelitis), intraosseous lipoma
-
N: non-ossifying fibroma (NOF)
-
E: enchondroma or eosinophilic granuloma (EG)
-
S: simple (unicameral) bone cyst
-
An accurate diagnosis of ABC is essential because of its close resemblance to some malignant conditions. Biopsy and histological examination is mandatory for accurate diagnosis. Incisional biopsy is the current gold standard of diagnosis. Although fine-needle aspiration biopsy is tempting, it lacks the specificity to accurately diagnose ABC [94,95,96].
“An accurate and timely diagnosis of ABC is critical because the differential diagnosis includes both benign and malignant lesions. Biopsy is mandatory for accurate diagnosis”.
Non-operative Management
There is a limited role for non-operative treatment in ABC. Although spontaneous healing has been reported, it is uncommon. There are two essential conditions to meet before pursuing non-operative treatment for ABC:
-
1.
The diagnosis has been confirmed without a doubt using the gold standard incisional biopsy (fine needle biopsy is considered to be suboptimum).
-
2.
The site and the stage of the lesion do not pose a substantive risk of fracture or further destruction. For lesions involving the proximal femur, weakening of the bone predisposes to pathologic fracture which should likely be treated prophylactically rather than watching and waiting.
Essential Non-operative Management Methods
Aneurysmal bone cyst can be treated expectantly if:
-
1.
The diagnosis has been confirmed without a doubt by biopsy.
-
2.
The risk of pathologic fracture or further destruction is very low.
Non-operative Pitfalls
-
1.
The diagnosis is not confirmed or delayed
-
2.
Relying on suboptimal methods to confirm diagnosis
-
3.
Underestimating how fast some ABC can expand
Operative Management
Operative treatment is recommended for ABC involving the hip. Several techniques have been described with variable success rates. Early and accurate diagnosis is essential for successful treatment. Secondary ABC may be associated with malignant primary conditions which need urgent referral to an orthopaedic oncologist. In this section we described the commonly recommended surgical techniques for the surgical management of primary ABC.
Curettage and Adjunctive Treatments
Cyst curettage and bone graft remains the current standard of treatment with a reported recurrence rates 14–59% [12, 97]. The high recurrence rates have prompted a barrage of adjunctive treatments which can be summarised as follow:
High Speed Burr
Successful curettage requires complete removal of the abnormal tissues. Most benign bone lesion including ABC have the outer rim closely adherent to the host bone. The bases of high-speed burr are to destroy the abnormal tissues to the level of the circumscribing bone. Several authors reported a success rates between 80 and 90% [98, 99]. (Fig. 33.23).
Treatment of 15-year old male with a symptomatic primary aneurysmal bone cyst of the left pubic ramus. Surgical treatment included curettage, high speed burring of the cyst walls, and calcium sulfate pellet packing, through an ilioinguinal approach (a–c). Excellent healing of the cyst has occurred by 2 years post-operatively (d). (Courtesy Jason J. Howard)
The use of high speed burr may not be possible in ABC that is close to the physis (juxtaphyseal cyst) or when the bone is very thin with an important structure in the vicinity such as cyst in the spine and certain pelvic areas.
Bone Cement
Bone cement has been used in malignant bone tumours to fill voids and provide immediate stabilisation of the involved bone. This has been successful in providing immediate pain relief and mobility. Its use has been expanded to benign but aggressive bone tumours such as ABC [100]. The exothermic reaction associated with curing of the cement is believed to damage abnormal cells and acts as a recurrence adjuvant [101]. Several authors have reported that recurrent rates had been reduced by half using bone cement [101,102,103]. There are several disadvantages to using bone cement in children. Bone cement is not osteoconductive—nor osteogenic—and thus there is no potential for osseous incorporation. Although it provides immediate structural support for axial loading, it does weaken the bone against torsional forces. It can lead to stress shielding—increasing the risk of future pathologic fracture—and as a foreign body which may act as a nidus for infection.
Phenol
Phenol has been used in several medical and surgical fields. It is well known in treating in-growing toe nails by chemically destroying the germinal matrix which is responsible for regenerating a new nail [104,105,106]. its use has been extended to treat benign bone lesions such as ABC. After a thorough curettage, phenol is used to wash the lesion, removing remaining tumour cells [107]. Bitzan and colleagues reported an overall recurrence rate of 11.8% in 34 patients who underwent surgery for ABC. Of these, 9 patients who received local phenol therapy after curettage and bone graft, no recurrence was observed [108]. Capanna and colleagues studied 165 cases of benign bone tumours (ABC, chondroblastoma and giant cell tumour) and reported a 7% recurrence rate following curettage and phenol versus 41% with curettage alone [107]. However, in another retrospective comparative study of 85 patients, Kececi and colleagues found no extra advantage of using Phenol over curettage and high-speed burr [109].
Cryosurgery
Cryosurgery has been successful used to treated some skin tumours and non-tumorous conditions. The principle of cryotherapy or cryosurgery is that the freezing temperature destroys pathological cells and help prevent recurrence. Enthusiasts reported low recurrence rate using cryosurgery (5%); however, the treatment is not widely adopted because of high complication rates which include postoperative fractures (14%), skin necrosis and wound infection (8%) [110,111,112,113].
Argon Plasma Coagulation
Argon Plasma Coagulation (APC) technique was adopted by gastroenterologists to control bleeding in the gastrointestinal tract. APC involves the use of a jet of ionised argon gas that is directed through a probe passed through the endoscope. The probe is placed at some distance from the bleeding lesion, and argon gas is emitted then ionised by a high voltage discharge (approximately 6 kV). High-frequency electric current is then conducted through the jet of gas, resulting in coagulation of the bleeding lesion on the other end of the jet. The depth of coagulation is usually only a few millimetres and it is pretty safe even in a very thin bowel walls such in caecum [114].
The same principle has been utilised in the treatment of ABC and other benign bone lesions. Directing APC beam therapy on the edges of the remaining lesion after curettage has been shown to reduce recurrence rates. Cummings and colleagues compared 17 patients who were treated with curettage and APC and 12 who were treated with curettage with or without phenol. None of the 17 patients treated with curettage and APC had a recurrence, whereas four patients treated without ABC had recurrences. They did not find differences between the two groups with regards to intraoperative complications, neurovascular injury, or bone graft incorporation [115]. Steffner and colleagues published their experience in treating 96 patients with ABC with a median duration of follow-up of 29.5 months. The overall rate of recurrence rate was 11.5%. The rate of recurrence was 20.6% after curettage and high-speed-burr treatment alone and 7.5% after curettage and high-speed-burr treatment plus APC. The 5-year Kaplan-Meier survival estimate was 92% for patients managed with curettage and adjuvant treatment with a high-speed burr and argon beam coagulation, compared with 73% for patients managed with curettage and a high-speed burr only (p = 0.060). However, this low recurrence rate was offset by the higher postoperative fracture rate of 12.5% with APC compared to 0% in the curettage and burr group. The authors attributed this to the APC; resulting in desiccation and osteonecrosis [116].
Arterial Embolization
Arresting the blood supply to ABC by embolization has been employed in areas that cannot be accessed surgically without high risk of complications. These include the spine, pelvis and sacrum most commonly. Embolization has been used as a solo treatment or adjuvant therapy in the treatment of these lesions. It requires special expertise and recurrence rate is high after first attempt but it decreases with further attempts. Rossi and colleagues showed that selective arterial embolization in 36 patients (of these, 17 were in the pelvis and 5 were in the sacrum) was effective in 94% of the cases; although 39% required more than one attempt. They reported two cases of associated skin necrosis and one transient paresis [117].
En Bloc Excision
This treatment modality has been fallen out favour as the resultant morbidity is unacceptably high. There are a few exceptions when en bloc excision or total resection is advised. Recurrent and/or refractory ABC can be treated by en bloc resection for areas that can be easily sacrificed without impairing function (such as the ribs or toes) but this is a rare occurrence for ABC involving the hip and pelvis (except, perhaps, lesions of the iliac wing). Several studies reported a very high success rates of 95–100% using the en bloc approach [90, 102, 118]. Flont and colleagues reported no recurrence after en bloc excision in comparison to curettage. However, patient morbidity was increased with en bloc procedures compared to curettage; with pain (en bloc 20% versus curettage 6.25%), limb length differences (en bloc 20% versus curettage 12.5%), reduced range of motion (en bloc 20% versus curettage 6.25%) and muscle strength impairment (en bloc 50% versus curettage 31.2%) being relevant. These differences, however, were not statistically significant (P > 0.05) [118].
Radiotherapy
Radiotherapy is another treatment of ABC that has fallen out of favour due to adverse side effects—namely radiation-induced sarcoma [110, 119]. Radiotherapy was used either as an adjuvant therapy or the main treatment. There are limited indications for radiotherapy for ABC management, but those that are recurrent, inoperable, or located in areas that are difficult to access may benefit [12]. It consists of external beam radiation to induce cellular death. The dose should be minimized (approximately 3000 to 5000 cGy) to decrease the risk of radiation induced sarcoma. In growing children, the physes are at risk of radiation-induced damage and subsequent growth disturbance(s).
“Radiotherapy should be limited to ABCs that are recurrent, inoperable, or located in areas that are difficult to access”
Emerging Treatments
ABC treatment remains challenging. None of the present treatments have been proven to be superior to others, whereas more aggressive approaches (complete excision and radiotherapy) have been associated with unacceptable complications. The search for a better treatment has been ongoing. The following treatments are being evaluated:
Curopsy
Curopsy is a mixture of the two words “Cure” and “Biopsy”. Reddy and colleagues popularised this novel biopsy technique which consists of a percutaneous, limited curettage at the time of biopsy, obtaining the lining membrane from various quadrants of the cyst leading to consolidation (“curopsy” = biopsy with intention to cure). This technique is gaining popularity for 3 main reasons:
-
1.
It is minimally invasive with low complication rates
-
2.
It provides favourable rates of local control
-
3.
It provides a confirmatory diagnosis of ABC
The principle of the technique is based on a well-known observation that some (but not all) ABCs heal following biopsy alone [120]. Reddy and colleagues reported on their experience using this technique on 102 patients with ABC and compared to 88 patients who were treated with curettage after a core needle biopsy. Of the 102 patients who had curopsy and observation, 83 (81%) required no additional treatment and the lesion resolved. Of the 88 patients who underwent curettage (with or without adjuvant therapy), the success rate was 90% (79 of 88). The recurrence rate was 19% with curopsy and 10% with traditional curettage, a statistically significant difference. However, curopsy patients avoided the morbidity associated with curettage and the use of adjuvants.
Percutaneous Doxycycline
Doxycycline is an antibiotic belonging to the Tetracycline class which were introduced in the 1960s. They work through inhibition of protein synthesis of bacteria. Doxycycline was found to have several anti-neoplastic properties including the inhibition of matrix metalloproteinase, angiogenesis, collagenase (MMP-1), and gelatinases that are implicated with the development of ABC and other neoplasms [121,122,123,124]. Therefore, doxycycline has recently been used to treat ABC. Preliminary reports of the use of doxycycline showed that all treated cysts exhibited signs of healing with recurrence rates of 5% over a short follow up of 20 months [125, 126]. It will be interesting to see the outcomes after a longer follow up period and whether the results can be replicated by other centres.
Bisphosphonate Medical Therapy
Since they were introduced over 3 decades ago, bisphosphonates have been increasingly used for various bone disorders. They act by inhibiting osteoclast-mediated bone loss due to osteoporosis, Paget disease of bone, malignancies metastatic to bone, multiple myeloma, and hypercalcemia of malignancy [127]. Bisphosphonates have also shown some anti-neoplastic activities through promoting apoptosis, inhibiting tumour cell adhesion, invasion, and angiogenesis [128].
Bisphosphonates have been used in several type of malignant and benign bone tumours including ABC with favourable outcomes reported. In a small study of 5 patients with ABC, Cornelis and colleagues reported varying degrees of lesion ossification and near universal pain relief following bisphosphonate treatment for symptomatic, unrespectable ABCs [129].
RANKL Inhibition
RANKL is a member of the tumour necrosis factor (TNF) cytokine family. It stands for Receptor Activator of Nuclear factor Kappa-Β Ligand. It is secreted by osteoblasts and binds to the RANK receptors on osteoclast cells (and their precursors) promoting bone resorption. RANKL expression has been associated with various bone tumours including ABC [130]. RANKL inhibitors such as Denosumab has been proposed to treat osteolytic bone lesions including ABCs [131]. Two case reports (5 year old and 27 year old) with large sacral ABCs who were treated with RANKL inhibitors demonstrated pain relief and cyst healing [132, 133].
Denosumab is associated with similar side effects to bisphosphonates including infection, eczema, and hypocalcaemia and, rarely, jaw osteonecrosis. The long-term effects of denosumab in skeletally immature pediatric patients are unknown.
Essential Surgical Techniques
Surgical techniques are similar to that employed for the treatment of SBC in terms of curettage and bone grafting.
However, because of the more aggressive nature of ABC as compared to SBC, aggressive approaches are more commonly adopted.
In weight-bearing bones such as the proximal femur, surgical excision.
Operative Pitfalls
-
Delaying treatment allowing for cyst expansion
-
Inadequate removal of the abnormal tissues
Classic Papers
Neer, C. S., 2nd, et al. (1966). “Treatment of unicameral bone cyst. A follow-up study of one hundred seventy-five cases.” J Bone Joint Surg Am 48(4): 731–745. One of the earliest, most comprehensive reviews with over 175 simple bone cysts. They highlighted the demographic distribution of SBC and they noted that fractures did not result in spontaneous healing.
Kaelin, A. J. and G. D. MacEwen (1989). “Unicameral bone cysts. Natural history and the risk of fracture.” Int Orthop 13(4): 275–282. Kaelin brought the attention to the frequency of fractures in patients with SBC. They also introduced the ‘cyst index’ as a predictor for future fracture. They reported that there were no fractures in 40 patients who had a cyst index lower than 3.5 even when they did not curtail their activities.
Reynolds, J. (1969). “The “fallen fragment sign“ in the diagnosis of unicameral bone cysts.” Radiology 92(5): 949–953 passim. Reynolds described the fallen fragment sign, which for long time was considered pathognomonic for SBC.
Scaglietti, O., et al. (1979). “The effects of methylprednisolone acetate in the treatment of bone cysts. Results of three years follow-up.” J Bone Joint Surg Br 61-B(2): 200–204. Scaglietti showed that synovial fluid is similar to SBC fluid and he was first to use intralesional steroids to treat these lesions.
Campanacci, M., et al. (1986). “Unicameral and aneurysmal bone cysts.” Clin Orthop Relat Res(204): 25–36. One of the largest series of SBC (319 patients) and ABC (198 patients). The authors introduced a new classification and highlighted the need to treat ABC surgically.
Key Evidence
Wright, J. G., et al. (2008). “A randomized clinical trial comparing intralesional bone marrow and steroid injections for simple bone cysts.” J Bone Joint Surg Am 90(4): 722–730. The first RCT comparing bone marrow injection with steroid (methylprednisolone acetate) injection in 90 patients. They reported that sixteen (42%) of the 38 cysts treated with methylprednisolone acetate healed, and 9 (23%) of the 39 cysts treated with bone marrow healed (p = 0.01). There were no significant differences between the groups with respect to function, pain, number of injections, additional fractures, or complications.
Kadhim, M., et al. “Treatment of unicameral bone cyst: systematic review and meta-analysis.” J Child Orthop 8(2): 171–191. A recent systematic review and meta-analysis of 62 studies that were selected for from a total of 463 articles. The cumulative sample size was 3211 patients with 3217 SBC. Authors concluded that active treatment for SBC provided variable healing rates and the outcomes were favorable relative to non-operative treatment. Due to the heterogeneity of the studies and reporting bias, the interpretation of these findings should be handled with caution.
Zhao, J.-G., et al. (1996). Interventions for treating simple bone cysts in the long bones of children. Cochrane Database of Systematic Reviews, Wiley-Blackwell. This is a Cochrane review on the best interventions for SBC. The authors used a very robust searching strategy but found only one RCT [64]. They concluded that the available evidence was insufficient to determine the relative effects of bone marrow versus steroid injections, although the bone marrow injections were more invasive. They also highlighted that the rate of radiographically assessed healing of the bone cyst at two years was well under 50% for both interventions.
Panoutsakopoulos, G., et al. (1999). “Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts.” Genes, Chromosomes and Cancer 26(3): 265–266. This study highlighted the high prevalence of a clonal translocation between chromosome 16 and 17 in primary ABC which enhance our understanding of the condition and its aetiology.
Capanna, R., et al. (1985). “Phenol as an adjuvant in the control of local recurrence of benign neoplasms of bone treated by curettage.” Ital J Orthop Traumatol 11(3): 381–388. Highlighted the value of phenol in reducing the recurrence rate of ABC.
References
Alshryda S, Wright J. In: Alshryda H, editor. Chapter 44: Evidence based treatment for simple bone cyst. pediatric orthopaedics: an evidence-based approach to clinical questions. Banaszkiewicz: Springer; 2016. p. 51–75.
Virchow, R. Ueber die bildung von knochencysten ([On the formation of bony cysts) . Berlin, Germany: Monatsber d Kgl Akad D Wissenschaften. Sitzung der Phisikalischen-mathemat Klasse vom; 12 Juni; 1876. p. 369–381.
Lagier R, Kramar C, Baud CA. Femoral unicameral bone cyst in a medieval child. Radiological and pathological study. Pediatr Radiol. 1987;17(6):498–500.
Cohen J. Simple bone cysts. Studies of cyst fluid in six cases with a theory of pathogenesis. J Bone Joint Surg Am. 1960;42-A:609–16.
Chigira M, Maehara S, Arita S, Udagawa E. The aetiology and treatment of simple bone cysts. J Bone Joint Surg Br. 1983a;65(5):633–7.
Mirra JM, Bernard GW, Bullough PG, Johnston W, Mink G. Cementum-like bone production in solitary bone cysts (so-called “Cementoma” of long bones) report of three cases. electron microscopic observations supporting a synovial origin to the simple bone cyst. Clin Orthop Relat Res. 1978;135:295–307.
Yu CL, D'Astous J, Finnegan M. Simple bone cysts. The effects of methylprednisolone on synovial cells in culture. Clin Orthop Relat Res. 1991;262:34–41.
Richkind KE, Mortimer E, Mowery-Rushton P, Fraire A. Translocation (16;20)(p11.2;q13). Sole cytogenetic abnormality in a unicameral bone cyst. Cancer Genet Cytogenet. 2002;137(2):153–5.
Vayego SA, De Conti OJ, Varella-Garcia M. Complex cytogenetic rearrangement in a case of unicameral bone cyst. Cancer Genet Cytogenet. 1996;86(1):46–9.
Komiya S, Minamitani K, Sasaguri Y, Hashimoto S, Morimatsu M, Inoue A. Simple Bone Cyst. Clin Orthop Relat Res. 1993a;287:204–11.
Komiya S, Minamitani K, Sasaguri Y, Hashimoto S, Morimatsu M, Inoue A. Simple bone cyst. Treatment by trepanation and studies on bone resorptive factors in cyst fluid with a theory of its pathogenesis. Clin Orthop Relat Res. 1993b;287:204–11.
Herring JA. Tachdjians Pediatric Orthopaedics. Philadelphia: Saunders Elsevier; 2013.
Doganavsargil B, Ayhan E, Argin M, Pehlivanoglu B, Kececi B, Sezak M, Basdemir G, Oztop F. Cystic bone lesions: histopathological spectrum and diagnostic challenges. Turk Patoloji Derg. 2015;31(2):95–103.
Shindell R, Connolly JF, Lippiello L. Prostaglandin levels in a unicameral bone cyst treated by corticosteroid injection. J Pediatr Orthop. 1987;7(2):210–2.
Shindell R, Huurman WW, Lippiello L, Connolly JF. Prostaglandin levels in unicameral bone cysts treated by Intralesional Steroid Injection. J Pediatr Orthop. 1989a;9(5):516–9.
Hart PH, Burgess DR, Vitti GF, Hamilton JA. Interleukin-4 stimulates human monocytes to produce tissue-type plasminogen activator. Blood. 1989;74(4):1222–5.
Kobza Black A, Greaves MW, Hensby CN. The effect of systemic prednisolone on arachidonic acid, and prostaglandin E2 and F2 alpha levels in human cutaneous inflammation. Br J Clin Pharmacol. 1982;14(3):391–4.
Gerasimov AM, Toporova SM, Furtseva LN, Berezhnoy AP, Vilensky EV, Alekseeva RI. The role of lysosomes in the pathogenesis of unicameral bone cysts. Clin Orthop Relat Res. 1991;266:53–63.
Komiya S, Inoue A. Development of a solitary bone cyst—a report of a case suggesting its pathogenesis. Arch Orthop Trauma Surg. 2000;120(7–8):455–7.
Komiya S, Tsuzuki K, Mangham DC, Sugiyama M, Inoue A. Oxygen scavengers in simple bone cysts. Clin Orthop Relat Res. 1994;308:199–206.
Neer CS 2nd, Francis KC, Marcove RC, Terz J, Carbonara PN. Treatment of unicameral bone cyst. A follow-up study of one hundred seventy-five cases. J Bone Joint Surg Am. 1966;48(4):731–45.
Wright JG. A randomized clinical trial comparing intralesional bone marrow and steroid injections for simple bone cysts. J Bone Joint Surg Am. 2008;90(4):722.
Ahn JI, Park JS. Pathological fractures secondary to unicameral bone cysts. Int Orthop. 1994;18(1):20–2.
Galasko CS. Letter: the fate of simple bone cysts which fracture. Clin Orthop Relat Res. 1974;101:302–4.
Garceau GJ, Gregory CF. Solitary unicameral bone cyst. J Bone Joint Surg Am. 1954;36(A:2):267–80.
Neer CS, Francis KC, Johnston AD, Kiernan HA. Current concepts on the treatment of solitary unicameral bone cyst. Clin Orthop Relat Res. 1973;97:40–51.
Kaelin AJ, MacEwen GD. Unicameral bone cysts. Natural history and the risk of fracture. Int Orthop. 1989;13(4):275–82.
Abdel-Wanis ME, Tsuchiya H. Simple bone cyst is not a single entity: point of view based on a literature review. Med Hypotheses. 2002;58(1):87–91.
Vasconcellos DA, Yandow SM, Grace AM, Moritz BM, Marley LD, Fillman RR. Cyst index: a nonpredictor of simple bone cyst fracture. J Pediatr Orthop. 2007;27(3):307–10.
Leong NL, Anderson ME, Gebhardt MC, Snyder BD. Computed tomography-based structural analysis for predicting fracture risk in children with benign skeletal neoplasms: comparison of specificity with that of plain radiographs. J Bone Joint Surg Am. 2010;92(9):1827–33.
Cohen J. Unicameral bone cysts. a current synthesis of reported cases. Orthop Clin North Am. 1977;8(4):715–36.
Haims AH, Desai P, Present D, Beltran J. Epiphyseal extension of a unicameral bone cyst. Skelet Radiol. 1997;26(1):51–4.
Hashemi-Nejad A, Cole WG. Incomplete healing of simple bone cysts after steroid injections. J Bone Joint Surg Br. 1997;79(5):727–30.
Stanton RP, Abdel-Mota'al MM. Growth arrest resulting from unicameral bone cyst. J Pediatr Orthop. 1998;18(2):198–201.
Tey IK, Mahadev A, Lim KB, Lee EH, Nathan SS. Active unicameral bone cysts in the upper limb are at greater risk of fracture. J Orthop Surg (Hong Kong). 2009;17(2):157–60.
Violas P, Salmeron F, Chapuis M, Sales de Gauzy J, Bracq H, Cahuzac JP. Simple bone cysts of the proximal humerus complicated with growth arrest. Acta Orthop Belg. 2004;70(2):166–70.
Cho HS, Seo SH, Park SH, Park JH, Shin DS, Park IH. Minimal invasive surgery for unicameral bone cyst using demineralized bone matrix: a case series. BMC Musculoskelet Disord. 2012;13:134.
Lokiec F, Ezra E, Khermosh O, Wientroub S. Simple bone cysts treated by percutaneous autologous marrow grafting. A preliminary report. J Bone Joint Surg Br. 1996;78(6):934–7.
Lokiec F, Wientroub S. Simple bone cyst: etiology, classification, pathology, and treatment modalities. J Pediatr Orthop B. 1998;7(4):262–73.
Chew FS, Bui-Mansfield LT, MJ K. Musculoskeletal imaging. Philadelphia: Lippincott Williams & Wilkins; 2003.
Reynolds J. The "fallen fragment sign" in the diagnosis of unicameral bone cysts. Radiology. 1969;92(5):949–53.. passim
Alyas F, Tirabosco R, Cannon S, Saifuddin A. Fallen fragment sign in Langerhans' cell histiocytosis. Clin Radiol. 2008;63(1):92–6.
Maas EJ, Craig JG, Swisher PK, Amin MB, Marcus N. Fluid-fluid levels in a simple bone cyst on magnetic resonance imaging. Australas Radiol. 1998;42(3):267–70.
Margau R, Babyn P, Cole W, Smith C, Lee F. MR imaging of simple bone cysts in children: not so simple. Pediatr Radiol. 2000;30(8):551–7.
Sullivan RJ, Meyer JS, Dormans JP, Davidson RS. Diagnosing aneurysmal and unicameral bone cysts with magnetic resonance imaging. Clin Orthop Relat Res. 1999;366:186–90.
Zhao JG, Ding N, Huang WJ, Wang J, Shang J, Zhang P. Interventions for treating simple bone cysts in the long bones of children. Cochrane Database Syst Rev. 2014;9:CD010847.
Scaglietti O, Marchetti PG, Bartolozzi P. The effects of methylprednisolone acetate in the treatment of bone cysts. Results of three years follow-up. J Bone Joint Surg Br. 1979;61-B(2):200–4.
Scaglietti O, Marchetti PG, Bartolozzi P. Final results obtained in the treatment of bone cysts with methylprednisolone acetate (depo-medrol) and a discussion of results achieved in other bone lesions. Clin Orthop Relat Res. 1982;165:33–42.
Campanacci M, Capanna R, Picci P. Unicameral and aneurysmal bone cysts. Clin Orthop Relat Res. 1986;204:25–36.
Campanacci M, De Sessa L, Trentani C. Scaglietti's method for conservative treatment of simple bone cysts with local injections of methylprednisolone acetate. Ital J Orthop Traumatol. 1977;3(1):27–36.
Capanna R, Campanacci DA, Manfrini M. Unicameral and aneurysmal bone cysts. Orthop Clin North Am. 1996;27(3):605–14.
Capanna R, Monte AD, Gitelis S, Campanacci M. The natural history of unicameral bone cyst after steroid injection. Clin Orthop Relat Res. 1982;166:204–11.
Capanna R, Albisinni U, Caroli GC, Campanacci M. Contrast examination as a prognostic factor in the treatment of solitary bone cyst by cortisone injection. Skelet Radiol. 1984;12(2):97–102.
Carrata A, Garbagna P, Mapelli S, Zucchi V. The treatment of simple bone cysts by topical infiltrations of methylprednisolone acetate: technique and results. Eur J Radiol. 1983;3(1):3–8.
Fernbach SK, Blumenthal DH, Poznanski AK, Dias LS, Tachdjian MO. Radiographic changes in unicameral bone cysts following direct injection of steroids: a report on 14 cases. Radiology. 1981;140(3):689–95.
Goel AR, Kriger J, Bronfman R, Lauf E. Unicameral bone cysts: treatment with methylprednisone acetate injections. J Foot Ankle Surg. 1994;33(1):6–15.
Pavone V, Caff G, Di Silvestri C, Avondo S, Sessa G. Steroid injections in the treatment of humeral unicameral bone cysts: long-term follow-up and review of the literature. Eur J Orthop Surg Traumatol. 2013;24(4):497–503.
Thawrani D, Thai CC, Welch RD, Copley L, Johnston CE. Successful treatment of unicameral bone cyst by single percutaneous injection of α-BSM. J Pediatr Orthop. 2009;29(5):511–7.
Arazi M, Senaran H, Memik R, Kapicioglu S. Minimally invasive treatment of simple bone cysts with percutaneous autogenous bone marrow injection. Orthopedics. 2005;28(2):108–12.
Kose N, Gokturk E, Turgut A, Gunal I, Seber S. Percutaneous autologous bone marrow grafting for simple bone cysts. Bull Hosp Jt Dis. 1999;58(2):105–10.
Yandow SM, Lundeen GA, Scott SM, Coffin C. Autogenic bone marrow injections as a treatment for simple bone cyst. J Pediatr Orthop. 1998;18(5):616–20.
Delloye C, Docquier PL, Cornu O, Poilvache P, Peters M, Woitrin B, Rombouts JJ, De Nayer P. Simple bone cysts treated with aspiration and a single bone marrow injection. Int Orthop. 1998;22(2):134–8.
Chang CH, Stanton RP, Glutting J. Unicameral bone cysts treated by injection of bone marrow or methylprednisolone. J Bone Joint Surg Br. 2002;84(3):407–12.
Wright JG, Yandow S, Donaldson S, Marley L. A randomized clinical trial comparing intralesional bone marrow and steroid injections for simple bone cysts. J Bone Joint Surg Am. 2008;90(4):722–30.
Kadhim M, Thacker M, Kadhim A, Holmes L. Treatment of unicameral bone cyst: systematic review and meta analysis. J Child Orthop. 2014;8(2):171–91.
Shinozaki T, Arita S, Watanabe H, Chigira M. Simple bone cysts treated by multiple drill-holes. 23 cysts followed 2-10 years. Acta Orthop Scand. 1996;67(3):288–90.
Chigira M, Watanabe H, Arita S, Udagawa E. Simple bone cyst--pathophysiology and treatment. Nihon Seikeigeka Gakkai Zasshi. 1983b;57(8):759–66.
Chuo CY, Fu YC, Chien SH, Lin GT, Wang GJ. Management strategy for unicameral bone cyst. Kaohsiung J Med Sci. 2003;19(6):289–95.
Santori F, Ghera S, Castelli V. Treatment of solitary bone cysts with intramedullary nailing. Orthopedics. 1988;11(6):873–8.
Tsuchiya H, Abdel-Wanis ME, Uehara K, Tomita K, Takagi Y, Yasutake H. Cannulation of simple bone cysts. J Bone Joint Surg Br. 2002;84(2):245–8.
McKay DW, Nason SS. Treatment of unicameral bone cysts by subtotal resection without grafts. J Bone Joint Surg Am. 1977;59(4):515–9.
Chaves D. Treatment of solitary cysts of the humerus. Treated by diaphyseal resection and bone grafting. Int Orthop. 1980;3(4):253–6.
Sturz H, Zenker H, Buckl H. Total subperiosteal resection treatment of solitary bone cysts of the humerus. Arch Orthop Trauma Surg. 1979;93(3):231–9.
Donaldson S, Wright JG. Recent developments in treatment for simple bone cysts. Curr Opin Pediatr. 2011;23(1):73–7.
Bonnel F, Canovas F, Faure P. Treatment of a simple bone cyst of the calcaneus by endoscopic curettage with cancellous bone injection. Acta Orthop Belg. 1999;65(4):528–31.
Otsuka T, Kobayashi M, Sekiya I, Yonezawa M, Kamiyama F, Matsushita Y, Matsui N. A new treatment of aneurysmal bone cyst by endoscopic curettage without bone grafting. Arthroscopy. 2001;17(7):1–10.
Yildirim C, Akmaz I, Sahin O, Keklikci K. Simple calcaneal bone cysts: a pilot study comparing open versus endoscopic curettage and grafting. J Bone Joint Surg Br. 2011;93(12):1626–31.
Yıldırım C, Mahiroğulları M, Kuşkucu M, Akmaz İ, Keklikci K. Treatment of a unicameral bone cyst of calcaneus with endoscopic curettage and percutaneous filling with corticocancellous allograft. J Foot Ankle Surg. 2009;49(1):93–7.
Jaffe HL. Solitary unicameral bone cyst. Arch Surg. 1942;44(6):1004.
Mascard E, Gomez-Brouchet A, Lambot K. Bone cysts: unicameral and aneurysmal bone cyst. Orthop Traumatol Surg Res. 2015;101(1 Suppl):S119–27.
Guseva NV, Jaber O, Tanas MR, Stence AA, Sompallae R, Schade J, Fillman AN, Miller BJ, Bossler AD, Ma D. Anchored multiplex PCR for targeted next-generation sequencing reveals recurrent and novelUSP6fusions and upregulation ofUSP6expression in aneurysmal bone cyst. Genes Chromosomes Cancer. 2016;56(4):266–77.
Lau AW, Pringle LM, Quick L, Riquelme DN, Ye Y, Oliveira AM, Chou MM. TRE17/Ubiquitin-specific protease 6 (USP6) oncogene translocated in aneurysmal bone cyst blocks osteoblastic maturation via an autocrine mechanism involving bone morphogenetic protein dysregulation. J Biol Chem. 2010;285(47):37111–20.
Leithner A, Lang S, Windhager R, Leithner K, Karlic H, Kotz R, Haas OA. Expression of insulin-like growth factor-I (IGF-I) in aneurysmal bone cyst. Mod Pathol. 2001;14(11):1100–4.
Oliveira AM, Hsi BL, Weremowicz S, Rosenberg AE, Dal Cin P, Joseph N, Bridge JA, Perez-Atayde AR, Fletcher JA. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004;64(6):1920–3.
Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999;26(3):265–6.
Toescu SM, Alalade AF, Steele L, Bhargava D, Hunter R. Frontal skull osteoblastoma with aneurysmal bone cyst-like changes associated with trauma during pregnancy: a case report. Acta Neurochir. 2017;159(2):393–6.
Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol. 1995;164(3):573–80.
Nielsen GP, Fletcher JA, Oliveira AM. Aneurysmal bone cyst. In: Fletcher BJA, Hogendoorn PCW, Mertens F, editors. WHO classification of tumours of soft tissue and bone. Lyon: Elsevier Science; 2013. p. 348–9.
Gardner ROE, Summers GR, Hopyan S. Chpater 12: Tumours. postgraduate pediatric orthopaedics: the candidate's guide to the FRCS (Tr and Orth) examination. Cambridge: Cambridge University Press; 2014.
Campanacci M. Aneurysmal bone cyst. Padova: Piccin; 1999.
Cottalorda J, Bourelle S. Modern concepts of primary aneurysmal bone cyst. Arch Orthop Trauma Surg. 2006;127(2):105–14.
Cottalorda J, Kohler R, Sales de Gauzy J, Chotel F, Mazda K, Lefort G, Louahem D, Bourelle S, Dimeglio A. Epidemiology of aneurysmal bone cyst in children: a multicenter study and literature review. J Pediatr Orthop B. 2004;13(6):389–94.
Murphey MD, wan Jaovisidha S, Temple HT, Gannon FH, Jelinek JS, Malawer MM. Telangiectatic osteosarcoma: radiologic-pathologic comparison. Radiology. 2003;229(2):545–53.
Creager AJ, Madden CR, Bergman S, Geisinger KR. Aneurysmal bone cyst: fine-needle aspiration findings in 23 patients with clinical and radiologic correlation. Am J Clin Pathol. 2007;128(5):740–5.
Layfield LJ, Armstrong K, Zaleski S, Eckardt J. Diagnostic accuracy and clinical utility of fine-needle aspiration cytology in the diagnosis of clinically primary bone lesions. Diagn Cytopathol. 1993;9(2):168–73.
Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. J Am Acad Orthop Surg. 2012;20(4):233–41.
Biesecker JL, Marcove RC, Huvos AG, Mike V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615–25.
Dormans JP, Hanna BG, Johnston DR, Khurana JS. Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin Orthop Relat Res. 2004;421:205–11.
Gibbs CP Jr, Hefele MC, Peabody TD, Montag AG, Aithal V, Simon MA. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J Bone Joint Surg Am. 1999;81(12):1671–8.
Nakatsuka A, Yamakado K, Maeda M, Yasuda M, Akeboshi M, Takaki H, Hamada A, Takeda K. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol. 2004;15(7):707–12.
Wallace MT, Henshaw RM. Results of cement versus bone graft reconstruction after intralesional curettage of bone tumors in the skeletally immature patient. J Pediatr Orthop. 2014;34(1):92–100.
Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol. 2005;23(27):6756–62.
Ozaki T, Hillmann A, Lindner N, Winkelmann W. Cementation of primary aneurysmal bone cysts. Clin Orthop Relat Res. 1997;337:240–8.
Herold N, Houshian S, Riegels-Nielsen P. A prospective comparison of wedge matrix resection with nail matrix phenolization for the treatment of ingrown toenail. J Foot Ankle Surg. 2001;40(6):390–5.
Misiak P, Terlecki A, Rzepkowska-Misiak B, Wcislo S, Brocki M. Comparison of effectiveness of electrocautery and phenol application in partial matricectomy after partial nail extraction in the treatment of ingrown nails. Pol Przegl Chir. 2014;86(2):89–93.
Zaraa I, Dorbani I, Hawilo A, Mokni M, Ben Osman A. Segmental phenolization for the treatment of ingrown toenails: technique report, follow up of 146 patients, and review of the literature. Dermatol Online J. 2013;19(6):18560.
Capanna R, Sudanese A, Baldini N, Campanacci M. Phenol as an adjuvant in the control of local recurrence of benign neoplasms of bone treated by curettage. Ital J Orthop Traumatol. 1985;11(3):381–8.
Bitzan P, Windhager R, Lang S, Richling B, Kotz R. Incidence of recurrence of aneurysmal bone cysts following surgical treatment and adjuvant therapy with phenol. Z Orthop Ihre Grenzgeb. 1995;133(5):422–8.
Kececi B, Kucuk L, Isayev A, Sabah D. Effect of adjuvant therapies on recurrence in aneurysmal bone cysts. Acta Orthop Traumatol Turc. 2014;48(5):500–6.
Marcove RC, Sheth DS, Takemoto S, Healey JH. The treatment of aneurysmal bone cyst. Clin Orthop Relat Res. 1995;311:157–63.
Marcove RC, Weis LD, Vaghaiwalla MR, Pearson R, Huvos AG. Cryosurgery in the treatment of giant cell tumors of bone. A report of 52 consecutive cases. Cancer. 1978;41(3):957–69.
Peeters SP, Van der Geest IC, de Rooy JW, Veth RP, Schreuder HW. Aneurysmal bone cyst: the role of cryosurgery as local adjuvant treatment. J Surg Oncol. 2009;100(8):719–24.
Schreuder HW, Veth RP, Pruszczynski M, Lemmens JA, Koops HS, Molenaar WM. Aneurysmal bone cysts treated by curettage, cryotherapy and bone grafting. J Bone Joint Surg Br. 1997;79(1):20–5.
Ben-Soussan E, Antonietti M, Savoye G, Herve S, Ducrotte P, Lerebours E. Argon plasma coagulation in the treatment of hemorrhagic radiation proctitis is efficient but requires a perfect colonic cleansing to be safe. Eur J Gastroenterol Hepatol. 2004;16(12):1315–8.
Cummings JE, Smith RA, Heck RK Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231–7.
Steffner RJ, Liao C, Stacy G, Atanda A, Attar S, Avedian R, Peabody TD. Factors associated with recurrence of primary aneurysmal bone cysts: is argon beam coagulation an effective adjuvant treatment? J Bone Joint Surg Am. 2011;93(21):e1221–9.
Rossi G, Rimondi E, Bartalena T, Gerardi A, Alberghini M, Staals EL, Errani C, Bianchi G, Toscano A, Mercuri M, Vanel D. Selective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2-butyl cyanoacrylate. Skelet Radiol. 2010;39(2):161–7.
Flont P, Kolacinska-Flont M, Niedzielski K. A comparison of cyst wall curettage and en bloc excision in the treatment of aneurysmal bone cysts. World J Surg Oncol. 2013;11(1):109.
Papagelopoulos PJ, Currier BL, Shaughnessy WJ, Sim FH, Ebersold MJ, Bond JR, Unni KK. Aneurysmal bone cyst of the spine. Spine (Phila Pa 1976). 1998;23(5):621–8.
Reddy KI, Sinnaeve F, Gaston CL, Grimer RJ, Carter SR. Aneurysmal bone cysts: do simple treatments work? Clin Orthop Relat Res. 2014;472(6):1901–10.
Cakir Y, Hahn KA. Direct action by doxycycline against canine osteosarcoma cell proliferation and collagenase (MMP-1) activity in vitro. In Vivo. 1999;13(4):327–31.
Duivenvoorden WC, Hirte HW, Singh G. Use of tetracycline as an inhibitor of matrix metalloproteinase activity secreted by human bone-metastasizing cancer cells. Invasion Metastasis. 1997;17(6):312–22.
Fife RS, Rougraff BT, Proctor C, Sledge GW Jr. Inhibition of proliferation and induction of apoptosis by doxycycline in cultured human osteosarcoma cells. J Lab Clin Med. 1997;130(5):530–4.
Onoda T, Ono T, Dhar DK, Yamanoi A, Fujii T, Nagasue N. Doxycycline inhibits cell proliferation and invasive potential: combination therapy with cyclooxygenase-2 inhibitor in human colorectal cancer cells. J Lab Clin Med. 2004;143(4):207–16.
Shiels WE, Beebe AC, Mayerson JL. Percutaneous doxycycline treatment of juxtaphyseal aneurysmal bone cysts. J Pediatr Orthop. 2016;36(2):205–12.
Shiels WE, Mayerson JL. Percutaneous doxycycline treatment of aneurysmal bone cysts with low recurrence rate: a preliminary report. Clin Orthop Relat Res. 2013;471(8):2675–83.
Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–45.
Morgan G, Lipton A. Antitumor effects and anticancer applications of bisphosphonates. Semin Oncol. 2010;37(Suppl 2):S30–40.
Cornelis F, Truchetet ME, Amoretti N, Verdier D, Fournier C, Pillet O, Gille O, Hauger O. Bisphosphonate therapy for unresectable symptomatic benign bone tumors: a long-term prospective study of tolerance and efficacy. Bone. 2014;58:11–6.
Yamagishi T, Kawashima H, Ogose A, Ariizumi T, Sasaki T, Hatano H, Hotta T, Endo N. Receptor-activator of nuclear KappaB ligand expression as a new therapeutic target in primary bone tumors. PLoS One. 2016;11(5):e0154680.
Dubory A, Missenard G, Domont J, Court C. Interest of denosumab for the treatment of giant-cells tumors and aneurysmal bone cysts of the spine. About nine cases. Spine (Phila Pa 1976). 2016;41(11):E654–60.
Pelle DW, Ringler JW, Peacock JD, Kampfschulte K, Scholten DJ 2nd, Davis MM, Mitchell DS, Steensma MR. Targeting receptor-activator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res. 2014;164(2):139–48.
Skubitz KM, Peltola JC, Santos ER, Cheng EY. Response of aneurysmal bone cyst to denosumab. Spine (Phila Pa 1976). 2015;40(22):E1201–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Deriu, L., Alshryda, S., Wright, J. (2019). Bone Cysts Involving the Hip. In: Alshryda, S., Howard, J., Huntley, J., Schoenecker, J. (eds) The Pediatric and Adolescent Hip. Springer, Cham. https://doi.org/10.1007/978-3-030-12003-0_33
Download citation
DOI: https://doi.org/10.1007/978-3-030-12003-0_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-12002-3
Online ISBN: 978-3-030-12003-0
eBook Packages: MedicineMedicine (R0)