Abstract
Aortic arch aneurysms present a special challenge to the cardiovascular surgeon because of the complicated anatomy of the aortic arch and critical need to protect the brain from ischemic and embolic injury during aortic arch aneurysm repair. Over the past 30 years, advances in operative technique and neuroprotective strategies have made routine repair of aortic arch aneurysms feasible. The development of endostents to treat aortic arch aneurysms has been delayed by anatomic constraints, such as the curvature of this aortic segment and critical branches to the brain that need to be reconstructed. The initial use of endostents to treat aortic arch pathology has been primarily in the context of hybrid endovascular-open procedures. Ongoing development of branched endografts will add to the therapeutic options for the treatment of the aortic arch aneurysms and improve the safety and outcomes of this procedure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aneurysm
- Aortic arch
- Aortic dissection
- Atherosclerosis
- Connective tissue disorder
- Deep hypothermic circulatory arrest
- Cystic medial degeneration
- Endostent
- Hybrid procedures
- Neuroprotection
- Selective antegrade perfusion
Introduction
Repair of aortic arch aneurysms is especially challenging because of the complicated anatomy of this aortic segment. The need to reconstruct critical branches supplying the brain and upper extremities, the curved shape, orientation from anterior to posterior, and frequent associated aneurysm involvement with the ascending and descending aorta impose anatomic constraints that limit exposure during open procedures and challenge the tolerances of endovascular devices. Add to these challenges the critical need to perform aortic arch aneurysm repair while protecting the brain from ischemic and embolic injury. Because of these anatomic, pathologic, and technical factors, aortic arch surgery has been associated with significant morbidity and mortality and is frequently performed in specialized centers that have developed expertise in treatment of this complex entity. Modern operative techniques and endovascular approaches have reduced these risks and allowed safer, more complete repair for this challenging group of patients.
Anatomy
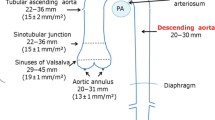
The aortic arch is contained within the superior mediastinum (Fig. 12.1). It is continuous with the ascending aorta and begins at the level of the second sternocostal articulation on the right side of the sternum. Its most proximal portion is directed superiorly and posteriorly, across the anterior surface of the trachea. It then travels posteriorly to the left side of the trachea before turning downward to give rise to the descending aorta at the level of the fourth thoracic vertebra.
Anterior view of the aortic arch . (Reprinted from [1], with permission from Springer)
The aortic arch normally has three branches. The most proximal and largest branch is the brachiocephalic artery, which is anterior to and to the right of the other arch vessels. The second branch is the left common carotid artery, which originates distal to and to the left of the brachiocephalic trunk. The third and most distal branch of the arch is the left subclavian artery.
Aortic arch branch vessel anomalies are common [1]. A bovine arch is the most common variant and occurs when the brachiocephalic artery shares a common origin with the left common carotid artery. A bovine arch occurs in 10–20% of the population. A thyroidea ima artery, supplying the inferior aspect of the thyroid gland, occurs in 4–10% of the population and can arise from the brachiocephalic artery, right common carotid artery, or directly from the aortic arch. Variant left vertebral arteries are present in 2–6% of the population and can arise directly from the aortic arch.
Pathophysiology
Aneurysms
The aortic arch is affected by the same pathophysiologic processes that cause aortic aneurysms in other portions of the thoracic aorta [2]. Aneurysms of the ascending aorta are most often the result of cystic medial degeneration, which appears histologically as loss of smooth muscle cells and elastic fibers within the artery wall media. Cystic medial degeneration normally occurs to some extent with aging. Hypertension and tobacco use are thought to accelerate the process.
The most common inherited disorders that cause accelerated cystic medial degeneration are Marfan syndrome and familial thoracic aortic aneurysm syndrome. The accelerated degenerative process usually leads to early presentation during young adulthood in these patients. At least 20% of thoracic aortic aneurysms are thought to be genetically caused. Other genetic disorders associated with cystic medial degeneration and early aneurysm formations include bicuspid aortic valve, Ehlers–Danlos syndrome, Loeys–Dietz syndrome, and Turner syndrome.
In contrast to the ascending aorta, cystic medial degeneration is not typically the cause of true aneurysms involving the descending aorta. Atherosclerosis is the predominant etiology of aneurysms of the descending aorta. These aneurysms typically originate just distal to the origin of the left subclavian artery and can involve the distal aortic arch. Risk factors for atherosclerotic thoracic aneurysms include advanced age, hypertension, and tobacco use.
Aneurysms of the aortic arch are less common than aneurysms of the ascending or descending aorta. This is likely related to the pattern of involvement of the thoracic aorta with the predominant aortic pathologies, cystic medial degeneration, and atherosclerosis. Cystic medial degeneration predominantly affects the aortic root and ascending aorta while atherosclerosis predominantly affects the descending aorta. Involvement of the proximal aortic arch with cystic medial degeneration or involvement of the distal aortic arch with atherosclerosis can lead to involvement of a portion of the aortic arch with contiguous aneurysms, but whole arch involvement or isolated arch involvement is rare. Aneurysm involvement of the whole aortic arch typically signals extensive aortic involvement because it is associated with diffuse medial degenerative disease or atherosclerosis in most cases.
Syphilis was once perhaps the most common cause for ascending aortic aneurysms, but in the era of common antibiotic treatment, such aneurysms are rarely seen today. The latent period from initial spirochetal infection to aortic aneurysm formation is 10–30 years. Aneurysm formation is related to direct sprichete infection of the aortic media. Aneurysms are often saccular and involve the ascending aorta but can involve the aortic root and arch.
Chronic inflammation of the aorta is a rare cause of aortic aneurysm. Takayasu’s arteritis is a rare disease of unknown etiology that causes a chronic aortitis. The disease affects women much more often than men and is diagnosed in early adulthood. It typically causes obliterative changes of the aorta or aortic branches but can cause aortic aneurysms in up to 15% of cases. Giant-cell arteritis and ankylosing spondylitis are other rare causes of aortitis that can lead to aortic aneurysm formation.
Dissections
Chronic aortic dissection is an important cause of aortic dilatation [3]. In many surgery practices, chronic dissection of the aortic arch after Stanford Type A or DeBakey Type I aortic dissection is the most common underlying pathology leading to aortic arch replacement (Fig. 12.2). When an aortic dissection occurs, it causes direct weakening of the aortic wall by forming a false lumen that is contained only by the media and adventitia of the aorta. This false lumen is typically exposed to systemic arterial pressures through fenestrations in the intimal flap between the true and false lumens. In addition, because the dissected aorta is acutely enlarged at the time of dissection, the aortic wall is subjected to higher wall stress as dictated by LaPlace’s Law. Further aortic growth over time begets further aortic growth by the same mechanism. Finally, many patients with chronic dissection have an underlying aortopathy that causes cystic medial degeneration and aortic growth.
Classification of aortic dissection by extent of aortic involvement. (From Isselbacher [29]. Reprinted with permission from Springer)
Trauma
Patients suffering severe blunt chest trauma can also suffer aortic arch injuries. Typically, these injuries are the result of rapid deceleration after motor vehicle accidents or falls, leading to partial aortic transection. The aortic injury usually occurs at a point of aortic fixation within the chest, most commonly the aortic isthmus of the distal aortic arch at the site of the ligamentum arteriosum. Aortic injuries resulting from blunt trauma are typically associated with a constellation of other thoracic, abdominal, orthopedic, and central nervous system injuries. Many patients with blunt aortic injuries die from hemorrhage or other associated injuries before they can be evaluated. When the aortic injury is contained, leading to an aortic pseudoaneurysm, patients may survive to be evaluated in an emergency department. Rarely, aortic transections are not diagnosed initially and patients may develop chronic pseudoaneurysms. These pseudoaneurysms are frequently saccular and discrete. They are subject to growth over time because of their inherent weakness and high wall stress.
Presentation
Most patients with thoracic aortic aneurysms are asymptomatic. Because of this, most thoracic aneurysms are discovered incidentally at the time of chest X-ray, CT scan, MRI, or echocardiogram. Patients with aortic arch aneurysms associated with ascending aortic or aortic root aneurysms may have secondary aortic regurgitation, leading to a diastolic murmur or congestive heart failure. Patients with aortic arch aneurysms associated with bicuspid valve may also present with valve dysfunction, either aortic stenosis or regurgitation. Patients with large aortic arch or associated thoracic aneurysms may suffer a local mass effect, such as compression of the trachea or left mainstem bronchus (causing cough, wheezing, pneumonia, or pneumonitis), the esophagus (causing dysphagia), or the recurrent laryngeal nerve (causing hoarseness). Rarely, patients with nondissecting aneurysms may present with chest or back pain related to direct compression of other intrathoracic structures or erosion into bone.
The major aortic complication caused by thoracic aneurysms is dissection or rupture. Patients are typically symptomatic and have a sense of foreboding, acute onset chest or back pain, hypotension, and malperfusion of any aortic branch vessel.
Imaging
Management of thoracic aortic aneurysms is highly dependent on sizing and morphology, making imaging a critical component of the evaluation of these patients. CT and MR angiography are the preferred modalities to define aneurysm size and morphology and aortic branch anatomy. CT scans should be gated to eliminate motion artifact to allow precise aortic measurement, as changes as small as several millimeters may influence treatment decisions. When measuring aortic diameters from axial images, great care must be taken to obtain true aortic short axis measurements. This is particularly true when evaluating the aortic arch because the arch is a curved structure that is imaged off-axis in typical axial CT images. Off-axis measurements tend to overestimate the true short axis diameter of the aorta. Imaging software packages allow three-dimensional reconstruction of the aorta and manipulation to measure different segments of even a tortuous aorta along its true short axis.
When evaluating patients with ascending aortic and proximal arch aneurysms, we find CT and MR angiography of the chest to be sufficient to guide management and treatment decisions. For patients with distal arch and descending aortic involvement, cross-sectional imaging of the aorta and distal runoff vessels should be obtained through the pelvis so that the entire aorta and distal branches can be evaluated for aneurysm involvement. Such a study also shows the suitability of the femoral and pelvic vessels for perfusion support during open repair or vascular access for transcatheter approaches.
We routinely obtain transthoracic echocardiograms in patients being considered for virtually any thoracic aortic aneurysm repair. These studies demonstrate aortic valve morphology and function. The association of aortic valve dysfunction as well as bicuspid aortic valve with thoracic aortic aneurysms makes evaluation of the aortic valve an important component in the workup of patients with thoracic aortic disease. The presence of a bicuspid aortic valve or aortic valve dysfunction may influence decisions on timing and extent of surgery.
Indications
Acute
Acute indications for aortic arch replacement include rupture of an aortic aneurysm or pseudoaneurysm. The most common indication for emergency surgery on the aortic arch is Stanford Type A dissection [4]. Emergency Type A dissection repair is undertaken to prevent free rupture of the ascending aorta or aortic root into the pericardial sac, causing cardiac tamponade. Emergency Type A dissection repair also effectively treats acute aortic regurgitation and coronary malperfusion. The standard repair for Type A dissection also involves replacement of the inferior aortic hemiarch. More extensive repair or replacement of the aortic arch in the setting of extensive dissection involvement of the arch is controversial; however, several centers support this approach. Addition of antegrade deployment of a stent graft into the proximal descending aorta (frozen elephant trunk) to the standard Type A dissection repair is another area of ongoing investigation.
Chronic
Elective aortic arch replacement is recommended for patients with large aneurysms (>6 cm) [2]. Close consideration for elective repair is given to patients with rapidly growing aneurysms (>0.5 cm/year), saccular aneurysms, and for symptomatic patients (pain or hoarseness). Smaller aneurysms (>5 cm) are considered for repair in patients with a genetically transmitted aortopathy or family history of aortic rupture or dissection. Replacement of the aortic arch is also considered at a smaller size when there is extensive aneurysm involvement of the ascending or descending aorta that mandates operative repair.
Open Aortic Arch Aneurysm Repair
Development
The advent of extracorporeal circulation and the use of hypothermia, coupled with the invention of synthetic aortic prostheses, allowed pioneers in the field to develop operations to repair thoracic aortic aneurysms. With regard to the repair of aortic arch aneurysms, the implementation of deep hypothermic circulatory arrest (DHCA) by Griepp and colleagues was a necessary development to allow neuroprotection during aortic arch surgery and this innovation was responsible for significant improvement in aortic arch surgery outcomes compared to discouraging early results [5].
Further advancements were made by Hans G. Borst, and later Lars Svenssoon, who revolutionized complex repairs of the aortic arch and proximal segments of the descending thoracic aorta by introducing the concept of the elephant trunk technique in the 1980s [6]. This consists of an open distal aortic arch anastomosis performed under DHCA with a continuous segment of aortic graft material (8–10 cm in length) left “hanging” in the proximal descending thoracic aorta aneurysm. This graft is then used to reconstruct the aortic arch to complete the first stage of the operation (Fig. 12.3). In the second stage of the repair, the proximal descending thoracic aorta is replaced through a posterolateral thoracotomy (Fig. 12.4). Control of the otherwise treacherous proximal descending thoracic aorta is greatly facilitated by the presence of the elephant trunk, which can be directly clamped and sewn to an aortic graft replacing the most distal portion of the descending thoracic aorta. In current practice, the second stage can be performed with endovascular techniques by landing an endostent within the elephant trunk segment proximally and distally in an area of normal aorta prior to the takeoff of the mesenteric vessels (Fig. 12.5).
Second stage of “elephant trunk” repair. The second stage procedure is done through a posterolateral thoracotomy or thoracoabdominal approach. The elephant trunk is accessed for proximal control of the descending aorta and used to complete aneurysm repair of the descending thoracic or thoracoabdominal aorta
Neuroprotective Strategies
Neurologic injury remains the most devastating complication of aortic arch surgery and there has been tremendous focus on developing neuroprotective strategies for use during these complex operations. There are three primary approaches used to achieve this goal. The first one is DHCA, which requires systemic cooling using cardiopulmonary bypass to below 20 °C. At this low temperature, brain metabolism is reduced to the point that circulatory support can be discontinued for 30–40 min without permanent brain injury. Once circulatory support is discontinued, the aortic cross clamp can be removed and the patient is partially exsanguinated. This allows for the distal anastomosis and arch vessel reconstruction to be performed in a bloodless field. Expediency is critical during this portion of the operation to limit the likelihood of postoperative neurocognitive deficits.
The other two approaches utilize selective brain perfusion strategies, either retrograde cerebral perfusion via the superior vena cava or selective antegrade cerebral perfusion (ACP) through the axillary artery or direct cannulation of the brachiocephalic and left carotid ostia when the aorta is splayed open [7, 8]. When ACP is used, deep hypothermia is not required and the patient can be cooled to only 23–26 °C as long as antegrade perfusion to the brain is maintained at rates between 10 and 20 ml/kg/min. Up to 90 min of moderate hypothermia (23–26 °C) is generally tolerated when selective ACP is used. The authors prefer the selective ACP method as a neuroprotective strategy; however, there is no data to suggest an advantage of one over another [9,10,11].
Dissections and Connective Tissue Pathologies
More recently, complex aortic arch repair techniques have been used with some modifications on different types of aortic pathologies. These include complex Type A aortic dissections involving the aortic arch and supra-aortic trunks. In these cases, as well as in patients with connective tissue disorders, direct implantation of the supra-aortic trunk vessels as an island of aortic arch tissue is not advised. Rather, a branched graft is used thereby removing as much of the diseased tissue involved as possible (Fig. 12.6). Also, care must be taken to obliterate the false lumen of the distal anastomosis by using a technique such as the “Felt Sandwich” (Fig. 12.7). A more recent modification of the elephant trunk technique, the “frozen elephant trunk,” offers several advantages to assure false lumen exclusion, depressurization of the aorta downstream, obliteration, and thromboexclusion, leading to shrinking of the false lumen (Fig. 12.8). This technique has potential to reduce leads the incidence of future re-interventions on the residual dissected aorta in this patient population, however prospective studies are required to demonstrate this theoretical benefit.
Conduct of the Operation
The operation is performed with the patient in the supine position. All of the appropriate monitoring equipment is placed including a PA catheter and bilateral radial arterial lines. Adequacy of cerebral perfusion is assessed using Near-Infrared Spectroscopy (NIRS). Baseline levels are obtained and continuously monitored throughout the case. The patient is widely prepped and draped including the right shoulder for axillary cannulation. An incision is made over the right deltopectoral groove. Through this incision the axillary artery is dissected and encircled with a vessel loop. The patient is then given 5000 units of heparin intravenously and an 8 mm Dacron graft is anastomosed in an end-to-side fashion onto the right axillary artery (Fig. 12.9). This graft will then be connected to the arterial line of the cardiopulmonary bypass (CPB) circuit. The arterial line of the CPB circuit has a “Y” configuration to provide flow to the axillary artery and the left carotid during selective ACP. A median sternotomy is then performed to gain access to the mediastinum and the brachiocephalic vein is mobilized circumferentially, dividing the thymus branches between suture ligatures. Once this is accomplished, an umbilical tape is placed around it and used for traction. Next, the proximal portions of the brachiocephalic trunk and left carotid and left subclavian arteries are mobilized. This dissection is carried as close to the aortic arch as possible to avoid injury to the recurrent laryngeal nerves bilaterally. An umbilical tape with a Rummel tourniquet is placed around the brachiocephalic trunk. After this is accomplished, the rest of the thymus is divided caudally, the pericardium is opened and a pericardial well is created. The patient is then given pump dose heparin and a dual-stage venous cannula is placed through a purse-string suture in the right atrial appendage. This venous cannula is then connected to the venous line of the CPB circuit. A retrograde cardioplegia catheter is placed through the right atrium into the coronary sinus and secured.
After the Activated Clotting Time (ACT) is higher than 500 s, CPB is initiated and the patient is slowly cooled to a core temperature (bladder) of 26 °C. Once on CPB, a left ventricular (LV) vent can be placed through the right superior pulmonary vein to prevent left ventricular distension. While cooling, the heart can be arrested and any necessary procedures on the ascending aorta or aortic root can be performed. Coronary artery bypass grafting can also be performed at this time as indicated. Ten minutes prior to achieving a core temperature of 26 °C, the patient is given a bolus of steroids, mannitol, and propofol, and the head is packed in ice. After the target temperature is reached, the patient is placed in steep Trendelenburg. The pump is stopped and the Rummel on the brachiocephalic trunk is tightened. The CPB pump is then restarted at 10 ml/kg/min, thereby providing flow to the right-sided circulation of the brain. The aortic clamp is then removed and pump suckers are placed in the arch and down the descending thoracic aorta to clear the blood. With the aorta opens, the left carotid ostium is visualized and a 13 Fr balloon tipped Gundry perfusion catheter, which is attached to the “Y” arterial line of the CPB circuit, is placed into the left carotid artery. CPB flow is then increased to 20 ml/kg/min (Fig. 12.10). During this part of the operation cerebral perfusion to both brain hemispheres is monitored closely using NIRS and the antegrade flow to the brain is adjusted accordingly to maintain baseline venous saturation values.
Once adequate antegrade cerebral perfusion is established, an island of the supra-aortic trunks is created. An appropriate sized Dacron tube is invaginated, assuring at least a 10 cm segment of graft material will remain in the proximal segment of the descending thoracic aorta as the so-called elephant trunk. This invaginated tube is then sewn into the neck of the aneurysm just distal to the left subclavian with nonabsorbable suture. The invaginated segment is then retracted or pulled out of the anastomosis and a keyhole graftotomy is made along the greater curvature of the graft to sew the supra-aortic trunk island in an end-to-side fashion. Prior to completing the anastomosis, the left carotid perfusion cannula is removed and the anastomosis is completed. The patient is kept on steep Trendelenburg and the pump is stopped again. The Rummel on the brachiocephalic trunk is loosened and the pump is started again slowly, allowing the blood to exit the graft. Once de-airing of the neo-arch is completed, the aortic cross clamp is placed on the aortic graft proximal to the supra-aortic island anastomosis and full CPB is reinstituted, rewarming the patient. Rewarming is slow, even, and thorough. During this time, any necessary remaining work on the proximal ascending aorta or aortic root, including the anastomosis to the proximal end of the aortic arch graft, can be performed.
Outcomes
Using selective ACP and mild-to-moderate hypothermia, Svensson showed a 2% risk of stroke in patients undergoing aortic arch surgery [12, 13]. Although there are several cannulation strategies available, right axillary cannulation provides appropriate access for ACP and allows for performance of the entire procedure with a single arterial cannulation site. Studies have demonstrated that it is accessible in 97% of aortic cases [14]. Selective ACP allows arrest of systemic circulation at a higher temperature while maintaining neuroprotection through antegrade brain perfusion. Several studies have demonstrated the safety of performing a distal anastomosis at 28 °C without increased risk of stroke or lower-body morbidity [7, 15]. When comparing outcomes of renal failure, bleeding and reoperation of DHCA versus moderate hypothermic circulatory arrest with ACP there were no differences: renal failure (13.3% vs. 12.6%, p = 0.32) and bleeding and reoperation (10.9% vs. 13.3%, p = 0.65) were similar between groups. Advantages of ACP include lengthening of the period of safe circulatory arrest up to 90 min allowing the performance of complex arch repair without increased risk of brain injury [15]. Despite these advancements, complex aortic arch repairs remain a challenge and are associated with a high risk of complications. However, with careful planning and a systematic approach that utilizes modern operative and neuroprotection strategies, morbidity and mortality are reduced, allowing complex aortic arch reconstructions to be performed with acceptable risk.
Endovascular and Hybrid Aortic Arch Aneurysm Repair
Open surgery remains the gold standard of treatment for aneurysms of the aortic arch. Many high volume centers have demonstrated excellent results [16]. However, these maximally invasive surgeries are often performed in elderly patients, sometimes with significant comorbid disease. These operations are technically very challenging, require a sternotomy and cardiopulmonary bypass, and frequently require hypothermic circulatory arrest. High risk patients defined as either physiologically high risk with significant comorbidities, or anatomically high risk, such as patients who have had previous sternotomy or multiple sternotomies, or patients with challenging anatomy such as anastomotic aneurysms, are often turned down for elective repair.
With proven success in the thoracic aorta with endovascular aortic stent grafting for the treatment of aneurysms and dissections, it is logical that thoracic endovascular aortic repair (TEVAR) techniques be extended to treat conditions of the aortic arch [17]. Endovascular repair of arch aneurysms often consists of hybrid operations that combine elements of endovascular stent grafting with open surgery or more recently, near-total endovascular repair of arch aneurysms.
The aortic arch presents complex anatomic challenges for endovascular repair, with curvatures and angulations that can be extreme. Inadequate apposition or conformability of the endograft in the inner curve of the aortic arch may cause difficulty with proximal seal and fixation, resulting in perioperative or postoperative Type I endoleak [18]. After placement, stent grafts in the aortic arch are subject to great dynamic strain, owing to a curved configuration, high blood flow, and pulsatile movement of the aorta, which may potentially cause migration, fracture, or disconnection of device components. Exclusion of the aneurysm sac, maintenance of cerebral perfusion, and avoidance of emboli are the primary intraoperative objectives in endovascular aortic arch aneurysm repair. Important supra-aortic vessels, such as the carotid and vertebral arteries, or coronary artery conduits arising from the internal mammary artery, must remain perfused. In addition, excessive manipulation of catheters, wires, and intravascular devices should be avoided within the confines of the aortic arch to avoid cerebral embolization with subsequent stroke or vessel wall injury, which could result in thrombus formation and dissection. These issues are particularly important for the new generation of side branch prostheses.
The success of endovascular exclusion depends on the adequacy of seal of the endograft to the aortic wall, proximal and distal to the aortic arch aneurysm. Suitable landing zones require a minimum length of 20 mm of healthy aorta. The geometry of the ascending aorta can pose specific challenges owing to a shortened inner curvature length compared to the outer curvature as well as resulting in occasional “bird-beaking” of the proximal stent–aorta interface, which can result in Type 1 endoleak. Proximal positioning must take into account the location of the coronary ostia, and oftentimes, distally placed origins of coronary artery bypass grafts. The distal landing zone can reside anywhere in the descending thoracic aorta provided it is of suitable length and diameter. Frozen elephant trunk placement of arch endografts can provide landing sites for subsequent thoracic or thoracoabdominal repair with stent grafts, or anastomotic sites for open repair. Sizing of the stent graft in the proximal landing zone requires at least 6 mm of oversizing. When the size of the ascending aorta exceeds 38 mm and is intended to be a proximal landing zone in a hybrid Zone 0 case, consideration should be given to ascending aorta replacement. Near-total arch branched grafts are relatively contraindicated in ascending aortas greater than 38 mm as this represents a potential unhealthy and unstable seal zone. Placement of an endostent in such a landing zone may result in Type 1 endoleak, aortic rupture, pseudoaneurysm formation, or retrograde Type A aortic dissection [19].
Hybrid Procedures
In hybrid repair, supra-aortic debranching is performed to provide an appropriate landing zone for the stent graft and to preserve perfusion to the supra-aortic trunks followed by stent graft deployment across the aortic arch pathology. Ischimura’s classification of zones of the aortic arch is widely used to determine the preferred option of hybrid endovascular repair (Fig. 12.11). Cases are often referred to as Zone 0, 1, or 2 endovascular arch aneurysm repairs. In “Zone 0” cases, the proximal landing zone involves the orifice of the innominate artery and a prophylactic revascularization from the ascending aorta to the innominate trunk, and left common carotid artery is required with concomitant endovascular exclusion of the aneurysm commencing in the ascending aorta distal to the debranching proximal anastomosis and ending distal to the arch or descending thoracic aortic aneurysm. In “Zone 1” cases, the proximal landing zone involves the orifices of the left common carotid artery, necessitating revascularization of this vital artery, usually with an extra-anatomic carotid–carotid artery bypass along with endovascular stent grafting starting close to the distal edge of the innominate artery. In “Zone 2” cases, the proximal landing zone involves the orifice of the left subclavian artery.
Hybrid procedures thus avoid the need for cardiopulmonary bypass or hypothermic circulatory arrest. Modifications in existing technology and new-generation devices, such as the Conformable TAG thoracic device (C-TAG, Gore & Associates), Valiant thoracic stent graft (Medtronic), Relay thoracic device (Bolton Medical), and Zenith Alpha thoracic endovascular graft (Cook Medical), have resulted in more reliable trackability and precise deployment at the distal margin of the innominate, left common carotid, and left subclavian arteries (LSA), or in the ascending aorta.
The arm’s rich collateral blood supply obviates the need for mandatory revascularization, especially in an emergency setting, but restoring direct arterial flow to the subclavian artery can be important in stroke prevention as well as in the prevention of paraplegia, depending on the extent of coverage of the thoracic aorta. The decision to revascularize the left subclavian with left carotid–subclavian artery bypass has been a subject of much debate in the literature [20]. As mentioned, often the decision is based on the amount of thoracic aortic coverage required or the presence of a dominant or solitary left vertebral artery, or the presence of a functioning left internal mammary artery coronary artery bypass graft, all of which necessitate left subclavian revascularization. Clinical trials are currently underway examining the safety and feasibility of the Gore TAG Thoracic Branch Endoprosthesis (TBE device), as well as the Valiant Mona LSA Thoracic Stent Graft System, both of which feature endovascular revascularization as part of their thoracic stent graft via branches attached to their stent graft platform (Fig. 12.12). If surgical left carotid subclavian bypass is performed, either proximal surgical ligation of the left subclavian artery or endovascular proximal occlusion of this artery is generally required to prevent retrograde endoleak.
Chimney or snorkel grafts (Fig. 12.13) have been proposed to extend the proximal fixation zone in the aortic arch during TEVAR repairs [21]. They have the advantage of using standard, off-the-shelf materials and being technically less demanding, but their durability and ability to effect exclusion of an arch aneurysm in the aortic arch remains questionable, despite reported early success [22, 23]. Thoracic stent graft technology is not being developed with chimney and snorkel grafts in mind, and consequently, there are presently no ideal stent grafts for this application. Until longer and more rigorous follow-up are available, chimney grafts should only be considered in emergency patients who are poor candidates for open repair or in cases of inadvertent coverage of the supra-aortic trunks.
Near-Total Arch Branched Endovascular Grafts
Near-total branched arch stent grafting offers several advantages over other approaches including avoiding the need for sternotomy, eliminating exposure to cardiopulmonary bypass, and circulatory arrest, and minimizing the extent of extra-anatomic bypass of supra-aortic vessels [24]. Several iterations of graft designs exist with the latest generation grafts being flexible enough to accommodate most arch anatomy. Branched arch endografts from Cook Medical and Bolton are available outside the United States under special access (Fig. 12.14).
The author (C.A.) has one of the largest experiences (15 cases) using the Cook Medical Arch Branched Graft in North America. Over 200 cases have been performed worldwide, and the global experience has provided valuable insight into improving stroke and mortality rates [25]. Supra-aortic branch target vessels must be of suitable diameter. The innominate artery should have a minimum diameter of 8 mm, while the left common carotid artery should have a minimum diameter of 6 mm. A custom-made branch extension limb provided by Cook Medical is usually required for the innominate artery in order to accommodate the larger diameter of the distal innominate artery. Commercially available covered stents are suitable for most carotid or subclavian arteries. Because of its accuracy and versatility, our preference is for the Atrium/ICast covered stent graft; although Bard Fluency™ and Gore Viabahn™ covered self-expanding stents are also used worldwide. The balloon expandable covered stents require lining with a bare metal self-expanding stent to add support and mitigate against tortuosity or possible kinking.
Conduct of the procedure has been described elsewhere [24]. Salient points include the need for a stiff wire buried in the left ventricle, deployment during asystole, achieved with either rapid ventricular pacing or right atrial venous balloon occlusion, and attention to the relationship of the endograft and its branches to the coronary arteries and supra-aortic vessels (Fig. 12.15).
Angiogram aortic arch demonstrating relationship of branch markings (yellow and red arrows), to origins of supra-aortic vessels (open white arrows), and proximal arch branch graft stent to origins of coronary arteries (solid white and black arrows). Note double curved lunderquist wire buried in left ventricle
The largest published series of arch branch endografting was a global experience that included 38 patients with median follow-up of 12 months [25]. The 30-day mortality of the entire cohort was 13.2%. Comparative analysis of the early experience (first 10 patients) and late experience demonstrated an interesting but not statistically significant difference in 30-day mortality (30% vs. 7.1%, p = 0.06). No late mortality was observed during the follow-up period. Cause of death included perioperative cardiac arrest, myocardial infarction, hemorrhagic shock, and pulmonary complications. Early procedural success was 84.2%. Failures included proximal type 1 endoleak, failure to catheterize the innominate branch, and conversion to chimney technique. On discharge, 28.8% of patients were diagnosed with an endoleak (5 Type I, 3 Type II, Type III, and 2 indeterminate), with 10.5% of patients requiring a secondary procedure. Neurological complications occurred in 16% of patients who survived the procedure. This compares to 4–12% neurological event rates seen in larger series of traditional open and hybrid repairs [26,27,28]. All patients in the branched arch endograft series had a full neurologic recovery (4 transient ischemic attacks, 1 stroke, 1 subarachnoid hemorrhage). In comparative analysis of the early experience (first 10 patients) compared to the late experience, there were significantly fewer intraoperative complications, less need for secondary procedures, less need for interventions for endoleaks, less operative time, and less radiation exposure. This highlights the importance of the learning curve involved with this complex procedure as well as the importance of confining these procedures to high volume regional centers.
Although a viable alternative to traditional open and hybrid repair, near-total endovascular arch stent grafting is still in its early development. The complexity of arch geometry and its branches poses unique challenges for endovascular devices and necessitates an individualized approach. These complex endovascular procedures should be reserved for patients who are not able to tolerate open or hybrid procedures for anatomic reasons, or in patients who have significant comorbidities precluding open surgery. Stroke remains an important risk in these procedures. Despite satisfactory early results, mid- to long-term studies are needed before we can recommend this treatment as a comparable alternative to standard open arch reconstruction or hybrid arch repair.
References
Berdajs D, Turina M. Surgical anatomy of the aorta. In: Berdajs D, Turina M, editors. Operative anatomy of the heart. 1st ed. Berlin: Springer-Verlag; 2011.
Moon MR, Sundt TM 3rd. Aortic arch aneurysms. Coron Artery Dis. 2002;13(2):85–92. PubMed PMID: 12004260. English.
Moon MR, Miller DC. Aortic arch replacement for dissection. Oper Tech Thorac Cardiovasc Surg. 1999;4:33–57.
DeBakey ME, McCollum CH, Crawford ES, Morris GC Jr, Howell J, Noon GP, et al. Dissection and dissecting aneurysms of the aorta: twenty-year follow-up of five hundred twenty-seven patients treated surgically. Surgery. 1982;92(6):1118–34. PubMed PMID: 7147190. English.
Griepp RB, Stinson EB, Hollingsworth JF, Buehler D. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg. 1975;70(6):1051–63. PubMed PMID: 1186283. English.
Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac Cardiovasc Surg. 1983;31(1):37–40. PubMed PMID: 6189250. English.
Kamiya H, Hagl C, Kropivnitskaya I, Bothig D, Kallenbach K, Khaladj N, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg. 2007;133(2):501–9. PubMed PMID: 17258589. English.
Ueda Y, Miki S, Kusuhara K, Okita Y, Tahata T, Yamanaka K. Surgical treatment of aneurysm or dissection involving the ascending aorta and aortic arch, utilizing circulatory arrest and retrograde cerebral perfusion. J Cardiovasc Surg. 1990;31(5):553–8. PubMed PMID: 2229147. English.
Okita Y, Minatoya K, Tagusari O, Ando M, Nagatsuka K, Kitamura S. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg. 2001;72(1):72–9. PubMed PMID: 11465234. English.
Safi HJ, Miller CC 3rd, Lee TY, Estrera AL. Repair of ascending and transverse aortic arch. J Thorac Cardiovasc Surg. 2011;142(3):630–3. PubMed PMID: 21269650. English.
Svensson LG, Nadolny EM, Penney DL, Jacobson J, Kimmel WA, Entrup MH, et al. Prospective randomized neurocognitive and S-100 study of hypothermic circulatory arrest, retrograde brain perfusion, and antegrade brain perfusion for aortic arch operations. Ann Thorac Surg. 2001;71(6):1905–12. PubMed PMID: 11426767. English.
Svensson LG, Blackstone EH, Rajeswaran J, Sabik JF 3rd, Lytle BW, Gonzalez-Stawinski G, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg. 2004;78(4):1274–84; discussion –84. PubMed PMID: 15464485. English.
Svensson LG, Crawford ES, Hess KR, Coselli JS, Raskin S, Shenaq SA, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg. 1993;106(1):19–28; discussion –31. PubMed PMID: 8321002. English.
Sabik JF, Nemeh H, Lytle BW, Blackstone EH, Gillinov AM, Rajeswaran J, et al. Cannulation of the axillary artery with a side graft reduces morbidity. Ann Thorac Surg. 2004;77(4):1315–20. PubMed PMID: 15063259. English.
Okita Y, Okada K, Omura A, Kano H, Minami H, Inoue T, et al. Total arch replacement using antegrade cerebral perfusion. J Thorac Cardiovasc Surg. 2013;145(3 Suppl):S63–71. PubMed PMID: 23266252. English.
Khullar V, Schaff HV, Dearani JA, Daly RC, Greason KL, Joyce LD, et al. Open surgical repair remains the gold standard for treating aortic arch pathology. Ann Thorac Surg. 2016;30:30. PubMed PMID: 27914636. English.
Abraha I, Romagnoli C, Montedori A, Cirocchi R. Thoracic stent graft versus surgery for thoracic aneurysm. Cochrane Database Syst Rev. 2016;(6):CD006796. PubMed PMID: 27265222. English.
Kanaoka Y, Ohki T, Maeda K, Baba T. Analysis of risk factors for early type I endoleaks after thoracic endovascular aneurysm repair. J Endovasc Ther. 2016;19:19. PubMed PMID: 27760812. English.
Kent WD, Appoo JJ, Bavaria JE, Herget EJ, Moeller P, Pochettino A, et al. Results of type II hybrid arch repair with zone 0 stent graft deployment for complex aortic arch pathology. J Thorac Cardiovasc Surg. 2014;148(6):2951–5. PubMed PMID: 25125209. English.
Hajibandeh S, Antoniou SA, Torella F, Antoniou GA. Meta-analysis of left subclavian artery coverage with and without revascularization in thoracic endovascular aortic repair. J Endovasc Ther. 2016;23(4):634–41.
Hogendoorn W, Schlosser FJ, Moll FL, Sumpio BE, Muhs BE. Thoracic endovascular aortic repair with the chimney graft technique. J Vasc Surg. 2013;58(2):502–11. PubMed PMID: 23697513. English.
Mangialardi N, Ronchey S, Malaj A, Fazzini S, Alberti V, Ardita V, et al. Value and limitations of chimney grafts to treat arch lesions. J Cardiovasc Surg. 2015;56(4):503–11. PubMed PMID: 25765852. English.
Sugiura K, Sonesson B, Akesson M, Bjorses K, Holst J, Malina M. The applicability of chimney grafts in the aortic arch. J Cardiovasc Surg. 2009;50(4):475–81. PubMed PMID: 19734832. English.
Lioupis C, Abraham CZ. Results and challenges for the endovascular repair of aortic arch aneurysms. Perspect Vasc Surg Endovasc Ther. 2011;23(3):202–13. PubMed PMID: 21821619. English.
Haulon S, Greenberg RK, Spear R, Eagleton M, Abraham C, Lioupis C, et al. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg. 2014;148(4):1709–16. PubMed PMID: 24685375. English.
Bachet J, Guilmet D, Goudot B, Dreyfus GD, Delentdecker P, Brodaty D, et al. Antegrade cerebral perfusion with cold blood: a 13-year experience. Ann Thorac Surg. 1999;67(6):1874–8; discussion 91–4. PubMed PMID: 10391330. English.
Harrington DK, Walker AS, Kaukuntla H, Bracewell RM, Clutton-Brock TH, Faroqui M, et al. Selective antegrade cerebral perfusion attenuates brain metabolic deficit in aortic arch surgery: a prospective randomized trial. Circulation. 2004;110(11 Suppl 1):II231–6. PubMed PMID: 15364868. English.
Westaby S, Katsumata T, Vaccari G. Arch and descending aortic aneurysms: influence of perfusion technique on neurological outcome. Eur J Cardiothorac Surg. 1999;15(2):180–5. PubMed PMID: 10219551. English.
Isselbacher EM. Aortic dissection. In: Creager MA, editor. Atlas of vascular disease. London: Current Medicine Group; 2003.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rodriguez, V.M., Abraham, C., Song, H.K. (2019). Aortic Arch Aneurysms. In: Dieter, R., Dieter Jr., R., Dieter III, R. (eds) Diseases of the Aorta . Springer, Cham. https://doi.org/10.1007/978-3-030-11322-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-11322-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11321-6
Online ISBN: 978-3-030-11322-3
eBook Packages: MedicineMedicine (R0)