Abstract
Medical treatment through the use of pharmaceuticals is dependent on the ability of therapeutic agents to reach their intended targets while evading unintended interactions, endosomal sequestration, and degradation. By developing targeted therapies, our treatments of different diseases can be tremendously improved in ways that not only enhance the functionality of relevant drugs, but also improve the patients’ experiences. Liposomes are nanocarriers that encapsulate their payloads, protecting active ingredients from biological environments and degradation. Their use in nanomedicine has the ability to reshape drug administration, from improved specificity and prolonged circulation to decreased cytotoxicity and fewer negative side effects. The efficacy and functionality of liposomes can be further refined and enhanced through surface modification. By conjugating liposomes with various moieties, drug delivery can become a much more targeted process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Surface modification
- Nanocarriers
- Nanotechnology
- Cell targeting
- Drug targeting
- Drug delivery
- Liposomes
- Stealth
- Targeted therapy
- PEG
1 Introduction
Nanocarriers have introduced a unique and promising method of drug delivery . One significant challenge of drug administration is ensuring that the drug not only reaches the correct destination but also does so without damaging or affecting the surrounding organs, cells, and/or tissues. Nanocarriers can maneuver around the problems typically associated with conventional drug delivery. One of their most exciting capabilities is specifically targeting diseased organs or cells while circumventing healthy, nondiseased organs and cells in the body [1]. Drug delivery through nanoparticles and nanocarriers allows higher efficacy with fewer side effects [1]. Nanocarriers, which range from 1 to 1000 nm, offer several advantageous properties, including high surface to volume ratio, increased tissue and membrane penetration, targeted and controlled drug release, and biological mobility [2].
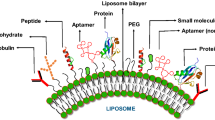
Liposomes are nanocarriers that protect the active ingredient they are carrying from degradation [3]. They are currently considered to be the most successful drug-carrier system [4]. Liposomes’ lipid compositions determine their chemical properties [3]. The efficacy of liposomes can be altered by tweaking their physiochemical properties, such as their size, surface charge, and lipid organization [4]. Much of the research on nanocarriers now focuses on surface modifications that can improve the efficacy of drug targeting . Surface modifications and functionalization with moieties, which alter the range of stimuli recognized, can further fine-tune the liposomes are nanocarriers [1]. Different surface modifications present different benefits. For example, polyethylene glycol (PEG) modification of liposomes can potentially improve blood circulation and reduce nonspecific interactions while avoiding the reticuloendothelial system (RES) that usually poses a significant challenge in intravenous administration [5].
Surface modifications and functionalization of liposomes can tremendously improve the treatment of solid tumors and cancer by presenting ways to overcome the current physiological and biological barriers [6]. Altering these liposomes opens the door for finding new and efficient methods of delivery for anticancer agents [6]. Treating malignant tumors through conventional treatment approaches is a particular obstacle due to their unique physiology. Uncontrollable growth of mutated cells that initiate within an organism’s own cells contributes to the difficulty of selective treatment of cancer [6]. However, with the use of nanocarriers and nanoparticles, site-specific delivery of anticancer drugs is a possibility. Considering the tremendous number of deaths per year that cancer is responsible for, improving anticancer drug delivery is vital. This review will explore the current barriers in selective drug targeting , examples of surface modifications of liposomes, the limitations of functionalized and modified liposomes, and how these alterations aid in overcoming the present barriers.
2 Liposomes
Liposomes are spherical artificial vesicles that contain at least one phospholipid bilayer between aqueous phases [7]. These favorable drug delivery systems vary considerably in terms of properties and compositions. Liposomes have contributed significantly to the advancement of drug delivery due to their biocompatibility , ability to encapsulate hydrophilic and hydrophobic compounds, low toxicity , size, surface charge , improved penetrability, and site-specific targeting [7]. However, liposomes must overcome certain limitations and barriers as well, such as their short half-life, low solubility, and their tendency to occasionally undergo oxidation and hydrolysis-like reactions [7]. By overcoming these barriers, advantages such as biocompatibility and site-specific drug delivery to tumors can be utilized [8].
2.1 Liposome Circulation Time Obstacles
Typical liposomes are taken up by the reticuloendothelial system (RES), which contributes to their shorter half-life [5]. The RES is a crucial defense mechanism of the body [9]. Upon intravenous administration, a liposome is absorbed by Opsonin , a serum protein which recognizes the liposome as a foreign substance [5, 9]. Once a liposome is opsonized, it is demolished by phagocytes that are a part of the RES [9]. The interaction of the RES and liposomes affects organs such as the liver, spleen, and bone marrow [10]. Another challenge that typically faces liposomes is their tendency to release their contents during circulation. This problem can be circumvented through surface modification; as an effort to increase the circulation time of a liposome, it can be modified with a hydrophilic polymer, polyethyleneglycol (PEG) [5, 9]. These liposomes are referred to as PEGylated or stealth liposomes [9]. Once a liposome’s surface is PEGylated, it becomes more stable and evasive, hence the name “stealth” liposome .
3 PEGylated Liposomes
3.1 Advantages of PEGylated Liposomes
PEGylated liposomes have several advantages. These modified nanocarriers have increased circulation time in systemic circulation and a decreased uptake by the phagocytes in the RES [11]. As opposed to non-PEGylated liposomes, stealth liposomes have a much greater ease of entry due to the enhanced permeability and retention (EPR) effect , and improved riddance of tumors due to the increased accumulation in the tumor tissue [11]. PEGylated molecules have a heavier weight, which lessens its clearance through glomerular filtration [12]. This in turn augments the drug efficacy [12]. Additionally, PEGylation modifies and disguises the protein surface, which minimizes the body’s immune response [12].
Following the discovery of stealth liposomes, site-specific liposomes came into existence. By conjugating the open ends of PEG polymers with different moieties, such as antibodies and peptides, the liposomes can engage in more specific targeting [13]. This surface functionalization allows the liposomes to interact with particular overexpressed receptors that are located on the cell surface [13]. Neutral PEGylated liposomes can be easily modified and have multifunctional proteins on their surface [14].
In addition to their stability and increased circulation time, PEGylated liposomes can also avoid enzymatic degradation [12]. The PEG-liposomes are eliminated from the body in a different way that is related to molecular mass, which is heavier than a normal liposome due to its increased water solubility [12]. The increased weight also allows for reduction in clearance via glomerular filtration [15, 16]. PEGylated liposomes with a weight below 20 kDa are eradicated through renal filtration, whereas the heavier ones are primarily eliminated by the liver [16].
3.2 Targeted Therapy Through PEGylation
Because of the EPR effect that PEGylation brings about, stealth liposomes have the ability to specifically target tumors [17]. In a study done by Matsumura and coworkers, the EPR effect was first introduced. The study characterized tumors as having leaky vasculature and poor lymphatic systems [18]. While fenestrated endothelium gaps normally range in size from 200 to 900 nm, the openings within tumor vasculature are even larger [19]. This allows nanoparticles to favorably accumulate in tumors [19]. This preferential accumulation improves the efficacy of drug delivery by allowing more targeted delivery.
The creation of nanoparticles that can target virtually anything in the body is an exciting feat for pharmaceuticals. However, this poses its own challenges as well, including the issue of systemic toxicity [17]. During circulation, nanoparticles may encounter erythrocytes. Their interactions can lead to erythrocyte aggregation and/or hemolysis [17]. This is of particular significance with cationic nanoparticles that are attracted to negatively charged cell surfaces due to electrostatic interactions between the two [17]. However, PEGylation can decrease hemolysis and erythrocyte aggregation through conjugation [20]. When conjugated to PEG, polymers such as polylysine (PLL) and poly(ethyleneimine) (PEI) showed decreased cytotoxicity [21].
3.3 PEGylation and Surface Modification as a Method of Penetrating Mucus
PEGylation is also useful for increasing penetration efficacy through mucus [22]. Mucus is a barrier that protects the human body from a plethora of foreign invaders, including viruses and bacteria [22]. Its mesh-like structure and intricate composition make mucus a challenging barrier for pharmaceutical advancement [22]. For issues such as mucus permeation, several alternative polymers (in addition to PEG) have been researched. Polymers such as poly(2-alkyl-2-oxazolines), polysarcosine, poly(vinyl alcohol), zwitterionic polymers, and mucolytic enzymes are some of the alternatives that have been considered as methods of improving and enabling mucus permeation of nanoparticles [22]. Certain polymers are able to adhere to the surfaces of mucosal membranes, thus improving the drug’s bioavailability [23]. This is of particular relevance when dealing with drug delivery to the airways; overcoming the dilemma of efficient drug delivery through mucus in the airways could open the door to new treatments for serious conditions such as cystic fibrosis [24]. In a series of studies conducted by Hanes et al., a method of enhancing mucosal penetration of nanoparticles was discovered [25, 26]. Nanoparticles with poor diffusion abilities in mucus were functionalized using PEG and the result was superior penetration and diffusion [25, 26]. This improvement is due to the reduction of mucoadhesion, preventing hydrophobic or electrostatic interactions , thus allowing the nanoparticles to permeate the mucus [22].
4 The Effects of Surface Functionalization on Liposomes
4.1 Interactions in the Body
A disadvantage of a nanoparticle is its interactions with biological fluids , which have high ionic strengths [27]. Due to the nature of nanoparticles and the interactions in biological fluids, colloidal stability may be weakened, leading to aggregation of nanoparticles [27]. Colloidal stability is especially weakened in the presence of aggregation. The two primary approaches used when addressing colloidal stabilization are stabilization through electrostatic repulsion and steric stabilization [27]. Functionalization is achieved by attaching polymers to the surface of the liposome. Attaching polymers such as PEG or poly(vinyl pyrrolidone) (PVP) can increase the stability of nanoparticles in biological fluid [28].
4.2 Liposomal Surface Chemistry
Surface functionalization of a liposome also requires considering surface chemistry. Incorporation of a given ligand onto the surface of a nanoparticle requires considering the nanoparticle composition and surface affinity [27]. There are three general classes of chemical groups when dealing with surface functionalization. The first class is noble metals, such as Au and Ag, that are typically functionalized with thiols, amines, and cyanides [27]. The second class is oxides, which are primarily used with magnetic nanoparticles and can be coated with acidic or hydroxyl groups using oxygen bonding [27]. Lastly, there are binary compounds (elements from Groups 12–16 on the Periodic Table), which have high affinities toward thiols and hydroxyl groups, but also may use amino groups [27].
PEG head groups can be modified in a variety of ways, making these molecules extremely versatile and advantageous. This presents several opportunities and possibilities for further bio-functionalization of liposomal surfaces [29]. Examples of terminal functional moieties that are used to modify PEG head groups include carboxylic (–COOH) and amine (–NH2) due to the fact that they can be introduced into PEG molecules without compromising the PEGylated nanoparticle’s colloidal stability in blood and plasma [29, 30]. The ease of PEG head group modification combined with the hydrophilic nature of PEG that enables steric stabilization is why PEG is the preferred, and most widely used, ligand [31].
4.3 PEG Coating
PEG coating is done in different ways, depending on the class of the nanoparticle. As mentioned, the first class is plasmonic nanoparticles (the noble metals). Plasmonic nanoparticles’ surfaces are commonly coated with thiol-terminated derivatives (PEG-SH) through covalent bonding [27]. For citrate-stabilized nanoparticles, this is simply accomplished by adding a solution of PEG-SH to the nanoparticles, which eventually leads to ligand exchange with PEG-SH, creating solutions that are extremely stable in solutions with high ionic strengths and also in biological fluids [27, 32].
4.4 Alternative Ligands to PEG
Although PEG is the most widely used ligand, alternative ligands are also used to increase the stability of nanoparticles in biological fluids. Zwitterionic ligands, ligands that have mixed charge functional groups that contribute to a net charge of zero, have also been used with nanoparticles. In fact, nanoparticles with zwitterionic ligands show even lower levels of opsonization than PEGylated liposomes [33]. In a study conducted by Bawendi et al., the importance of surface charge arrangement was considered [34]. The study demonstrated that zwitterionic nanoparticles with positive charges on their outermost surface displayed nonspecific accumulation and absorption, whereas zwitterionic nanoparticles with negative surface charges showed minimal nonspecific absorptions and fewer protein interactions [34]. There were also differences between the in vivo behavior of zwitterionic nanoparticles and nonionic nanoparticles. The study elucidated that exposed charged groups enhanced the interactions between zwitterionic nanoparticles and the environment [34]. This study highlighted the importance of considering spatial arrangement of charge when creating nanoparticles to be used in vivo. In order to minimize nonspecific interactions, specific attention to the spatial arrangement of positive charges needs to be considered [34].
5 Variables Affecting PEG Liposomes
5.1 PEG Molecular Weight
The circulation time of PEGylated liposomes is dependent on several different variables, such as PEG molecular weight, content, conformation, and surface density [17]. The molecular weight of a PEG chain is proportional to the length of its polymer chain, thus making the molecular weight a pertinent element of surface shielding ability [17]. In a study performed by Duvall et al. using 50D mixed micelles administered intravenously in vivo, results indicated that increasing the PEG molecular weight in the corona significantly increased its half-life for blood circulation [35]. In a different study, PEGylated liposomes were coated with 750 kDa PEG and compared to non-PEGylated liposomes [36]. Although the two were initially comparable, increasing the PEG molecular weight to 5 kDa resulted in extended circulation time and decreased uptake by the mononuclear phagocyte system [36]. Since opsonin proteins in the blood serum easily bind to non-PEGylated nanoparticles, they can be quickly recognized and eliminated by the mononuclear phagocytic system , preventing the nanoparticles to effectively do their jobs [37, 38]. By modifying the surface of a nanoparticle with the addition of a PEG polymer, a hydrophilic layer is formed around the nanoparticle that creates steric repulsion forces, protecting the nanoparticle from opsonin proteins [38]. Generally, the minimum PEG molecular weight required to effectively shield nanoparticle surfaces from opsonin proteins and opsonization by the mononuclear phagocyte system is 2 kDa or greater [38]. However, this number is subject to change in certain circumstances. For example, according to a different study, human monocytic leukemia cell line-derived macrophages (THP-1) require a PEG molecular weight of at least 10 kDa in order to achieve effective shielding of nanoparticles [39]. However, PEG molecular weight may alter the molecule’s density, thus creating a possible confounding factor that can influence results.
5.2 PEG Surface Density and Conformation
In addition to PEG molecular weight, PEG surface density and conformation are important factors influencing circulation time and varying interactions with components of the blood. In a study conducted by Yang et al., it was discovered that a “dense brush” conformation was necessary in order to avoid uptake by human monocytic THP-1 cells in vitro [40]. Brush conformations are assumed by the PEG chains at higher grafting densities when the Flory radius (R F) of the PEG coils divided by the grafting distance (D) equals greater than 1 [40]. The “dense brush” conformation required for evasion of THP-1 cells is adopted when the PEG layer thickness is greater than the Flory radius by at least twofold (R F/D > 2.8) [40]. The authors of the study also discovered that increases in PEG surface density were necessary for increased blood circulation time in vivo [40]. Yang and coworkers concluded that nanoparticle interactions with the mononuclear phagocyte system cells significantly depend on PEG chain conformations and that a highly dense brush conformation is critical for increasing circulation time [40].
6 Liposome Drug Entrapment
Another challenge with liposomes is drug entrapment efficiency. Liposomes entrap lipophilic drugs in the lipid film and hydrophilic drugs in the aqueous part [4]. While entrapment of lipophilic drugs is typically easily accomplished, hydrophilic drugs exhibit encapsulation efficiency with liposomes [41]. A promising method of accomplishing entrapment of hydrophilic drugs into liposomes is the microencapsulation vesicle (MCV) method, which uses “water-in-oil-in-water” emulsion [42]. In a study conducted by Kaimoto and coworkers, the MCV method was used to prepare surface-modified liposomes with PEG and a site-directed ligand [42]. In the last phase of the experiment, a peptide ligand with an affinity to adipose tissue vasculature was utilized [42]. After injecting mice with the fluorescent-labeled liposomes, there was considerable liposomal accumulation in the adipose tissue vessels, indicating that the MCV method with solvent optimization may be a very useful technique for achieving high drug entrapment efficiency and targeting delivery in surface-modified liposomes [42].
7 Disadvantages of Surface Modification of Liposomes
7.1 The Effects of PEGylation on Liposomal Stability
Although surface modification via PEGylation is advantageous and offers several improvements in nanoparticle drug delivery systems, some drawbacks do exist. PEG chains have both hydrophobic and hydrophilic tendencies [43]. Because of the hydrophobic characteristics PEG exhibits, the hydration of membrane phospholipid head groups cannot occur [43, 44]. In a study performed by Tirosh et al., it was concluded that hydration in part contributes to the thermodynamic stability of PEG liposomes [44]. Thus, these hydrophobic restraints can lead to destabilization of the liposomes and problems regarding drug entrapment and loading [43, 44]. Following a study that evaluated the effect cholesterol has on preventing the PEG-induced phase separation of lipid 1-palmitoyl-2[6-(pyren-1-yl)]decanoyl-sn-glycero-3-phosphocholine (PPDPC), it was suggested that excess cholesterol be used in PEGylated liposomes due to its ability to lessen PEG chain–chain interactions [45]. PEGylation can also negatively affect the stability of liposome preparation in relation to storage conditions [43]. However, long-term stability is possible through freezing and freeze-drying in conjunction with the use of a cryoprotective agent [46]. Cryoprotective agents aid in the prevention of liposomal fusion or degradation during the freezing process [46].
7.2 Immunogenic Responses of PEGylated Liposomes
In addition to these drawbacks of using PEGylated liposomes , it has been shown that they can also prompt adverse immunogenic responses, such as acute immune toxicity, that arise not through IgE like immediate type I hypersensitivity reactions, but rather the activation of the complement system [47, 48]. These responses present themselves through hypersensitivity reactions [47]. These infusion reactions that may be induced by PEGylated liposomes are referred to as complement activation-related pseudoallergy (CARPA) [47, 48]. There are a multitude of factors that play a role in a PEG-liposome’s ability to activate the complement system, including hydrophobicity, the inclusion of cholesterol, and particle size [47,48,49]. In addition to these, the presence of preexisting PEG antibodies in people have been considered and implicated in the manifestation of infusion reactions [48, 50]. The presence of these anti-PEG antibodies can affect the targeting, efficacy, and clearance rate of a liposome-encapsulated drug [51], thus making it an important area for research. The consideration of the possible limitations, disadvantages, and consequences of PEG liposomes has increased interest in the discovery and utilization of different lipopolymers as PEG substitutes.
7.3 In Vivo Effects of Surface-Modified Liposomes
The goal of surface modification of liposomes is to improve their overall functionality by overcoming some of the properties of regular liposomes while avoiding or minimizing the effects to the clearance mechanisms of the body [43]. However, this has not been entirely perfected yet. Although PEG liposomes exhibit higher circulation times, the PEG-induced stealth properties of liposomes have finite extents [52]. As the stealth properties of these polymer coatings diminish, these surface-modified liposomes are eventually recognized and cleared by the mononuclear phagocyte system [52], thus limiting the in vivo stabilization effects of PEGylation .
Another in vivo consequence of extended system circulation is an increase in the possibility of drug interaction and exposure of the other nontargeted tissues, potentially leading to toxicity [53]. In some cases, this potential for toxicity challenges the very use of PEGylated liposomes in certain treatments [54].
To improve the in vivo efficacy of PEGylated liposomes, targeting strategies can be utilized. For example, the addition of a ligand to a PEG liposome can improve its activity in comparison to a PEG liposome that is without a ligand. This has been demonstrated in a variety of ways, using a variety of ligands. One example is a study conducted by Yamada et al. in which in vivo antitumor efficacy was assessed through PEGylation and targeting [55]. As a result of PEGylated-induced steric hindrance , the association between a liposome-bound ligand with its receptor can be hampered [55]. This was tested by Yamada and group using three types of folate-linked PEGylated liposomal doxorubicin: untargeted PEGylated doxorubicin-loaded liposomes, non-PEGylated liposomes concealing folate, and PEGylated liposomes with surface exposure to folate [55]. Their research found that the PEGylated, folate-linked, doxorubicin-loaded liposomes exhibited the greatest antitumor efficacy.
8 Conclusion
The use of liposomes in drug delivery is a developing and promising field of nanotechnology that offers a plethora of advancements . As research grows, ways of circumventing the restrictions, limitations, and disadvantages of traditional liposomes appear. A major method of fine-tuning these nanocarriers is surface modification. The most commonly seen routine for this is the addition of a PEG molecule to a liposome. PEGylated liposomes are generally seen as safe, effective, and extremely useful. With their proper usage, drug delivery systems can be redefined to include enhancements such as site-specific delivery of drugs, improved biocompatibility , greater drug efficacy, increased permeation, decreased contact with nontargeted tissues and organs, and decreased toxicity. PEGylated liposomes are currently being used to treat a variety of diseases and ailments. PEG liposomes are being more intensely studied, leading to the discovery of the benefits of ligand attachment. Surface modification of liposomes has facilitated further refinement of these small drug delivery vesicles. The introduction of ligands to PEG liposomes has further expanded the uses and advantages of liposomes, leading to new discoveries in methods of drug delivery and therapeutic treatments.
Although liposomes are generally effective vesicles for drug delivery on a nanoscale, PEG liposomes have some drawbacks that must be considered and continuously researched as an effort to improve the safety and efficacy of surface-modified liposomes as drug delivery systems. However, the benefits of stealth liposomes cannot and should not be ignored. For improving issues such as circulation time, PEGylation is considered to be the gold standard [43]. Despite this, the increase in research on PEG substitutes has led to the discovery of several alternatives that provide a whole gamut of refinements and improvements to surface-modified liposomes .
9 Future Trends
Surface-modified liposomes have proven to be effective and useful vesicles for systemic delivery of drugs. By altering traditional liposomes via PEGylation , these nanoparticles have managed to overcome several of the limitations and barriers that liposomes have faced. PEGylation is one of the primary methods of modification being studied. PEG coatings on liposomes have increased overall function, safety, and efficacy of drug agents. These nanocarriers are certainly going to continue being applied in clinical settings.
The expansion of research on ligand additions to PEGylated liposomes will lead to the discovery of safer, more specific, and more efficacious systems of drug delivery. Surface-modified liposomes offer several advantages over traditional liposomes, such as prolonged circulation, preferential accumulation, subcellular targeting, decreased toxicity, enhanced cellular uptake, and new treatment strategies for countless diseases, including cancer. This may fundamentally change the outcomes and overall experiences of afflicted individuals. In addition, more efficacious vaccines can be delivered, improving general public health, leading to a decrease in healthcare costs. Lower concentrations of therapeutic agents required to achieve intended effects, new methods of disease prevention, and early detection and diagnostic abilities can significantly lower the costs of medical treatment for patients, practitioners, and pharmaceutical companies. However, more research is required before nanocarriers become widespread. In the future, there will most likely be an increase in the versatility of and demand for surface-modified nanocarriers and particles in medical, agricultural, electronic, and environmental fields. Nanocarriers , albeit small, offer huge incentives and possibilities that may reshape our approach to life entirely. Thus, it is in the interest of both economics and innovation to continue researching and improving nanocarriers.
References
Fakhar ud, D., et al. (2017). Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. International Journal of Nanomedicine, 12, 7291–7309. PMC. Web: August 28, 2018, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5634382/
Siafaka, P. I., Okur, N. Ü., Karavas, E., & Bikiaris, D. N. (2016). Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: Current status and uses. International Journal of Molecular Sciences, 17(9), 1440. MDPI. Accessed August 28, 2018, from http://www.mdpi.com/1422-0067/17/9/1440/htm
Kothalawala, N., Mudalige, T. K., Sisco, P., & Linder, S. W. (2018). Novel analytical methods to assess the chemical and physical properties of liposomes. Journal of Chromatography B, 1091, 14–20.
Bozzuto, G., & Molinari, A. (2015). Liposomes as nanomedical devices. International Journal of Nanomedicine, 975–999. https://doi.org/10.2147/ijn.s68861.
Riaz, M., et al. (2018). Surface Functionalization and targeting strategies of liposomes in solid tumor therapy: A review. International Journal of Molecular Sciences, 19, 195.
Sriraman, S. K., Aryasomayajula, B., & Torchilin, V. P. (2014). Barriers to drug delivery in solid tumors. Tissue Barriers, 2, e29528. https://doi.org/10.4161/tisb.29528.
Akbarzadeh, A., et al. (2013). Liposome: Classification, preparation, and applications. Nanoscale Research Letters, 8(1), 102. https://doi.org/10.1186/1556-276X-8-102. PMC. Web: August 22, 2018.
Hofheinz, R. D., Gnad-Vogt, S. U., Beyer, U., & Hochhaus, A. (2005). Liposomal encapsulated anti-cancer drugs. Anti-Cancer Drugs, 16, 691–707. https://doi.org/10.1097/01.cad.0000167902.53039.5a.
Hatakeyama, H., Akita, H., & Harashima, H. (2013). The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biological and Pharmaceutical Bulletin, 36, 892–899.
Immordino, M. L., Dosio, F., & Cattel, L. (2006). Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. International Journal of Nanomedicine, 1(3), 297–315. Print.
Liu, X., Peng, H., & Wang, Q. (2014). Surface engineering of liposomal formulations for targeted drug delivery. Chemical Engineering and Process Techniques, 2(1), 1024.
Milla, P., Dosio, F., & Cattel, L. (2012). PEGylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Current Drug Metabolism, 13, 105. https://doi.org/10.2174/138920012798356934.
Shen, Z., Ye, H., Kröger, M., & Li, Y. (2018). Aggregation of polyethylene glycol polymers suppresses receptor-mediated endocytosis of PEGylated liposomes. Nanoscale, 10, 4545–4560.
Fisher, R. K., et al. (2017). Improving the efficacy of liposome-mediated vascular gene therapy via lipid surface modifications. Journal of Surgical Research, 219, 136–144.
Harris, J. M., Martin, N. E., & Modi, M. (2001). Pegylation: a novel process for modifying pharmacokinetics. Clinical Pharmacokinetics, 40(7), 539–551.
Roberts, M. J., Bentley, M. D., & Harris, J. M. (2002). Chemistry for peptide and protein PEGylation. Advanced Drug Delivery Reviews, 54(4), 459–476.
Suk, J. S., et al. (2016). PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Advanced Drug Delivery Reviews, 99(Pt A), 28–51. PMC. Web: August 23, 2018 from https://www.ncbi.nlm.nih.gov/pubmed/26456916
Matsumura, Y., & Maeda, H. (1986). A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Research, 46, 6387–6392.
Hobbs, S. K., et al. (1998). Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proceedings of the National Academy of Sciences of the United States of America, 95(8), 4607–4612. Print.
Qi, R., Gao, Y., Tang, Y., He, R. R., Liu, T. L., He, Y., Sun, S., Li, B. Y., Li, Y. B., & Liu, G. (2009). PEG-conjugated PAMAM dendrimers mediate efficient intramuscular gene expression. The AAPS Journal, 11, 395–405.
Jevprasesphant, R., Penny, J., Jalal, R., Attwood, D., McKeown, N. B., & D'Emanuele, A. (2003). The influence of surface modification on the cytotoxicity of PAMAM dendrimers. International Journal of Pharmaceutics, 252, 263–266. https://doi.org/10.1016/S0378-5173(02)00623-3.
Khutoryanskiy, V. V. (2018). Beyond PEGylation: Alternative surface-modification of nanoparticles with mucus-inert biomaterials. Advanced Drug Delivery Reviews, 124, 140–149.
Sosnik, A., das Neves, J., & Sarmento, B. (2014). Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Progress in Polymer Science, 39, 2030–2075.
Schneider, C. S., et al. (2017). Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Science Advances, 3, e1601556.
Mert, O., et al. (2012). A poly(ethylene glycol)-based surfactant for formulation of drug-loaded mucus penetrating particles. Journal of Controlled Release, 157, 455–460.
Xu, Q. G., Boylan, N. J., Cai, S. T., Miao, B., Patel, H., & Hanes, J. (2013). Scalable method to produce biodegradable nanoparticles that rapidly penetrate human mucus. Journal of Controlled Release, 170, 279–286.
Guerrini, L., Alvarez-Puebla, R. A., & Pazos-Perez, N. (2018). Surface modifications of nanoparticles for stability in biological fluids. Materials, 11, 1154.
Gref, R., Lück, M., Quellec, P., Marchand, M., Dellacherie, E., Harnisch, S., Blunk, T., & Müller, R. H. (2000). ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids and Surfaces B: Biointerfaces, 18, 301–313.
Thanh, N. T. K., & Green, L. A. W. (2010). Functionalization of nanoparticles for biomedical applications. Nano Today, 5, 213–230.
Carril, M., Padro, D., Del Pino, P., Carrillo-Carrion, C., Gallego, M., & Parak, W. J. (2017). In situ detection of the protein corona in complex environments. Nature Communications, 8, 1542.
Zhang, G., Yang, Z., Lu, W., Zhang, R., Huang, Q., Tian, M., Li, L., Liang, D., & Li, C. (2009). Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials, 30, 1928–1936.
Rahme, K., Nolan, M. T., Doody, T., McGlacken, G. P., Morris, M. A., O’Driscoll, C., & Holmes, J. D. (2013). Highly stable pegylated gold nanoparticles in water: Applications in biology and catalysis. RSC Advances, 3, 21016–21024.
Longmire, M., Choyke, P. L., & Kobayashi, H. (2008). Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine, 3, 703–717.
Han, H.-S., et al. (2013). Spatial charge configuration regulates nanoparticle transport and binding behavior in vivo. Angewandte Chemie (International edition in English), 52(5), 1414–1419. PMC. Web: August 24, 2018.
Miteva, M., et al. (2015). Tuning PEGylation of mixed micelles to overcome intracellular and systemic siRNA delivery barriers. Biomaterials, 38, 97–107. PMC. Web: August 25, 2018.
Mori, A., Klibanov, A. L., Torchilin, V. P., & Huang, L. (1991). Influence of the steric barrier activity of amphipathic poly(ethyleneglycol) and ganglioside GM1on the circulation time of liposomes and on the target binding of immunoliposomes in vivo. FEBS Letters, 284, 263–266.
Gref, R., Domb, A., Quellec, P., et al. (1995). The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Advanced Drug Delivery Reviews, 16, 215–233.
Owensiii, D., & Peppas, N. (2006). Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International Journal of Pharmaceutics, 307, 93–102.
He, Q., Zhang, J., Shi, J., Zhu, Z., Zhang, L., Bu, W., Guo, L., & Chen, Y. (2010). The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials, 31, 1085–1092.
Yang, Q., Jones, S. W., Parker, C. L., Zamboni, W. C., Bear, J. E., & Lai, S. K. (2014). Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Molecular Pharmaceutics, 11, 1250–1258.
Eloy, J. O., et al. (2014). Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids and Surfaces B: Biointerfaces, 123, 345–363.
Kajimoto, K., Katsumi, T., Nakamura, T., Kataoka, M., & Harashima, H. (2018). Liposome microencapsulation for the surface modification and improved entrapment of cytochrome c for targeted delivery. Journal of the American Oil Chemists Society, 95, 101–109.
Nag, O. K., & Awasthi, V. (2013). Surface engineering of liposomes for stealth behavior. Pharmaceutics, 5(4), 542–569. PMC. Web: August 27, 2018.
Tirosh, O., et al. (1998). Hydration of polyethylene glycol-grafted liposomes. Biophysical Journal, 74(3), 1371–1379.
Lehtonen, J. Y., & Kinnunen, P. K. (1995). Poly(ethylene Glycol)-induced and temperature-dependent phase separation in fluid binary phospholipid membranes. Biophysical Journal, 68(2), 525–535. PMC. Web: August 27, 2018.
Stark, B., Pabst, G., & Prassl, R. (2010). Long-term stability of sterically stabilized liposomes by freezing and freeze-drying: Effects of cryoprotectants on structure. European Journal of Pharmaceutical Sciences, 41, 546–555. https://doi.org/10.1016/j.ejps.2010.08.010.
Szebeni, J. (2005). Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology, 216, 106–121. https://doi.org/10.1016/j.tox.2005.07.023.
Neun, B., Barenholz, Y., Szebeni, J., & Dobrovolskaia, M. (2018). Understanding the role of anti-PEG antibodies in the complement activation by doxil in vitro. Molecules, 23, 1700.
Szebeni, J., Alving, C. R., Rosivall, L., Bunger, R., Baranyi, L., Bedocs, P., Toth, M., & Barenholz, Y. (2007). Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. Journal of Liposome Research, 17, 107–117.
Chen, B. M., Su, Y. C., Chang, C. J., Burnouf, P. A., Chuang, K. H., Chen, C. H., Cheng, T. L., Chen, Y. T., Wu, J. Y., & Roffler, S. R. (2016). Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Analytical Chemistry, 88, 10661–10666.
Yang, Q., Ma, Y., Zhao, Y., She, Z., Wang, L., Li, J., Wang, C., & Deng, Y. (2013). Accelerated drug release and clearance of pegylated epirubicin liposomes following repeated injections: A new challenge for sequential low-dose chemotherapy. International Journal of Nanomedicine, 8, 1257–1268.
Nag, O. K., Yadav, V. R., Hedrick, A., & Awasthi, V. (2013). Post-modification of preformed liposomes with novel non-phospholipid poly(ethylene glycol)-conjugated hexadecylcarbamoylmethyl hexadecanoic acid for enhanced circulation persistence in vivo. International Journal of Pharmaceutics, 446, 119–129. https://doi.org/10.1016/j.ijpharm.2013.02.026.
Gabizon, A., Goren, D., Horowitz, A. T., Tzemach, D., Lossos, A., & Siegal, T. (1997). Long-circulating liposomes for drug delivery in cancer therapy: A review of biodistribution studies in tumor-bearing animals. Advanced Drug Delivery Reviews, 24, 337–344. https://doi.org/10.1016/S0169-409X(96)00476-0.
Cui, J., Li, C., Guo, W., Li, Y., Wang, C., Zhang, L., Zhang, L., Hao, Y., & Wang, Y. (2007). Direct comparison of two pegylated liposomal doxorubicin formulations: Is auc predictive for toxicity and efficacy? Journal of Controlled Release, 118, 204–215. https://doi.org/10.1016/j.jconrel.2006.12.002.
Yamada, A., Taniguchi, Y., Kawano, K., Honda, T., Hattori, Y., & Maitani, Y. (2008). Design of folate-linked liposomal doxorubicin to its antitumor effect in mice. Clinical Cancer Research, 14(24), 8161–8168.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Milani, D., Athiyah, U., Hariyadi, D.M., Pathak, Y.V. (2019). Surface Modifications of Liposomes for Drug Targeting. In: Pathak, Y. (eds) Surface Modification of Nanoparticles for Targeted Drug Delivery. Springer, Cham. https://doi.org/10.1007/978-3-030-06115-9_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-06115-9_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-06114-2
Online ISBN: 978-3-030-06115-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)