Abstract
The lung has numerous roles, including gas exchange, immune surveillance, and barrier function. Being a highly vascularized organ, the lung receives dual blood supply from both the pulmonary and bronchial circulation. Therefore, pericytes likely play a prominent role in lung physiology given their localization in the perivascular niche. New genetic approaches have increased our understanding of the origin and the diverse functions of lung pericytes. Lung pericytes are myofibroblast progenitors, contributing to development of fibrosis in mouse models. Lung pericytes are also capable of responding to danger signals and amplify the inflammatory response through elaboration of cytokines and adhesion molecules. In this chapter, we describe the molecular, anatomical, and phenotypical characterization of lung pericytes. We further highlight their potential roles in the pathogenesis of lung diseases including pulmonary fibrosis, asthma, and pulmonary hypertension. Finally, current gaps in knowledge and areas of ongoing investigation in lung pericyte biology are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lung pericytes
- PDGFRβ
- Lung development
- Lung myofibroblasts
- Pulmonary fibrosis
- Lung injury
- Lung inflammation
3.1 Introduction
A critical but vastly understudied cell in the lung is the pericyte. Pericytes are mesenchymal cells defined by their anatomical association with endothelial cells (Armulik et al. 2011; von Tell et al. 2006). Pericytes directly interact with microvascular endothelium, including capillaries, precapillary arterioles, postcapillary, and collecting venules, and are critical to blood vessel maturation and stability. However, it is increasingly clear that lung pericytes play important roles not only in vascular homeostasis but in lung injury and repair. In this chapter, we will review what is known about lung pericytes in normal lung homeostasis, including their developmental origins, discuss characterization of these cells in vitro and in vivo, and highlight current knowledge of lung pericytes in lung diseases, such as pulmonary fibrosis and pulmonary hypertension . Finally, we will speculate on the potential role of pericytes as a regenerative niche in the lung.
3.2 Definition of Lung Pericytes
Anatomic Characterization
Pericytes are classically defined by ultrastructural localization demonstrating direct contact with microvascular endothelial cells through gaps in the shared basement membrane. Using transmission electron microscopy, Epling was the first to observe pericytes as “filament-containing cells” in bovine and porcine lungs (Epling 1966), and Weibel confirmed the presence of pericytes in capillaries of the lungs of humans and other mammals (Weibel 1974). Compared to organs such as the heart or pancreas, pericytes are more rare in the lung, and their frequency correlates with body size. For example, pericytes were readily detected in human and dog lungs but were sparser in rodent lungs and could not be found in lungs of the Etruscan shrew, the smallest mammal known (Weibel 1974). Pericytes are sensitive to oxygen levels and thus are less abundant in areas of gas exchange (Shepro and Morel 1993). The pericyte-to-endothelial ratio in the lung has been estimated to be about 1:10 (Shepro and Morel 1993), less than that in the retina and central nervous system (~1:1 to 1:3), where barrier function is paramount and pericytes play a key role in regulating permeability. By genetically tracing pericytes in mice, Kato et al. found a pericyte-to-endothelial ratio from 1:7 to 1:9 (Kato et al. 2018). Coverage of the abluminal surface of capillaries also varies by organ and ranges between 18 and 26% in bovine lungs, depending on developmental stage of the animal (Sims and Westfall 1983).
Perivascular stromal cells are found along all levels of the bronchovascular bundle. Classically, lung pericytes are defined at the level of the alveolar capillary bed (Armulik et al. 2011). Electron microscopy lungs show pericytes embedded within the capillary basement membrane of alveoli in humans, mice, and other mammals (Hung et al. 2013) (Epling 1966; Weibel 1974). In human lung samples, Weibel observed that while most of the pericyte cell body and its large cellular processes are separated from endothelial cells by the intervening basement membrane, finer processes penetrate the basement membrane and make contacts with the endothelial cells in capillaries (Weibel 1974). Some pericytes were even seen to bridge separate capillary segments (Weibel 1974). The nature of pericyte interactions with endothelial cells in larger microvessels (i.e., the arterioles and venules) of the lung has not yet been described at the ultrastructural level.
Developmental Origins
Current understanding of lung pericytes indicates that they primarily originate from the mesoderm and mesothelium (Que et al. 2008). In the mouse, a multipotent cardiopulmonary progenitor positive for the transcription factors Gli1 and Isl1 and the signaling molecule Wnt2 gives rise to mesodermal lineages in the lung, including pericytes (Peng et al. 2013). We showed that the forkhead transcription factor FoxD1 is transiently expressed in pulmonary mesenchymal progenitors as they infiltrate the lung buds during mouse embryogenesis between days 11.5 and 12.5 (Hung et al. 2013). Expression of FoxD1 is then silenced by day 15.5 once the progenitors differentiate into mature mesenchymal cells, making this factor a useful marker for fate mapping these progenitors in the mouse lung (see section on Molecular Identification below). For most organs, including the lung, the PDGF-B-PDGFRβ signaling axis is critical in recruiting pericytes to their proper anatomical niche within the microvascular environment during embryogenesis (Armulik et al. 2011). Disruption of this axis by knockout of PDGFRβ or PDGF-B results in virtually total absence of pericytes in the mouse embryonic lung (Hellstrom et al. 1999). The developmental origin of human lung pericytes is not known, but we presume that they derive from a mesodermal precursor as well. As discussed below, PDGFRβ is a key, robust marker of lung pericytes.
Molecular Identification and Pericyte Subtypes
Whereas identification of pericytes in early studies of lung tissue specimens used electron microscopy to define the cells at the ultrastructural level, this method is cumbersome and difficult to carry out in most laboratories. Advances in molecular characterization of pericytes and in fate-mapping transgenic models have enabled investigators to study lung pericytes with greater ease and flexibility than in the past. Nevertheless, lack of a truly pericyte-specific marker means that researchers need to use multiple criteria including location relative to endothelial cells in situ, morphology, and genetic markers to identify pericytes.
Only in the last decade or so have a limited number of studies been undertaken to isolate and characterize pericytes from “normal” human lung (typically from failed donor organs or tissue adjacent to carcinoma). The commonalities in these studies were that pericytes were selected from dissociated lung cells using magnetic bead-linked antibodies against one or two markers and then expanded in commercial medium especially designed for growth of pericytes. In culture, isolated lung pericytes have a stellate or elongated morphology with long extensive cellular processes (Yuan et al. 2015; Wilson et al. 2018) (Fig. 3.1). In addition, lung pericytes form primitive tubelike structures on the basement membrane preparation Matrigel (Wilson et al. 2018; Bagley et al. 2006) (Fig. 3.1).
Cell morphology and characteristics of isolated human lung pericytes. Representative phase-contrast light microscopic images of pericytes from normal lungs after plating on plastic (a) or Matrigel™ (b). Note the extensive number of elongated cellular processes emanating from the cells on plastic. On Matrigel™, pericytes assemble into primitive networks composed of nodes of cells from which cellular processes project and connect with other nodes (b). Human lung pericytes are positive for PDGFRβ (c) and NG2 (d) by immunofluorescence. Scale bar = 500 μm in (a), (c), and (d) and 100 μm in (b)
Table 3.1 summarizes the markers that were used for isolation of human lung pericytes and the other markers that were detected in these cells in different studies. Expression of the proteoglycan neural glial antigen-2 (NG2 or chondroitin sulfate proteoglycan 4) (Yuan et al. 2015; Wilson et al. 2018; Bagley et al. 2005; Bichsel et al. 2015; Ricard et al. 2014) was reported in all the studies (Fig. 3.2). NG2+ cells localized to perivascular regions in human lung tissue by immunofluorescence (Sava et al. 2017; Rock et al. 2011). Most studies showed that lung pericytes are PDGFRβ+, as expected (Fig. 3.2), and we used this marker in our own work for cell selection from both human and mouse lungs (Wilson et al. 2018; Hung et al. 2017a).
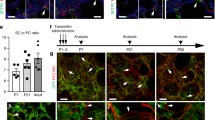
Fate-mapping of Foxd1 progenitor-derived pericytes identifies them as a major source of myofibroblast precursors in lung injury. (a, b) Confocal images showing fibrotic foci on d7 and d14 after bleomycin lung injury in Foxd1-Cre; Rs26-TdT-R mice stained for the myofibroblast marker αSMA (green). Co-expression of myofibroblast marker αSMA and the tdTomato fate marker of Foxd1-derived pericytes indicated by ( ). Graph indicating proportion of tdTomato+ cells co-expressing αSMA+ in fibroblastic foci at indicated time points after bleomycin injury (mean ± SEM, n = 3). (c, d) Confocal images showing fibrotic foci on d7 after bleomycin lung injury in triple transgenic mouse Foxd1-Cre; Rs26-TdT-R; Coll-GFPTg and graph summarizing proportion of tdTomato cells co-expressing Coll-GFP in fibrotic foci (mean ± SEM, n = 3) (Bar = 50 μm). Modified and reprinted with permission of the American Thoracic Society. Copyright © 2018 American Thoracic Society (Hung et al. 2013). The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society
). Graph indicating proportion of tdTomato+ cells co-expressing αSMA+ in fibroblastic foci at indicated time points after bleomycin injury (mean ± SEM, n = 3). (c, d) Confocal images showing fibrotic foci on d7 after bleomycin lung injury in triple transgenic mouse Foxd1-Cre; Rs26-TdT-R; Coll-GFPTg and graph summarizing proportion of tdTomato cells co-expressing Coll-GFP in fibrotic foci (mean ± SEM, n = 3) (Bar = 50 μm). Modified and reprinted with permission of the American Thoracic Society. Copyright © 2018 American Thoracic Society (Hung et al. 2013). The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society
Other prominent mesenchymal markers found in isolated human lung pericytes include CD73 (ecto-5′-nucleotidase), CD90 (Thy1, also a fibroblast marker), and the hyaluronan receptor CD44, although not all studies examined the cells for these markers. Expression of endoglin (CD105) and PDGFRα is also characteristic of human lung pericytes. The monoclonal antibody 3G5-defined ganglioside antigen has emerged as a useful pericyte marker for the lung as well as the skin (Helmbold et al. 2001a, b) and was used by two investigator teams to isolate these cells (Yuan et al. 2015; Ricard et al. 2014). There are discrepant reports on CD146 (also known as MCAM or melanoma cell adhesion molecule) expression: one study found that the cells are positive for CD146 (Yuan et al. 2015), whereas two others did not (Wilson et al. 2018; Bichsel et al. 2015). The lack of significant CD146 expression is surprising as it is a typical marker of pericytes from other organs, such as the placenta and brain (Crisan et al. 2008), and was detected in mouse lung pericytes (Hung et al. 2013). Potential explanations for these conflicting data on CD146 positivity include the presence of subpopulations of lung pericytes, differences in underlying pathology of donor lung tissue, and/or differences in culture conditions. Also, while α-SMA has been described as a pericyte marker, in general, only mural cells associated with precapillary arterioles and postcapillary venules express this contractile protein, whereas quiescent capillary pericytes do not (Nehls and Drenckhahn 1991). Further complicating interpretations, while pericytes on arterioles and capillaries are NG2+, venular pericytes are NG2−, at least in rat mesenteric and subcutaneous tissue and skeletal muscle (Murfee et al. 2005). NG2 is induced in venular pericytes during angiogenesis and vascular remodeling (Murfee et al. 2006). Although this differential expression of NG2 has not yet been established in the microvasculature of the human lung, it raises the possibility that a cell selection strategy based on NG2 may not include the entire spectrum of pericytes. Because the markers are not pericyte specific [e.g., CD146 is expressed by endothelial cells (Bardin et al. 2001) and 3G5 by mast cells (Gushi et al. 2008)], it is equally important to exclude expression of other cell-specific markers such as CD31 and CD144 (endothelial cell markers PECAM and VE-cadherin, respectively), CD45 (pan leukocyte marker), and CD326 (EpCAM, an epithelial cell marker).
Animal Models
Much of our knowledge on the origin and fate of lung pericytes comes from genetic approaches in mouse models, mostly using Cre-LoxP technology (Table 3.2). In these models, Cre recombinase expression is driven by a promoter selective for pericytes or pericyte progenitors, and the recombinase activates expression of a reporter allele by targeting LoxP sequences that flank a transcriptional stop site upstream of the reporter. This technique irreversibly labels cells with active promoter expression and their progeny. We showed that expression of the transcriptional factor FoxD1 marks progenitors destined to become pericytes in the lung (Hung et al. 2013). Using triple transgenic mice expressing Cre recombinase from the FoxD1 promoter and GFP from the collagen I promoter (Yata et al. 2003), in conjunction with a tdTomato reporter allele at the ROSA locus, we found that FoxD1-derived progenitors give rise to vascular smooth muscle cells and two populations of pericytes. The major population does not express collagen I or α-SMA and is positive for PDGFRβ; a second, minor population expresses collagen I and PDGFRα rather than PDGFRβ (Hung et al. 2013). In this study, all FoxD1-derived cells were negative for CD31, an important distinction given that a group has reported that FoxD1 positivity also marks endothelial progenitors in the mouse lung (Sims-Lucas et al. 2013).
One important limitation in lung pericyte research using lineage tracing animal models is the lack of a definitive marker for pericytes. Commonly recognized markers such as PDGFRβ, NG2, and CD146 are not uniformly expressed in all pericytes. Moreover, their expression may be spatially and temporally dynamic throughout development and after injury. For example, PDGFRβ promoter is active in multiple cell lineages during embryogenesis and is not specific to pericytes (Guimaraes-Camboa et al. 2017). Furthermore, activated myofibroblasts derived from non-pericyte populations following lung injury may upregulate expression of PDGFRβ (Henderson et al. 2013; Hung et al. 2018).
Pulse labeling of pericyte populations circumvents the dynamic expression of pericyte markers that are observed in other cell lineages during development and with physiologic stress. Modification of Cre recombinase to make its activity inducible allows temporal control in labeling pericytes. For example, a tamoxifen-inducible PDGFRβ-CreER transgenic animal enables postnatal marking of PDGFRβ+ cells; tamoxifen administration at days 1 through 3 after birth labeled cells tightly juxtaposed to endothelial cells in the lung (Kato et al. 2018). Isolated labeled cells were positive for expression of PDGFRβ and NG2 and were negative for markers of endothelial and epithelial cells (Kato et al. 2018).
There are important limitations to Cre-loxP models. As discussed previously, promoter activity driving Cre recombinase expression may not be restricted to pericytes, depending on the developmental stage and the presence of physiologic stress. Secondly, Cre recombinase activity is rarely 100% efficient. Promoter activity, accessibility of the target loxP sequences in the genome, and experimental conditions used to induce Cre activity in inducible models can all influence labeling efficiency. In one report, postnatal tamoxifen administration labeled only ~15% of the NG2+ lung cells in NG2-CreER mice (Rock et al. 2011), highlighting a potential drawback of these types of models. Furthermore, bioavailability of tamoxifen can influence labeling efficiency in tamoxifen-inducible Cre models. Some investigators have leveraged this property to study lineage-labeled single cells distributed throughout the lung by administering low-dose tamoxifen (Kato et al. 2018; Barkauskas et al. 2013). Finally, there is currently no single marker that identifies the entire lung pericyte population. The commonly used markers do not completely overlap, suggesting heterogeneity within the lung pericyte population. We showed that only a subset of PDGFRβ+ cells (~60%) express NG2 (Hung et al. 2013), suggesting that NG2 may not be an all-inclusive marker of mouse lung pericytes. Indeed, NG2 has been described as a marker of pericytes in angiogenesis and tissue remodeling rather than homeostasis (Murfee et al. 2006). Interestingly, Rock et al. found that a FoxJ1-CreER transgene also marks NG2+ pericytes in the lung (Rock et al. 2011), although there have been no further reports in the literature using this transgenic mouse for pericyte fate mapping. Our group investigated NG2 and CD146 expression in human pericytes. We found that PDGFRβ+ human lung pericytes have low CD146 expression unlike mouse lung pericytes (Hung et al. 2017a, b). Taken together, the studies suggest that the lung pericyte population is heterogeneous. Understanding functional differences in lung pericyte subpopulations is an area of ongoing investigation.
Cre-loxP technology can also induce specific gene deletions in pericyte-lineage lung cells. A cross of mice with loxP-flanked Yap1 and Wwtr1 (encoding TAZ protein) alleles to PDGFRβ-CreER mice generated pericyte-specific inducible deletions of these genes in postnatal lung (Kato et al. 2018). The disruptions in YAP1 and TAZ signaling in pericytes altered postnatal lung morphogenesis. Another novel application of Cre-loxP technology involves targeted ablation of pericytes within the lung. Mice are inherently insensitive to diphtheria toxin (DT) as they lack its receptor (DTR). We crossed ROSA26iDTR mice, in which the expression of the simian diphtheria toxin receptor (iDTR) is Cre inducible, to FoxD1-Cre mice to generate the double transgenic FoxD1-Cre;ROSA26iDTR model. In this mouse model, iDTR is expressed in FoxD1-derived cells, rendering them sensitive to ablation upon exposure to DT. Conventional delivery of DT by intraperitoneal injection resulted in neurologic side effects such as seizures within 72 hours of DT administration (unpublished data). This observation reflected the critical role of brain pericytes in the regulation of blood-brain barrier (Hung et al. 2017b). To circumvent this limitation, we administered low-dose DT by oropharyngeal aspiration. Using this method, we achieved approximately 40% ablation of PDGFRβ+ stromal cells in the lung at 7 days after DT delivery (Hung et al. 2017b). Long-term ablation, however, remains elusive as progenitor populations replenish ablated cells.
3.3 Pericytes During Lung Homeostasis and Repair
The exact roles of pericytes in lung homeostasis remain largely uncharacterized. Functional studies that disrupt the pericyte-endothelial signaling axis (e.g., PDGF-PDGFRß) result in early embryonic lethality due to abnormal vasculogenesis. Most models that induce pericyte loss focus on developing vessels rather than fully mature pericyte-endothelial units. In one study, pericytes were depleted by administering a PDGFRβ-blocking antibody in postnatal pups, which caused abnormal retinal capillary formation (Ogura et al. 2017) and demonstrated a critical role of pericytes in retinal angiogenesis. However, administration of the same antibody in adult mice did not result in pericyte coverage loss, suggesting the PDGF-PDGFRβ signaling axis is dispensable in the developed microvascular unit. Akt/Jagged1 signaling maintains perivascular stromal cell coverage in homeostasis in the heart and retina as disruption of endothelial Akt production led to pericyte apoptosis and breakdown of perivascular matrix (Kerr et al. 2016). Whether blockade of the Akt/Jagged1/Notch signaling axis results in similar findings in the lung remains unknown. When retinal pericytes were ablated with DT in adult mice, microangiopathy developed, suggesting that maintenance of vascular integrity is a critical function of retinal pericytes (Valdez et al. 2014). In our model of lung pericyte ablation with DT, we did not observe changes in lung permeability (Hung et al. 2017b), suggesting that lung pericytes may not be as critical to normal barrier function as in other organs. Thus, our understanding of pericyte biology in mature lungs under homeostatic conditions is limited.
The lung has limited ability to regenerate compared to skin and gut epithelium. Acute lung injury from a variety of insults leads to denudement of damaged alveolar epithelium and a concurrent local inflammatory response. This is normally followed by resolution of inflammation, transient scarring, and repopulation of the lost epithelial barrier. In some instances, a pathological response results in persistent scarring or fibrosis, leading to distortion of normal architecture and impairment of lung function. The precise cellular mechanisms that control regenerative versus pathological responses in the lung remain an area of intense interest.
Accumulating evidence suggests pericytes are important in regulating lung repair as progenitors of myofibroblasts and mesenchymal progenitor cells. The multidrug resistance transporter ATP-binding cassette G (ABCG2) was identified as a marker in lineage-tracing experiments that labeled mesenchymal progenitor cells (MPCs) that give rise to pericytes (Jun et al. 2011; Marriott et al. 2014; Chow et al. 2013; Gaskill et al. 2017). Differentiation of ABCG2-lineage cells to mature pericytes was important in attenuation of bleomycin-induced lung fibrosis and restoration of normal vascular function following injury (Gaskill et al. 2017). Overexpression of the Wnt/ß-catenin pathway in ABCG2-lineage cells led to increased pericyte specification without maturation. These mice developed worse lung fibrosis and increased microvascular dysfunction, suggesting MPC differentiation into mature, functional pericytes is integral to normal repair in the lung. There may be other pools of progenitors that give rise to renewal of pericytes in different physiologic contexts such as aging and apoptosis. Identification of the progenitor pool and understanding the molecular signals that direct regeneration will greatly advance the field’s understanding of normal and abnormal repair in the lung interstitium, with relevance to many chronic pulmonary diseases such as COPD and interstitial lung diseases where effective medical therapy continues to be lacking.
One aspect of the regenerative response that has garnered significant attention is the identity of adult progenitor cells that are capable of self-renewal or even renewal of other cell lineages (multipotency). These elusive adult progenitor cells are thought to be of mesenchymal origin and are often referred as mesenchymal stem cells (MSCs). Pericytes have been postulated to be a source of MSCs in multiple organs (Crisan et al. 2008). MSCs are marked by expression of the transcription factor Gli1 and ABCG2 and comprise a small subset of the PDGFRβ+ cells in the lung (Marriott et al. 2014; Chow et al. 2013; Kramann et al. 2015). Although these MSCs have been called “pericytes,” this term primarily reflects their anatomical location, as they do not express other markers associated with mature pericytes in the mouse, such as NG2 and CD146. Using a genetic tracing approach to isolate ABCG2+ cells, Chow et al. showed that lung MSCs can differentiate into NG2+ pericytes and endothelial cells in vitro (Chow et al. 2013). In contrast, a study using the transcription factor Tbx18 as a marker of pericytes found that Tbx18-lineage cells did not exhibit multipotency in vivo (Guimaraes-Camboa et al. 2017). Likewise, we did not detect expression of MSC markers on human lung pericytes (defined by NG2+ PDGFRß+) in vitro (Wilson et al. 2018). One possibility to account for the conflicting reports on pericyte multipotency is that cell culture environments often introduce non-physiologic cues that may induce phenotypic changes in pericytes, cues that are not representative of the native tissue environment where pericytes reside. To date, there is no evidence to suggest lung pericytes possess multipotent cell fate plasticity in vivo.
3.4 Pericytes in Lung Disease
Lung Inflammation and Injury
Historically, focus on pericytes has been limited to their functions as structural mural cells that support vascular maturation and endothelial homeostasis. Accumulating evidence, however, suggests these specialized cells may have broader biological functions. One area that has captured the interest of investigators is the potential role of pericytes in immunity. Much of the evidence for pericytes in inflammation comes from studies in the central nervous system where pericytes elaborate chemoattractants, adhesion molecules, and paracrine signaling to amplify local inflammation (Jansson et al. 2014; Rustenhoven et al. 2017).
Similar to findings in other organs, our group has shown that mouse lung pericytes respond to pro-inflammatory signaling. Murine lung pericytes possess multiple functional TLRs and elaborate cytokines in response to specific TLR agonists in vitro (Table 3.3) (Hung et al. 2017a). Similarly, cultured human lung pericytes respond to inflammatory cues and upregulate cytokine expression. Compared to mouse lung pericytes, human lung pericytes exhibited a narrower range of TLR responses, with TLR 2/6 and TLR 4 signaling leading to the most robust pro-inflammatory responses (Table 3.3). We showed that depletion of pericytes by DT exposure attenuated the acute inflammatory response in a sterile lung injury model (Hung et al. 2017a, b). How lung pericyte ablation affects the inflammatory profile in other models of sterile lung injury and infectious models is yet to be defined. Based on our experience, we speculate that pericytes play an important supportive role in mediating local lung inflammation. Their perivascular position in the alveoli suggests they may play important roles in the activation of the endothelium through paracrine signaling and direct contact during inflammation. Furthermore, they reside in a prime location to sense pro-inflammatory alveolar components and may be one of the very first responders to alveolar damage. Beyond the production of inflammatory cytokines, pericytes may also direct trafficking of inflammatory cells that exit systemic circulation through upregulation of adhesion molecules as well as modification of the extracellular matrix in the perivascular space and the lung interstitium.
Pulmonary Fibrosis
During pulmonary fibrosis , the main effector cell for matrix production is the lung myofibroblast. However, the origin of lung myofibroblasts remains controversial. In our transgenic mouse model work, we found that FoxD1-derived cells express common markers used to identify pericytes such as PDGFRß, NG2, and CD146, while they lack expression of endothelial-, epithelial-, or hematopoietic-specific markers. In addition, FoxD1-derived cells are located adjacent to endothelial cells, suggesting they are indeed pericytes (Fig. 3.2) (Hung et al. 2013). Importantly, they contributed to >50% of α-smooth muscle actin (αSMA)-positive myofibroblasts in the bleomycin model of lung fibrosis (Hung et al. 2013) (Fig. 3.2). Our study and Rock et al. (Rock et al. 2011) found other cell types contribute significantly to the myofibroblast pool in the lung. In contrast, in both kidney and liver models of fibrosis, pericytes were the predominant source of myofibroblasts (Lin et al. 2008; Humphreys et al. 2010; Mederacke et al. 2013). These findings establish the novel concept that pericytes are key effectors and potential therapeutic targets in pulmonary fibrosis.
Pulmonary Arterial Hypertension
Pulmonary Arterial Hypertension (PAH) , defined by elevated mean arterial pressure ≥ 25 mmHg at rest, is characterized by progressive remodeling of the distal pulmonary arterials with resultant increase in pulmonary vascular resistance, leading to right heart failure and ultimately death. Pulmonary endothelial dysfunction is a key feature of PAH pathogenesis. The pulmonary artery wall includes a secondary layer of vascular smooth muscle cells (SMCs) or smooth muscle-like pericytes (Hemnes and Humbert 2017). Pericytes isolated from PAH lungs had impaired association with endothelial cells (Yuan et al. 2015). Another study showed increased number of pericytes (defined by 3G5 staining) surrounding pulmonary vessels of all sizes in explanted human PAH lungs and in rodent models of pulmonary hypertension (Ricard et al. 2014). Furthermore, the increased number of pericytes was detected before changes in arterial pressures were detected, suggesting the changes in pericyte were not secondary to hemodynamic derangements. Their data suggested that dysregulated FGF2 and IL-6 signaling from endothelial cells drive pericyte proliferation during PAH and contribute to pathogenesis.
Interestingly, there were two case reports of infants with Adams-Oliver syndrome , a rare genetic disorder characterized by congenital scalp defects and limb defects of unknown pathogenesis, who developed severe infantile pulmonary hypertension, intracranial bleeding, and cutis marmorata telangiectatica congenita (Patel et al. 2004). At autopsy, lack of pericyte coverage was found areas of vascular dilatation, while increased pericyte coverage and proliferation were found in association with vessel stenosis. They hypothesized that aberrant pericyte recruitment caused pulmonary hypertension in this syndrome (Patel et al. 2004). These studies support the role of pericyte recruitment and proliferation in the pathogenesis of pulmonary arterial hypertension.
Hereditary Hemorrhagic Telangiectasia
Hereditary Hemorrhagic Telangiectasia (HHT) also known as Osler-Weber-Rendu syndrome is an autosomal dominant disorder characterized by arteriovenous malformations (AVM) affecting major organs, particularly the lung, liver, and brain. Lung AVMs are more commonly seen in HHT1 patients. Most HHT1 patients have mutations in ENG encoding endoglin (McAllister et al. 1994), a receptor for transforming growth factor-β (TGFβ)/bone morphogenetic protein (BMP) expressed primarily in endothelial cells and pericytes. Impaired communication between endothelial cell and pericyte, including loss of normal TGFβ activation and signaling, is postulated to lead to impaired pericyte differentiation and vessel maturation in HHT (Thalgott and Lebrin 2015). One group reported that thalidomide increased pericyte number, α-SMA expression, and their recruitment to blood vessels in a mouse model of HHT, leading to vasculature stabilization (Thalgott and Lebrin 2015). A speculated mechanism was a thalidomide-induced increase of PDGF-B expression by endothelial cells.
Pericytes and Asthma
Asthma is a common disease characterized by recurrent inflammatory episodes with airway and vascular remodeling. The airway remodeling includes myofibroblast accumulation, collagen deposition, and increased smooth muscle mass leading to subepithelial fibrosis and airway narrowing. Our understanding of the contributions of pericytes in asthma is limited. In a murine model of chronic house dust mite (HDM) exposure, investigators noted decreased capillary-associated pericyte coverage (defined by NG2 positivity) and increased subepithelial (peribronchial) pericytes. These findings were exaggerated when mice were treated with a PDFGRβ inhibitor and resulted in worsened airway hyperresponsiveness and airway smooth muscle thickening (Johnson et al. 2015). Given evidence supporting pericyte transition to myofibroblast in other conditions, it is interesting to speculate that pericytes likewise contribute to subepithelial fibrosis in asthma . However, more studies are needed to define a role of pericytes in asthma pathogenesis.
3.5 Conclusion
We are only beginning to appreciate the multiple roles pericytes play in lung disease pathogenesis. Studies in animal models suggest lung pericytes exhibit a degree of biological plasticity that was previously underappreciated. They participate in inflammation and fibrosis, and they also regulate microvascular permeability and stability (Fig. 3.3). Clinically, some disorders such as pulmonary hypertension and HHT are associated with changes in pericyte coverage. So are pericytes beneficial or harmful in disease? To date, there is not enough information on lung pericytes to answer this question. The timing of pericyte activation during disease, the interaction of pericytes with their cellular neighbors and their microenvironmental niche when homeostasis is disturbed, and the environmental cues that activate pericytes are some of the important knowledge gaps in lung pericyte biology that require further study. Refining methods to identify, isolate, and manipulate lung pericytes in the laboratory will be essential to advancing knowledge on these cells in pulmonary health and disease.
References
Akamatsu T, Arai Y, Kosugi I, Kawasaki H, Meguro S, Sakao M, Shibata K, Suda T, Chida K, Iwashita T (2013) Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences. Fibrogenesis Tissue Repair 6(1):15. https://doi.org/10.1186/1755-1536-6-15. PubMed PMID: 23927729; PMCID: PMC3751789
Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21(2):193–215. https://doi.org/10.1016/j.devcel.2011.07.001. PubMed PMID: 21839917
Bagley RG, Weber W, Rouleau C, Teicher BA (2005) Pericytes and endothelial precursor cells: cellular interactions and contributions to malignancy. Cancer Res 65(21):9741–9750. https://doi.org/10.1158/0008-5472.CAN-04-4337. PubMed PMID: 16266995
Bagley RG, Rouleau C, Morgenbesser SD, Weber W, Cook BP, Shankara S, Madden SL, Teicher BA (2006) Pericytes from human non-small cell lung carcinomas: an attractive target for anti-angiogenic therapy. Microvasc Res 71(3):163–174. https://doi.org/10.1016/j.mvr.2006.03.002. Epub 2006/04/19. PubMed PMID: 16624341
Bardin N, Anfosso F, Masse JM, Cramer E, Sabatier F, Le Bivic A, Sampol J, Dignat-George F (2001) Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood 98(13):3677–3684. PubMed PMID: 11739172
Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL (2013) Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123(7):3025–3036. https://doi.org/10.1172/JCI68782. Epub 2013/08/08. PubMed PMID: 23921127; PMCID: PMC3696553
Bichsel CA, Hall SR, Schmid RA, Guenat OT, Geiser T (2015) Primary human lung pericytes support and stabilize in vitro perfusable microvessels. Tissue Eng Part A 21(15–16):2166–2176. https://doi.org/10.1089/ten.TEA.2014.0545. PubMed PMID: 25891384
Chow K, Fessel JP, Kaoriihida S, Schmidt EP, Gaskill C, Alvarez D, Graham B, Harrison DG, Wagner DH Jr, Nozik-Grayck E, West JD, Klemm DJ, Majka SM (2013) Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulm Circ 3(1):31–49. https://doi.org/10.4103/2045-8932.109912. PubMed PMID: 23662173; PMCID: PMC3641738
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3):301–313. https://doi.org/10.1016/j.stem.2008.07.003. PubMed PMID: 18786417
Epling GP (1966) Electron microscopic observations of pericytes of small blood vessels in the lungs and hearts of normal cattle and swine. Anat Rec 155:513–530
Gaskill CF, Carrier EJ, Kropski JA, Bloodworth NC, Menon S, Foronjy RF, Taketo MM, Hong CC, Austin ED, West JD, Means AL, Loyd JE, Merryman WD, Hemnes AR, De Langhe S, Blackwell TS, Klemm DJ, Majka SM (2017) Disruption of lineage specification in adult pulmonary mesenchymal progenitor cells promotes microvascular dysfunction. J Clin Invest 127(6):2262–2276. https://doi.org/10.1172/JCI88629. Epub 2017/05/04. PubMed PMID: 28463231; PMCID: PMC5451236
Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM (2017) Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20(3):345–359.e5. https://doi.org/10.1016/j.stem.2016.12.006. PubMed PMID: 28111199; PMCID: PMC5337131
Gushi A, Tanaka M, Tsuyama S, Nagai T, Kanzaki T, Kanekura T, Matsuyama T (2008) The 3G5 antigen is expressed in dermal mast cells but not pericytes. J Cutan Pathol 35(3):278–284. https://doi.org/10.1111/j.1600-0560.2007.00809.x. PubMed PMID: 18251741
Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C (1999) Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126(14):3047–3055. PubMed PMID: 10375497
Helmbold P, Nayak RC, Marsch WC, Herman IM (2001a) Isolation and in vitro characterization of human dermal microvascular pericytes. Microvasc Res 61(2):160–165. https://doi.org/10.1006/mvre.2000.2292. PubMed PMID: 11254395
Helmbold P, Wohlrab J, Marsch WC, Nayak RC (2001b) Human dermal pericytes express 3G5 ganglioside—a new approach for microvessel histology in the skin. J Cutan Pathol 28(4):206–210. PubMed PMID: 11426828
Hemnes AR, Humbert M (2017) Pathobiology of pulmonary arterial hypertension: understanding the roads less travelled. Eur Respir Rev 26(146). https://doi.org/10.1183/16000617.0093-2017
Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D (2013) Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19(12):1617–1624. https://doi.org/10.1038/nm.3282. PubMed PMID: 24216753; PMCID: PMC3855865
Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176(1):85–97. https://doi.org/10.2353/ajpath.2010.090517. PubMed PMID: 20008127; PMCID: PMC2797872
Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS (2013) Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188(7):820–830. https://doi.org/10.1164/rccm.201212-2297OC. PubMed PMID: 23924232; PMCID: 3826269
Hung CF, Mittelsteadt KL, Brauer R, McKinney BL, Hallstrand TS, Parks WC, Chen P, Schnapp LM, Liles WC, Duffield JS, Altemeier WA (2017a) Lung pericyte-like cells are functional interstitial immune sentinel cells. Am J Physiol Lung Cell Mol Physiol 312(4):L556–L567. https://doi.org/10.1152/ajplung.00349.2016. PubMed PMID: 28188224; PMCID: PMC5407093
Hung CF, Chow YH, Liles WC, Altemeier WA, Schnapp LM (2017b) Ablation of Pericyte-like cells in lungs by oropharyngeal aspiration of diphtheria toxin. Am J Respir Cell Mol Biol 56(2):160–167. https://doi.org/10.1165/rcmb.2016-0083MA. PubMed PMID: 27779900
Hung CF, Wilson CL, Chow YH, Schnapp LM (2018) Role of integrin alpha8 in murine model of lung fibrosis. PLoS One 13(5):e0197937. https://doi.org/10.1371/journal.pone.0197937. Epub 2018/05/29. PubMed PMID: 29813125
Jansson D, Rustenhoven J, Feng S, Hurley D, Oldfield RL, Bergin PS, Mee EW, Faull RL, Dragunow M (2014) A role for human brain pericytes in neuroinflammation. J Neuroinflammation 11:104. https://doi.org/10.1186/1742-2094-11-104. PubMed PMID: 24920309; PMCID: PMC4105169
Johnson JR, Folestad E, Rowley JE, Noll EM, Walker SA, Lloyd CM, Rankin SM, Pietras K, Eriksson U, Fuxe J (2015) Pericytes contribute to airway remodeling in a mouse model of chronic allergic asthma. Am J Physiol Lung Cell Mol Physiol 308(7):L658–L671. https://doi.org/10.1152/ajplung.00286.2014. Epub 2015/02/01. PubMed PMID: 25637607; PMCID: PMC4385988
Jun D, Garat C, West J, Thorn N, Chow K, Cleaver T, Sullivan T, Torchia EC, Childs C, Shade T, Tadjali M, Lara A, Nozik-Grayck E, Malkoski S, Sorrentino B, Meyrick B, Klemm D, Rojas M, Wagner DH, Jr., Majka SM. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells 2011;29(4):725–735. doi: https://doi.org/10.1002/stem.604. Epub 2011/02/12. PubMed PMID: 21312316; PMCID: PMC3322548
Kato K, Dieguez-Hurtado R, Park DY, Hong SP, Kato-Azuma S, Adams S, Stehling M, Trappmann B, Wrana JL, Koh GY, Adams RH (2018) Pulmonary pericytes regulate lung morphogenesis. Nat Commun 9(1):2448. https://doi.org/10.1038/s41467-018-04913-2. PubMed PMID: 29934496; PMCID: PMC6015030
Kerr BA, West XZ, Kim YW, Zhao Y, Tischenko M, Cull RM, Phares TW, Peng XD, Bernier-Latmani J, Petrova TV, Adams RH, Hay N, Naga Prasad SV, Byzova TV (2016) Stability and function of adult vasculature is sustained by Akt/Jagged1 signalling axis in endothelium. Nat Commun 7:10960. https://doi.org/10.1038/ncomms10960. Epub 2016/03/15. PubMed PMID: 26971877; PMCID: PMC4793084
Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD (2015) Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16(1):51–66. https://doi.org/10.1016/j.stem.2014.11.004. PubMed PMID: 25465115; PMCID: PMC4289444
Lin SL, Kisseleva T, Brenner DA, Duffield JS (2008) Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173(6):1617–1627. https://doi.org/10.2353/ajpath.2008.080433. ajpath.2008.080433 [pii]. Epub 2008/11/15. PubMed PMID: 19008372; PMCID: 2626374
Marriott S, Baskir RS, Gaskill C, Menon S, Carrier EJ, Williams J, Talati M, Helm K, Alford CE, Kropski JA, Loyd J, Wheeler L, Johnson J, Austin E, Nozik-Grayck E, Meyrick B, West JD, Klemm DJ, Majka SM (2014) ABCG2pos lung mesenchymal stem cells are a novel pericyte subpopulation that contributes to fibrotic remodeling. Am J Physiol Cell Physiol 307(8):C684–C698. https://doi.org/10.1152/ajpcell.00114.2014. PubMed PMID: 25122876; PMCID: PMC4200000
McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J et al (1994) Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8(4):345–351. https://doi.org/10.1038/ng1294-345. PubMed PMID: 7894484
Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF (2013) Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 4:2823. https://doi.org/10.1038/ncomms3823. Epub 2013/11/23. PubMed PMID: 24264436; PMCID: PMC4059406
Murfee WL, Skalak TC, Peirce SM (2005) Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation 12(2):151–160. https://doi.org/10.1080/10739680590904955. PubMed PMID: 15824037
Murfee WL, Rehorn MR, Peirce SM, Skalak TC (2006) Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation 13(3):261–273. https://doi.org/10.1080/10739680600559153. PubMed PMID: 16627368
Nehls V, Drenckhahn D (1991) Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol 113(1):147–154. PubMed PMID: 2007619; PMCID: PMC2288926
Ogura S, Kurata K, Hattori Y, Takase H, Ishiguro-Oonuma T, Hwang Y, Ahn S, Park I, Ikeda W, Kusuhara S, Fukushima Y, Nara H, Sakai H, Fujiwara T, Matsushita J, Ema M, Hirashima M, Minami T, Shibuya M, Takakura N, Kim P, Miyata T, Ogura Y, Uemura A (2017) Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight 2(3):e90905. https://doi.org/10.1172/jci.insight.90905. Epub 2017/02/15. PubMed PMID: 28194443; PMCID: PMC5291729 employees of Astellas Pharma Inc.
Patel MS, Taylor GP, Bharya S, Al-Sanna'a N, Adatia I, Chitayat D, Lewis MES, Human DG (2004) Abnormal pericyte recruitment as a cause for pulmonary hypertension in Adams–Oliver syndrome. Am J Med Genet A 129A(3):294–299. https://doi.org/10.1002/ajmg.a.30221
Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, Evans SM, Morrisey EE (2013) Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature 500(7464):589–592. https://doi.org/10.1038/nature12358. PubMed PMID: 23873040; PMCID: PMC3758448
Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL (2008) Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A 105(43):16626–16630. https://doi.org/10.1073/pnas.0808649105. PubMed PMID: 18922767; PMCID: PMC2567908
Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, Sattler C, Fadel E, Seferian A, Montani D, Dorfmuller P, Humbert M, Guignabert C (2014) Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation 129(15):1586–1597. https://doi.org/10.1161/CIRCULATIONAHA.113.007469. PubMed PMID: 24481949
Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL (2011) Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A 108(52):E1475–E1483. https://doi.org/10.1073/pnas.1117988108. PubMed PMID: 22123957; PMCID: PMC3248478
Rustenhoven J, Jansson D, Smyth LC, Dragunow M (2017) Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci 38(3):291–304. https://doi.org/10.1016/j.tips.2016.12.001. Epub 2016/12/27. PubMed PMID: 28017362
Sava P, Ramanathan A, Dobronyi A, Peng X, Sun H, Ledesma-Mendoza A, Herzog EL, Gonzalez AL (2017) Human pericytes adopt myofibroblast properties in the microenvironment of the IPF lung. JCI Insight. 2(24). https://doi.org/10.1172/jci.insight.96352. PubMed PMID: 29263297; PMCID: PMC5752282
Shepro D, Morel NM (1993) Pericyte physiology. FASEB J 7(11):1031–1038. PubMed PMID: 8370472
Sims DE, Westfall JA (1983) Analysis of relationships between pericytes and gas exchange capillaries in neonatal and mature bovine lungs. Microvasc Res 25(3):333–342. PubMed PMID: 6855632
Sims-Lucas S, Schaefer C, Bushnell D, Ho J, Logar A, Prochownik E, Gittes G, Bates CM (2013) Endothelial progenitors exist within the kidney and lung mesenchyme. PLoS One 8(6):e65993. https://doi.org/10.1371/journal.pone.0065993. PubMed PMID: 23823180; PMCID: PMC3688860
Thalgott J, Lebrin F (2015) Pericytes as targets in hereditary hemorrhagic telangiectasia. Front Genet 6:37
Valdez CN, Arboleda-Velasquez JF, Amarnani DS, Kim LA, D'Amore PA (2014) Retinal microangiopathy in a mouse model of inducible mural cell loss. Am J Pathol 184(10):2618–2626. https://doi.org/10.1016/j.ajpath.2014.06.011. S0002-9440(14)00366-6 [pii]. Epub 2014/08/06. PubMed PMID: 25092275
von Tell D, Armulik A, Betsholtz C (2006) Pericytes and vascular stability. Exp Cell Res 312(5):623–629. https://doi.org/10.1016/j.yexcr.2005.10.019. PubMed PMID: 16303125
Weibel ER (1974) On pericytes, particularly their existence on lung capillaries. Microvasc Res 8(2):218–235. PubMed PMID: 4140459
Wilson CL, Stephenson SE, Higuero JP, Feghali-Bostwick C, Hung CF, Schnapp LM (2018) Characterization of human PDGFRβ-positive pericytes from IPF and non-IPF lungs. Am J Physiol Lung Cell Mol Physiol. https://doi.org/10.1152/ajplung.00289.2018
Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA (2003) DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology 37(2):267–276. https://doi.org/10.1053/jhep.2003.50067. PubMed PMID: 12540776
Yuan K, Orcholski ME, Panaroni C, Shuffle EM, Huang NF, Jiang X, Tian W, Vladar EK, Wang L, Nicolls MR, Wu JY, de Jesus Perez VA (2015) Activation of the Wnt/planar cell polarity pathway is required for pericyte recruitment during pulmonary angiogenesis. Am J Pathol 185(1):69–84. https://doi.org/10.1016/j.ajpath.2014.09.013. PubMed PMID: 25447046; PMCID: PMC4278244
Acknowledgments
The authors thank all the members of the Schnapp and Hung laboratories who have contributed to studies described in this chapter. We also thank Seth Bollenbecker for providing the artwork in Fig. 3.3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hung, C.F., Wilson, C.L., Schnapp, L.M. (2019). Pericytes in the Lung. In: Birbrair, A. (eds) Pericyte Biology in Different Organs. Advances in Experimental Medicine and Biology, vol 1122. Springer, Cham. https://doi.org/10.1007/978-3-030-11093-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-11093-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11092-5
Online ISBN: 978-3-030-11093-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)