Abstract
Chronic hepatitis C is one of the major causes of chronic liver disease worldwide with a progressive course that leads to the development of liver cirrhosis, liver failure, and hepatocellular carcinoma if remains untreated. The landscape of chronic hepatitis C treatment has evolved rapidly since 2011 with the arrivals of multiple combinations of direct-acting antiviral (DAA) therapies with the excellent sustained virological response, in the 90%. Despite the outstanding efficacy, antiviral resistance and treatment failure still remain in a small fraction of patients in the current direct-acting antiviral therapies. Recently approved DAAs and future combination therapies focus on targeting the viral resistance and treatment failure using complementary antiviral regimens.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
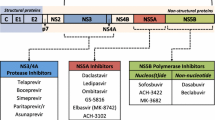
Chronic hepatitis C causes a serious global liver disease including liver cirrhosis and hepatocellular carcinoma. An estimate of 71 million people worldwide are chronically infected with the hepatitis C [1]. In the USA, chronic hepatitis C is the leading cause of liver-related mortality and liver transplantation, surpassing human immunodeficiency (HIV) infection [2]. Since the arrival of high response rate of HCV protease inhibitor agents in 2011, the landscape of hepatitis C treatment has evolved rapidly given the introduction of numerous combination therapies over the past few years and many more are expected in near future. The current gold standard for hepatitis C treatment is interferon-free direct-acting antiviral (DAA) combination therapy with or without ribavirin (RBV). Three major DAA drug classes that interfere with HCV replication and posttranslational processing are as follows [3]:
-
1.
NS3/4A protease inhibitors (“-previrs”) interfere with the proteolytic processing of the HCV polyprotein by blocking the NS3/4A serine protease. Current FDA approvals are simeprevir (approved in 2013), paritaprevir (approved in 2014), and grazoprevir (in 2016). In 2017, two pan-genotypic protease inhibitors, voxilaprevir and glecaprevir, were approved.
-
2.
NS5B polymerase inhibitors (“-buvirs”) target the viral RNA replication by inhibition of the RNA-dependent RNA polymerase (RdRp). Two subgroups are nucleoside/-tide analogue; current agent is sofosbuvir (available in 2013) and non-nucleoside inhibitors; the available agent is dasabuvir (in 2014).
-
3.
NS5A inhibitors (“-asvirs”) affect the viral replication and assembly by blocking the NS5A protein. Current agents are ledipasvir and ombitasvir (approved in 2014), daclatasvir (in 2015), elbasvir and velpatasvir (available in 2016), and pibrentasvir (approved 2017).
A summary of current DAA drug classes is listed in Table 8.1.
The current DAAs broaden the different groups of patients who were not considered for treatment under interferon era such as those with advanced liver disease (Child-Pugh B, C), autoimmune diseases, renal failure, or postorgan transplant patients. In addition, DAA therapies demonstrate high virological response, on average >90%, compared to 40–50% with interferon and ribavirin in genotypes 1 and 4, 60–70% in genotypes 5 and 6, and 80–90% in genotypes 2 and 3 [4].
8.2 Challenges Under DAA Era
Despite the excellent efficacy under current modern all-oral DAA combination therapy, there are still a small percentage, up to about 5%, of patients who fail combination therapy [5]. Failures to DAA regimens are usually related to relapse defined as rebound of HCV RNA to pretreatment levels once therapy is discontinued [6]. There are multiple factors affecting the outcome and hepatitis C resistance including virus-related factors, host-related factors, and drug-related factors [4].
With virus-related factors, hepatitis C has six different genotypes worldwide and the RNA sequence can vary up to 35% between genotypes [4]. Given highly diversified genetic and rapid rate of replication, significant genetic errors occur and affect the treatment outcome. Failure to direct antiviral therapy is often associated with the development of resistance-associated substitutions (RASs). RASs are viral resistance to DAA by selecting viral variants that have amino acid substitution which alters the drug target used in the therapeutic regimen and therefore becomes less susceptible to drug’s inhibitory activity [7, 8] but also affects subsequent salvage treatment [5].
Regarding host-related factors, adherence to therapy with proper administration of the drug at the regular time is a key factor to achieve best drug response. In addition, the presence of fibrosis stages as the presence of cirrhosis is negatively associated with achievement of SVR [9], or presence of comorbidities (i.e., HIV, postorgan transplant status, BMI) also affects treatment outcome [4].
One reasonable approach for retreatment after DAA failure in the presence of RASs is to switch DAA class (due to lack of cross-resistance among different DAA classes) [10, 11]. However, retreatment could be challenging when viruses harbor RASs in multiple DAA targets [12] which is a drug-related factor noticeable in posttreatment RASs. The frequent selected RASs are observed in patients that failed NS3/4A or NS5A inhibitor-containing regimens [13]. NS3/4A RASs seems to be less permanent and could disappear from peripheral blood within weeks to months while NS5A RASs tend to linger for years and impact treatment and retreatment [14,15,16,17]. Quite contrary, even after exposure to NS5B inhibitor-containing DAA regimen, only 1% of NS5B nucleotide RASs are selected [18, 19]. Both baseline and selected RASs have a variable negative impact on virological response depending on prior DAA regimen, whether ribavirin was included, and the duration of treatment as those factors would shape the retreatment regimen. However, the RASs are not always absolute as the same class of drug that has resistance can be used with modification (adding ribavirin or increasing duration) [13]. Therefore, RAS testing alone does not dictate the optimal DAA regimen and should only be done in a certain patient characteristic and certain DAA regimen [13]. In fact, given the high barrier resistance of the latest DAA therapy, especially the aforementioned new pan-genotypic agents, the presence of RASs has no impact on sustained virological response.
8.3 Retreatment of DAA Failure
There has been a great influx of new direct-acting antiviral combination therapies targeting patient groups that failed prior DAAs. Below is the summary of AASLD guideline on retreatment of patients with or without cirrhosis that failed to achieve sustained virological response to prior non-interferon direct-acting antiviral therapy listed according to genotype. The management of decompensated cirrhotic patients will not be discussed here.
8.3.1 Genotype 1
Even in patients that have no prior exposure to NS5A inhibitors, an estimation of 10–15% of genotype 1 patients have detectable NS5A RASs [13] which have variable clinical impacts depending on DAA regimen and patient characteristics [13]. Therefore, testing for RASs prior to treatment decision is only recommended in a selected population [20].
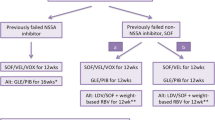
For genotype 1a, non-cirrhotic patients that failed non-NS5A inhibitor, sofosbuvir-containing regimen-experienced, a daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) for 12 weeks was recommended. The overall SVR rate from POLARIS-4 clinical trial was 97% [13]. And for genotype 1b who met similar criteria of prior sofosbuvir-containing regimen failure, sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks was recommended [13]. The regimen of daily fixed dose of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks was for any genotype 1 non-cirrhotic patients that failed non-NS5A inhibitor, sofosbuvir-containing regimen experienced though clinical data was limited [13]. In non-cirrhotic or cirrhotic patients that failed sofosbuvir-containing regimen (excluding simeprevir), a retreatment regimen with a daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) with weight-based ribavirin for 12 weeks was recommended due to high SVR 12 in one clinical trial [13].
A summary of recommended regimens for non-cirrhotic genotype 1 patients that failed non-NS5A inhibitor, sofosbuvir-containing regimen is listed in Table 8.2.
Retreatment recommended regimens for compensated cirrhotic genotype 1 patients that were non-NS5A inhibitor, sofosbuvir-containing regimen failure are similar to the aforementioned non-cirrhotic patients except that ledipasvir (90 mg)/sofosbuvir (400 mg) + weight-based ribavirin were not recommended.
In non-cirrhotic or compensated cirrhotic, genotype 1 patients who were NS5A inhibitor DAA experienced, there were no approved DAA regimens for this failure group until July 2017 when a daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) given for 12 weeks was recommended. The overall SVR12 rate in POLARIS-1 trial was 97%. In addition, the presence of cirrhosis and baseline RASs was not affecting the virological response rate [13]. Followed the sofosbuvir/velpatasvir/voxilaprevir’s approval, in August 2017, another daily fixed-dose combination including glecaprevir (300 mg)/pibrentasvir (120 mg) for 16 weeks was approved for genotype 1-infected patients who were experienced with an NS5A inhibitor but not concomitantly treated with an NS3/4A protease inhibitor. The reported SVR12 rate was 94% in MAGELLAN-1 trial [13].
8.3.2 Genotype 2
In genotype 2 patients with or without compensated cirrhosis who experienced sofosbuvir and ribavirin, two daily fixed-dose combination regimens were recommended. One regimen was sofosbuvir (400 mg)/velpatasvir (100 mg) for 12 weeks. The SVR12 rate in POLARIS-4 clinical trial was 97% though one patient in the trial experienced virologic breakthrough and found to have the presence of NS5B RAS [13]. The other daily fixed-dose regimen recommended was glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks. The SVR12 rate was 99–100% in ENDURANCE-2 and EXPEDITION-1 clinical trials [13].
8.3.3 Genotype 3
Genotype 3-infected patients who were treatment experienced had been the most challenging group for retreatment until the arrival of daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg)/voxilaprevir (100 mg) in July 2017. This regimen was approved for 12 weeks in genotype 3, with or without cirrhotic patients who failed DAA regimen but not an NS5A inhibitor. The SVR12 in POLARIS-4 study was 96%. For genotype 3 cirrhotic patients who failed prior DAA regimen containing an NS5A inhibitor, weight-based ribavirin was added to the sofosbuvir/velpatasvir/voxilaprevir combination for 12 weeks due to high rate of relapse noted in cirrhotic patients [22].
8.3.4 Genotype 4
The same combination of sofosbuvir/velpatasvir/voxilaprevir for 12 weeks was also recommended for genotype 4, DAA-experienced (including NS5A inhibitors) patients with or without cirrhosis. The SVR12 rate was 100% in patients that had a history of treatment failure with DAA regimen not containing an NS5A inhibitor compared to 91% in patients that failed prior DAA containing NS5A inhibitor regimen [22].
8.3.5 Genotypes 5 and 6
Regarding genotype 5 or 6 patients with or without cirrhosis who failed prior DAA regimen including NS5A inhibitors, clinical data are limited though the daily fixed-dose combination of sofosbuvir/velpatasvir/voxilaprevir for 12 weeks was also recommended in this group [22].
8.4 Future DAAs
The next DAA combination therapy coming down the pipeline is the fixed-dose combination of grazoprevir/ruzasvir and uprifosbuvir [23]. This regimen was given either 16 weeks with ribavirin or 24 weeks without ribavirin that has shown a high efficacy in genotype 1 patients with cirrhosis who failed prior ledipasvir/sofosbuvir or elbasvir/grazoprevir with baseline NS5A RASs [5].
8.5 Conclusion
The ultimate goal of hepatitis C elimination is to prevent the progression of liver disease, cirrhosis, and hepatocellular carcinoma. The current direct-acting antiviral therapies offer excellent efficacy and with the future arrivals of combination therapies that target antiviral resistance at multiple sites it would not be too long before hepatitis C is completely eradicated.
References
World Health Organization. Hepatitis C. Media centre. Fact sheet; Oct 2017.
Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8.
Pawlotsky J-M, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. 2007;132(5):1979–98.
Hassany M, Elsharkwawy A. Chapter 6: HCV treatment failure in the era of DAAs. In: Allam N, editor. Advances in treatment of hepatitis C and B, Infectious diseases. London: IntechOpen; 2017. p. 1–15.
Rockstroh J. Global implementation of hepatitis C (HCV) treatment: what are the successes, what are the remaining challenges? EASL; 2017.
Buti M, Esteban R. Management of direct antiviral agent failures. Clin Mol Hepatol. 2016;22:432–8.
Clavel F, Hance AJ. HIV drug resistance. N Engl J Med. 2004;350:1023–35.
Pawlotsky JM. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology. 2016;151:70–86.
Gy I, Dieterich DT. Direct-acting antiviral agents in patients with hepatitis C cirrhosis. Gastroenterol Hepatol. 2012;8:727–65.
Lontok E, Harrington P, Howe A, et al. Hepatitis C virus drug resistance-associated substitutions: state of the art summary. Hepatology. 2015;62:1623–32.
European Association for Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63:199–236.
Di Maio VC, Cento V, Lenci I, Aragri M, Rossi P, Barbaliscia S, Melis M, Verucchi G, Magni CF, Teti E, Bertoli A, Antonucci F, Bellocchi MC, Micheli V, Masetti C, Landonio S, Francioso S, Santopaolo F, Pellicelli AM, Calvaruso V, Gianserra L, Siciliano M, Romagnoli D, Cozzolongo R, Grieco A, Vecchiet J, Morisco F, Merli M, Branccio G, Di Biagio A, Loggi E, Mastroianni CM, Pace Palitti V, Tarquini P, Puoti M, Taliani G, Sarmati L, Picciotto A, Vullo V, Caporaso N, Paoloni M, Pasquazzi C, Rizzardini G, Parruti G, Craxi A, Bbudieri S, Andreoni M, Angelioi M, Perno CF, Ceccherini-Siberstein F, HCV Italian Resistance Network Study Group. Multiclass HCV resistance to direct-acting antiviral failure in real-life patients advocates for tailored second-line therapies. Liver Int. 2017;37:514–28.
Hepatitis C Guidance: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed 14 Nov 2017.
Yoshimi S, Imamura M, Murakami E, et al. Long term persistence of NS5A inhibitor-resistant hepatitis C virus in patients who failed daclatasvir and asunaprevir therapy. J Med Virol. 2015;87:1913–20.
Krishnan P, Tripathi R, Schnell G, et al. Long-term follow-up of treatment-emergent resistance-associated variants in NS3, NS5A and NS5B with paritaprevir/r-, ombitasvir- and dasabuvir-based regimens. J Hepatol. 2015;62:S220.
Dvory-Sobol H, Wyles D, Ouyang W, et al. Long-term persistence of HCV NS5A variants after treatment with NS5A inhibitor ledipasvir. J Hepatol. 2015;62:S221.
Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol. 2016;64:486–504.
Svarovskaia ES, Dvory-Sobol H, Parkin N, Hebner C, Gontcharova V, Martin R, et al. Infrequent development of resistance in genotype 1-6 hepatitis C virus-infected subjects treated with sofosbuvir in phase 2 and 3 clinical trials. Clin Infect Dis. 2014;59(12):1666–74.
Wyles D, Mangia A, Cheng W, Shafran S, Schwabe C, Ouyang W, et al. Long-tern persistence of HCV NS5A resistance associated substitutions after treatment with the HCV NS5A inhibitors, ledipasvir, without sofosbuvir. Antivir Ther. 2018;23(3):229–38.
Zeuzem S, Rockstroh JK, Kwo PY, et al. Predictors of response to grazoprevir/elbasvir among HCV genotype 1 (GT1)-infected patients: integrated analysis of phase 2-3 trials. AASLD, Nov 13–17. San Francisco, CA; 2015.
European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461–511.
Bourliere M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2134–46.
Wedemeyer H, et al. Safety and Efficacy of the fixed-dose combination regimen of MK-3682/grazoprevir/ruzasvir in cirrhotic or non-cirrhotic patients with chronic HCV GT1 infection who previously failed a direct acting antiviral regimen (C-SURGE). 52nd EASL; Amsterdam, Netherlands; Apr 19–23, 2017. Abstract PS-15.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tran, KD., Albarrak, A.A., Tahan, V. (2019). Management of Interferon-Free Direct-Acting HCV Antiviral Therapy Failure. In: Ozaras, R., Salmon-Ceron, D. (eds) Viral Hepatitis: Chronic Hepatitis C. Springer, Cham. https://doi.org/10.1007/978-3-030-03757-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-03757-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-03756-7
Online ISBN: 978-3-030-03757-4
eBook Packages: MedicineMedicine (R0)