Abstract
Acute hepatitis C virus (HCV) infections remain one of the main causes of liver disease worldwide with a varying incidence among different countries. Acute HCV infection classically refers to the first 6 months after exposure to the virus. During recent years, incidence seems to be changing in many countries: lowering rates due to improved case detection and improved prevention of transmission (i.e., unsafe medical procedures) or higher due to emerging of prevalent routes especially among injection drug users and men who have sex with men (MSM). Diagnosis of acute infection could be challenging and is mainly based on serological and molecular assays, since clinical symptoms are not present in most patients. Spontaneous viral clearance occurs in about 25% of individuals, generally in the first 3–6 months of infection due to complex interaction between virus and host, which is only partially understood. HCV reinfections have been described in people who injected drugs (PWID) and MSM who cleared the infection spontaneously or were successfully treated. Treatment of acute HCV is now based on a combination of direct-acting antivirals (DAAs) and it seems promising even if clear recommendations regarding optimal regimen and treatment duration are currently unavailable. Data are lacking also on pre-exposure and post-exposure prophylaxis. Implementation of screening, information campaigns, and community awareness together with early treatment could be the strategies to control the spread of HCV infection.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute HCV
- Injection drug use (IDU)
- People who inject drugs (PWID)
- Men who have sex with men (MSM)
- Direct-acting antivirals (DAAs)
11.1 Epidemiology
11.1.1 Incidence
Hepatitis C virus (HCV) infections remain one of the main causes of chronic liver disease worldwide [1]. According to the last World Health Organization (WHO) Hepatitis Report, in 2015, 71 million people worldwide are living with chronic hepatitis C (Fig. 11.1). Although several studies suggest a global decline in incidence of HCV infections since the second half of the twentieth century, a rise again was noted in the early twenty-first century with still 1.75 million new HCV infections occurring worldwide in 2015 [2]. For example, a decline in acute hepatitis C was described in the United States of America (USA) until the first years of the 2000s [3]. A great contribution to this trend surely came from improvements in injection safety, which has led to a reduction of infections transmitted through unsafe medical procedures [4, 5]. Despite this, during recent years this trend of declining incidence seems to be changing in many countries, related to emerging prevalent routes of transmission (i.e., iv drug use) and improved case detection.
In the USA 3.5 million people are estimated to be living with hepatitis C. The most common risk factor for transmission, responsible for the majority of infected cases, is injection drug use (IDU). Since the late 1980s, acute hepatitis C incidence has declined because of instituted risk-reduction practices among people who injected drugs (PWIDU) [6]. However, in recent years, from 2010 onwards a 2.9-fold rise in acute HCV infections was noted in the USA which is mainly attributed to an increase in IDU in young people, particular in rural area. For example, a large increase in incidence was observed east of the Mississippi river, especially in Kentucky, Tennessee, Virginia, and West Virginia [7]. A similar situation was reported for Massachusetts, Wisconsin, and New York state. Transmission among men having sex with men (MSM) has also become relevant, especially between those coinfected with HIV [8].
Between 7 and 9 million people live with hepatitis C in Latin America and the Caribbean [9]. There is scarce data pertaining to acute hepatitis C infection in South America. A survey conducted in Argentina, Uruguay, and Paraguay found that the most common risk factors for acute hepatitis C were related to nosocomial exposure [10]. In another survey conducted in Brazil, the main risk factors for acute hepatitis C transmission were identified in hospital procedures especially hemodialysis, while it was low IN intravenous drug users [11].
In Europe it is estimated that around 19 million people are living with chronic HCV [12]. Incidence rates fluctuate between 2010 and 2014 but are overall steadily increasing. However, the annual epidemiological report of 2015 by the European Centre of Disease Prevention and Control (ECDC) reports 34,651 new cases in Europe, which is a decrease of 4% compared to the previous year. The reason for this is currently not clear but it might be speculated that it is caused by the new direct-acting antiviral agents (DAAs) being used in the treatment of chronic HCV [13]. It is estimated that 1% of all cases are classified as acute infection, mostly due to IDU and health-care-associated transmission. An increase in acute (re)infection is also observed in MSM, coinfected with HIV living in large European cities [14].
Approximately 210,000 people live with chronic HCV infection in Australia, with an estimated 80% having acquired their infection through IDU [15]. From 1996 to 2001 a steady increase in new infections was registered with the majority of the cases diagnosed in the age group 20–39 years old related to injection drug use [16]. Again sexual transmission was found to be relevant among HIV positive MSM [17].
The estimated prevalence of chronic HCV in the whole Asian continent is 2.8%, accounting over 60% of the estimated cases worldwide. In the Asia-Pacific region prevalence recently has been scaled down, since better seroprevalence studies demonstrate lower rates of active infection in China than previously believed. There are no firm estimates on the incidence of acute HCV around the Asian continent due to the lack of systematic population-based estimates or national surveillance reporting system. Epidemiology is often described by isolated studies or blood bank data [18]. So available data are mostly about chronic HCV infection. China, a large Asian country, has approximately 25–50 million HCVinfected individuals, accounting for 1.8–3.7% of the overall Chinese population [19]. Blood transfusion and IDU are the main routes of transmission. A similar situation was shown for India where transmission is mainly related to unsafe health-care procedures, although it appears to be highly variable according to the geographical site or the population analyzed (0.09–7.89%) [20]. In Thailand there is no national HCV reporting system. Approximately 759,000 individuals are currently anti-HCV-positive and that 357,000 individuals have viremic HCV infection. In Indonesia the prevalence of anti-HCV is estimated to be 0.8% [21] and it is found to be higher in Java then Sumatra, which is probably due to the more dense population in Java [22]. Japan, however, is considered a low prevalence country for HCV with also a low incidence, but also here acute cases among MSM HIV positive patients were recently reported [23]. In Asia, the first study describing a transmission network among HIV-infected MSM was performed in Taiwan, finding an incidence of 9.25 per 1000 PY [24].
In North Africa, accurate assessment of the burden of hepatitis C infection is difficult due to the lack of adequate surveillance data and poor resources for proper data collection and management. Despite the geographic proximity of these countries and longstanding interaction between them, the prevalence and complications of HCV greatly differ between them. According to current estimates, the lowest prevalence of HCV is in Libya (0.9–1.6%) whereas the highest is in adjacent Egypt (12.5–26.6%) [25]. In the latter in 2015, it was estimated that 5.3 million individuals were anti-HCV positive. With this high seroprevalence rate, Egypt ranks among the highest in the world [26]. From 2008 to 2015 a significant reduction in prevalence was observed (from 14.7% to 10.0%) because behavioral changes with respect to promotion and expansion of infection prevention and control programs (including safe injections and blood transfusions) led to a decline in HCV incidence in the younger age groups [27].
Sub-Saharan Africa has a substantial HCV disease burden, but detailed epidemiology is limited due to the scarcity of reliable prevalence data and population-based studies. A meta-analysis published in 2015 suggested an overall HCV a seroprevalence of 3% [28] with substantial variation between regions, related to the quality of serological tests used in various studies, the variability of populations screened (e.g., blood donors vs injecting drug users), and the HIV seroprevalence within the countries [29]. Prevalence was found to be the highest in Central Africa region (4.34%).
11.1.2 Definition of Acute HCV
Acute infection classically refers to the first 6 months after exposure to hepatitis C virus [30], though definition varies, mainly because of the absence of specific markers of acute infection and additionally because most patients experience no symptomatology of an acute infection [31]. As is shown in Fig. 11.2, a diagnosis of acute HCV can also be established by seroconversion to anti-HCV, or with the detection of hepatitis C virus nucleic acid (HCV RNA) in serum/plasma in the absence of the specific Ig antibody (anti-HCV Ig) [32].
Identification of acute HCV infections is important from an individual patient’s point of view since 70–75% of the patients progress to chronic infection with a possible long-term risk of developing cirrhosis, hepatocellular carcinoma, and decompensated liver disease [33]. From a public health point of view, identification of an acute infection is equally important because of the high risk of transmission these patients have in the acute phase of the infection and therefore spreading of the virus among others. Starting therapy during the acute phase is of particular importance since treatment could reduce transmission and could improve clinical outcome and possibly could also be cost-effective compared to deferring treatment until the chronic phase of infection [34].
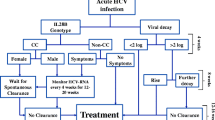
Identifying those who are able to spontaneously clear the infection from those who will develop chronic HCV is important. One proposed way of identification of spontaneous clearance was proposed by Vogel et al. [35] who in an intent-to-treat (ITT) analysis evaluated spontaneous viral clearance rates in 92 HIV-infected patients with acute HCV. Those patients who did not develop a 2 log10 drop in HCV-RNA at week 4 after the diagnosis had an 85% chance of becoming chronically infected with HCV Since this observation has not been firmly established in prospective randomized trials, this 2 log drop rule has not been adopted in the clinical management of acute HCV [36].
11.1.3 Routes of Transmission and Related Risk Group
Although mode of transmission of acute HCV varies among different regions and within countries, injection drug use (IDU) and unsafe health-care practices remain the leading modes of transmission [37]. Areas with high rates of infection related to unsafe health-care transmission are located in the Eastern Mediterranean Region (62.5 per 100,000, usually related to unsafe health-care transmission) [38, 39] and in the European Region (61.8 per 100,000) where IDU accounts for a substantial proportion of the new cases each year [40]. Worldwide, acute HCV infections are more frequent among male young adults, reflecting the demographic profile of injection drug users [41, 42]. According to a study published in 2013, all forms of drug dependence and related disease were highest in men aged 20–29 years and the majority of new infections are related to illicit drug use [43]. The three countries with the largest populations of IDUs living with HCV are China (1.6 million, range 1.1–2.2), Russia (1.3 million, range 0.7–2.3), and the USA (1.5 million, range 1.0–2.2) [44]. In the early 1990s the incidence of HCV was extremely high in people who injected drugs. The implementation of HIV sexual transmission prevention programs, methadone substitution, and needle exchange services reduced transmission rates in many countries [12]. But, the rate of new infection remains high or is again rising since iv drug use still remains the primary mode of transmission due to poor health-care services (i.e., Eastern Europe/Russia) or the availability of cheap drugs (USA) [45].
Transmission related to unsafe medical care practice has diminished over time. Before the advent of blood screening assays before transfusion, in the early 1990s most infections were due to transfusions with infected blood and its derivatives or to unsafe medical and surgical procedures. The introduction of large-scale screening assays reduced the risk to less than 1 per 100,000 units of blood [46]. Although the implementation even though the implementation of blood screening strategies in a lot of countries worldwide is successful, the situation still remains alarming in some resources-limited settings (Africa and Americas WHO areas). According to the WHO database on blood safety in 2012, 39 countries did not perform routine screening of HCV in blood products and 47% of all worldwide blood (derivate) donations were tested in settings without any quality assurance [47]. However, a significant decrease of HCV infection was observed in hemodialysis patients in the USA and Europe [48]. Transmission related to dialysis depends on reuse of lines, hygiene and sterilization of equipment, patient rotation of machines, and the undertaking of rigorous universal precaution rules [49]. In addition to transmission via blood transfusions, unsafe injections using contaminated syringes or needles were the most common way to acquire infection in the past and continue to be responsible for a large amount of nosocomial HCV transmissions, both in developed and developing countries [50]. A case report published in 2016 reported acute hepatitis C infection after accidental needle stick injury with a used blood glucose lancet of a diabetic patient with a chronic hepatitis C infection [51]. Egypt’s mass campaigns for schistosomiasis treatment may represent the world’s largest iatrogenic transmission of HCV [52]. The parenteral anti-schistosomal therapy with tartar emetic injections was administered in a nationwide campaign during the 1950s until the 1980s. Over the 18 years of treatment, 36 million injections were administered to >6 million people, almost all with unsterilized and shared syringes and needles. This intensive transmission established a large reservoir of chronic HCV infection genotype 4, responsible for the high prevalence of HCV infection and current high rates of transmission [53].
Finally, a far less common but incidentally described way of HCV transmission is between hepatitis C and acupuncture. A modest association has been reported in some countries, stressing the importance of exclusively using disposable acupuncture needles [54]. In addition, investigators have reported methods such as tattooing, piercing, coke straw sharing, and cupping as additional agents for HCV transmission [55, 56].
Another possible way of HCV transmission includes sexual contacts with a person infected with HCV. Sexual transmission was long considered an inefficient mode to spread the infection [57]. Indeed, heterosexual transmission of HCV is estimated to occur at a very low rate of 0–0.6% per year between sero-discordant heterosexual partners [58]. Moreover, a lack of association between specific sexual practices and HCV acquisition was observed. This transmission rate is somewhat higher for heterosexuals with multiple partners or in the context of coexistent STI (0.4–1.8% per year) [59]. For men having sex with men (MSM) however, this is totally different as was learned over the past decade. The first reports of acute infection between MSM mostly associated with sexual and non-IDU behavior date from the beginning of the twenty-first century in London [60, 61] and were soon thereafter followed by additional cases from other European countries, Australia, the USA, and Asia [14, 62, 63]. In the Swiss Cohort Study, HCV incidence increased 18-fold in MSM between 1998 and 2011, while it declined in PWID and remained <1 per 100 in heterosexuals [64]. A similar increase was reported in MSM for the Netherlands (32–53 corresponding to a 65.6% increase) [65]. Similar situation is described in Australia and Asia. Sexual behavior is also responsible for the increase in acute cases in the USA. Several factors have been attributed to this increase such as increased cheap air travel, rising popularity of the internet, increase in extreme sexual techniques, and use of drugs and stimulants for sexual pleasure. However, this does not fully explain the finding in a recent meta-analysis that HIV positive MSM have higher rates of acute HCV infection than HIV negative MSM [66]. It has been hypothesized that the destruction of gut-associated lymphoid tissue (GALT) early in an acute HIV infection might be responsible for a lower immunological barrier in the gut during anal intercourse between MSM leading to increased susceptibility for HCV.
Finally, acute hepatitis has been rarely reported during pregnancy [67]. Vertical transmission from mother to child is the primary route of transmission of HCV infection among children. Infection can occur in utero, intrapartum, and postpartum. It is estimated that the prevalence of Ig antibodies to HCV in pregnant women is 0.1–2.4% and the proportion of women with anti-HCV who have active infection with viremia is approximately 70% [68]. A recent meta-analysis by Benova et al. suggested that vertical risk transmission appears to be limited to infants of viremic mothers, ranging from 5.8% to 10.8% depending on their HIV status and HCV viremia [69]. Spontaneous clearance of the HCV virus has been reported in up to 25–30% of HCV-infected children [70]. Diagnosis could be difficult because specific antibodies (not the ones of the mother) appear 12 months after birth [71] and HCV RNA can be detected only 1 or 2 months after birth and has a low sensitivity [72].
In conclusion, the global incidence of HCV infection seems to decrease and the introduction of DAAs could significantly modify the natural history of the disease due to large-scale treatment implementation programs. At this time IDUs and HIV+ MSM share the largest incidence of acute HCV infections. It would be important to identify if screening strategies in particular populations together with therapy of acute infection, as was recently suggested in a publication for the Netherlands, could indeed contribute to a final stop in the spread of the disease [13].
11.1.4 Spontaneous Clearance and Predictive Factors
Spontaneous viral clearance occurs in about 25% of individuals, generally in the first 3–6 months of infection [73], although cases have been reported after 1 year [74]. A recent study by Ragonnet et al. [75] showed a median spontaneous clearance rate of 184 days after diagnoses.
The outcome of acute HCV is affected by complex interactions between virus and host, which is only partially understood. Diversity of HCV viral quasispecies and HCV genotype might be linked with clearance. Host factors such as female sex, initial cellular immune response, virus-specific neutralizing antibodies, and host genetics such as polymorphism of interleukin-28 gene (IL28B) have been associated with clearance of acute infection [76]. In particular individuals with an IL28B type CC genotype are more likely to clear the infection spontaneously than those with a type CT or TT. There is evidence to support that spontaneous viral clearance is better for building up immunological memory compared to chemically induced clearance. For the latter strong host innate and adaptive immune responses are necessary. For example, Thimme et al. demonstrated that in patients spontaneously clearing their acute HCV infection, a strong, broadly specific, and sustained adaptive cellular immune response is necessary [77]. Long-term follow-up of patients spontaneously clearing HCV infection showed detectable HCV-specific memory CD4 and CD8 T cells up to 20 years after resolution [78]. Similarly, in chimpanzees, memory CD4 and CD8 T cells were detectable in the peripheral blood and liver 7 years after clearing an acute HCV infection [79]. Upon re-challenge with HCV, chimpanzees demonstrated no sterilizing immunity but were characterized by a shorter duration of viremia and lower viral loads. Further evidence to support partial memory after clearance of acute HCV comes from epidemiological studies in high-risk injecting drug users (PWID). Several studies have shown that in those PWID spontaneously clearing one infection it was less likely for them to become reinfected compared to HCV-naïve individuals in the same high-risk circumstances, although this has not been shown for MSM [80, 81]. In HIV-infected patients with acute HCV chances of spontaneous viral clearance were lower (around 10–15%) before C ART, but also highest within the first 12 weeks after the diagnosis [82].
11.1.5 Reinfection
In the presence of maintained risk behaviors, HCV reinfections have been described in PWID and MSM who cleared the infection spontaneously or were successfully treated with either interferon-based therapy or new direct-acting antivirals [76]. In a recent meta-analysis of 61 studies, the 5-year risk of HCV reinfection in HIV-infected MSM was as high as 15% and higher than in studies on PWID [83]. A large cohort study of HIV-infected MSM conducted in Western Europe demonstrated a high reinfection incidence among this population (7.3/100 PY) [84]. It has been suggested in some studies that individuals who spontaneously clear their acute infection may be at lower risk of future HCV reinfection when compared to those who are treated and achieve SVR due to a stronger immunological response [80, 85]. This indicates that a degree of protective immunity may develop for some patients. An effective immune response against HCV through multiple infections has been shown in animal models [86]. However, studies among PWID have failed to consistently demonstrate a protective effect [87]. This is highlighted by the observation that MSM can be repeatedly infected by acute HCV with either the same genotype or with a different genotype [84].
Other studies demonstrated no difference in incidence of HCV infection in individuals with no previous infection and in those with previous HCV clearance [88]. However, it has been shown that the chance of spontaneous clearance of HCV in case of a reinfection is higher. This is possibly due to a lower HCV RNA concentration, which is generally more transient and shorter in duration than during initial infection [89].
HCV reinfection is a critical health concern among HIV MSM and PWID after successful treatment or spontaneous clearance of acute HCV infection. Prevention strategies—both treatment and behavioral—are needed to target high-risk groups to reduce morbidity and treatment costs.
11.2 Diagnostics
11.2.1 Definition
According to the latest EASL guidelines published in 2016, diagnosis of acute infection is based on seroconversion to anti-HCV, or with the detection of hepatitis C virus nucleic acid (HCV RNA) or hepatitis C virus core antigen (HCV core) in serum/plasma in the absence of the specific antibody (anti-HCV). The incubation period is within 7–21 days after viral transmission and when HCV RNA becomes detectable in serum (Fig. 11.3). Usually, the qualitative detection of HCV RNA confirms the diagnosis [90]. However, the exact time course of virological and immunological markers of HCV infection is not well defined, particularly during the first months of infection, due to differences in the host immune response, specific properties of the infecting virus, and sensitivity of the assays used to determine the appearance of HCV markers.
Following an initial phase (window period) of 1–2 weeks when no virological or serological markers of infection can be detected, the natural course of HCV infection is characterized by the appearance of HCV RNA and subsequently the HCV core p22 Antigen (Ag) in the absence of an antibody response (Ab) by the host. Seroconversion is defined by the development of a specific antibody response in a previously seronegative person occurring within 4–10 weeks after infection.
In Western countries, acute hepatitis C is most often diagnosed in the setting of post-exposure surveillance, or seroconversion in high-risk individuals (e.g., health-care professionals or injecting drug users) previously known to be seronegative. Seroconversion is most frequently documented in the setting of needle stick injuries, when the exposed individual is followed prospectively, or during surveillance of high-risk individuals [91].
In HIV-infected people, who are usually followed frequently for HIV, an unusual ALAT elevation is a sign of alert in favor of a recent HCV infection. In the other cases diagnosis could be difficult due to different reasons. Firstly, most of the patients do not exhibit symptoms within the first 6 months [92]. The classic clinical picture of an acute hepatitis with jaundice is observed in only 10–15% of the cases. Less commonly, the presentation of acute infection could include constitutional symptoms like nausea, loss of appetite, fatigue, and abdominal pain [31]. A large increase in alanine aminotransferase (ALT), which often can peak around 1000 UI/mL is usually an indicator of an acute hepatic illness. It could however be the presentation of another acute process, such as alcohol-induced hepatitis or a second viral infection superimposed upon a chronic HCV infection [93]. It is therefore likely that these symptomatic patients will be diagnosed as having acute HCV. This is opposite to asymptomatic patients. Since the latter also have a much lower probability to clear the infection spontaneously, they most likely will be diagnosed at some point during their chronic infection [23]. Rates of spontaneous clearance are higher in symptomatic patient and occur usually during the first 3 months after the onset of the symptoms [94].
11.2.2 Assays
Assays detecting HCV infection can be broadly divided into molecular tests, which can directly identify the virus or partial sequences, and include qualitative and quantitative determination of HCV RNA or antigen, and serological tests, which identify antibodies or viral proteins (Table 11.1) [90].
11.2.2.1 Serological Assays
Serological assays are based on the immunoassay principle and are available in the form of rapid diagnostic tests (RDTs) or laboratory-based enzyme immunoassays (EIAs), chemiluminescence immunoassays (CLIAs), and electro-chemiluminescence immunoassays (ECLs). Most of these tests have a sensitivity and specificity close to 100% [95]. False negative results may occur in the setting of severe immunosuppression such as infection with HIV, solid organ transplant recipients, hypo- or a-gammaglobulinemia, or in patients on hemodialysis. False positive results are more likely to occur among populations where the prevalence of hepatitis C is low [96]. Current serological markers cannot reliably distinguish acute hepatitis from an exacerbation of chronic infection [97]. The anti-HCV IgM antibody has not proven useful because they are present in similar level in both acute and chronic disease [93], even if someone suggested that IgM levels are undetectable or present with low steady level in reactivation of chronic hepatitis [98]. In recent years much work has been done to develop a test for measuring avidity of the HCV antibody which was proven in other infections to be a more reliable marker for distinction of a recent viral infection. Avidity increases progressively with time after exposure to immunogen due to rapid mutation in the DNA coding for the variable part of the antibody [99]. It was confirmed that IgG anti-HCV avidity increases with time after primary infection [100] and if detected very early after onset of symptoms it could be useful to distinguish acute process from exacerbation of chronic infection [101]. It has been shown that testing for IgG antibody avidity allows diagnosis in up to 90% of acute hepatitis C [100]. These promising assays still require further evaluation and validation in various clinical settings [102].
11.2.2.2 Molecular Assays
Qualitative and quantitative methods for the detection of HCV RNA—including reverse transcriptase (RT) PCR, branched DNA (bDNA) assays, and transcription-mediated amplification (TMA)—are the most sensitive means to document viremia [91]. Qualitative assays detect the presence of HCV RNA but they cannot measure HCV viral load. The lower limit of detection varies by the method used. The newer real-time PCR detection assays, such as the Cobas TaqMan assay (Roche Diagnostics) and Abbott Real-Time HCV assay (Abbott Laboratories), have very low limits of detection (15 IU/mL and 10 IU/mL, respectively). The Bayer TMA assay (Bayer Laboratories) can detect HCV at limits of 5 IU/mL.
HCV RNA can be quantified by target amplification techniques (competitive PCR or real-time PCR) or signal amplification techniques (branched DNA (bDNA) assay). Five standardized assays are commercially available. Ranges of quantification of the assays refer to the HCV RNA intervals within which quantification is accurate in the corresponding assay [103]. HCV RNA levels falling above the upper limit of quantification of the assay are underestimated and the samples must be retested after 1/10 to 1/100 dilution in order to achieve accurate quantification. It is recommended not to take into account HCV RNA load variations of less than threefold (i.e., ±0.5 log10), which may be related to the intrinsic variability of the assays. In contrast, variations of more than threefold (i.e., 0.5 log10) can reliably be considered to reflect significant differences in HCV RNA load. The newer assays are extremely reliable with a very high sensitivity and specificity (both >95%). However, detecting HCV RNA by PCR is not cost-effective in a low-risk population and is not recommended as a screening test for chronic infection [93].
NAT (nucleic acid amplification technology) test is a molecular technique that detects the presence of viral nucleic acid—DNA or RNA—through targeting a specific segment of the virus, which is then amplified. Amplification step enables the detection of low levels of the virus earlier than the other screening methods, thus narrowing the window period to only 4 days. It is used for screening blood donation to reduce the risk of transfusion-transmitted infections in the recipients [104].
Finally, it has been demonstrated that the HCV core antigen level strongly correlates with the HCV RNA level for various genotypes. Currently, core antigen can be easily detected and quantified by means of a chemiluminescent microparticle immunoassay in the fully automated Architect HCV Core antigen test (Abbott Laboratories) [105]. The Architect HCV Ag assay had a specificity of 100%, with a lower limit of detection of 3 fmol/L corresponding to approximately 1000 IU/mL of HCV RNA [106]. Although a study conducted in the Netherlands suggested that HCV core antigen assay could also be used in the diagnosis of acute HCV infection among coinfected HIV patients [107], as it is a sensitive and specific test, so far it has still not gained a role in the diagnostics of an acute HCV infection.
11.2.3 Assays in Clinical Practice
Since there is no definite test as proof of an acute infection, physicians usually rely on clinical judgments (i.e., the presence of symptoms) in combination with abnormal laboratory results such as elevated aminotransferases, and a positive HCV-RNA or serology in combination with a prior seronegative assay. In specific circumstances, for example in HIV-infected patients with an acute HCV, antibody generation by the host immune response could be initially absent or delayed for months [108]. Also, successful viral clearance may occur in the absence of antibody production or be associated with rapid antibody loss [93]. This suggests that clearance of viremia may be related both to humoral and cellular responses [109]. Among IDUs some studies suggested an average interval from first injection and HCV infection to the development of HCV Ig antibodies of 1 year or even longer. Factors associated with a shorter interval to seroconversion included injecting every day, the shared use of syringes to inject, and the shared use of a cooker or cotton to prepare drugs for injection [110]. In another study conducted on IDUs a delayed and low titers antibody response was observed during acute hepatitis C [111].
Another pitfall in the diagnosis of acute HCV in high-risk HIV+ MSM is the distinction between relapse of infection after DAA therapy or reinfection. When the same genotype again is present, it still might be a reinfection. Only phylogenetic analysis can firmly distinguish between these two entities [84].
11.2.4 Treatment
In a chapter about treatment of acute HCV, a distinction should be made in the era before and after availability of direct-acting antivirals (DAAs). Similar to chronic HCV, DAAs proved to be highly efficacious in the treatment of patients with acute HCV, leading experts in the field to believe there is no distinction anymore between acute and chronic HCV. Therefore, current HCV treatment guidelines recommend a treatment with a combination of two DAAs based on genotype for a total duration of 8 weeks. Patients with acute hepatitis C and HIV coinfection and/or a baseline HCV RNA level >1 million IU/L may need to be treated for 12 weeks with the same combination regimens [32]. However, the question remains if this is totally true. There have been several issues in the pre-DAA era which remain intriguing still today, as we mentioned above. It has been common clinical practice to await spontaneous clearance before considering starting therapy. The publication of the landmark study by Jaeckel et al. [112] clearly demonstrated that treatment of patients with an acute HCV mono-infection with at that time standard interferon-alfa (trice weekly) for 24 weeks resulted in a sustained virological response (i.e., HCV-RNA negativity 24 weeks after discontinuation of therapy) rate of 98%. Then SVR rates achieved by treating the acute stage of the infection were superior to SVR rates achieved with treatment in the chronic phase. Over time, treatment regimen changed similar to those being administered in chronic HCV. For a long time pegylated interferon-alfa for 24 weeks was the recommended course of therapy for acute HCV mono-infection while ribavirin was added in case of coinfection with HIV [113, 114].
With the availability of DAAs for chronic HCV and its demonstration of high efficacy, it is obvious to use DAAs in cases of acute HCV as well. However, to date none of the currently available DAAs formally registered for the treatment of acute HCV. This is because there is insufficient data about efficacy of particular regimens and treatment durations in acute HCV infection mostly due to the low number of patients per study in the field. An overview of studies regarding treatment of acute hepatitis C is provided in Table 11.2. The largest study to date in patients with an acute HCV treated with DAAs is running in the Netherlands. An interim analysis showed a SVR of 98% after a short course of 8 weeks grazoprevir/elbasvir (an NS3/NS5a combination) (personal communication) [120].
Taken together, the use of DAAs in patients with an acute HCV infection seems promising based on a couple of case reports and small cohort studies but clear recommendations regarding optimal regimen and treatment duration are currently unavailable.
11.2.5 Post-exposure Prophylaxis
Ever since the notion of blood-born transmission of viruses through needle stick injuries, HCV (previously called non-A, non-B hepatitis) has been one of the viruses recognized for causing acute hepatitis syndrome. Early on post-exposure prophylaxis with then available interferon-based therapies was tried but shown to be unsuccessful in the case of a Japanese health-care worker who was treated with a short course of interferon after a needle stick injury [121].
In the current DAA era there are no data on the efficacy or cost-effectiveness of antiviral therapy for pre-exposure or post-exposure prophylaxis of HCV infection.
11.2.6 Prevention of Acute Hepatitis
In the era of highly effective DAAs that promises an individual as well as a collective benefit, the World Health Organization (WHO) launched the first global health strategy on the elimination of viral hepatitis as a public health threat by 2030 [122]. Targets by 2030 are to achieve a 90% reduction of new viral hepatitis infections, a 65% reduction of liver-related deaths, and a 90% diagnosis rate of those being infected. Studies have shown that increased capacity for treatment as well as screening is going to be critical in several countries [123]. However, the former is difficult since the high cost of DAAs in many countries continues to lead to prioritization of therapy [124]. In addition, there are several barriers to scaling-up of HCV treatment in high-risk populations, especially in PWID [125]. To reduce HCV incidence in PWID, combining universal introduction of DAAs with increased diagnosing rates and enhanced prevention measures such as opioid substitution treatment and needle and syringe exchange programs, provided in multidisciplinary settings, was shown to be crucial [126].
Strategies to control the spreading of infection are needed also among MSM. The ECDC published a document focusing on communication strategies for the prevention of hepatitis and other STI, which stressed the importance of counseling information and public awareness of the disease [127]. In Amsterdam a local program (MC Free) organized by the Amsterdam Institute for Global Health and Development (AIGHD) developed online and offline interventions to increase knowledge and awareness of HCV infection among MSM population by increasing regular HCV testing and earlier diagnosis and to stimulate risk reduction behavior. The early treatment of acute infection also seems to reduce the incidence of hepatitis C in this population [13].
An increase in appropriate prevention measures such as safe medical procedures, safe sexual practices, and prevention of mother-to-child transmission needs to be encouraged [128]. Improving public health surveillance could help state-run and local programs to identify and address HCV-related health disparities by documenting and monitoring the impact of testing, care, and treatment services [129].
References
Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81.
World Health Organization. Global hepatitis report, 2017. 2017. ISBN: 978-92-4-156545-5.
Williams IT, Bell BP, Kuhnert W, Alter MJ. Incidence and transmission patterns of acute hepatitis C in the United States, 1982–2006. Arch Intern Med. 2011;171:242–8.
Pépin J, Abou Chakra CN, Pépin E, Nault V, Valiquette L. Evolution of the global burden of viral infections from unsafe medical injections, 2000–2010. PLoS One. 2014;9:e99677.
Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300.
Surveillance for Acute Viral Hepatitis—United States. 2005. https://www.cdc.gov/mmwr/preview/mmwrhtml/ss5603a1.htm. Accessed 29 Nov 2017.
Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64:453–8.
Matthews GV, Hellard M, Kaldor J, Lloyd A, Dore GJ. Further evidence of HCV sexual transmission among HIV-positive men who have sex with men: response to Danta et al. AIDS. 2007;21:2112–3.
Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–15.
Dirchwolf M, Marciano S, Mauro E, et al. Clinical epidemiology of acute hepatitis C in South America. J Med Virol. 2017;89:276–83.
Ferreira A de SP, Perez R de M, Ferraz MLG, et al. Acute hepatitis C in Brazil: results of a national survey. J Med Virol. 2011;83:1738–43.
Negro F. Epidemiology of hepatitis C in Europe. Dig Liver Dis. 2014;46(Suppl 5):S158–64.
Boerekamps A, van den Berk G, Lauw F, Leyten EMS, Arends JE. Substantial decline in acute HCV infections among Dutch HIV+MSM after DAA roll out. In: CROI 2017; 2017. http://www.croiconference.org/sessions/substantial-decline-acute-hcv-infections-among-dutch-hivmsm-after-daa-roll-out. Accessed 29 Nov 2017.
van de Laar TJW, Matthews GV, Prins M, Danta M. Acute hepatitis C in HIV-infected men who have sex with men: an emerging sexually transmitted infection. AIDS. 2010;24:1799–812.
Dore GJ, Law M, MacDonald M, Kaldor JM. Epidemiology of hepatitis C virus infection in Australia. J Clin Virol. 2003;26:171–84.
Law MG, Dore GJ, Bath N, et al. Modelling hepatitis C virus incidence, prevalence and long-term sequelae in Australia, 2001. Int J Epidemiol. 2003;32:717–24.
Matthews GV, Pham ST, Hellard M, et al. Patterns and characteristics of hepatitis C transmission clusters among HIV-positive and HIV-negative individuals in the Australian trial in acute hepatitis C. Clin Infect Dis. 2011;52:803–11.
Sievert W, Altraif I, Razavi HA, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(Suppl 2):61–80.
Peng J, Lu Y, Liu W, et al. Genotype distribution and molecular epidemiology of hepatitis C virus in Hubei, Central China. PLoS One. 2015;10:e0137059.
Mukhopadhyaya A. Hepatitis C in India. J Biosci. 2008;33:465–73.
Indonesia Basic Health Research 2010—GHDx. http://ghdx.healthdata.org/record/indonesia-basic-health-research-2010. Accessed 31 Jan 2018.
Mulyanto. Viral hepatitis in Indonesia: past, present, and future. Euroasian J Hepatogastroenterol. 2016;6:65–9.
Ishikane M, Watanabe K, Tsukada K, et al. Acute hepatitis C in HIV-1 infected Japanese cohort: single center retrospective cohort study. PLoS One. 2014;9:e100517.
Sun H-Y, Chang S-Y, Yang Z-Y, et al. Recent hepatitis C virus infections in HIV-infected patients in Taiwan: incidence and risk factors. J Clin Microbiol. 2012;50:781–7.
Riou J, Aït Ahmed M, Blake A, et al. Hepatitis C virus seroprevalence in adults in Africa: a systematic review and meta-analysis. J Viral Hepat. 2016;23:244–55.
Liakina V, Hamid S, Tanaka J, et al. Historical epidemiology of hepatitis C virus (HCV) in select countries—volume 3. J Viral Hepat. 2015;22:4–20.
Kandeel A, Genedy M, El-Refai S, Funk AL, Fontanet A, Talaat M. The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int. 2017;37:45–53.
Rao VB, Johari N, du Cros P, Messina J, Ford N, Cooke GS. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:819–24.
Sonderup MW, Afihene M, Ally R, et al. Hepatitis C in sub-Saharan Africa: the current status and recommendations for achieving elimination by 2030. Lancet Gastroenterol Hepatol. 2017;2:910–9.
European Association for Study of Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–64.
Irving WL, Salmon D, Boucher C, Hoepelman IM. Acute hepatitis C virus infection. Euro Surveill 2008;13(21). pii: 18879.
European Association for Study of Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66:153–94.
Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C. JAMA. 2014;312:631.
Bethea E, Chen Q, Hur C, Chung RT, Chhatwal J. Should we treat acute hepatitis C? A decision and cost-effectiveness analysis. Hepatology. 2018;67:837–46. https://doi.org/10.1002/hep.29611. Published Online 23 Oct 2017.
Vogel M, Boesecke C, Rockstroh JK. Acute hepatitis C infection in HIV-positive patients. Curr Opin Infect Dis. 2011;24:1–6.
The American Association for the Study of Liver Diseases, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C; 2017. p. 247.
Kalafateli M, Buzzetti E, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Pharmacological interventions for acute hepatitis C infection. In: Gurusamy KS, editor. Cochrane database of systematic reviews. Chichester: Wiley; 2017. p. CD011644.
Mohsen A, Bernier A, LeFouler L, et al. Hepatitis C virus acquisition among Egyptians: analysis of a 10-year surveillance of acute hepatitis C. Trop Med Int Heal. 2015;20:89–97.
Khan AJ, Luby SP, Fikree F, et al. Unsafe injections and the transmission of hepatitis B and C in a periurban community in Pakistan. Bull World Health Organ. 2000;78:956–63.
Mitruka K, Tsertsvadze T, et al. Launch of a nationwide hepatitis C elimination program—Georgia, April 2015. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6428a2.htm. Accessed 23 Jan 2018.
Centers for Disease Control and Prevention. Viral hepatitis surveillance, United States, 2015. 2015. p. 25–36.
European Centre for Disease Prevention and Control. Annual epidemiological report 2015—hepatitis C. 2017. p. 23–6.
Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21:34–59.
Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet (London, England). 2011;378:571–83.
Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67.
Pomper GJ, Wu Y, Snyder EL. Risks of transfusion-transmitted infections: 2003. Curr Opin Hematol. 2003;10:412–8.
World Health Organization (WHO). Global status report on blood safety and availability; 2016.
Mangia A, Burra P, Ciancio A, et al. Hepatitis C infection in patients with chronic kidney disease. Int J Artif Organs. 2008;31:15–33.
Engel M, Malta FM, Gomes MM, et al. Acute hepatitis C virus infection assessment among chronic hemodialysis patients in the Southwest Parana State, Brazil. BMC Public Health. 2007;7:50.
Pozzetto B, Memmi M, Garraud O, Roblin X, Berthelot P. Health care-associated hepatitis C virus infection. World J Gastroenterol. 2014;20:17265–78.
Inayat F, Rai AB. Acute hepatitis C virus infection related to capillary blood glucose meter. Saudi Med J. 2016;37:93–5.
Frank C, Mohamed MK, Strickland GT, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet (London, England). 2000;355:887–91.
Elgharably A, Gomaa AI, Crossey MM, Norsworthy PJ, Waked I, Taylor-Robinson SD. Hepatitis C in Egypt—past, present, and future. Int J Gen Med. 2017;10:1–6.
Ernst E, Sherman KJ. Is acupuncture a risk factor for hepatitis? Systematic review of epidemiological studies. J Gastroenterol Hepatol. 2003;18:1231–6.
Moosavy SH, Davoodian P, Nazarnezhad MA, Nejatizaheh A, Eftekhar E, Mahboobi H. Epidemiology, transmission, diagnosis, and outcome of hepatitis C virus infection. Electron Physician. 2017;9:5646–56.
Carney K, Dhalla S, Aytaman A, Tenner CT, Francois F. Association of tattooing and hepatitis C virus infection: a multicenter case-control study. Hepatology. 2013;57:2117–23.
Chan DPC, Sun H-Y, Wong HTH, Lee S-S, Hung C-C. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47–58.
Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881–9.
Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:s99–105.
Ghosn J, Pierre-François S, Thibault V, et al. Acute hepatitis C in HIV-infected men who have sex with men. HIV Med. 2004;5:303–6.
Browne R, Asboe D, Gilleece Y, et al. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sex Transm Infect. 2004;80:326–7.
Urbanus AT, van de Laar TJ, Stolte IG, et al. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS. 2009;23:F1–7.
Gambotti L, Batisse D, Colin-de-Verdiere N, et al. Acute hepatitis C infection in HIV positive men who have sex with men in Paris, France, 2001–2004. Euro Surveill. 2005;10:115–7.
Wandeler G, Gsponer T, Bregenzer A, et al. Hepatitis C virus infections in the Swiss HIV cohort study: a rapidly evolving epidemic. Clin Infect Dis. 2012;55:1408–16.
Hullegie SJ, van den Berk GEL, Leyten EMS, et al. Acute hepatitis C in the Netherlands: characteristics of the epidemic in 2014. Clin Microbiol Infect. 2016;22:209.e1–3.
Yaphe S, Bozinoff N, Kyle R, Shivkumar S, Pai NP, Klein M. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect. 2012;88:558–64.
Prasad M, Honegger J. Hepatitis C virus in pregnancy. Am J Perinatol. 2013;30:149–60.
Roberts EA, Yeung L. Maternal-infant transmission of hepatitis C virus infection. Hepatology. 2002;36:S106–13.
Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765–73.
Yeung LTF, To T, King SM, Roberts EA. Spontaneous clearance of childhood hepatitis C virus infection. J Viral Hepat. 2007;14:797–805.
Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–39.
Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32.
Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41.
Mosley JW, Operskalski EA, Tobler LH, et al. The course of hepatitis C viraemia in transfusion recipients prior to availability of antiviral therapy. J Viral Hepat. 2007;15:120–8.
Ragonnet R, Deuffic-Burban S, Boesecke C, et al. Estimating the time to diagnosis and the chance of spontaneous clearance during acute hepatitis C in HIV-infected individuals. Open Forum Infect Dis. 2016;4:ofw235.
Grebely J, Prins M, Hellard M, et al. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. Lancet Infect Dis. 2012;12:408–14.
Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–406.
Takaki A, Wiese M, Maertens G, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–82.
Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–55.
Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–83.
Grebely J, Conway B, Raffa JD, Lai C, Krajden M, Tyndall MW. Hepatitis C virus reinfection in injection drug users. Hepatology. 2006;44:1139–45.
Schnuriger A, Dominguez S, Guiguet M, et al. Acute hepatitis C in HIV-infected patients: rare spontaneous clearance correlates with weak memory CD4 T-cell responses to hepatitis C virus. AIDS. 2009;23:2079–89.
Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis. 2016;62:683–94.
Ingiliz P, Martin TC, Rodger A, et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J Hepatol. 2017;66:282–7.
Martin TCS, Martin NK, Hickman M, et al. Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS. 2013;27:2551–7.
Lanford RE, Guerra B, Chavez D, et al. Cross-genotype immunity to hepatitis C virus. J Virol. 2004;78:1575–81.
Sacks-Davis R, Grebely J, Dore GJ, et al. Hepatitis C virus reinfection and spontaneous clearance of reinfection—the InC3 study. J Infect Dis. 2015;212:1407–19.
Aitken CK, Lewis J, Tracy SL, et al. High incidence of hepatitis C virus reinfection in a cohort of injecting drug users. Hepatology. 2008;48:1746–52.
Osburn WO, Fisher BE, Dowd KA, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315–24.
Pawlotsky J-M. Use and interpretation of virological tests for hepatitis C. Hepatology. 2002;36:S65–73.
Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet. 2008;372:321–32.
Blackard JT, Shata MT, Shire NJ, Sherman KE. Acute hepatitis C virus infection: a chronic problem. Hepatology. 2007;47:321–31.
Orland J, Wright TL, Cooper S. Acute hepatitis C. Hepatology. 2001;33:321–7.
Santantonio T, Medda E, Ferrari C, et al. Risk factors and outcome among a large patient cohort with community-acquired acute hepatitis C in Italy. Clin Infect Dis. 2006;43:1154–9.
Colin C, Lanoir D, Touzet S, et al. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat. 2001;8:87–95.
Ghany MG, Strader DB, Thomas DL, Seeff LB, American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74.
Craxì A, Licata A. Acute hepatitis C: in search of the optimal approach to cure. Hepatology. 2006;43:221–4.
Sagnelli E, Tonziello G, Pisaturo M, Sagnelli C, Coppola N. Clinical applications of antibody avidity and immunoglobulin M testing in acute HCV infection. Antivir Ther. 2012;17:1453–8.
Inouye S, Hasegawa A, Matsuno S, Katow S. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J Clin Microbiol. 1984;20:525–9.
Coppola N, Pisapia R, Tonziello G, et al. Improvement in the aetiological diagnosis of acute hepatitis C: a diagnostic protocol based on the anti-HCV-IgM titre and IgG avidity index. J Clin Virol. 2009;46:222–9.
Coppola N, Pisapia R, Marrocco C, et al. Anti-HCV IgG avidity index in acute hepatitis C. J Clin Virol. 2007;40:110–5.
Ciotti M, D’Agostini C, Marrone A. Advances in the diagnosis and monitoring of hepatitis C virus infection. Gastroenterol Res. 2013;6:161–70.
Chevaliez S, Pawlotsky J-M. Hepatitis C virus serologic and virologic tests and clinical diagnosis of HCV-related liver disease. Int J Med Sci. 2006;3:35–40.
Hans R, Marwaha N. Nucleic acid testing-benefits and constraints. Asian J Transf Sci. 2014;8:2–3.
Morota K, Fujinami R, Kinukawa H, et al. A new sensitive and automated chemiluminescent microparticle immunoassay for quantitative determination of hepatitis C virus core antigen. J Virol Methods. 2009;157:8–14.
Chevaliez S, Soulier A, Poiteau L, Bouvier-Alias M, Pawlotsky J-M. Clinical utility of hepatitis C virus core antigen quantification in patients with chronic hepatitis C. J Clin Virol. 2014;61:145–8.
Hullegie SJ, GeurtsvanKessel CH, van der Eijk AA, Ramakers C, Rijnders BJA. HCV antigen instead of RNA testing to diagnose acute HCV in patients treated in the Dutch acute HCV in HIV study. J Int AIDS Soc. 2017;20:21621.
Thomson EC, Nastouli E, Main J, et al. Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV. AIDS. 2009;23:89–93.
Eckels DD, Wang H, Bian TH, Tabatabai N, Gill JC. Immunobiology of hepatitis C virus (HCV) infection: the role of CD4 T cells in HCV infection. Immunol Rev. 2000;174:90–7.
Thomas DL, Vlahov D, Solomon L, et al. Correlates of hepatitis C virus infections among injection drug users. Medicine (Baltimore). 1995;74:212–20.
Netski DM, Mosbruger T, Depla E, et al. Humoral immune response in acute hepatitis C virus infection. Clin Infect Dis. 2005;41:667–75.
Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–7.
Arends JE, Lambers FAE, van der Meer JTM, et al. Treatment of acute hepatitis C virus infection in HIV+ patients: Dutch recommendations for management. Neth J Med. 2011;69:43–9.
Boesecke C, van Assen S, Stellbrink H-J, et al. Peginterferon-alfa mono-therapy in the treatment of acute hepatitis C in HIV-infection. J Viral Hepat. 2014;21:780–5.
Naggie S, Marks KM, Hughes M, et al. Sofosbuvir plus ribavirin without interferon for treatment of acute hepatitis C virus infection in HIV-1-infected individuals: SWIFT-C. Clin Infect Dis. 2017;64:1035–42.
Rockstroh JK, Bhagani S, Hyland RH, et al. Ledipasvir-sofosbuvir for 6 weeks to treat acute hepatitis C virus genotype 1 or 4 infection in patients with HIV coinfection: an open-label, single-arm trial. Lancet Gastroenterol Hepatol. 2017;2:347–53.
Deterding K, Spinner CD, Schott E, et al. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect Dis. 2017;17:215–22.
He YL, Yang SJ, Hu CH, et al. Safety and efficacy of sofosbuvir-based treatment of acute hepatitis C in end-stage renal disease patients undergoing haemodialysis. Aliment Pharmacol Ther. 2018;47:526–32.
Brancaccio G, Sorbo MC, Frigeri F, et al. Treatment of acute hepatitis C with ledipasvir and sofosbuvir in patients with hematological malignancies allows early re-start of chemotherapy. Clin Gastroenterol Hepatol. 2018;16:977–8. https://doi.org/10.1016/j.cgh.2017.10.032. Published online 28 Nov 2017.
Dutch Acute HCV in HIV Study (DAHHS-2): Grazoprevir/Elbasvir for acute HCV—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02600325?term=grazoprevir&cond=Acute+Hepatitis+C&rank=2. Accessed 1 Feb 2018.
Nakano Y, Kiyosawa K, Sodeyama T, et al. Acute hepatitis C transmitted by needlestick accident despite short duration interferon treatment. J Gastroenterol Hepatol. 1995;10:609–11.
World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Global Hepatitis Programme Department HIV/AIDS. 2016. p. 56.
Alfaleh FZ, Nugrahini N, Matičič M, et al. Strategies to manage hepatitis C virus infection disease burden—volume 3. J Viral Hepat. 2015;22:42–65.
European Liver Patients Association. The 2016 Hep-CORE report. 2016.
Alavi M, Micallef M, Fortier E, et al. Effect of treatment willingness on specialist assessment and treatment uptake for hepatitis C virus infection among people who use drugs: the ETHOS study. J Viral Hepat. 2015;22:914–25.
Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57:S39–45.
European Centre for Disease Prevention and Control (ECDC). Communication strategies for the prevention of HIV, STI and hepatitis among MSM in Europe. 2016.
World Health Organization. Guidelines for the screening care and treatment of persons with chronic hepatitis C infection. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection. Updated version 2016. p. 140.
Ward JW, Valdiserri RO, Koh HK. Hepatitis C virus prevention, care, and treatment: from policy to practice. Clin Infect Dis. 2012;55:S58–63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Arends, J.E., Leoni, M.C., Salmon-Ceron, D. (2019). Acute Hepatitis C. In: Ozaras, R., Salmon-Ceron, D. (eds) Viral Hepatitis: Chronic Hepatitis C. Springer, Cham. https://doi.org/10.1007/978-3-030-03757-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-03757-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-03756-7
Online ISBN: 978-3-030-03757-4
eBook Packages: MedicineMedicine (R0)