Abstract
This chapter describes EEG abnormalities in infectious diseases of the central nervous system. Meningitis, encephalitis and meningoencephalitis of bacterial, viral, parasitic, or mycotic origin are conditions in which EEG represents an important diagnostic tool, especially in emergency, sometimes showing specific patterns. EEG features may indeed range from normal activity to diffuse or lateralized abnormalities and also present epileptiform discharges.
Herpes Virus Encephalitis (HVE) and Subacute Sclerosing Pan Encephalitis (SSPE) are the ones showing the most specific EEG abnormalities. Creutzfeldt-Jakob Disease (CJD) is a prion encephalitis and, due to its characteristic and diagnostic EEG features, is treated separately and more extensively.
Finally, EEG abnormalities in bacterial abscesses and other infectious disorders will also be described, although these are often non-specific.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Infectious Meningitis
EEG studies on meningitis reveal that EEG abnormalities are absent or only mild in viral (aseptic) meningitis, while they are more pronounced in bacterial meningitis [1].
1.1 Bacterial Meningitis
Bacterial infections of the meninges (dura mater, arachnoid and pia mater) are distinguished in acute meningitis, also defined purulent, and subacute/chronic meningitis that often affect the basal portion of the brain. These latter may be caused also by non-bacterial organisms, such as fungi and parasites.
Acute bacterial meningitis is a life-threatening infection that requires prompt diagnosis and treatment. Ninety-five percentage of patients present with two of the following four symptoms: fever, nuchal rigidity, altered mental status and headache [2]. Its incidence is about 5 per 100,000 adults per year in developed countries. The principal causative pathogens in adults are Streptococcus pneumoniae (pneumococcus) and Neisseria meningitidis (meningococcus), responsible for about 80% of all cases [3]. The organisms causing meningitis in hospitalized patients (nosocomial meningitis) differ from those of community-acquired meningitis and they include Gram-negative rods (e.g., Escherichia coli, Klebsiella spp., Pseudomonas aeruginosa, Acinetobacter spp., Enterobacter spp.), staphylococci and streptococci other than S. pneumoniae.
Although EEG may be heterogeneous, the most common finding in purulent meningitis consists of diffuse slow-wave abnormalities of variable severity. A single EEG may not be pivotal for the specific diagnosis, but seriated recordings are useful to follow the course of the disease, for the early detection of complications or relapses, and for indication of the presence of additional brain damage in the case when the meningitis evolves into a meningo-encephalitis. Fuvrthermore, EEG allows recognizing epileptiform discharges even before the development of clinical epileptic seizures, correlating with a poor prognosis and a higher mortality. Thus, EEG provides clinicians an instrument to eventually establish a preventive antiepileptic therapy.
Other EEG abnormal patterns observed in bacterial meningitis include triphasic waves and occasional frontal delta wave activity (Frontal Intermittent Rhythmic Delta Activity, FIRDA), although these are more typical of other etiologies [4]. It is reasonable to consider slowings in the delta range as the only abnormality that may suggest clinicians to a likely etiological diagnosis, more often observed in bacterial than in viral meningitis (Fig. 39.1) [1]. Lateralized Periodic Discharges (LPDs) have been described in pneumococcal infections, especially when a brain lesion occurs [5]. Another possibility is the formation of bacterial brain abscesses that may represent either the cause or the consequence of bacterial meningitis. In such cases, EEG shows focal unilateral delta slowings [6], although other EEG changes are possible, including epileptiform focal abnormalities, focal suppression of the background activity, and LPDs [4].
Subacute and chronic infectious meningitides are commonly defined as inflammations of the meninges evolving during weeks to months, without resolution of Cerebro Spinal Fluid (CSF) abnormalities.
Also called “basal meningitis”, due to their tendency to involve the basal regions of the brain and the brainstem, several pathogens are recognized. Bacterial causes of subacute and chronic meningitis include Mycobacterium tuberculosis and syphilis. Mycobacterium tuberculosis represents the most common bacterial agent.
Tuberculous Meningitis (TBM) is an aggressive form of extrapulmonary disease, more common in patients coinfected with HIV. Mycobacterium tuberculosis may reach the meninges and the CNS through the blood, 4–8 weeks after primary infection, giving rise to an inflammation that involves the cranial nerves and that may generate a blockage of CSF flow with obstructive hydrocephalus. However, although it may follow a primary infection, TBM is more frequently due to reactivation of an old subclinical tuberculoid focus that had previously been inactive, sometimes even for many years [7].
TBM in immunocompetent adults is typically preceded by non-specific symptoms like anorexia, fatigue, weight loss, fever, myalgia and headache. Characteristic clinical features are vomiting, meningeal signs, focal deficits, vision loss, cranial nerve palsies and increased intracranial pressure [8].
The EEG findings in TBM vary according to the site of the inflammatory process and to the presence of other conditions, such as hydrocephalus and increased intracranial pressure.
In basal meningitis, EEG may be normal or may show only mild, non-specific changes. The involvement of the cerebral cortex and of the meninges results in moderate to severe slowing of EEG activity. The degree of the slowings depends on several factors, which include the magnitude of cortical involvement, the rate of progression of the process, the level of consciousness and the medications administered.
Some authors report heterogeneous EEG patterns and their presence in the early phase appear to be associated with a poor outcome. Among them, the most frequent are the diffuse slowing of background activity and the Intermittent Rhythmic Delta Activity (IRDA); on the other hand, lateralized epileptiform discharges (consisting in spike or multiple spike and wave complexes) appear to be rare (Fig. 39.2). IRDA is a non-specific finding and may occur in increased intracranial pressure, being attributed to a diffuse gray matter disease, involving both cortical and subcortical areas. Significant right to left asymmetry in voltage and frequency has also been observed [7, 9, 10].
1.2 Viral Meningitis
Viral meningitis, also known as aseptic meningitis, is a non-bacterial form of meninges infection. The most commonly involved viruses are enterovirus, Herpes Simplex Virus (HSV), and Varicella-Zoster Virus (VZV) [11].
Clinically, viral meningitis is characterized by fever, headache and signs of meningeal irritation, although a mild degree of drowsiness is not uncommon [12]. As the clinical picture of bacterial and viral meningitis are similar, CSF analysis is essential for their distinction, to identify the virus, and to decide whether or not to initiate antiviral therapy [11].
EEG is usually normal. However, non-specific EEG changes occur in 33–50% of patients especially in young children, consisting essentially in generalized, sometimes prevalent in posterior regions, continuous or intermittent slowings (Fig. 39.3). Epileptiform abnormalities are infrequent, and their occurrence suggests a diagnosis of encephalitis [12].
2 Infectious Encephalitis
Encephalopathies due to infections may result from the direct invasion of brain parenchyma by various agents (bacteria, viruses, parasites, fungi), from the effects of a systemic infection during sepsis (“Sepsis-Associated Encephalopathies,” SAE) or, indirectly, from the aberrant immunologic reaction to the infectious pathogen (“post-infectious encephalitis”). The latter may occur either as a consequence of an otherwise benign infectious disease or after a vaccination. Most infectious encephalitides have non-specific EEG patterns. Among those with characteristic EEG abnormalities, Herpes Virus Encephalitis (HVE), Subacute Sclerosing Pan Encephalitis (SSPE) and prion diseases are described.
Herpes virus encephalitis is an acute encephalitis characterized by fever, altered mental status, focal neurological symptoms and generalized or focal epileptic seizures. Symptoms usually appear in hours to days. Differently from the other acute encephalitides (VZV and arboviruses, including West Nile, Toscana, tick-borne, Japanese and others) that cause widespread infection in the brain and tend to determine only diffuse delta to theta slowing [9, 13], HVE induces characteristics focal or multifocal EEG abnormalities likewise epileptic discharges. Besides providing a diagnostic support, EEG in HVE appears to have a prognostic role, as an early normal EEG seems to be predictive of good outcome [14, 15]. HVE is caused in 90% of all cases by HSV-1, whereas HSV-2 leads to disseminated infection especially in immunocompromised individuals and neonates [15].
EEG findings in HVE may consist in focal or diffuse slow waves, focal epileptiform abnormalities, electrical seizures, attenuation/disorganization of background activity, or periodic lateralized discharges [16]. The EEG activity in HVE reflects the virus tendency to affect temporal and orbital frontal lobes and the cingulate gyrus, although it may also cause a more widespread cerebral involvement.
EEG shows initially delta slowings in the temporal lobe. Later, isolated sharp waves appear on this slow activity and, as the disease progresses - between the 2nd and 14th day of progression -, LPDs appear. The periodic complexes consist of sharply shaped slow waves or polyphasic spikes with a duration of complexes of 200–600 ms and an amplitude of 100 to about 500 μV, recurring at a rather constant interval of 1.5–2.5 s, but sometimes ranging from 1 to 5 s. These lateralised alterations may become bilateral as the disease involves the contralateral hemisphere [16]. When bilateral, they can be bilateral independent (BIPDs) or multifocal (MfPDs) or generalised (GPDs) [13].
PLDs localised in the affected temporal lobe are then considered the most characteristic abnormalities for the diagnosis of HVE.
Seizure discharges are common in the affected brain areas, consisting of epileptiform fast rhythmic activity, with or without clinical correlate [4].
Attenuation of background activity is another EEG finding in HVE, coexisting or not with periodic patterns. Although periodic sharp waves and the other EEG findings are not pathognomonic of this condition, the localization of these abnormalities in the temporal lobe is considered very characteristic and helpful in the early diagnosis, enabling the prompt start of antiviral therapies [16].
In refractory or fatal cases, a burst suppression pattern may be observed, with generalized EEG attenuation following a burst of LPDs or polyspikes, indicating a poor prognosis. In nonfatal HVE, with the resolution of the infection, the EEG periodic abnormalities become infrequent and intermittent and they are replaced by a slow activity that may persist even for few months. However EEG recovers usually slowly to almost normal activity on seriated examinations during a period of 20–30 days, with the clinical improvement often preceding the electrographic normalization. Figs. 39.4 and 39.5 show the progression of EEG pattern in HVE in two cases.
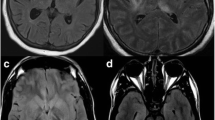
EEG evolution of a 64-year-old male patient with right temporal herpetic encephalitis. Four days after symptoms onset (fever, confusion and focal seizures), EEG showed right quasi-periodic spikes and sharp waves, with a tendency to contralateral transmission. After 7 days, the abnormalities were less represented, maintaining a quasi-periodic recurrence. After 14 days, sporadic slow sequences were only evident, especially in the contralateral hemisphere. After 20 days, EEG normalization was observed. MRI showed in the small boxes.
Herpes virus encephalitis EEG progression in a 56-year-old female patient. Two days after symptoms onset, EEG showed left temporal monomorphic slow activity with sporadic spikes. After 5 days, lateralized periodic discharges appeared in the same. Rare monomorphic delta activity in anterior regions was observed after 14 days. After 20 days, EEG normalization was observed. MRI showed in the small boxes.
Subacute sclerosing panencephalitis is a progressive and usually fatal encephalitis, secondary to measles virus infection, that causes a widespread CNS demyelination with a higher risk in subjects who had the infection earlier, before the age of 2 years. It is a late complication, occurring with a latency of about 6 years after the first infection. Its pathogenesis seems to be related to an abnormal immune response that may favor the permanence and replication of the mutant virus in the CNS [17].
The main clinical symptoms are represented by poor school performance and progressive intellectual deterioration, personality changes and behavior abnormalities, followed by stereotyped myoclonic jerks and other neurological symptoms such as pyramidal, extrapyramidal, visual, cerebellar and autonomic symptoms, seizures, and coma.
EEG recording is essential for the diagnosis. Typical Periodic Complexes (PCs) are found in 65–83% of individuals with SSPE and they are described as stereotyped, bilaterally synchronous and symmetrical 100–800 μV, 1–3 Hz slow-wave complexes, sometimes with sumperimposed spikes or sharp waves (Fig. 39.6). Their duration ranges from 0.5 to 3 s, and the interval between complexes varies from 3 to 20 s, although in the early phases, they can recur as long as every 5 min [17]. Due to the variable but long interval between them, periodic complexes in SSPE are suggested to be named as Periodic, Long-Interval Diffuse Discharges (PLIDDs), in contraposition with those observed in Creutzfeldt–Jakob disease presenting shorter (0.5–3 s) intervals (Periodic Short-Interval Diffuse Discharges, PSIDDs) [18]. PCs are usually present during awake and sleep; during sleep, however, there could be differences in their morphology and frequency and they may also disappear; in the early sleep phases, PCs could be elicited by external stimuli [17, 19]. PCs are recognizable throughout SSPE early stages and they are often related to the myoclonias, which may precede or succeed the PCs in the EEG. Less commonly, periodic complexes can disappear with high body temperatures, lose their rhythmicity with variable intervals between them, or be preceded by bisynchronous occipital spikes. Other atypical findings include, focal spikes, slow wave and random spikes in the frontal regions, and multifocal paroxysmal, high-amplitude slow waves alternating with fast waves [18].
Even if the PLIDDs are typically observed in SSPE, they are also reported in some toxic encephalopathies (like baclofen or ketamine intoxication) and in anoxic brain injury.
Periods of short-lasting (1–4 s) bioelectrical suppression may follow PCs. FIRDA are also recognized in more than half of the patients with SSPE during waking and light sleep. Although classically considered prominent in later stages of the disease, in the study conducted by Demir in 2013, [20] they appear especially in the early stages, so that they may be considered as a transitory phenomenon that could evolve into other types of EEG abnormalities. Independent from the PCs, epileptogenic discharges as focal or multifocal spikes and/or unilateral or bilateral generalized paroxysmal abnormalities have been reported in the EEGs of patients with SSPE [20].
3 Prion Diseases
Prion Diseases (PrDs) are a group of neurodegenerative disorders caused by an abnormally proteinaceous infectious particles, called prions. Prion diseases result from the conversion of a normal, cell-surface glycoprotein (PrPC) into a conformationally altered protein isoform (PrPSc) that, in the absence of the nucleid acids, becomes infectious. For the characteristic neuropathological changes that include spongiform vacuolation, neuronal loss, PrPSc deposition, amyloid plaque formation, and gliosis, prionic diseases are also called spongiform encephalitis. PrDs are classified into three groups: sporadic, genetic and acquired. The sporadic form is called sporadic Creutzfeldt-Jakob Disease (sCJD). The genetic forms are subdivided into genetic CJD (gCJD), Gerstmann-Sträussler-Scheinker Disease (GSSD), and Familial Fatal Insomnia (FFI). The acquired forms include kuru, iatrogenic CJD (iCJD), and variant CJD (vCJD).
3.1 Sporadic Creutzfeldt–Jakob Disease (sCJD)
sCJD is the most common subtype, accounting for 85% of the spongiform encephalitis, with an incidence of 1 to 1.5 per million per year [21].
Clinical symptoms rapidly include progressive dementia, cerebellar dysfunction and visual, speech and gait abnormalities. Dementia is the major symptom, followed by spontaneous or induced myoclonia. Pyramidal and extrapyramidal dysfunction, as well as behavioral changes with agitation, confusion and depression may also be observed. At the late stage of the disease, patients become unresponsive to external stimuli [22]. In almost 40% of patients, the initial symptom at presentation is represented by cognitive deterioration while cerebellar symptoms - as well as behavioral symptoms and visual dysfunction - are the initial manifestation in approximately only 15–20% of cases. Extrapyramidal and pyramidal symptoms are usually observed during the disease progression. Although myoclonus is infrequent as a presenting symptom, it is observed during the clinical course in almost the totality of cases. Epileptic seizures, either focal or generalized, usually occur later in the progression of the disease, in the 8–9% of cases. Symptoms appear in a rapidly progressive course and death occurs often within 1 year.
EEG is an essential part of the diagnostic process and it is included, together with clinical and CSF features (presence of abnormal protein 14-3-3), both in the WHO and in the MRI-CJD diagnostic criteria of sCJD [21, 23].
EEG activity changes over the sCJD course with different features. In the early stage (first 8–9 weeks), characterized clinically by cognitive and behavioral disturbances, non-specific EEG abnormalities are usually observed; these are represented by diffuse slowing and disorganization of background activity and occasional predominantly frontal Generalized Rhythmic Delta Activity (GRDA).
In the middle stage, after a time average of 10 weeks and clinically dominated by abundant myoclonic jerks and sensorimotor and cerebellar dysfunction, an almost pathognomonic EEG pattern appears: continuous generalized bisynchronous Periodic Sharp Wave Complexes (PSWCs) (Fig. 39.7).
In the last stage, usually lasting 3 to 4 months and clinically characterized by a severe dementia evolving to akinetic mutism or even coma, unreactive EEG tracings with predominantly slow waves, low-voltage activity, or—rarely—an alpha-coma pattern may be observed. In more advanced stages, burst suppression and then diffuse suppression patterns are likely evident (Fig. 39.8) [4].
EEG evolution in a 71-year-old female patient with sporadic Creutzfeldt-Jacob disease. EEG performed 3 days after hospital admission showed left temporal slow activity with rare sharp waves. At that phase, she appeared disoriented and manifested behavioral alterations, headache and visual disturbances. EEG performed after 24 days demonstrated left quasi-periodic epileptiform abnormalities. After 36 days, EEG showed a diffuse periodic activity predominant in the left hemisphere. From days 52 and 96, periodic complexes were more evident in the right hemisphere and the EEG activity was poorly reactive to acoustic and nociceptive stimuli; the patient also did not show reactions to external stimuli and had myoclonic jerks, not always correlated to periodic EEG abnormalities. Three days before exitus (day 147), electrocortical diffuse attenuation with low-amplitude periodic abnormalities was recorded
Atypical EEG patterns may also be observed, with bifrontal sharp waves that are quasi-periodic or even with multifocal spike-and-wave discharges [4].
However, PSWCs are the hallmark EEG finding in patients with sCJD and may be missed if the EEG is performed too early or, less commonly, too late in the course of the disease [24]. For this reason, it is essential to perform seriated EEG recordings overall the disease course.
Typical PSWCs may consist of both simple sharp waves, biphasic or triphasic, and complexes with mixed spikes, polyspikes and slow waves of 150–300 μV amplitude, with a typical 100–600 ms duration, recurring every 0.5–2 s. PSWCs occur, in average, 12 weeks after the first symptoms, typically together with the appearance of myoclonic jerks [24, 25]. Topographically, PSWCs commonly reveal a bilateral voltage distribution with a fronto-precentral midline maximum.
PSWCs may or may not be related to the myoclonic jerks. Myoclonus in CJD can be classified according to its time course as periodic, rhythmic, or irregular and, in relation to the EMG pattern, as positive or negative. Polygraphic study reveals that positive EMG bursts of periodic myoclonus are always time-locked with PSWCs [26, 27].
PSWCs may also occur lateralized, especially at early disease stage, but with usual development into the typical sCJD bilateral PSWCs during the course of the disease [23].
However, differently from the LPDs, sCJD-associated lateralized PSWCs are not consistent with the transitory EEG discharges occurring during an acute brain lesion and they are influenced by external stimulation and sleep, being prominent during wakefulness and usually decreasing-until disappearing-during sleep.
With regard to EEG reactivity, it is important to underline that in the early stages, when PSWCs may still be absent, they may appear after external stimuli. As the disease progresses, photic, acoustic and somatosensory stimulations may also induce modifications in PSWCs frequency or even determine their appearance. Particularly, low-frequency intermittent photic stimulation may induce the appearance of rhythmic periodic complexes with frequency identical to that of the stimulation.
Moreover, seizures have been reported during the late phases of disease, and seizure-like EEG activity, in particular nonconvulsive status epilepticus must be carefully distinguished from PSWCs. Most problems concern the responsiveness of both PSWCs and epileptic activity to antiepileptic drugs, such as benzodiazepines. Therefore, EEG response to AEDs alone is not sufficient to conclude that the periodic discharges represent the EEG correlates of seizures [28, 29].
The following table (Table 39.1) summarises the clinical-electrophysiological correlations during the progression of sCJD.
With regard to PolySomnoGraphic (PSG) analysis, sleep abnormalities in patients with CJD, with or without sleep complaints are frequently observed. The most commonly observed PSG abnormalities are loss of normal sleep EEG architecture and the presence of sleep-disordered breathing. The lack of sleep spindles, K-complexes and vertex waves seems to be the main finding. Furthermore, REM sleep is less represented, as well as slow-wave sleep [30]. Terzano and colleagues (1995) [31] published a case report of serial PSGs in a single patient with CJD, which suggested that loss of physiologic sleep patterns might be associated with disease progression and follow this order: Sequences of sporadic sharp waves; pseudo-periodic discharges cyclically alternating with slow pseudo-rhythmic theta-delta activity; slow activity at 0.5–4 Hz poorly rhythmic.
3.2 Genetic CJD (gCJD)
gCJD cases are associated with point mutations or insertions within the open reading frame of the PRioN Protein (PRNP) gene. Up to date, more than 20 mutations have been described. The clinical presentation of gCJD overlaps between mutations and may resemble patients with sCJD, although it seems to be associated with a younger age at onset and a longer clinical course [21, 24]. On the contrary, EEG abnormalities may be different . Although a typical CJD-EEG is more frequent in gCJD than in the other forms of genetic spongiform encephalopathies (Familial Fatal Insomnia and Gerstmann-Sträussler-Scheinker syndrome), overall PSWCs are uncommon in patients with gCJD, occurring in only about 10% of them [24].
3.2.1 Variant Creutzfeldt-Jakob Disease (vCJD)
vCJD, also known as “mad cow disease,” is strictly associated with Bovine Spongiform Encephalopathy (BSE), currently believed to be the only non-human PrD to be directly transmissible to humans. Transmission from affected cattle occurs through meat consumption. Compared to patients with sCJD, those affected by vCJD are usually younger, with a median age of onset at around 27 years (range 12–74 years). The mean disease duration is longer, ≈14.5 months compared with sCJD. Even if psychiatric symptoms may characterize the early stages of sCJD, prominent psychiatric symptoms are regularly the presenting symptoms in vCJD and they may be the only clinical feature for more than 6 months before clear neurologic symptoms arise [21].
PSWCs do not occur in patients with vCJD and this absence is considered characteristic, so that their absence is one of the diagnostic criteria for vCJD [32]. Normal EEG or non-specific slow-wave activity may be found.
3.2.2 Iatrogenic CJD (iCJD)
iCJD cases are very rare and may result from the use of cadaveric-derived human pituitary hormones, dura mater grafts, corneal transplants, reuse of EEG implanted depth electrodes, or other neurosurgical equipment.
EEG shows an evolutive pattern similar to that of the sCJD. Both PSWCs, lateralized or generalized, and slow-wave activity, focal or diffuse, have been described. Obviously, since this form is the result from the direct inoculation of the pathogen, the presence of prevailing EEG abnormalities in a specific cerebral site is more frequent, especially in the initial phases. In two patients contaminated with bifrontal deep electrodes, Wieser et al. (2004) [33] described the evolutionary phases of the EEG pattern during the course of the disease, from incubation to death, consisting of:
-
Regional progressive unspecific slowing of background activity at the site of the infected electrode.
-
Regional sharper slow theta-activity.
-
Occasional frontal delta activity.
-
PSWCs, first intermittent and with regional accentuation, then more stereotyped and finally symmetrical and generalized with prefrontal-midline maximum.
-
Progressive disappearance of periodic complexes with attenuation/suppression of EEG activity.
The best solution to eradicating cross-contamination risk should be to remove and incinerate all materials coming in contact with any tissue other than the skin. However, due to the elevated costs of some invasive instruments, hospitals are generally reluctant to discard and replace them. On the other hand, some expensive instruments cannot endure the hard treatment that has been shown to ensure complete inactivation of prions (e.g., 1-h immersion in bleach or lye followed by 1-h autoclaving at 132 °C). Finally, the accuracy of more recently studied “gentle” methods to inactivate the proteins (e.g., immersion in detergents and proteinases in an alkaline solution or exposure to gaseous H2O2) still lacks verification in multiple independent laboratories.
In this situation, the best solution could be the institution of regional centers with a set of instruments dedicated for use on patients with proven or highly probable CJD. Hospitals should be able to access to these instruments, sharing the cost of their purchase, storage and handling. After their use, the instruments would be returned to the centers and decontaminated using “harsh” methods for durable instruments and one or more “gentle” methods for fragile instruments. Therefore, in order to avoid disease transmission through invasive instruments, patients with proven or highly probable CJD should undergo invasive procedures conducted with appropriate isolation protocols and dedicated instruments. Patients with possible CJD, even if unlikely, should be operated using the same isolation protocols, but with non-dedicated instruments. The instruments should be labeled and quarantined until a correct diagnosis had been established and then either sent to the regional center for dedicated instruments or returned to hospital service, depending on the diagnosis [34].
4 Neurosyphilis
Syphilitic involvement of the CNS can occur early or late in the course of the disease. According to the clinical pictures, Neuro Syphilis (NS) can present under the following forms: asymptomatic NS, syphilitic meningitis, meningovascular NS and parenchymal NS (including General Paresis (GP) and tabes dorsalis). GP has a peak incidence in 10–20 years after syphilitic infection. The clinical spectrum of GP is very wide and may include cognitive impairment, a delusional or apathetic state, dysarthria, myoclonus, intention tremor, seizures, hyperreflexia, and Argyll Robertson pupils [35].
EEG abnormalities depend on the brain structures involved, the rate of progression of the disease and the age of the patient. Epileptic discharges have been described, as seizures are known to occur in GP and meningovascular NS with a frequency from 14 to 60% [36]. Background EEG activity is slowed with bursts of diffuse or predominant anterior slow waves, which increase in relation to the progression of the disease. In some patients, LPDs have also been described, mostly on the fronto-temporal areas, in the absence of lesions detectable with neuroimaging. After pharmacological therapy, the improvement of the clinical conditions is generally observed, not necessarily related to the normalization of EEG abnormalities.
5 HIV-Related CNS Diseases
Human Immunodeficiency Virus (HIV), the viral pathogen responsible for Acquired Immuno Deficiency Syndrome (AIDS), is a neuroinvasive, neurotrophic and neurovirulent virus. Thus, it is able to determine meningoencephalitis independently from the occurrence of secondary opportunistic CNS infection, during the entire progression of the disease. The early CNS infection is usually asymptomatic, but CSF and imaging studies can detect abnormalities even during the “asymptomatic” period that presage later neurological events.
The most common CNS manifestation of HIV is the so-called HIV-Associated Neurocognitive Disorder (HAND), a chronic neurodegenerative condition characterized by cognitive, central motor, and behavioral abnormalities [37].
EEG in the asymptomatic phase of the disease is mostly normal, although a focal or diffuse slowing of background activity may be appreciated. As the disease progresses, slow activity increases, and rarely epileptic discharges may be found.
Furthermore, through opportunistic infections of the CNS, HIV increases the risk of other neurological manifestations. Toxoplasmosis, CNS lymphoma, cryptococcal or tuberculous meningitis and Progressive Multifocal Leukoencephalopathy (PML) are strongly associated with the occurrence of acute or chronic repeated seizures.
Although new-onset seizures are reported in a variable percentage of patients with HIV infection, ranging from 3 to 17% according to different studies, most of patients have unspecific EEG abnormalities, while epileptiform discharges are seen infrequently. This means that EEG is relatively insensitive in the setting of seizures associated with HIV infection. However, patients with more advanced HIV disease and those with neuroimaging abnormalities seem to be more likely to have abnormal EEG [38].
6 Brain Abscess and Subdural Empyema
Brain abscess and subdural empyema are severe life-threatening infectious diseases caused by bacteria, fungi or parasites. Both can result from contiguous site infections (such as skull osteomyelitis, chronic otitis, mastoiditis, sinusitis and dental infections), from the direct access of microorganisms after head trauma or neurosurgical procedures or from metastatic inoculation of the brain from distant extracranial sources (pulmonary, infection, endocarditis). The microbial dissemination tends to provoke multiple cerebral abscesses, while specific localizations are related to the area of primary infection or altered tissue integrity.
Neurological function can be impaired in different ways: direct destruction of nervous tissue, infarction after inflammatory occlusion of veins and arteries, or compression caused by a mass effect. EEG abnormalities vary according to several factors, such as the localization of the abscess, which may be supratentorial or subtentorial, and its severity. Supratentorial abscesses may be characterized by slow-wave focal activity, sometimes intermixed with epileptiform abnormalities (spikes or sharp waves) and associated with a diffuse slowing or attenuation/suppression of background activity on the interested hemisphere (Fig. 39.9). Clinical and/or electrographic seizures are not uncommon [4]. EEG in subtentorial abscesses is often normal, although non-specific slowing of background activity, sometimes with sequences of bilateral, monomorphic delta-theta waves, predominant in the anterior regions can be observed . In subdural empyema, EEG can show a depression of background activity in the infectious site in the late stages, with slow and epileptiform focal abnormalities, indicating a cortical involvement.
7 Parasitic Brain Infections
Parasites that are able to affect the CNS include cestodes [(Taenia solium: neurocysticercosis), Echinococcus granulosus (cerebral cystic echinococcosis), E. multilocularis (cerebral alveolar echinococcosis)], nematodes (Toxocara canis and T. cati neurotoxocariasis), and protozoa [(Toxoplasma gondii: neurotoxoplasmosis), Plasmodium falciparum (cerebral malaria), and Trypanosoma brucei gambiense/rhodesiense (sleeping sickness) or Trypanosoma cruzi (cerebral Chagas disease)]. Adults or larvae of helminths or protozoa enter the CNS and cause meningitis, encephalitis, ventriculitis, myelitis, ischemic stroke, bleeding, venous thrombosis, or cerebral abscess, clinically manifesting as headache, epilepsy, weakness, cognitive decline, impaired consciousness, confusion, coma, or focal neurological deficits [39].
7.1 Neurotoxoplasmosis
Neurotoxoplasmosis is caused by the obligate intracellular protozoal parasite Toxoplasma gondii; the primary hosts are the cats and humans may represent an intermediate host, infected through the oral or the transplacental route.
Common sources of human infection are the consumption of undercooked meat from lamb or pork that contains viable tissue cysts or direct ingestion of oocytes from contaminated soil, water, goat’s milk, or unwashed vegetables. In adults, most T. gondii infections are subclinical, but severe clinical manifestations occur in immune-compromised patients, especially in HIV-infected patients, in whom toxoplasmic encephalitis is particularly common. Involvement of the cerebral gray or white matter in these patients results in encephalitis with cerebral tissue destruction or abscess formation. Furthermore, it can result in a CNS mass lesion, presenting with headache, epilepsy, hemiparesis, psychosis and cognitive dysfunction. The basal ganglia, the thalami and the cortico-medullary junction are the most frequently affected structures [39].
EEG in infants with congenital infections may show several abnormalities: slowing of background activity; sequences of delta slow activity, both focal and diffuse; epileptiform patterns (even hypsarrhythmia). In adults, especially when toxoplasmosis gives rise to a mass lesion, EEG is demonstrative of focal slow abnormalities, sometimes intermixed with sharp waves.
7.2 Neurocysticercosis
Neurocysticercosis is caused by encysted larval stages of the tapeworm Taenia solium. Ingestion of eggs from the feces of tapeworm carriers is followed by their spread to neural, muscular or ocular tissue. Neurocysticercosis is the most common helminthic disease of the CNS, particularly in endemic areas where the parasite exchange between humans and pigs is maintained. Cerebral manifestations of neurocysticercosis include epilepsy (50–70%); headache (40%); hydrocephalus (33%); meningitis; aneurysm formation; stroke. Ischemic stroke occurs in 4–12% of the neurocysticercosis patients. Seizures, predominantly focal, are caused by the perilesional inflammation, stroke, vasculitis, or calcified granulomas [39].
EEG slow and epileptiform abnormalities may be focal or diffuse and their entity depends on the type, site and severity of the cerebral lesion.
7.3 Neurotoxocariasis
The genus Toxocara includes Toxocara canis and Toxocara cati, for which the definitive host is, respectively, the upper digestive tract of dogs and foxes. Human infection is the result of accidental ingestion of embryonated eggs from soils or ingestion of infected raw fruits and vegetables. These embryonated eggs then hatch in the small intestine and release immature larvae, which migrate to the liver via the portal circulation, then lungs and left heart, from where they further disseminate through the systemic circulation, especially to the muscles, optic nerves and, in rare cases, to the central nervous system [38]. Asymptomatic CNS infection is probably common. Clinically evident neurotoxocariasis consists of a wide spectrum of neurological manifestations such as eosinophilic meningitis, encephalitis, meningoencephalitis, ependymitis, arachnoiditis, vasculitis, meningomyelitis, meningoradiculitis, epilepsy, dementia or optic neuritis [39,40,41].
Encephalic/meningeal toxocariasis involvement was associated with a broad range of clinical manifestations including headache, seizures, focal deficits, confusional state and cognitive impairment, with or without fever [41].
EEG abnormalities, slow and epileptiform, depend on the lesion characteristics, especially on their location, that may be multiple, cortical, or subcortical.
7.4 Cerebral Cystic Echinococcosis (Cystic Hydatidosis)
Cerebral cystic echinococcosis is caused by infection with larvae of Echinococcus granulosus, a tapeworm which requires canines as definitive hosts and herbivores or humans as intermediate hosts.
Cystic echinococcosis is a self-limiting but potentially fatal disease, with CNS involvement in only 1–3% of cases [39]. Generally, cysts are located within the parenchyma, the subarachnoid space, or the ventricles. Patients may present with headache, vomiting, hemiparesis and other focal neurological deficits, or seizures. EEG generally shows polymorphic focal slow-wave delta activity or sequences of monomorphic slow rhythms. Rarely, epileptiform discharges can be appreciated.
7.5 Trypanosomiasis
African trypanosomiasis (sleeping sickness) is caused by Trypanosoma brucei, which is transmitted via the bite of the tsetse fly. African trypanosomiasis follows two stages. In stage one the parasite proliferates within the hemolymphatic system, followed by stage two, in which the parasite invades the CNS, resulting in meningoencephalitis with progressive neurological dysfunction and a disrupted sleep–wake pattern. Patients are sleepy by day, but awake and restless by night. Encephalopathy may progress to an apallic syndrome/vegetative state/unresponsive wakefulness [39].
Wake EEG shows a diffuse slowing of background activity with bursts of bisynchronous slow waves recurring every 5–10 s. In more advanced stages of the disease, EEG activity becomes slower, background activity tends to disappear, and epileptic discharges are often observed, associated or not with clinical seizures. Sleep EEG doesn’t show the typical NREM sleep graphoelements and a disorganization of sleep architecture is observed. Sleep is fragmented with frequent arousals, associated with muscle tone, respiratory and cardiac alterations, especially in the deepest sleep stages.
American trypanosomiasis (Chagas disease) is caused by Trypanosoma cruzi. In the acute stages, Chagas disease presents mainly with myocarditis and with megaesophagus and megacolon in the chronic stages. CNS involvement manifests as acute meningitis, which is a hallmark of the disease, or as multifocal, nodular encephalitis due to reactivation, occasionally complicated by a brain abscess. Also, patients with cardio-embolic stroke have been described. Isolated cases with CNS involvement may also present with dementia, confusion, or sensory or motor deficits. Cerebral Chagas disease is frequently associated with HIV [39]. As in other parasitoses, EEG slow and epileptiform abnormalities may be focal or diffuse, and their entity depends on the type, site, and severity of the cerebral lesion.
7.6 Cerebral Malaria
Cerebral malaria is the most severe complication of malignant tertian malaria. It is caused by Plasmodium falciparum, developing within the red blood cells.
It is clinically defined as unarousable coma in the absence of other potential causes but the presence of malaria-infected erythrocytes. Impaired consciousness with coma may occur spontaneously or may develop at least 1 h after termination of a seizure, following 1–3 days of fever, or after correction of hypoglycemia. Some patients develop headache, delirium or coma, following progressive weakness or prostration due to cerebral edema manifesting as brainstem signs or retinal changes, often associated with systemic complications. Some patients develop cortical infarcts or cerebral sinus venous thrombosis. Survivors have an increased risk of long-term complications such as cognitive impairment, speech and language problems, behavioral difficulties, or epilepsy [39].
Even in patients with minimal neurological manifestations, EEG may show mild alterations. When CNS involvement is wide, EEG abnormalities are significant and consist in bilateral theta-delta waves and, rarely, spikes and sharp waves. Electrical and clinical seizures are frequently observed.
References
Bartel P, Schutte CM, Becker P, van der Meyden C. Discrimination between viral and nonviral meningitis by visually analyzed and quantitative electroencephalography. Clin Electroencephalogr. 1999;30:35–8.
VanDemark M. Acute bacterial meningitis: current review and treatment update. Crit Care Nurs Clin North Am. 2013; 25: 351–61.
Heckenberg SG, Brouwer MC, van de Beek D. Bacterial meningitis. Handb Clin Neurol. 2014;121:1361–75.
Galovic M, Schmitz B, Tettenborn B. EEG in inflammatory disorders, cerebrovascular diseases, trauma and migraine. In: Shomer DL, Lopes da Silva FH, editors. Niedermeyer’s electroencephalography: basic principles, clinical applications, and related fields. 7th ed. New York: Oxford University Press; 2018. p. 371–412.
Pardal-Fernández JM, Bengoa M, Carrascosa-Romero MC. Periodic Lateralized Epileptiform Discharges (PLEDs) and pneumococcal meningoencephalitis. Eur J Paediatr Neurol. 2012;16:749–52.
Sáez-Llorens X, Nieto-Guevara J. Brain abscess. Handb Clin Neurol. 2013;112:1127–34.
Konno S, Sugimoto H, Nemoto H, et al. Triphasic waves in a patient with tuberculous meningitis. J Neurol Sci. 2010;291:114–7.
Zunt JR, Baldwin KJ. Chronic and subacute meningitis. Continuum (Minneap Minn). 2012;18:1290–318.
Kalita J, Misra UK. EEG changes in tuberculous meningitis: a clinicoradiological correlation. Electroencephalogr Clin Neurophysiol. 1998;107:39–43.
Patwari AK, Aneja S, Ravi RN, Singhal PK, Arora SK. Convulsions in tuberculous meningitis. J Trop Pediatr. 1996;42:91–7.
Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372.
Pollak L, Klein C, Schiffer J, Flechter S, Rabey J. Electroencephalographic abnormalities in aseptic meningitis and noninfectious headache. A comparative study. Headache. 2001;41:79–83.
Young GB. Encephalopathy of infection and systemic inflammation. J Clin Neurophysiol. 2013;30:454–61.
Sutter R, Kaplan PW, Cervenka MC, et al. Electroencephalography for diagnosis and prognosis of acute encephalitis. Clin Neurophysiol. 2015;126:1524–31.
Jordan B, Kösling S, Emmer A, Koch A, Müller T, Kornhuber M. A study on viral CNS inflammation beyond herpes encephalitis. J Neurovirol. 2016;22:763–73.
Lai CW, Gragasin ME. Electroencephalography in herpes simplex encephalitis. J Neurophysiol. 1988;5:87–103.
Gutierrez J, Issacson RS, Koppel BS. Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol. 2010;52:901–7.
Yemisci M, Gurer G, Saygi S, Ciger A. Generalised periodic epileptiform discharges: clinical features, neuroradiological evaluation and prognosis in 37 adult patients. Seizure. 2003;12:465–72.
Ekmekci O, Karasoy H, Gokcay A, Ulku A. Atypical EEG findings in subacute sclerosing panencephalitis. Clin Neuro- physiol. 2005;116:1762–7.
Demir N, Cokar O, Bolukbasi F, et al. A close look at EEG in subacute sclerosing panencephalitis. J Clin Neurophysiol. 2013;30:348–56.
Takada LT, Geschwind MD. Prion diseases. Semin Neurol. 2013;33:348–56.
Imran M, Mahmood S. An overview of human prion diseases. Virol J. 2011;8:559.
Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132:2659–8.
Wieser HG, Schindler K, Zumsteg D. EEG in Creutzfeldt–Jakob disease. Clin Neurophysiol. 2006;117:935–51.
Marquetand J, Knake S, Strzelczyk A, et al. Periodic EEG patterns in sporadic Creutzfeldt-Jakob-disease can be benzodiazepine-responsive and be difficult to distinguish from non-convulsive status epilepticus. Seizure. 2017;53:47–50.
Hashimoto T, Iwahashi T, Ishii W, Yamamoto K, Ikeda S. EEG–EMG polygraphic study of dystonia and myoclonus in a case of Creutzfeldt–Jakob disease. Epilepsy Behav Case Rep. 2015:4:30–2.
Binelli S, Agazzi P, Canafoglia L, et al. Myoclonus in Creutzfeldt-Jakob disease: polygraphic and video-electroencephalography assessment of 109 patients. Mov Disord. 2010;25:2818–27.
Lapergue B, Dermeret S, Denys V, et al. Sporadic Creutzfeldt-Jacob disease mimicking nonconvulsive status epilepticus. Neurology. 2010;74:1995–9.
Fernandez-Torre JL, Solar DM, Astudillo A, Cereceda R, Acebes A, Calatayud MT. Creutzfeldt-Jakob disease and nonconvulsive status epilepticus: a clinical and electroencephalographic follow-up study. Clin Neurphysiol. 2004;115:316–9.
Kang P, de Bruin GS, Wang LH, et al. Sleep pathology in Creutzfeldt-Jakob disease. J Clin Sleep Med. 2016;12:1033–9.
Terzano MG, Parrino L, Pietrini V, et al. Precocious loss of physiologic sleep in a case of Creutzfeldt Jakob disease: a serial polygraphic study. Sleep. 1995;18:849–58.
World Health Organisation. The revision of the surveillance case definition for variant Creutzfeldt–Jakob Disease (vCJD). Report of a WHO consultation Edinburgh, United Kingdom. 2001. http://whqlibdoc.who.int/hq/2002/WHO_CDS_CSR_EPH_2001.5.pdf.
Wieser HG, Schwarz U, Blättler T, et al. Serial EEG findings in sporadic and iatrogenic CreutzfeldtJakob disease. Clin Neurophysiol. 2004;115:2467–78.
Brown P, Farrell M. A practical approach to avoiding iatrogenic Creutzfeldt-Jakob disease (CJD) from invasive instruments. Infect Control Hosp Epidemiol. 2015;36:844–8.
Chen YY, Zhang YF, Qiu XH, et al. Clinical and laboratory characteristics in patients suffering from general paresis in the modern era. J Neurol Sci. 2015;15:79–83.
Noone ML, Sinha S, Taly AB, Chandrika S. Periodic lateralized epileptiform discharges in neurosyphilis. Epilepsia. 2007;48:390–3.
Singer EJ, Valdes-Sueiras M, Commins D, Levine A. Neurologic presentations of AIDS. Neurol Clin. 2010;28:253–75.
Siddiqi OK, Elafros MA, Sikazwe I, et al. Acute EEG findings in HIV-infected Zambian adults with new-onset seizure. Neurology. 2015;84:1317–22.
Finsterer J, Auer H. Parasitoses of the human central nervous system. J Helminthol. 2013;87:257–70.
Deshayes S, Bonhomme J, de La Blanchardière A. Neurotoxocariasis: a systematic literature review. Infection. 2016;44:565–74.
Sánchez SS, García HH, Nicoletti A. Clinical and magnetic resonance imaging findings of Neurotoxocariasis. Front Neurol. 2018;9:53.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Davassi, C., Pulitano, P., Mecarelli, O. (2019). Cerebral Infectious Diseases. In: Mecarelli, O. (eds) Clinical Electroencephalography. Springer, Cham. https://doi.org/10.1007/978-3-030-04573-9_39
Download citation
DOI: https://doi.org/10.1007/978-3-030-04573-9_39
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04572-2
Online ISBN: 978-3-030-04573-9
eBook Packages: MedicineMedicine (R0)