Abstract
Novel hydrangea-like VS4 nanomaterials as high-performance electrode materials of supercapacitors were successfully synthesized by hydrothermal method using Na3VO4 · 12H2O2, citric acid and TAA (thioacetamide). The prepared samples were characterized by XRD, SEM and BET, CV (cyclic voltammetry) and GCD (galvanostatic charge and discharge). The results revealed that VS4 are hollow microspheres with a diameter of ~5 μm and made of nanosheets , the microspheres formed from diffusion growth of solid microspheres. These materials exhibited a tremendous specific capacitance of 533 F/g, at 0.1 A/g in the potential range of −0.9 to 0 V when used as supercapacitor electrodes in a solution of 1 M Na2SO3. The energy density is as high as 60 Wh/kg, which is much higher than those of many other symmetrical supercapacitors. In addition, the capacity retention of 80% was achieved even after 500 cycles, demonstrating the high performance of vanadium oxide nanomaterials used in supercapacitors.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Supercapacitors are one of the critical energy storage devices because of the high-power density, lightweight, and long cycle life [1,2,3]. Among the various electrode materials, transition metal sulfides, such as MoS2, VS2, VS4 and TiS2, etc., have been investigated widely as materials for energy storage [4,5,6,7]. The high theoretical specific capacitance and electrical conductivity , fast ion diffusion rate, and tunable properties of transition metal sulfides have to attract people’s attention in recent years. Owning to the multiple valances from V(II) to V(V), vanadium sulfides own high energy density [8]. Furthermore, most of the vanadium sulfides are lamellar structure, which contributes to ions insertion/extraction during the charge/discharge process, improving the specific capacitance of supercapacitors. Therefore, vanadium sulfides are promising materials as electrode materials for electrochemical capacitors.
VS4 is usually made into various structures to prevent the nanosheets stacking together and increase the specific surface to improve electrochemical properties of supercapacitors. For example, urchin-like VS4 , octopus-like VS4 , sea grass-like VS4 , and VS4 nanodendrites have been synthesized and applied in LIBs [9]. However, the study on VS4 as electrode materials of electrochemical capacitors is rare, it is necessary to study the electrochemical performance of VS4 applies to supercapacitors.
In this work, novel hydrangea-like VS4 microspheres, have been synthesized. These microspheres also possess high specific surface and high density and thus exhibits a high specific capacitance (533 F/g) and high mass energy density (60 Wh/kg). The cycling stability of these microspheres is good due to the stable nanostructure of hydrangea-like VS4 microspheres. This work proves a strategy to improve the conductivity and stability of electrode materials, which has potential application in the future.
Experiment
Synthesis of Samples
These VS4 microspheres were prepared as follows: 3.9 mM Na3VO4 · 12H2O2 powder and 9.8 mM citric acid was dissolved in 30 mL H2O solution with vigorously magnetic stirring at 90 °C for 1 h. And then cool down the solution temperature to 25 °C and maintain the solution to constant volume of 30 ml. 27.2 mM TAA (Thioacetamide) powder was added into the solution with vigorously magnetic stirring at 25 °C for 1 h. After that, the mixture was transferred into a 50 mL Teflon container and sealed in an autoclave, which was placed in an electrical oven and heated at 160 °C for 48 h, which was then cooled down to room temperature in the air. The black precipitates existed in the bottom of Teflon were collected by centrifugation, followed by washed with de-ionized water and anhydrous alcohol 3 times, respectively. The precipitates were dried in vacuum at 80 °C for 12 h. After cooling down to room temperature in a vacuum, VS4 microspheres were finally obtained. All reagents used in the experiments are of analytical grade and used without any further purification.
Characterization
The chemical composition of products was characterized by X-ray diffraction (XRD; Rigaku D/Max 2500 PC, Cu Kα, Japan). The morphology and microstructure of VS4 microspheres were characterized by field emission scanning electron microscopy (FESEM; FEI Nova 400, Netherland) and N2 gas adsorption-desorption method (Quadrasorb 2MP, USA).
Electrochemical Measurements
CHI660E electrochemical workstation was used to measure the electrochemical performance. The working electrode was prepared by mixing the active materials, acetylene black and polyvinylidene fluoride (PVDF) with a mass ratio of 80:10:10. The mixture then was made into slurry by using 1-methyl-2-pyrrolidinone (NMP) as a solvent. Subsequently, the slurry of the mixture was spread on a round foam nickel with an area of 1 cm2 and dried under vacuum at 120 °C for 12 h, the working electrode then was obtained, which contained about 1 mg active materials. Electrochemical tests were performed using a three-electrode cell, in which Platinum sheet and Ag/AgCl were used as the counter and the reference electrode, respectively the electrolyte was 1 M Na2SO3. The electrode should be kept static for 2 h so that the electrode materials were thoroughly wetted with the electrolyte. The cyclic voltammetry (CV) and galvanostatic charging/discharging (GCD) measurements were conducted on the electrochemical workstation. All the electrochemical tests were performed at room temperature.
Results and Discussion
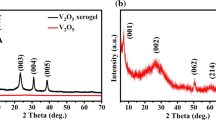
Chemical components of products were investigated by XRD. Figure 1 shows XRD patterns of the sample in the 2θ range of 5–80°. It can be seen in the XRD patterns that the shift of (110), (020) and (114) peaks from 2θ = 15.788°, 17.004° and 36.329° are well indexed to monoclinic VS4 (JCPDS no. 87-0603; a = 6.755 Å, b = 10.42 Å and c = 12.11 Å), and it is different All the observed diffraction peaks in the pattern of VS4 are well indexed according to the power diffraction card of monoclinic VS4 . Therefore, XRD patterns indicate that the major composition of products is VS4 crystals.
The morphology and microstructure of the as-prepared product were examined by scanning electron microscope (SEM) image. As shown in Fig. 2, SEM images for VS4 at various magnifications were displayed. It can be seen from Fig. 2a that the products are microspheres with a diameter of ~5 μm and stacking together with uniform shape. These microspheres are made up of ~500 nm long nanosheets , as shown in Fig. 2b, c, it is clear that 1–3 nanolayers combine together formed nanosheets , and each nanosheet link together with 1–4 nanosheets . To further figure out internal structure of the microsphere , the figure of microspheres amplified in Fig. 2d. It is clear that the surface and internal of microsphere is a combination of nanosheets , the average pore size between sheets and sheets are about 0.5 μm and thickness of the sheets is about 100 nm. The internal pores and surface pores of microspheres were arranged by a kind of staggered type, which illustrates that the microspheres formed from diffusion growth of solid microspheres. The combination of nanosheets provides high structural stability, high specific surface area and this surface structure made the microspheres possess loose construction, it is conducive to ion adsorption and desorption.
To further investigate the microstructure of products, N2 adsorption-desorption isotherms were used (Fig. 3). The isotherms showed a sharp capillary condensation step at high relative pressure and belong to type III isotherm according to IUPAC classification, indicating the existence of pores in microspheres, this result is the same as SEM images (Figs. 3a and 2d). The hysteresis loop of Type H4 in adsorption/desorption isotherms indicates that these pores are fractured pores formed by plate-like particles, verifying that pores are produced by a combination of nanosheets . They are all mesopores with an average pore size of 3 nm (Fig. 3b). The majority of these mesopores are several-nanometer sized (3–10 nm) while the minority is larger than 10 nm.
In order to evaluate the electrochemical properties of Hydrangea-like VS4 microspheres, the three-electrode system was used and the reference electrode is saturated Ag/AgCl electrode. Cyclic voltammetry (CV) and galvanostatic charging/discharging (GCD) measurements were conducted. Figure 4a exhibits the typical CV curve of Hydrangea-like VS4 microspheres at the scan rates of 10 mV/s, 30 mV/s, 50 mV/s and 100 mV/s, respectively, within the potential range from −0.9 V to 0 V. The CV curve is mirror symmetrical at the different scan rate. Redox peaks emerge in symmetrical positions indicates the redox reactions are reversible, and with the scan rate increased the redox peak became more and more obvious. The redox reactions associated with this pair of redox peaks as follows:
where x is the mole fraction of intercalated Na+ ions. This pair of redox peaks indicates the charge storage mechanism is pseudocapacitance, which has broad application prospect for electrode materials of supercapacitors [10]. This superior performance of the electrode materials is mainly due to VS4 crystals with porous structure that contribute to the electron transfer. Figure 4b shows the galvanostatic charging/discharging curve at different current densities. The specific capacitance of supercapacitors can be calculated through galvanostatic charging/discharging measurement, the corresponding formula as follows:
where C is the specific capacitance, I is the constant current; \( \Delta t \) is the discharging time; m is the mass of electrode material; \( \Delta V \) is the potential drop upon discharging. As show in Fig. 4c, the gravimetric specific capacitance of hydrangea-like VS4 microspheres is as remarkably high as 533, 261, 251, 178 and 191 F/g at the current density of 0.1, 0.2, 0.3, 0.5 and 1 A/g. The obtained specific capacitance of hydrangea-like VS4 microspheres significantly higher compared to that of MoS2/Mo binder-free electrodes (192.7 F/g), and its highest specific capacitance is much higher than most vanadium oxide or vanadium sulfide-based electrodes such as hollow V2O5 (488 F/g), nanoporous layer structured V2O5 (214 F/g) or VS2/G (211F/g). [11, 12] ENREF_11 [4, 13] ENREF_4 The reasons associated with such superior specific capacitance of supercapacitor-based VS4 microspheres are that combination of nanosheets leads to high specific area that provides a large amount of active surface sites for redox reaction to take place in, and porous structure exists mass mesopores which can facilitate the intercalation/deintercalation of electrolyte ions so that the rate of redox reactions on the surface can be increased, which is beneficial to increasing the redox reaction rate. It is also worthy to point out that VS4 microspheres possess higher mass density and power density, which can decrease the mass of electrodes and lead to higher specific discharge capacity.
a CV curves of the hydrangea-like VS4 microspheres at different scan rates; b Galvanostatic charging/discharging curves of hydrangea-like VS4 microspheres at different current densities; c Specific capacitance at different current density of hydrangea-like VS4 microspheres; d Cycling performance of hydrangea-like VS4 microspheres at current density of 1.0 A/g
According to the two-electrode configuration, E and P are calculated by the following formula:
where C is the specific capacitance, V is the cell voltage and Δt is the discharge time. The maximum energy density was calculated to be 60 Wh/kg at the power density of 12.5 W/kg and the maximal power density is 125 kW/kg at 1 A/g. This energy density is much higher than those of symmetrical supercapacitors based on VS2/G (46.93 Wh/kg at a power density of 910 W/kg) [11] and VS2//AC (42 Wh/kg at 700 W/kg) [14]. The high energy density is due to the high specific surface area of the hierarchical microspheres, the high conductivity and high density of VS4 in the microspheres.
Another important criterion for supercapacitor is cycling performance examined at 1.0 A/g as shown in Fig. 4d. Notably, 80% of initial specific capacitance has been maintained after 500 cycles, however, the specific capacitance increases sharply by 9% of initial value in the first 27 cycles, and then it decreased. The reason of specific capacitance increasing and decreasing is that the electrolyte was dissolved into interior pores and destroyed the external nanostructure of VS4 microspheres during the charging/discharging process, which is common in lithium-ion batteries based on VS4 -rGO materials. It is clear that the combination of nanosheets provide remarkable stability for VS4 microspheres.
Conclusions
In summary, the hydrangea-like VS4 microspheres have been successfully synthesized by hydrothermal method and it was testified by XRD. Both SEM and BET indicate that the VS4 microspheres are the combination of nanosheets and it was forming from combination of nanosheets . These nanosheets was formed by nanolayers, VS4 microspheres with a diameter of ~5 μm are made up of ~500 nm long nanosheets . The VS4 microspheres prepared for electrode materials of supercapacitors exhibit superior electrochemical performance with a high specific capacity of 533 F/g and outstanding energy density of 60 Wh/kg. 80% of initial specific capacitance has been maintained after 500 cycles. Further studies on VS4 may accelerate the development of transition metal sulfides for supercapacitors considering its outstanding performance.
References
Faraji S, Ani FN (2014) J Power Sources 263:338–360
González A, Goikolea E, Barrena JA, Mysyk R (2016) Renew Sustain Energy Rev 58:1189–1206
Krishnamoorthy K, Veerasubramani GK, Pazhamalai P, Kim SJ (2016) Electrochim Acta 190:305–312
Zhou J, Wang L, Yang M, Wu J, Chen F, Huang W, Han N, Ye H, Zhao F, Li Y, Li Y (2017) Adv Mater 29
Wang Y, Liu Z, Wang C, Yi X, Chen R, Ma L, Hu Y, Zhu G, Chen T, Tie Z, Ma J, Liu J, Jin Z (2018) Adv Mater 30:e1802563
Zhang L, Sun D, Kang J, Wang HT, Hsieh SH, Pong WF, Bechtel HA, Feng J, Wang LW, Cairns EJ, Guo J (2018) Nano Lett 18:4506–4515
Yan Y, Li B, Guo W, Pang H, Xue H (2016) J Power Sources 329:148–169
Yang G, Zhang B, Feng J, Wang H, Ma M, Huang K, Liu J, Madhavi S, Shen Z, Huang Y (2018) ACS Appl Mater Interfaces 10:14727–14734
Brodd MWR (2004) ChemInform 35(50):4245
Abureden S, Hassan FM, Yu A, Chen Z (2017) Energy Technol 5:1919–1926
Reddy RN, Reddy RG (2006) J Power Sources 156:700–704
Zhang Y (2017) Mater Sci Pol 35
Masikhwa TM, Barzegar F, Dangbegnon JK, Bello A, Madito MJ, Momodu D, Manyala N (2016) RSC Adv 6:38990–39000
Xu X, Jeong S, Rout CS, Oh P, Ko M, Kim H, Kim MG, Cao R, Shin HS, Cho J (2014) J Mater Chem A 2:10847–10853
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Nos. 51474041, 51674051), and Fundamental Research Funds for the Central Universities of China (106112015CDJZR465505, cqu2017hbrc1B08).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Peng, ZW., Jun, KF., Li, HY., Xie, B. (2019). Hydrangea-Like VS4 Microspheres: A Novel Structure Material for High-Performance Electrochemical Capacitor Electrode. In: TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-05861-6_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-05861-6_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05860-9
Online ISBN: 978-3-030-05861-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)