Abstract
Modern society has changed its diet composition, transitioning to a higher intake of saturated fat with a 50% increase of cardiovascular risk (CVD). Within the context of increased CVD, there is an induction of a prothrombotic phenotype mainly due to increased platelet reactivity as well as decreased platelet response to inhibitors. Platelets maintain haemostasis through both blood components and endothelial cells that secrete inhibitory or stimulatory molecules to regulate thrombus formation. There exist a correlation between platelets’ polyunsaturated fatty acid (PUFA) and the increase in platelet reactivity. The aim of this chapter is to review the metabolism of the main PUFAs involved in platelet function associated with the role that their enzyme-derived oxidized metabolites exert in platelet function and fate. Finally, how lipid metabolism in the organism affect platelet aggregation and activation and the pharmacological modulation of these processes will also be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
With increased industrialization, modern society has changed its diet composition, transitioning to a higher intake of saturated fat [1]. This coupled with an array of genetic mutations, prone humans to develop dyslipidemia , a disorder characterized by high levels of circulating lipids such as low-density lipoprotein (LDL), total cholesterol (TC) or triglycerides and/or decreased levels of high-density lipoprotein (HDL). The last survey conducted by the world health organization (WHO) in 2008 estimated the prevalence of dyslipidemia (characterized by TC > 5 mmol/L) to be 23.1% in Africa, 53.7% in Europe, with a global prevalence of 38.9% [2]. Such high prevalence developed onto a public health debate, given that a rise in 2 mmol/L in TC has been shown to increase cardiovascular risk (CVD) by 50% [3], while a more recent meta-analysis questioned this assumption [4]. Within the context of increased CVD, it is well established that dyslipidemia induces a prothrombotic phenotype mainly due to increased platelet reactivity as well as decreased platelet response to inhibitors [5,6,7,8].
Platelets are fragments of megakaryocytes circulating within the bloodstream to block leakages – an evolutionary adaptation required for mammal survival. These cells maintain haemostasis through both blood components and endothelial cells that secrete inhibitory (e.g. nitric oxide) or stimulatory (e.g. thromboxane) molecules to regulate thrombus formation, which involves the exposure of sub-endothelial collagen and fibrinogen after endothelial injury. Platelets will then bind to both collagen and fibrinogen through glycoprotein (GP) VI and GPIb-V-IX receptors, respectively culminating in platelet adhesion, secretion of stimulatory molecules such as adenosine diphosphate (ADP) and aggregation to other platelets – a process deemed irreversible through binding to the platelet fibrinogen receptor αIIbβ3 [9,10,11]. While these processes are important to maintain haemostasis in physiological environments, it has been described an up-regulation of stimulatory signals as well as down-regulation of inhibitory ones in chronic diseases such as dyslipidemia and atherosclerosis [12]. Therefore, increased platelet activity has been perceived as a maladaptation implicated in chronic non-communicable diseases, opening up perspectives of new scientific discoveries – according to Prof Barry Coller, this is the “Golden Age of Platelet Research” [13].
The rise in platelet reactivity is due to the actions of different lipids on platelet function, which will be discussed in the following sections of this chapter. We will review the metabolism of the main polyunsaturated fatty acid (PUFA) involved in platelet function, arachidonic acid (AA) , associated with the role that its enzyme-derived oxidized metabolites exert in platelet function and fate. Finally, how lipid metabolism in the organism affect platelet aggregation and activation and the pharmacological modulation of these processes will also be discussed.
2 Overview of Arachidonic Acid Metabolism
Arachidonic acid (AA, all-cis-5, 8, 11, 14-eicosatetraenoic acid) is an omega-6 PUFA . It is an essential constituent of cell membrane , necessary for membrane fluidity, flexibility and function in all cell types. The presence of its cis- four double bonds gives the compound a certain degree of flexibility to interact with proteins or to react against molecular oxygen, forming a range of bioactive oxygenated molecules via enzymatic and non-enzymatic mechanisms [14,15,16,17,18]. Esterified AA is usually localized in the glycerol backbone sn-2 position, constituting an important part of phospholipid-content in the membranes and cytosol of mammalian cells and tissues. In platelets, up to 25% of phospholipid fatty acids are AA [14,15,16,17,18], reaching levels near to 5 mM in resting platelets [19]. Arachidonic acid cellular concentration may influence both normal cellular functions and the development of platelet diseases. More important, it has been proposed that a low dietary intake of AA decrease the production of pro-inflammatory eicosanoids , which contributes to processes resolution in chronic inflammatory diseases [15, 17]. However, our body’s AA needs are higher than the concentration found in human diet. Thus, our tissues depend on endogenous formation of AA from its precursor, linoleic acid (LA 18: 2n-6), in a process regulated by the action of enzymes such as desaturases and elongases. The activity of Δ6 and Δ5 desaturases (d-5-d) converts LA to gamma-linolenic acid (GLA, 18: 3), dihomo-GLA (DGLA, 20:3) and AA [15, 17].
Arachidonic acid is released from its esterified form by the action of phospholipase A2 (PLA2) complex, which mediates the one-step hydrolysis of the AA present at the sn-2 position on phospholipid backbone [14, 20,21,22,23]. Cytosolic calcium (Ca+2)-dependent group IV PLA2 (cPLA2α) catalyzes that hydrolysis to generate a free fatty acid and a lysophospholipid. Secretory PLA2 (sPLA2), which is induced by the action of cPLA2, controls magnitude and duration of free AA release, as well as paracrinely propagates the inflammatory response to neighboring cells [24,25,26]. Lastly, cytosolic Ca+2-independent PLA2 plays a role on cellular homeostasis through generation of SPM (specialized pro-resolvins mediators) and reacylation of free AA in membranes as well [16, 27]. Nevertheless, there are other phospholipases equally able to release AA: phospholipase C (PLC) and phospholipase D (PLD). PLC participates in the formation of diacylglycerol (DAG) by the action of diacylglycerol lipase and lipid products containing AA by the action of monoacylglycerol lipases [15, 20, 26, 27]. Similarly, phosphatidic acid or DAG are formed from posphatidylcholine by PLD. The former can be further catalyzed by phosphatidate phosphohydrolase to form DAG. Then, DAG-lipase hydrolyzes DAG to generate AA. Upon stimulation, Gq protein-coupled receptor activates phospholipase C that cleaves phosphatidylinositol 4,5 bisphosphate (PIP2) to inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG). The presence of DAG and Ca2+ activates protein kinase C and IP3 opens ER Ca2+ channel [28,29,30]. The increase in intracellular Ca2+ ions levels guides the PLA2 enzyme translocation to the perinuclear membrane where exerts the explained release of AA from the membrane to let the AA-metabolizing enzymes their substrate [14, 20,21,22,23].
Free AA can undergo four possible enzymatic pathways: prostaglandin endoperoxide H synthase (PGHS), lipoxygenase (LOX), cytochrome p450 (CYP 450) and anandamide pathway, which create bioactive 20-carbon oxygenated PUFA generally denominated eicosanoids . These bioactive lipids act as local hormones and/or signaling molecules produced both in response to basal metabolism as well as in inflammatory sites upon regulation by normal immune response stimuli (IL-1, TNF-alpha, lipopolysaccharides, etc.). The metabolic fate subsequent to AA release into the cytosol depends on characteristics of each tissue type, although on different tissues the same precursor often gives rise to products with antagonistic function, e.g. PGE2 [14, 17]. Cyclooxygenase (COX), more precisely known as Prostaglandin Endoperoxide H synthase (PGHS), converts AA first into PGG2, via a COX function and then to PGH2 following a peroxidase reaction [31]. Two different PGHS isoforms (PGHS-1 and PGHS-2) are involved in the PGHS pathway to generate prostaglandin H2 (PGH2), an intermediate hub whose metabolization by downstream enzymes leads to different prostaglandins: PGE2, PGD2 and PGF2α, prostacyclin (PGI2), or thromboxaneA2 (TXA2). LOX pathway consists of AA oxidation by the enzyme isoforms LOX-5, LOX-8, LOX-12, and LOX-15 to generate their products, leukotrienes (LTA4, LTB4, LTC4, LTD4 and LTE4), lipoxins (LXA) and 8-, 12- or 15- hydroperoxyeicosatetraenoic (HpETE) acids or their corresponding reduced derivatives hydroxyeicosatetraenoic (HETE) acids. CYP 450 pathway involves two enzymes, CYP450 epoxygenase and CYP450 hydroxylase giving rise to epoxyeicosatrienoic acid (EETs) and 20-HETE, respectively. Finally, anandamide pathway comprises the FAAH (fatty acid amide hydrolase) to produce the endocannabinoid , anandamide [14,15,16,17, 20, 32,33,34,35,36,37].
Cell–cell interaction is important for eicosanoids synthesis since a donor cell has to transfer its unstable intermediate, e.g. PGH2, to a recipient cell to trigger eicosanoid biosynthesis in the latter. The single donor cell should have all the enzymes necessary to produce the different eicosanoids while the recipient cell does not necessarily have all the required enzymes for AA release. Thus, for inflammation initiation, the complete set of enzymes to initiate eicosanoids production must be present in at least two cells in the injured tissue. The AA intermediate metabolites are lipophilic and require a group of special proteins called fatty acid binding proteins (FABP), specific for each cell type, which are responsible for the increase of AA intermediates export via their stabilization and lengthening their half-life time to allow them to exert their biological activities. The major action of AA metabolites is promotion of acute inflammatory response, characterized by the production of pro-inflammatory mediators, e.g. PGE2 and PGI2, followed by a second phase in which lipid mediators with pro-resolution activities may be generated [14,15,16,17, 20, 32,33,34,35,36,37] Resolution of inflammation is no more considered a passive process, but rather an active programmed response regulated by mediators with pro-resolving capacity.
3 Arachidonic Acid Metabolism in Platelets
The activation or inhibition of platelets can be modulated by many agents with a central role being played by eicosanoids , being TxA2 and PGI2 the main eicosanoids affecting platelets’ function [38].
Arachidonic acid is metabolized to PGH2 in the cytosol of platelets and for this, PLA2 activity is necessary to release AA from platelet membrane [39, 40]. PGH2 in platelets undergoes further transformations catalyzed by Tx synthase, PGD isomerase or PGE synthase to form TxA2, PGD2 or PGE2 respectively (Fig. 7.1). In addition to the wide variety of eicosanoids formed by the COX pathway [41], 11-HETE and 15(S)-HETE-but not 5-HETE- are produced when AA is inserted at the active site of COX-1 in a different structural arrangement than the one necessary for PGH2 synthesis [42]. Both products can be formed at similar levels to TxA2 whose formation is associated with an increase in AA levels, e.g. at platelet hyperactivation where high concentrations of AA are released from platelet membranes. The participation of COX in 11-HETE and 15(S)-HETE formation was also demonstrated by the lack of decrease in 12-HETE when using only aspirin thus LOX is not the enzyme involved in these products formation [42]. Reduction of 12-HETE was only observed when both PGHS and P2Y12 inhibitors were used confirming the former proposal [42].
Arachidonic acid metabolism in platelets. Arachidonic acid is esterified at the platelet membrane and released to the cytosol due to the activity of PLA2. Then, AA is the substrate for PGHS-1 forming PGH2 which then is the substrate for the subsequent formation of TxA2, PGE2 or PGD2. If AA is oxidized by LOX, then 12-HETE is formed. When an increase of vasculature RNS formation or the appearance of an inflammatory process occur, AA is nitrated to NO2-AA which inhibit PGHS-1 thus decreasing the pro-aggregant TxA2 formation [40]. The increase of non-oxidized AA in the cytosol led to a subsequent oxidation of the fatty acid by the 12-LOX thus increasing the levels of 12-HETE [47]
Platelets mainly express PGHS-1, but traces of PGHS-2 have been detected, possibly carried over from megakaryocytes, the platelet precursor cells, or as a result of the transcription of residual mRNA into protein [22, 33, 43]. In addition to PGHS, AA can also be oxidized by the non-heme iron-containing enzymes LOXs. Different isoforms of LOXs are found depending on the carbon where the hydroperoxyl (-OOH) group is added. Platelets present 12-LOX which inserts oxygen primarily at C-12 of AA forming the 12S-hydroperoxy-5Z,8Z,10E,14Z-eicosatetraenoic acid [12(S)HpETE] [44]. The highly reducing environment present in the platelet rapidly reduce 12(S)-HpETE to its hydroxyl derivative 12-HETE [44]. The biological activity of 12-HETE in platelets in vivo remains under discussion; some authors suggest a pro-thrombotic activity by acting at vascular hypertension (Fig. 7.2) while others propose an anti-aggregant and anti-inflammatory action by decreasing the release of AA from the membrane and regulating integrin activation (Fig. 7.3). The pro-inflammatory leukotrienes precursor, being able to affect platelet activation during an inflammatory process, is formed by the 5-LOX present in neutrophils. One important difference between the AA-metabolizing enzymes due to their capacity to be inhibited is that while aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit PGHS activity [45, 46], LOX remain active in their presence being able to oxidize AA to HpETEs.
3.1 Role of Distinct Eicosanoids in Platelets
3.1.1 Thromboxane A2 (TxA2)
The most directly important prostanoid for platelet function is the PGHS-1-generated TxA2, which is synthesized by activated platelets acting in an autocrine and paracrine manner to induce thrombosis . On platelets, TxA2 binds to the thromboxane prostanoid (TP) receptor and initiates an amplification loop leading to further platelet activation, aggregation and TxA2 formation. In the vasculature, TxA2 induces vasoconstriction and the proliferation of vascular smooth muscle cells (Fig. 7.2) [21, 38, 47, 48].
3.1.2 12-Hydroxy-Eicosatetraenoic Acid (12-HETE)
12-HETE is the major 12-LOX-catalysed metabolite and the most abundant eicosanoid produced by platelets upon stimulation [41, 42], although its effects on platelet function are not completely understood. There are studies reporting that inhibition of 12-LOX led to decreased platelet aggregation correlated with a significant reduction of 12-HETE levels in response to collagen [49]. A recent review concluded that 12-HETE can exert both pro- and anti-aggregant effects (Figs. 7.2 and 7.3) on platelets, depending on 12-HETE concentration, stereospecificity and co-incubation with different agonists [50].
3.1.3 Prostacyclin (PGI2)
Endothelium-produced PGI2 binds to the Gs-coupled PGI2 receptor (IP) on platelets and generally reduces platelet reactivity, which can be critical to minimizing the risk for atherothrombotic events [51, 52]. Binding of PGI2 to the IP receptor, results in the activation of adenylate cyclase and a subsequent rise in cAMP levels in platelets [51, 52]. This stimulates phosphorylation of PKA, which suppresses various signaling pathways involved in platelet function such as adhesion, aggregation and granule secretion.
3.1.4 Prostaglandin D2 (PGD2)
PGD2 is well established as a macrophage product but, in lesser amounts, is also synthesized by platelets . By interaction with platelet DP1 receptors, PGD2 increases adenylyl cyclase activity and so, like PGI2, inhibits platelet activation [53, 54] (Fig. 7.3).
3.1.5 Prostaglandin E2 (PGE2)
PGE2 is released by endothelial cells (ECs) and, to some extent, by activated platelets. It acts on a range of prostanoid receptors, EP1 – EP4, that differently modulate second messengers, such as cAMP and free Ca2+, within platelets and exert contrasting effects on platelet function [55]. The effects on platelets of PGE2 acting through EP receptors are concentration dependent. At low concentrations (0.1–10 μM), PGE2 binds to Gi-coupled receptors (EP3) to enhance aggregation (Fig. 7.2), whereas at higher concentrations (100 μM), it activates Gs-coupled receptors (EP2, EP4) to inhibit aggregation [56,57,58] (Fig. 7.3). Stimulation of EP3 receptors by PGE2 decreases cAMP levels, thus favoring platelet aggregation, but the full effect is only seen in the presence of another platelet agonist [58]. On the other hand, the increased cAMP levels which accompany EP4 receptor activation correlate with suppressed platelet aggregation [57]. In addition to PGE2, PGF2α and PGD2 can also bind to EP3 and EP4 receptors but with lower affinity and reversible effects [57, 58]. As well as the well-characterized effects of PGE2 mediated through EP3 and EP4 receptors, EP1 receptors are also expressed on platelets [56, 59]. Although the signal transduction pathway is not clear, studies in several cell lines expressing EP1 receptors suggest that its activation increases Ca2+ influx and might thereby stimulate platelet aggregation [60]. While PGE2 seems to both inhibit and potentiate platelet aggregation in vitro, a study by Gross et al. has elegantly shown that, in vivo, PGE2 is produced by the vessel wall or after the rupture of a plaque. Under these conditions, PGE2 activates the EP3 receptors on platelets and clearly enhances, rather than reduces, thrombus formation in the arterial vessel wall [61].
4 Diversion of Normal AA Metabolism in Platelets: Formation of NO2-AA
Regarding AA metabolism in platelets and its oxidation by PGHS and/or LOXs, the participation of nitric oxide (•NO) and reactive nitrogen species (RNS) during platelet activation has been widely demonstrated. PGHS is a target for •NO and peroxynitrite, modulating prostaglandins as well as TxA2 synthesis [62, 63]. Inflammatory processes and PUFAs are linked by eicosanoids , which represent mediators and regulators of inflammatory processes. Many of the reported protective, anti-aggregant and signaling actions of •NO are due to its interaction with iron-containing enzymes. In fact, the heme-containing enzyme PGHS is a target for •NO, modulating prostaglandin synthesis [62]. Nitric oxide can act as a reducing co-substrate of the peroxidase (POX) activity of the enzyme favoring the catalytic cycle of POX [39] or as a precursor of peroxynitrite which may also be a POX substrate or an inhibitor of the enzyme by tyrosine nitration at the enzyme active site. Indeed, •NO enhanced COX inactivation by peroxynitrite [62]. During COX catalysis, AA is oxidized being formed arachidonyl radicals which are potential targets for •NO or RNS reactivity thus influencing enzyme activity. Our reports suggest that during COX catalysis, those AA-derived radicals can be “sequestered” by RNS to form nitrogen-containing AA products decreasing enzyme substrates thus diminishing enzyme activity [62].
We have extensively described the synthesis of the nitrated derivative of AA, which we propose that besides being formed by acidic gastric nitration, can be a byproduct of COX activity in the presence of RNS . Nitroarachidonic acid (NO2-AA) can be formed in biological membranes from AA, as explained above the most abundant fatty acid present in the 2-carbon position of phospholipids, to exert biological effects upon PLA2 cleavage. One of the currently accepted mechanisms for RNS (e.g. •NO2)-mediated oxidation and nitration for AA involve different routes including hydrogen atom abstraction and addition reactions [64]. Reaction of •NO2 with PUFAs leads to the generation of isomerized, oxidized and/or nitro-allylic, nitroalkene, dinitro, or nitro-hydroxy lipid derivatives. An arachidonyl carbon-centered lipid radical and nitrous acid (HONO) are formed, and under anaerobic conditions or when O2 tension is low, a second molecule of •NO2 reacts with the carbon-centered radical generating a nitroalkane (the -NO2 moiety is bound to a double bond) [64, 65].
As AA is the substrate for PGHS activity, we evaluated if nitration of the carbon chain of AA can exert any effects on PGHS normal activity of oxidizing AA. When analyzed in vitro, NO2-AA inhibited both POX and COX activities of PGHS-1 while only affected POX in PGHS-2 [40], while nitration of other fatty acids was unable to modify PGHS activity. The mechanism of inhibition involves the release of heme as a result of NO2-AA reaction with the protein [40]. Not only in vitro the effects of NO2-AA on PGHS were analyzed. The capacity of NO2-AA to modulate PGHS-1 activity was also evaluated in human platelets [40, 47] being a potent inhibitor of thrombin-mediated platelet aggregation with an inhibitory concentration 50 (IC50) of 1.3 μM (refs. [40, 47]). Inhibiton of PGHS in platelets lead to a decrease in TxA2 synthesis with a concomitant divertion of AA to the LOX pathway, thus AA is mainly oxidized by LOX forming 12-HETE [40, 47]. Importantly, platelet aggregation and activation was modulated by NO2-AA in response to several membrane receptors activation, i.e. protease activated receptors -PARs- for thrombin, P2Y receptors for ADP and TxA2 receptors for AA, by either TxA2 positive retroalimentation after its synthesis or through direct stimulation of protein kinase C (PKC), indicating that NO2-AA acts downstream membrane receptors to exert its antiplatelet effects [47].
The PKC family is centrally involved in platelet activation and aggregation. As explained previously, stimulation of PLC led to the generation of IP3 and DAG where the latter activates at the platelet membrane the cytosolic PKC isoforms (cPKC), which act in a synergistic manner with Ca2+. While NO2-AA did not mobilize Ca2+, it was able to inhibit α-granule secretion [47]. The effect of the nitroalkene on PKCα was analyzed in non-activated and thrombin activated platelets. PKCα was diffusively distributed in the cytosol of untreated platelets and migrated to the plasma membrane after thrombin-stimulation in a process that was abolished by NO2-AA in addition to a prevention of platelet shape change and cytoskeletal reorganization, characteristics of platelet activation [47]. It has been reported that the bioactive signaling activities of nitroalkenes are due to their capacity to perform Michael-addition reactions with nucleophilic Cys of His residues. cPKC isoforms contain Cys rich motifs that are duplicated as a tandem domain which are critical for its interaction with membrane phospholipids, suggesting that PKC inhibition could be mediated by electrophilic modifications of the enzyme. In addition, NO2-AA modulation of PKC route has been shown by its capacity to inhibit platelet responses downstream to PKC activation (α-granule secretion, Erk2 phosphorylation, PKC translocation to the membrane) while not affecting upstream responses (e.g. Ca2+ mobilization). Moreover, when platelet activation is directly activated by the PKC activator PMA, NO2-AA inhibited platelet aggregation. These observations provide a possible novel mechanism for platelet regulation under conditions where AA acts as a mild agonist for hemostasis, but adopts potent anti-platelet properties at inflammatory environments associated with increased •NO and RNS production when transformed into NO2-AA [47].
5 How Lipoproteins Modulate Platelet Function
5.1 Native and Oxidized LDL in Cardiovascular Disease
High levels of LDL are more prone to be altered by reactive oxygen species (ROS) , which are normally present in physiological processes acting as second messengers, but also induce oxidative stress in conditions such as ageing, smoking, diabetes mellitus, metabolic syndrome and dyslipidemia . ROS act on PUFAs (free or ester-bound) on the LDL particle, leading to hydroperoxides formation, which in turn, breaks down to form reactive particles as malondialdehyde (MDA) and 4-hydroxy-nonenal (4-HNE). MDA and 4-HNE react with lysine residues at the Apo lipoprotein B in LDL, together with amine groups in some phospholipids such as phosphatidylethanolamine and phosphatidylserine, forming Schiff-bases, generating neoepitopes. These processes produce oxidized LDL (OxLDL) particles, which are highly immunogenic. In fact, IgG autoantibodies against OxLDL have been found, although their role in atherogenesis and atherotrombosis remains to be elucidated [66].
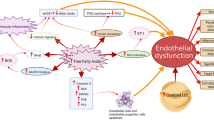
Oxidized LDL has been associated to atheromatous plaque formation through four mechanisms that complement each other: endothelial dysfunction, foam cell formation, smooth muscle cell migration and proliferation, and induction of platelet adhesion and aggregation. Endothelial dysfunction occurs when its anticoagulant and vascular regulatory properties are compromised and a pro-inflammatory phenotype is produced. OxLDL can be formed and retained in subendothelial space, where it can induce adhesion molecules as ICAM-1 and VCAM-1 and the secretion of chemotactic protein MCP-1 and mCSF, favoring monocyte recruitment. Also, OxLDL is associated with a decrease in •NO production, leading to endothelial dysfunction, facilitating the atheromatous plaque formation [67]. One of the main mechanisms is that OxLDL up-regulates arginase I expression in the vascular wall without altering eNOS expression, which contributes to endothelial dysfunction by reducing l-arginine availability to eNOS for •NO production [68].
The oxidation of LDL particles increases their uptake by macrophages , so OxLDL is considered highly atherogenic by inducing foam cells formation in the first steps of the atherosclerotic lesion. Indeed, macrophages of the sub-endothelial space express scavenger receptors, which have a high affinity for OxLDL (in contrast to normal LDL) and they are not downregulated, leading to a massive fat accumulation. In turn, the foam cells present pro-inflammatory and pro-oxidative features that promote more LDL oxidization in the sub-endothelial space, and the recruitment of more monocyte to form macrophages and then, foam cells. Finally, the excessive intracellular accumulation of oxLDL induces the apoptosis or necrosis of foam cells, forming an important amount of cell debris that constitute the core of atherosclerotic plaque [67].
The vascular smooth muscle cells migrate from tunica to intima and proliferate in sub-endothelial space, as part of the process of atheromatous plaque formation. OxLDL can favor this, due to its capacity to induce platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) release form endothelial cells and macrophages . Also, OxLDL induces LOX-1 expression, a scavenger receptor also present in macrophages, allowing the entry of OxLDL in smooth muscle cells, forming new foam cells, this time derived from smooth muscle [69].
5.2 Native and Oxidized LDL in Platelet Function
Relevant to dyslipidemia , several groups have described an increase in platelet activation after platelet exposure to native (nLDL) and oxLDL [5, 70, 71]. Given the pro-oxidant environment that develops in dyslipidemia and associated metabolic diseases, the accepted hypothesis is that LDL is partly oxidized into oxLDL, which is the main molecule responsible for potentiating platelet response. It has been shown the importance of platelets in atheromatous plaque formation, and OxLDL also have a role in it. As mentioned above, OxLDL induces an endothelial dysfunction, which in turn favors platelet activation. But also, OxLDL have direct effects on platelets that lead to activation and thrombus formation in the aterothrombosis context. We will review this last aspect with more detail next.
Studies demonstrated that specific oxidized phospholipids that accumulate in plasma in dyslipidemia promote platelet activation by interacting with different scavenger receptors: CD36, lectin-like oxidized LDL receptor-1, scavenger receptor class B type I (SR-BI), Toll-like receptor 2 (TLR2), scavenger receptor that binds phosphatidylserine and oxidized lipoprotein/chemokine (C-X-C motif) ligand [71,72,73,74,75]. Although it was known for some time that platelets possessed CD36, a class B scavenger receptor that binds to several unrelated molecules, such as thrombospondin-1 and -2, [76, 77] as well as oxLDL [78], it was only on the last decade that it was shown that CD36 is the main receptor for oxLDL in platelets. Upon binding to CD36, oxLDL enhances oxidative stress and pro-thrombotic phenotype. In fact, Podrez et al. [7] were the firsts to describe the importance of CD36 in dyslipidemia, showing that genetic deletion of CD36 in murine models of dyslipidemia restored arteriole and venule occlusion times back to control levels.
Less than a year later, the first signalling pathway for oxLDL-CD36 was described. Chen et al. [79] demonstrated for the first time that mitogen-activated protein kinase kinase 4 (MKK4) and downstream c-Jun N-terminal kinase (JNK)2 were phosphorylated in oxLDL-exposed platelets. Interestingly, src family kinases Fyn and Lyn, which are also important for collagen receptor GPVI signalling, were recruited to the cytoplasmic tail of CD36 after exposure to oxLDL. Also, immobilized oxLDL increased protein tyrosine kinase Syk activation and intracellular Ca2+ while enhancing thrombus formation under flow in a collagen-coated surface – an effect highly dependent on CD36 signalling [80]. Platelet-bound oxLDL was increased in patients with acute coronary syndromes, while incubation of oxLDL with platelets from healthy individuals was able to enhance platelet adhesion to collagen, as well as activate endothelial cells [81]. Overall, it seems CD36 and collagen signalling pathways are intrinsically connected in the context of dyslipidemia and other metabolic diseases, prompting the discovery of novel treatments targeting CD36 and its pathway components.
In addition to the strong body of evidence showing how oxLDL induce platelet activation, platelets may induce LDL oxidation per si, prompting a positive feedback loop. For instance, supernatant collected from collagen-activated platelets was able to enhance copper-induced LDL oxidation [82]. More direct evidence showed that AA-stimulated but not resting platelets were able to oxidize LDL and HDL in a concentration-dependent manner. Moreover, the authors ascribed the oxidative stress induced by platelets to be due to secretory PLA2, produced by activated platelets, that would then induce adverse modifications in lipoproteins [83]. Another group proposed that activated platelets oxidize LDL in an NADPH oxidase (Nox)2-dependent manner, by showing that oxLDL had no effect on platelet activation in Nox-2-deficient patients and that the addition of a Nox-2 inhibitor abrogated the production of oxLDL induced by activated platelets [84]. Finally, platelets contribute to the deleterious actions of oxLDL in other cells, such as monocytes, inducing oxLDL-dependant monocyte migration and thus contributing to vascular oxidative stress and atherosclerotic plaque instability [85]. Overall, it seems there is an intricate positive feedback loop in which platelets oxidize LDL that will then activate platelets in a continuum that also involves other blood cells and possibly the endothelium.
Besides increased oxLDL levels, patients with dyslipidemia and associated metabolic syndrome tend to produce more microparticles, derived from platelets (PMP), endothelial cells (EMP) as well as other blood components [86, 87]. Curiously, EMPs were found to bind to platelet surface CD36, since the inhibition of CD36 with monoclonal antibodies or the addition of competitive agonist oxLDL prevented EMP binding [88]. However, the majority of microparticles in blood is derived from platelets [89], which also induce a pro-thrombotic and pro-coagulant state due to PMP-induced platelet activation and the presence of negatively-charged phospholipids, like phosphatidylserine (PS) [86, 90, 91]. Hypothesizing that PS might be a ligand for CD36, Wang et al. [92] demonstrated that not only oxLDL leads to increased production of PMPs, but PMPs per se activate platelet through binding of CD36. Therefore, CD36 is a receptor for PMP binding and consequent pro-thrombotic outcome, while both contribute to the positive feedback installed in metabolic syndrome.
5.3 Native and Oxidized HDL in Platelet Function
Although LDL and its oxidized form oxLDL display pro-thrombotic effects through binding to CD36, HDL and oxidized HDL (oxHDL) have been shown to exert the opposite effect binding to a similar receptor. Population studies have shown that HDL is inversely correlated to thrombosis [93], whereas this observation was corroborated in vitro by showing that HDL reduces platelet aggregation in response to a variety of agonists, such as collagen and ADP [71]. Although HDL was firstly described to bind to platelet through fibrinogen receptor αIIbβ3 [94, 95], later reports refuted these findings using more refined techniques [96], proposing scavenger receptor B1 (SR-B1) as the binding site of HDL in platelets [97]. It is curious to notice that the initial data on SR-B1 knockout mice showed increased platelet activation, with elevated P-selectin binding at resting and increased adherence to immobilized fibrinogen, due to dyslipidemia and platelet cholesterol overload [73]. However, in ApoE−/− dyslipidemic mice, bone marrow transfusion from SR-B1−/− mice resulted in resistance to platelet hyperactivity as well as protected the pro-thrombotic phenotype present in these dyslipidemic animals [72]. It is now widely accepted that SR-B1 is the HDL receptor in platelets and that there is an inverse relationship between levels of this receptor and platelet response to ADP [98, 99].
Interestingly, oxHDL has been shown to inhibit platelet function more potently than native HDL [71]. Valiyaveettil [71] showed that oxHDL was able to inhibit platelet aggregation induced by collagen, ADP and thrombin – an effect that was dependent on platelet SR-BI. Another study using HDL from diabetic patients have found that glycoxidized HDL was also able to inhibit platelet aggregation induced by collagen [100]. However, others have shown that the oxidation of HDL by hypochlorite produces a different form of oxHDL that is seemingly more oxidized and able to potently activate platelets through binding to CD36 [101, 102]. Hence, it seems that the degree of HDL oxidation determines whether these lipoproteins will exert an anti- or pro-thrombotic effect [103]. It is still elusive which form of oxHDL is predominant in dyslipidemia and whether or not oxHDL functions as a negative loop for the already established oxLDL-platelet positive feedback system.
6 Dyslipidemias and Platelet Activation
There is evidence that distinct dyslipidemias (See Box 2 for dyslipidemias definition, classification and diagnosis) are associated with increased platelet activity. Hyperactive platelets with increased platelet cholesterol may contribute to accelerated atherogenesis associated with coronary artery disease. Plasma cholesterol levels appear to have a critical role in modulating platelet activity because hypercholesterolemia increases platelet activation (GPIIb/IIIa, P-selectin and phosphatidylserine expression), platelet FXa generation and platelet tissue factor activity, more potently than hypertriglyceridemia. Thus, plasma membrane cholesterol accumulation in platelets could potentially alter the membrane structure and affect signaling via surface receptors [104,105,106,107,108]. As compared with 26 normal subjects, platelets from patients with the Type II hyperlipoproteinemia aggregated in response to 1/25 the mean concentration of epinephrine, one third the concentration of collagen, and one third the concentration of ADP. In contrast, platelets from type IV hyperlipoproteinemia showed normal sensitivity to ADP and collagen, and normal secretion. This suggest that platelet activation is associated with the thrombotic complications of type II hyperlipoproteinemia [6].
Low-density lipoprotein particles sensitize platelets via binding of apoB-100 to a receptor on the platelet membrane and via transfer of lipids to the platelet membrane. Oxidative modifications of LDL (oxLDL) generate a platelet-activating particle, and this interaction might contribute to the development of the atherosclerotic plaque. Preincubation of isolated platelets with oxLDL, but not with native LDL, resulted in enhanced platelet adhesion to collagen and activated endothelial cells. The oxLDL has been shown to promote platelet activation by interact with different scavenger receptors: class A scavenger receptor, CD36, lectin-like oxidized LDL receptor-1, scavenger receptor class B type I (SR-BI), Toll-like receptor 2 (TLR2), scavenger receptor that binds phosphatidylserine and oxidized lipoprotein/chemokine (C-X-C motif) ligand. Also, oxidized choline glycerophospholipids are markedly increased in plasma of hyperlipidemic mice and in plasma of subjects with low HDL level, and promote platelet activation and hyperreactivity [7, 81, 109, 110].
6.1 Dyslipidemias: Definition, Classification and Diagnosis
Dyslipidemias are a group of abnormalities in the lipid metabolism that leads to altered blood levels of triglycerides, lipoproteins or phospholipids. More frequently, dyslipidemias are associated with high levels of blood lipids, although an altered lipoprotein pattern is also important and not only the total lipids levels in blood. Dyslipidemias can be classified regarding their causes: Genetic (familial) or Acquired (secondary). Most of dyslipidemias are hyperlipidemic, and between them, most are caused by a combination of genetic polymorphism and dietary and lifestyle factors. An iconic example of this is the apolipoprotein E2/E2 polymorphism, which is present in 1/200–1/500 frequency, but its dyslipidemia associated is present only in 1/5000, because it needs the association with certain lifestyles (sedentary, hypercaloric diets) and other disorders, such as obesity, diabetes and/or hypothyroidism. On another hand, only a 2% of hyperlipidemic disorders can be associated with identifiable mutations (as a strong genetic cause) and they are the most difficult to treat and with a high cardiovascular risk. Acquired dyslipidemias are hyperlipemic also, and they commonly associate with diabetes mellitus, hypothyroidism, nephrotic syndrome and use of drugs (thiazide diuretics, beta adrenergic blockers, glucocorticoids, etc.) [111].
Other important classification is based on the type of lipid and lipoprotein elevated in plasma, and it is applied to familial dyslipidemias (Table 7.1) [112]. A third classification (Table 7.2) can be made based on clinical phenotype independently of causes, and includes the most frequently dyslipidemia manifestation: the genetic and environmental factors combination. In general, this classification takes into account not only elevations in LDL, VLDL or Chylomicrons, but also HDL decrease, and some of them can be associated to other pathologies [113].
The diagnosis is based mainly on clinical tests for a lipid profile, searching for abnormal levels of triglycerides, LDLc (LDL cholesterol), VDLc (VLDL cholesterol), or HDLc (HDL cholesterol). Usually, low levels of LDLc and/or triglycerides are not considered of clinical importance by themselves. A decrease below normal levels of LDLc and triglycerides are usually considered as secondary to other disorders as liver disease, malabsorption syndromes, hyperthyroidism, etc. On the other hand, high levels of HDLc as an abnormal condition, constitutes a very rare genetic disorder with not well elucidated cardiovascular risk. For these reasons, we will focus on hyperlipidemic disorders [114]. The only clinical manifestation that helps to think specifically in hyperlipidemia is the presence of xanthomas, but they are observed mainly in genetic dyslipidemias with low frequency. In these patients, hepatosplenomegaly, lipemia retinalis and family history of pancreatitis (when triglycerides are elevated) can be present. Independently of the dyslipidemic origin, of special concern in all of them is the cardiovascular risk associated, and its determination and management is one of the main goals in dyslipidemia treatment [114]. To evaluate the cardiovascular risk in a dyslipidemic patient is important to consider: clinical manifestations of atherosclerosis (coronary, cerebral or peripheral), age (>45 years in males, >55 in females), family background with atherosclerosis in young, smoking, hypertension, diabetes mellitus and HDLc <40 mg/dl. Of course, the blood levels of triglycerides and total cholesterol are important, but of special relevance for cardiovascular risk, are the LDLc levels. A large amount of evidence support the idea that high levels of LDL in blood, in concert with an elevated oxidative stress in the organism, leads to a high risk to the development of atherosclerotic and atherotrombotic events [115].
7 Closing Remarks and Perspectives
Platelets are central workers in the cardiovascular function and hemodynamics, acting as watchers of changes within the bloodstream. As shown in this chapter, lipids play an essential role on platelet metabolism and reactivity, modulating its response in accordance to the serum lipid profile. Although the focus has been placed on the circulating levels of distinct lipoproteins, with LDL particles augmenting and HDL particles decreasing platelet reactivity (Fig. 7.4), the immunological essence of platelet lipid metabolism increases its importance in disorders characterized by low-grade inflammation . Of importance, metabolic syndrome allies pro-thrombotic and pro-inflammatory statuses with dyslipidemia , whereas constitutes itself an independent risk factor for cardiovascular disease [116, 117]. We have previously revised the relationship between platelet hyperactivity and metabolic syndrome, suggesting the redox-sensing protein disulfide isomerase as a central actor [118]. Notwithstanding, the role of lipids in platelet biology has been recently widened with the application of lipidomic approaches [119]. A study just released by Peng, Geue [120] showed how changes in the platelet lipidome may modulate platelet function, uncovering novel therapeutic targets and agents. On this matter, the NO2-AA might be additionally proposed as a potential target/agent in metabolic syndrome-derived platelet hyperactivity, since its formation depends on both redox and inflammatory processes. Thus, the tale of lipid role on platelet function and dysfunction seems to be an open novel with many interesting chapters to be written.
How lipoproteins affect platelet function. Oxidized low-density lipoprotein (oxLDL) or platelet-derived microparticles (PMPs) bind to CD36 on platelets, generating three concomitant responses. On the left, active fyn and lyn will activate mitogen-activated protein kinase kinase 4 (MKK-4) and downstream c-Jun N-terminal kinase (JNK-2). Upon activation, CD36 will also stimulate platelets through a Src kinase-dependent activation of Syk and downstream phospholipase C (PLCγ-2), which will then increase intracellular Ca2+. The third signaling pathway of CD36 involves activation of NADPH oxidase (Nox-2) , which will generate reactive oxygen species (ROS) that will act as second messenger, inducing extracellular-signal-regulated kinase (ERK-5) phosphorylation. All of the CD36 pathways culminate in platelet activation, which produces oxidant molecules on the extracellular environment that will oxidize native LDL (nLDL) into oxLDL and perpetrate the positive feedback loop. To counter balance, high-density lipoprotein (HDL) binds to its own receptor, scavenge receptor B1 (SR-B1) to inhibit platelet function. The mechanisms of such inhibition are still poorly understood
References
Popkin BM, Adair LS, Ng SW (2012) Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 70(1):3–21
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645
Libby P (2002) Inflammation in atherosclerosis. Nature 420(6917):868–874
Berger S, Raman G, Vishwanathan R, Jacques PF, Johnson EJ (2015) Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr 102(2):276–294
Berger M, Wraith K, Woodward C, Aburima A, Raslan Z, Hindle MS et al (2018) Dyslipidemia-associated atherogenic oxidized lipids induce platelet hyperactivity through phospholipase Cgamma2-dependent reactive oxygen species generation. Platelets:1–6
Carvalho AC, Colman RW, Lees RS (1974) Platelet function in hyperlipoproteinemia. N Engl J Med 290(8):434–438
Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M et al (2007) Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med 13(9):1086–1095
Colas R, Sassolas A, Guichardant M, Cugnet-Anceau C, Moret M, Moulin P et al (2011) LDL from obese patients with the metabolic syndrome show increased lipid peroxidation and activate platelets. Diabetologia 54(11):2931–2940
Massberg S, Gawaz M, Gruner S, Schulte V, Konrad I, Zohlnhofer D et al (2003) A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J Exp Med 197(1):41–49
Savage B, Saldivar E, Ruggeri ZM (1996) Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 84(2):289–297
Mancuso ME, Santagostino E (2017) Platelets: much more than bricks in a breached wall. Br J Haematol 178(2):209–219
Akkerman JW (2008) From low-density lipoprotein to platelet activation. Int J Biochem Cell Biol 40(11):2374–2378
Coller BS (2011) Historical perspective and future directions in platelet research. J Thromb Haemost 9(Suppl 1):374–395
Brash AR (2001) Arachidonic acid as a bioactive molecule. J Clin Invest 107(11):1339–1345
Das UN (2018) Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus: a review. J Adv Res 11:43–55
Das UN (2018) Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review. J Adv Res 11:57–66
Hanna VS, Hafez EAA (2018) Synopsis of arachidonic acid metabolism: a review. J Adv Res 11:23–32
Tsai IJ, Croft KD, Puddey IB, Beilin LJ, Barden A (2011) 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am J Physiol Heart Circ Physiol 300(4):H1194–H1200
Neufeld EJ, Majerus PW (1983) Arachidonate release and phosphatidic acid turnover in stimulated human platelets. J Biol Chem 258(4):2461–2467
Davi G, Patrono C (2007) Platelet activation and Atherothrombosis. N Engl J Med 357(24):2482–2494
Holinstat M, Boutaud O, Apopa PL, Vesci J, Bala M, Oates JA et al (2011) Protease-activated receptor signaling in platelets activates cytosolic phospholipase A2alpha differently for cyclooxygenase-1 and 12-lipoxygenase catalysis. Arterioscler Thromb Vasc Biol 31(2):435–442
Maskrey BH, Bermudez-Fajardo A, Morgan AH, Stewart-Jones E, Dioszeghy V, Taylor GW et al (2007) Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. J Biol Chem 282(28):20151–20163
Thomas CP, Morgan LT, Maskrey BH, Murphy RC, Kuhn H, Hazen SL et al (2010) Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J Biol Chem 285(10):6891–6903
Dana R, Leto TL, Malech HL, Levy R (1998) Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase. J Biol Chem 273(1):441–445
Han C, Demetris AJ, Michalopoulos G, Shelhamer JH, Wu T (2002) 85-kDa cPLA(2) plays a critical role in PPAR-mediated gene transcription in human hepatoma cells. Am J Physiol Gastrointest Liver Physiol 282(4):586–597
Hurt-Camejo E, Camejo G, Peilot H, Oorni K, Kovanen P (2001) Phospholipase A(2) in vascular disease. CircRes 89(4):298–304
Das UN (2018) Ageing: is there a role for arachidonic acid and other bioactive lipids? A review. J Adv Res 11:67–79
Baldassare JJ, Henderson PA, Burns D, Loomis C, Fisher GJ (1992) Translocation of protein kinase C isozymes in thrombin-stimulated human platelets. Correlation with 1,2-diacylglycerol levels. J Biol Chem 267(22):15585–15590
Kolesnick RN, Hemer MR (1990) Physiologic 1,2-diacylglycerol levels induce protein kinase C- independent translocation of a regulatory enzyme. J Biol Chem 265(19):10900–10904
Yamamoto Y, Kambayashi Y, Ito T, Watanabe K, Nakano M (1997) 1,2-diacylglycerol hydroperoxides induce the generation and release of superoxide anion from human polymorphonuclear leukocytes. FEBS Lett 412(3):461–464
Smith WL, DeWitt DL, Garavito RM (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182
Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR et al (2012) Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation. J Lipid Res 53(12):2546–2559
Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA (1999) Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J Biol Chem 274(33):22903–22906
Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D (2005) Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev 57(2):217–252
Murphy RC, Gijon MA (2007) Biosynthesis and metabolism of leukotrienes. Biochem J 405(3):379–395
Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Villela CG, Fierro IM (2005) Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am J Physiol Cell Physiol 289(3):C557–C563
O’Donnell VB, Maskrey B, Taylor GW (2009) Eicosanoids: generation and detection in mammalian cells. Methods Mol Biol 462:5–23
Jennings LK (2009) Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost 102(2):248–257
O’Donnell VB, Coles B, Lewis MJ, Crews BC, Marnett LJ, Freeman BA (2000) Catalytic consumption of nitric oxide by prostaglandin H synthase-1 regulates platelet function. J Biol Chem 275(49):38239–38244
Trostchansky A, Bonilla L, Thomas CP, O’Donnell VB, Marnett LJ, Radi R et al (2011) Nitroarachidonic acid, a novel peroxidase inhibitor of prostaglandin endoperoxide H synthases 1 and 2. J Biol Chem 286(15):12891–12900
Kirkby NS, Reed DM, Edin ML, Rauzi F, Mataragka S, Vojnovic I et al (2015) Inherited human group IVA cytosolic phospholipase A2 deficiency abolishes platelet, endothelial, and leucocyte eicosanoid generation. FASEB J Off Publ Fed Am Soc Exp Biol 29(11):4568–4578
Rauzi F, Kirkby NS, Edin ML, Whiteford J, Zeldin DC, Mitchell JA et al (2016) Aspirin inhibits the production of proangiogenic 15(S)-HETE by platelet cyclooxygenase-1. FASEB J Off Publ Fed Am Soc Exp Biol 30(12):4256–4266
Marnett LJ (2002) Recent developments in cyclooxygenase inhibition. Prostaglandins Other Lipid Mediat 68-69:153–164
Brash AR (1999) Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem 274(34):23679–23682
Kalgutkar AS, Crews BC, Rowlinson SW, Garner C, Seibert K, Marnett LJ (1998) Aspirin-like molecules that covalently inactivate cyclooxygenase-2. Science 280(5367):1268–1270
Kalgutkar AS, Kozak KR, Crews BC, Hochgesang GP Jr, Marnett LJ (1998) Covalent modification of cyclooxygenase-2 (COX-2) by 2-acetoxyphenyl alkyl sulfides, a new class of selective COX-2 inactivators. J Med Chem 41(24):4800–4818
Bonilla L, O’Donnell VB, Clark SR, Rubbo H, Trostchansky A (2013) Regulation of protein kinase C by nitroarachidonic acid: impact on human platelet activation. Arch Biochem Biophys 533(1–2):55–61
Rouzer CA, Marnett LJ (2008) Non-redundant functions of cyclooxygenases: oxygenation of endocannabinoids. J Biol Chem 283(13):8065–8069
Maskrey BH, Rushworth GF, Law MH, Treweeke AT, Wei J, Leslie SJ et al (2014) 12-hydroxyeicosatetraenoic acid is associated with variability in aspirin-induced platelet inhibition. J Inflamm 11(1):33
Porro B, Songia P, Squellerio I, Tremoli E, Cavalca V (2014) Analysis, physiological and clinical significance of 12-HETE: a neglected platelet-derived 12-lipoxygenase product. J Chromatogr B Analyt Technol Biomed Life Sci 964:26–40
Midgett C, Stitham J, Martin K, Hwa J (2011) Prostacyclin receptor regulation--from transcription to trafficking. Curr Mol Med 11(7):517–528
Stitham J, Midgett C, Martin KA, Hwa J (2011) Prostacyclin: an inflammatory paradox. Front Pharmacol 2:24
Gimenez-Bastida JA, Boeglin WE, Boutaud O, Malkowski MG, Schneider C (2018) Residual cyclooxygenase activity of aspirin-acetylated COX-2 forms 15 R-prostaglandins that inhibit platelet aggregation. FASEB J Off Publ Fed Am Soc Exp Biol:fj201801018R
Schuligoi R, Schmidt R, Geisslinger G, Kollroser M, Peskar BA, Heinemann A (2007) PGD2 metabolism in plasma: kinetics and relationship with bioactivity on DP1 and CRTH2 receptors. Biochem Pharmacol 74(1):107–117
Deeb RS, Upmacis RK, Lamon BD, Gross SS, Hajjar DP (2008) Maintaining equilibrium by selective targeting of cyclooxygenase pathways: promising offensives against vascular injury. Hypertension 51(1):1–7
Petrucci G, De Cristofaro R, Rutella S, Ranelletti FO, Pocaterra D, Lancellotti S et al (2011) Prostaglandin E2 differentially modulates human platelet function through the prostanoid EP2 and EP3 receptors. J Pharmacol Exp Ther 336(2):391–402
Glenn JR, White AE, Iyu D, Heptinstall S (2012) PGE(2) reverses G(s)-mediated inhibition of platelet aggregation by interaction with EP3 receptors, but adds to non-G(s)-mediated inhibition of platelet aggregation by interaction with EP4 receptors. Platelets 23(5):344–351
Friedman EA, Ogletree ML, Haddad EV, Boutaud O (2015) Understanding the role of prostaglandin E2 in regulating human platelet activity in health and disease. Thromb Res 136(3):493–503
Kauskot A, Hoylaerts MF (2012) Platelet receptors. In: Handbook of experimental pharmacology, vol 210. Springer-Verlag, Berlin Heidelberg, pp 23–57
Whittle BJ, Silverstein AM, Mottola DM, Clapp LH (2012) Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: treprostinil is a potent DP1 and EP2 agonist. Biochem Pharmacol 84(1):68–75
Gross S, Tilly P, Hentsch D, Vonesch JL, Fabre JE (2007) Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. J Exp Med 204(2):311–320
Trostchansky A, O’Donnell VB, Goodwin DC, Landino LM, Marnett LJ, Radi R et al (2007) Interactions between nitric oxide and peroxynitrite during prostaglandin endoperoxide H synthase-1 catalysis: a free radical mechanism of inactivation. Free Radic Biol Med 42(7):1029–1038
Trostchansky A, Souza JM, Ferreira A, Ferrari M, Blanco F, Trujillo M et al (2007) Synthesis, isomer characterization, and anti-inflammatory properties of nitroarachidonate. Biochemistry 46(15):4645–4653
O’Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M et al (1999) Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol 12(1):83–92
Trostchansky A, Rubbo H (2008) Nitrated fatty acids: mechanisms of formation, chemical characterization, and biological properties. Free Radic Biol Med 44(11):1887–1896
Koenig W, Karakas M, Zierer A, Herder C, Baumert J, Meisinger C et al (2011) Oxidized LDL and the risk of coronary heart disease: results from the MONICA/KORA Augsburg study. Clin Chem 57(8):1196–1200
Di Pietro N, Formoso G, Pandolfi A (2016) Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc Pharmacol 84:1–7
Wang W, Hein TW, Zhang C, Zawieja DC, Liao JC, Kuo L (2011) Oxidized low-density lipoprotein inhibits nitric oxide-mediated coronary arteriolar dilation by up-regulating endothelial arginase I. Microcirculation 18(1):36–45
Pirillo A, Norata GD, Catapano AL (2013) LOX-1, OxLDL, and atherosclerosis. Mediat Inflamm 2013:152786
Hackeng CM, Franke B, Relou IA, Gorter G, Bos JL, van Rijn HJ et al (2000) Low-density lipoprotein activates the small GTPases Rap1 and Ral in human platelets. Biochem J 349(Pt 1):231–238
Valiyaveettil M, Kar N, Ashraf MZ, Byzova TV, Febbraio M, Podrez EA (2008) Oxidized high-density lipoprotein inhibits platelet activation and aggregation via scavenger receptor BI. Blood 111(4):1962–1971
Ma Y, Ashraf MZ, Podrez EA (2010) Scavenger receptor BI modulates platelet reactivity and thrombosis in dyslipidemia. Blood 116(11):1932–1941
Korporaal SJ, Meurs I, Hauer AD, Hildebrand RB, Hoekstra M, Cate HT et al (2011) Deletion of the high-density lipoprotein receptor scavenger receptor BI in mice modulates thrombosis susceptibility and indirectly affects platelet function by elevation of plasma free cholesterol. Arterioscler Thromb Vasc Biol 31(1):34–42
Biswas S, Zimman A, Gao D, Byzova TV, Podrez EA (2017) TLR2 plays a key role in platelet Hyperreactivity and accelerated thrombosis associated with hyperlipidemia. Circ Res 121(8):951–962
Assinger A, Koller F, Schmid W, Zellner M, Koller E, Volf I (2010) Hypochlorite-oxidized LDL induces intraplatelet ROS formation and surface exposure of CD40L--a prominent role of CD36. Atherosclerosis 213(1):129–134
Silverstein RL, Nachman RL (1987) Thrombospondin binds to monocytes-macrophages and mediates platelet-monocyte adhesion. J Clin Invest 79(3):867–874
Simantov R, Febbraio M, Silverstein RL (2005) The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol 24(1):27–34
Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M et al (2002) Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem 277(41):38503–38516
Chen K, Febbraio M, Li W, Silverstein RL (2008) A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res 102(12):1512–1519
Nergiz-Unal R, Lamers MM, Van Kruchten R, Luiken JJ, Cosemans JM, Glatz JF et al (2011) Signaling role of CD36 in platelet activation and thrombus formation on immobilized thrombospondin or oxidized low-density lipoprotein. J Thromb Haemost 9(9):1835–1846
Stellos K, Sauter R, Fahrleitner M, Grimm J, Stakos D, Emschermann F et al (2012) Binding of oxidized low-density lipoprotein on circulating platelets is increased in patients with acute coronary syndromes and induces platelet adhesion to vascular wall in vivo--brief report. Arterioscler Thromb Vasc Biol 32(8):2017–2020
Aviram M, Dankner G, Brook JG (1990) Platelet secretory products increase low density lipoprotein oxidation, enhance its uptake by macrophages, and reduce its fluidity. Arteriosclerosis 10(4):559–563
Blache D, Gautier T, Tietge UJ, Lagrost L (2012) Activated platelets contribute to oxidized low-density lipoproteins and dysfunctional high-density lipoproteins through a phospholipase A2-dependent mechanism. FASEB J Off Publ Fed Am Soc Exp Biol 26(2):927–937
Carnevale R, Bartimoccia S, Nocella C, Di Santo S, Loffredo L, Illuminati G et al (2014) LDL oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis 237(1):108–116
Badrnya S, Schrottmaier WC, Kral JB, Yaiw KC, Volf I, Schabbauer G et al (2014) Platelets mediate oxidized low-density lipoprotein-induced monocyte extravasation and foam cell formation. Arterioscler Thromb Vasc Biol 34(3):571–580
Helal O, Defoort C, Robert S, Marin C, Lesavre N, Lopez-Miranda J et al (2011) Increased levels of microparticles originating from endothelial cells, platelets and erythrocytes in subjects with metabolic syndrome: relationship with oxidative stress. Nutr Metab Cardiovasc Dis 21(9):665–671
Zhang X, McGeoch SC, Johnstone AM, Holtrop G, Sneddon AA, MacRury SM et al (2014) Platelet-derived microparticle count and surface molecule expression differ between subjects with and without type 2 diabetes, independently of obesity status. J Thromb Thrombolysis 37(4):455–463
Ghosh A, Li W, Febbraio M, Espinola RG, McCrae KR, Cockrell E et al (2008) Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in mice. J Clin Invest 118(5):1934–1943
Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L et al (2008) Detection of microRNA expression in human peripheral blood microvesicles. PLoS One 3(11):e3694
Aleman MM, Gardiner C, Harrison P, Wolberg AS (2011) Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost 9(11):2251–2261
Geddings JE, Mackman N (2014) New players in haemostasis and thrombosis. Thromb Haemost 111(4):570–574
Wang H, Wang ZH, Kong J, Yang MY, Jiang GH, Wang XP et al (2012) Oxidized low-density lipoprotein-dependent platelet-derived microvesicles trigger procoagulant effects and amplify oxidative stress. Mol Med 18:159–166
Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW (2008) Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 117(1):93–102
Koller E, Koller F, Binder BR (1989) Purification and identification of the lipoprotein-binding proteins from human blood platelet membrane. J Biol Chem 264(21):12412–12418
Nofer JR, Walter M, Kehrel B, Seedorf U, Assmann G (1995) HDL3 activates phospholipase D in normal but not in glycoprotein IIb/IIIa-deficient platelets. Biochem Biophys Res Commun 207(1):148–154
Pedreno J, de Castellarnau C, Masana L (2001) Platelet HDL(3) binding sites are not related to integrin alpha(IIb)beta(3) (GPIIb-IIIa). Atherosclerosis 154(1):23–29
Brodde MF, Korporaal SJ, Herminghaus G, Fobker M, Van Berkel TJ, Tietge UJ et al (2011) Native high-density lipoproteins inhibit platelet activation via scavenger receptor BI: role of negatively charged phospholipids. Atherosclerosis 215(2):374–382
Imachi H, Murao K, Cao W, Tada S, Taminato T, Wong NC et al (2003) Expression of human scavenger receptor B1 on and in human platelets. Arterioscler Thromb Vasc Biol 23(5):898–904
Nofer JR, van Eck M (2011) HDL scavenger receptor class B type I and platelet function. Curr Opin Lipidol 22(4):277–282
Le QH, El Alaoui M, Vericel E, Segrestin B, Soulere L, Guichardant M et al (2015) Glycoxidized HDL, HDL enriched with oxidized phospholipids and HDL from diabetic patients inhibit platelet function. J Clin Endocrinol Metab 100(5):2006–2014
Assinger A, Schmid W, Eder S, Schmid D, Koller E, Volf I (2008) Oxidation by hypochlorite converts protective HDL into a potent platelet agonist. FEBS Lett 582(5):778–784
Assinger A, Koller F, Schmid W, Zellner M, Babeluk R, Koller E et al (2010) Specific binding of hypochlorite-oxidized HDL to platelet CD36 triggers proinflammatory and procoagulant effects. Atherosclerosis 212(1):153–160
van der Stoep M, Korporaal SJ, Van Eck M (2014) High-density lipoprotein as a modulator of platelet and coagulation responses. Cardiovasc Res 103(3):362–371
Wang N, Tall AR (2016) Cholesterol in platelet biogenesis and activation. Blood 127(16):1949–1953
Ravindran R, Krishnan LK (2007) Increased platelet cholesterol and decreased percentage volume of platelets as a secondary risk factor for coronary artery disease. Pathophysiol Haemost Thromb 36(1):45–51
Relou IA, Hackeng CM, Akkerman JW, Malle E (2003) Low-density lipoprotein and its effect on human blood platelets. Cell Mol Life Sci 60(5):961–971
Kumar S, Vikram A, Kim YR (2014) J SJ, Irani K. P66Shc mediates increased platelet activation and aggregation in hypercholesterolemia. Biochem Biophys Res Commun 449(4):496–501
Panes O, González C, Hidalgo P, Valderas JP, Acevedo M, Contreras S et al (2017) Platelet tissue factor activity and membrane cholesterol are increased in hypercholesterolemia and normalized by rosuvastatin, but not by atorvastatin. Atherosclerosis 257:164–171
Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS et al (2005) Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med 353(1):46–57
Chen K, Li W, Major J, Rahaman SO, Febbraio M, Silverstein RL (2011) Vav guanine nucleotide exchange factors link hyperlipidemia and a prothrombotic state. Blood 117(21):5744–5750
Ramasamy I (2016) Update on the molecular biology of dyslipidemias. Clin Chim Acta 454:143–185
Beaumont JL, Carlson LA, Cooper GR, Fejfar Z, Fredrickson DS, Strasser T (1970) Classification of hyperlipidaemias and hyperlipoproteinaemias. Bull World Health Organ 43(6):891–915
AACE (2017) Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 23(2):1–87
Tada H, Kawashiri MA, Yamagishi M (2017) Clinical perspectives of genetic analyses on dyslipidemia and coronary artery disease. J Atheroscler Thromb 24(5):452–461
Nelson RH (2013) Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 40(1):195–211
van Rooy MJ, Duim W, Ehlers R, Buys AV, Pretorius E (2015) Platelet hyperactivity and fibrin clot structure in transient ischemic attack individuals in the presence of metabolic syndrome: a microscopy and thromboelastography study. Cardiovasc Diabetol 14:86
van Rooy MJ, Pretorius E (2015) Metabolic syndrome, platelet activation and the development of transient ischemic attack or thromboembolic stroke. Thromb Res 135(3):434–442
Gaspar RS, Trostchansky A, Paes AM (2016) Potential role of protein disulfide isomerase in metabolic syndrome-derived platelet hyperactivity. Oxidative Med Cell Longev 2016:2423547
McFadyen JD, Peter K (2018) Platelet lipidomics and function: joining the dots. Blood 132(5):465–466
Peng B, Geue S, Coman C, Munzer P, Kopczynski D, Has C et al (2018) Identification of key lipids critical for platelet activation by comprehensive analysis of the platelet lipidome. Blood 132(5):e1–e12
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Paes, A.M.d.A., Gaspar, R.S., Fuentes, E., Wehinger, S., Palomo, I., Trostchansky, A. (2019). Lipid Metabolism and Signaling in Platelet Function. In: Trostchansky, A., Rubbo, H. (eds) Bioactive Lipids in Health and Disease. Advances in Experimental Medicine and Biology, vol 1127. Springer, Cham. https://doi.org/10.1007/978-3-030-11488-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-11488-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11487-9
Online ISBN: 978-3-030-11488-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)