Abstract
The successful surgical treatment of discogenic low back pain (LBP) requires a proper diagnosis and understanding of the pain generators. Over the past decades, many surgical treatment options have been evolved. Spinal fusion is considered as the gold standard for treating disabling LBP after failure of conservative management. Interbody fusion with removal of the diseased painful disc is a proper way to surgically stabilize painful spine unit. Interbody fusion can be done from posterior or anterior approach. Anterior approach can provide greater access for complete disc removal, better sagittal profile correction, and bigger cage application. Anterior lumbar interbody fusion (ALIF) complications are usually approach related, so access surgeon is usually recommended. Posterior approaches are more commonly used. Different posterior fusion techniques are described in the literature with good clinical and radiological results. Motion segment fusion and mobility elimination push the surgeon to think about motion preserving procedure to manage the disabling LBP. This led to the invention of lumbar total disc replacement (TDR) with many successful clinical trials. This chapter will provide cases as example of a patient with degenerative lumbar disc disease (DDD) in different stages from the pathology, and discusses different surgical treatment options. Detailed surgical techniques for preferred treatment for these patients are provided.
Medical knowledge and professional surgical practice in the spine field are continuously changing. Spine surgeons need to rely on their own surgical experience and knowledge in evaluating different surgical techniques. Patient selection and safe spine surgeon are the keys of success in spine procedures.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Chronic low back pain may be anticipated due to facet joints, ligaments, muscles, and intervertebral disc pathologies. Proper history taking, clinical examination, radiological investigations, and diagnostic injection techniques can help to identify the exact cause.
Over the past decades, many surgical options were described as treatment for disabling discogenic low back pain (LBP). Fusion was considered as gold standard with different techniques. Each has its advantages, tips, and tricks. Lumbar interbody fusion can be done through anterior or posterior approaches. Anterior approach can offer better removal of the disc, bigger lordotic cage can be applied, and better correction of sagittal balance. Approach-related complications are limiting cause for wide spread of this technique. Posterior approach is much common technique; most of the surgeons are familiar with such techniques. Currently different minimally invasive posterior or anterior approaches are used to treat LBP after failure of conservative treatment.
Motion preserving technology was the idea behind introduction of lumbar total disc replacement (TDR) aiming to surgically treat LBP with non-inferior results when compared to fusion.
This chapter will present different case scenarios; all have disabling LBP and managed surgically with different techniques. Each case will be managed according to the authors preferred method, tips, and tricks and best available evidence will be formulated in the discussion.
14.1 Case 1
Female patient 80 years old, presented with long-lasting disabling low back pain, not responsive to conservative treatment including medications, physical therapy, and local spinal injections. Her back pain visual analog scale (VAS) rating was eight out of ten and constant. The Oswestry Disability Index (ODI) score was 58%, with moderate lower extremity intermittent claudicating pain. Past medical and surgical histories are unremarkable.
Clinically, the patient has back muscle spasm, low back tenderness, negative straight leg raising test, and limited lumbar spine range of motion.
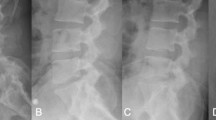
Radiological investigations: Plain radiographs (Fig. 14.1a) showed collapsed L4–L5–S1 disc spaces narrowing with double-level degenerative spondylolisthesis Grade one at the L4–5 and L5–S1 levels. Dynamic lateral flexion-extension lumbosacral x-ray shows fixed spondylolisthesis. Patient global sagittal profile is aligned and accepted. MRI scans show narrow spinal canal at these two levels with severe spondylosis and degeneration at the L4–5 and L5–S1 levels (Fig 14.1b).
(a) Eighty-year-old female patient, her plain X ray lateral, Ap, flexion and extension show grade 1 degenerative spondylolithesis at L4–5–S1 levels. (b) MRI Lumbosacral spine sagittal cuts show modic changes at the disc and spinal canal stenosis at L4–5–S1 with fixed spondylolithesis. (c) Postoperative lateral and A-p plain X ray show the screws in good position and the posterolateral bone graft
Posterior L4–L5–S1 decompression and instrumented posterolateral fusion was done (Fig 14.1c).
14.1.1 Technical Notes
Under spinal anesthesia, a urinary Foley catheter is placed and a preoperative prophylactic antibiotic (usually third-generation cephalosporin) is given. The patient is positioned prone over a four-bolster frame, and all pressure points of the face, torso, and extremities are carefully padded. The patient belly hangs free to limit pressure on the inferior vena cava, which aids in minimizing intraoperative blood loss. Tranexamic acid 10 mg/kg bolus is given and 1 mg/kg infusion till the wound closure. Patient hips kept extended to help in lumbar lordosis restoration. Elastic stocking is applied to both the legs. L4–L5–S1 image-guided transpedicular trajectory identification is used to mark incision site. The skin is sterilized and draped in hospital standard sterile fashion. Posterior midline skin incision is made. Bovie electrocautery is used to subperiosteally dissect the paraspinal muscles so that the facet capsules and transverse processes are exposed bilaterally at each level. Care is taken not to injure the facet capsules that do not need to be fused. Using mammilary process as a clinical intraoperative landmark, for the L4–L5–S1 pedicles identification. This can be confirmed radiologically by image intensifier in two views. Bilateral laminotomies (fenestration) from L4 through S1 were performed with flavectomy and partial facetectomies. Decompressions were done centrally and along the lateral recesses to improve patient intermittent claudication. While decompression, the neural structures with undercutting medial facetectomy, the surgeon should ensure that at least 50% of a functional facet joint complex remains, inclusive of a functioning superior facet and inferior facet. A nerve hook is used to palpate the pedicle and assess nerve root canal after decompression to ensure that adequate bilateral foraminotomies have been performed. Decortications of the transverse processes bilaterally and the facet complexes are done. Locally harvested bone autograft from the lamina and facets is morselized and placed in the lateral gutters to achieve posterolateral fusion (Fig 14.1c). The low back pain experienced by the patient described in the case presentation was related to the mechanical instability at L4 through S1, and posterior instrumentation with a pedicle screw and rod construct provides immediate fixation and symptomatic improvement.
14.2 Case 2
Male patient, 62 years old presented with long-lasting disabling low back pain, mechanical, increase with motion and standing, not responsive to conservative treatment including medications, physical therapy, and local spinal injections. His pain scale rating was nine out of ten. The Oswestry Disability Index (ODI) score was 60%, with lower extremity intermittent claudication pain. The patient is diabetic, hypertensive but controlled past surgical history is unremarkable.
Clinically, the patient has back muscle spasm, low back tenderness, negative straight leg raising test, and limited lumbar spine range of motion.
Radiological investigations: Plain radiographs was done, lumbosacral MRI (Fig. 14.2a) showed collapsed degenerated L4–L5 disc spaces with degenerative spondylolisthesis at the L4–L5. Patient sagittal profile showed kyphotic L4–L5 level with lost lumbar lordosis.
Posterior L4–L5 decompression and instrumented, transforaminal lumbar interbody fusion (TLIF) was the decision. The authors prefer TLIF as there is less traction to the neural structure, no need to do midline generous laminectomy, and more possible to apply the cage anteriorly and correcting the sagittal alignment (Fig. 14.2b).
TLIF is the technique recommended by the authors in revision cases as you can approach disc posterolaterally through the foramen away from the tethered or adherent neural midline structures.
Interbody fusion help to remove the degenerated disc and apply interbody cage with bone graft. Bone fusion is better in interbody fusion because bone graft under compression loads with big surface of cancellous bone anteriorly.
14.2.1 Surgical Technique
Under general or spinal anesthesia, the surgery can be done; we used spinal anesthesia for most of our patients, a urinary Foley catheter is placed and a preoperative prophylactic antibiotic (usually third-generation cephalosporin) is given. The patient is positioned prone over a four-bolster frame, and all pressure points are carefully padded. The patient belly hangs free. Tranexamic acid 10 mg/kg bolus is given and 1 mg/kg infusion till the wound closure. Patient hips kept extended to help in restoring the lumbar lordosis. Elastic stocking is applied to both the legs to decrease the incidence of deep venous thrombosis. To locate the incision over L4–L5 level, image intensifier is used to mark L4–L5 transpedicular line. The skin is sterilized and draped in hospital standard sterile fashion. Posterior midline skin incision is made. Bovie electrocautery is used to subperiosteally dissect the paraspinal muscles so that the facet capsules and transverse processes are exposed bilaterally at each level. Care is taken not to injure the facet capsules that do not need to be fused. While dissection, it is important to preserve supraspinous and interspinous ligaments to decrease incidence of adjacent level degeneration or failure. Using mammilary process as a clinical intraoperative landmarks, for the L4 and 5 pedicles identification. This can be confirmed radiologically using anteroposterior and lateral images by C-arm. We usually do facetectomy and decompression starting from the site of patient complaint. We may decompress (flavectomy and partial facetectomy) the contralateral side if stenotic or symptomatic.
In degenerative scoliosis, we prefer to apply the cage from the concave side. In lost lumbar lordosis, we may do bilateral facetectomies and putting higher cage anteriorly in the intersomatic space then compression at the end of the procedure posteriorly (deformity TLIF), aiming to restore the segmental lordosis.
After facetectomy and decompression, we remove the intervertebral disc and the cartilaginous endplate. Care should be taken while disc preparation for fusion; do not over distract between the screws, to decrease incidence of screw loosening. It is better to use intervertebral distracters gradual increase in reamers and shavers heights to remove disc material.
We used to apply locally harvested bone graft anterior to the cage and inside the cage for fusion.
The highest cage possible to be applied in the anterior part of the disc space is chosen. Care should be taken in revision cases to avoid over distraction and over stretch of tethered neural elements by previous scar tissue and fibrosis.
A nerve and disc hook are used to palpate the pedicle and assess nerve root canal after decompression to ensure that adequate bilateral foraminotomies have been performed. It is important to check there is no any bone fragments around the neural structures. Decortications of the transverse processes bilaterally and the contralateral facet complex if preserved are done. Locally harvested bone autograft from the lamina and facets is morselized and placed in the lateral gutters to achieve posterolateral fusion.
Proper hemostasis and irrigation of the wound by saline. We do not use suction drain. We close the wound in sequential manner.
Patient is allowed to move out of the bed after fully recovering from anesthesia. And usually discharged two days after surgery.
The low back pain experienced by the patient described in the case presentation was related to the mechanical instability and disc degeneration at L4–L5 disc level. Posterior instrumentation with a pedicle screw and rod construct with interbody cage provides immediate fixation and symptomatic improvement. Interbody cage provides maximum translational stability and provides tool to correct sagittal and coronal alignment.
14.3 Case 3
Male patient, 50 years old presented with long-standing disabling low back pain, mild bilateral leg pain, his back pain increase with motion and standing, not responsive to conservative treatment including medications and physical therapy. Local spinal injections give him pain relief for short time then his back pain recurred. His back pain scale rating was eight out of ten. The (ODI) score was 60%, with mild lower extremity intermittent claudication pain. The patient has no medical comorbidities and past surgical history is unremarkable.
Clinically, the patient has back muscle spasm, low back tenderness, negative straight leg raising test, and limited lumbar spine range of motion.
Radiological investigations: Plain radiographs show gas sign in L3–4–5 disc and abnormal upper endplate at L3–4–5 disc (Fig. 14.3a); Lumbosacral MRI (Fig. 14.3b) showed collapsed degenerated L3–L4–L5 disc spaces with degenerative disc disease (DDD). L3–4–5 posterior lumbar interbody fusion (PLIF) was done with mild improvement in the patient back pain, and the patient mild leg pain did not improve. Figure 14.3c with follow-up patient complaint was constant and no improvement with conservative medical and physiotherapy treatment. Local injection selective root block at L5–S1 was given with improvement of patient complaint.
(a) Male patient 50 years old with LBP, Plain X ray Lumbosacral spine show degenerated disc with gas sign at L3–4–5 with abnormal upper endplate. (b) MRI sagittal and axial T2 weighted images showed degenerated disc with no stenosis. (c) Postoperative lumbosacral plain x ray Ap-Lateral show pedicular screws and PLIF cages in place at L3–4–5 with restoration of segmental lordosis and good amount of bone graft in the disc space and posterolateral. (d) Postoperative lumbosacral MRI sagittal and axial T2 cuts show degenerated disc at L5–S1 with stenotic foramen with signs of screw at L3–4–5. (e) immediate postoperative lumbosacral plain X ray Ap-Lateral show L3–S1 pedicular screws and PLIF cages in place at L3–4–5 with posterolateral instrumented fusion at L5–S1. Skin stables and portovac can be seen in the X ray. (f) Immediate postoperative (debridement and change of implant) lumbosacral plain X ray Ap-Lateral show removal of the infected loose s1 screws and left L4 screw. L3–S2 alariliac fixation and PLIF cages in place at L3–4–5 with posterolateral instrumented fusion at L5–S1. Skin stables and portovac can be seen in the X ray. (g) Last lumbosacral plain X ray Ap-Lateral show big anterior L5-S1 cages after ALIF. With posterolateral instrumented fusion at L5–S1. (h) Prior to skin incision, intraoperative image intensifier photo to lumbosacral region lateral projection show trajectory of L5–S1 disc at patient skin. (i) intraoperative image intensifier photo to lumbosacral region AP and lateral projection to confirm proper cage position at L5–S1 disc prior to skin closure
New MRI was done, and narrow L5–S1 foramen in the sagittal cut was diagnosed. Figure 14.3d Extension of fusion to L5–S1 was decided. Intraoperative conjoint nerve root was diagnosed and to do interbody fusion from posterior approach was impossible. L5–S1 extension of fusion with posterolateral fusion using local autograft was done (Fig. 14.3e).
Postoperatively patient improve clinically but one month later he got surgical site infection with significant back pain and leg pain; so, debridement and change implant was the decision, S1 screws was loose so we removed it and the fixation was extended distally to S2-alar-iliac screw as distal anchorage point (Fig. 14.3f).
The patient improved but still has back pain, he scheduled for anterior lumbar interbody fusion (ALIF) L5–S1 with improvement of his back and leg pain (Fig. 14.3g).
ALIF at L5–S1 was the decision taken by the authors. The authors prefer ALIF as there is less traction to the neural structure, bigger diameter, and higher anterior cages can be applied under better physiological condition for fusion. Indirect decompression of the neural foramen can be achieved by restoring disc height and indirectly increasing the foraminal height. Better removal of the entire disc can be achieved from anterior approach. Higher cage in the disc can better restore segmental lumbar lordosis.
Absolute contraindications for ALIF approach are significantly calcified aorta or prior reconstructive vascular surgery. Relative contraindications are morbid obesity, previous intraabdominal surgery, history of severe pelvic inflammatory disease (PID), and previous anterior spinal surgery.
14.3.1 Surgical Technique
PLIF is a posterior procedure for interbody fusion, while patient in prone positioning, after general or spinal anesthesia, paravertebral muscle dissection with exposure of the spinous processes and lamina over the appropriate levels midline laminotomy (medial to the facet) were done, and the dural sleeve was retracted to approach disc. After disc space preparation, we apply bullet-like cage in the disc space, unlike TLIF more retraction to the neural midline structure is needed.
In this case scenario, we will discuss the anterior L5–S1 lumbar interbody fusion (ALIF), which allows better disc space clearance. Under general anesthesia, a urinary Foley silicone catheter was placed under complete aseptic condition, and a preoperative prophylactic antibiotic was delayed till harvesting culture from L5–S1 disc space. (Usually third-generation cephalosporin is given.) The patient is positioned supine, and all pressure points are carefully padded. Elastic stocking to the patient legs was applied. Tranexamic acid 10 mg/kg bolus is given and 1 mg/kg infusion till the wound closure. Sterilization and draping according to the hospital standards. The L5/S1disc space is reached through a Pfannenstiel surgical incision. An 8-cm transverse skin incision is performed after localization of the corridor by image intensifier in A-P and lateral view (Fig. 14.3h). A right retroperitoneal route to L5/S1 was performed through the linea alba of the rectus sheath. The incidence of retrograde ejaculation is much lower when a retroperitoneal approach is used compared to when a transperitoneal approach is used. Beside we keep the transperitoneal approach for revision anterior surgery. The arcuate line is identified and released. The peritoneum and ureter are retracted from lateral to medial with a special soft-tissue retractor. The psoas muscle is identified and great vessels. L5–S1 approached in between the bifurcation. The median sacral vessels are ligated and dissected, and it is important to use only the bipolar cautery at the anterior surface of L5–S1 disc and by soft gauze dissect the superior hypogastric plexus to avoid retrograde ejaculation. Once the anterior circumference of L5/S1 intervertebral disc is exposed, interbody fusion using titanium mesh cage filed with autologous iliac crest bone graft is performed after debridement of the disc material. Assessment of cage location was done clincally and by the use of image intensifier (Fig. 14.3i). Hemostasis then closure of wound in layers.
14.4 Case 4
Female patient, 46 years old presented with long-lasting disabling low back pain, not responsive to conservative treatment including medications, physical therapy, and local spinal injections. But she showed improvement on disc analgesia test. Visual analog pain scale rating was 8 out of 10 and constant. The Oswestry Disability Index (ODI) score was 60%, with no lower extremity radicular or intermittent claudication pain. The patient is medically free.
Clinically, the patient has back muscle spasm, low back tenderness, negative straight leg raising test, and limited lumbar spine range of motion.
Radiological investigations: Plain radiographs showed narrow L4–5, L5–S1 disc space. Lumbosacral MRI showed collapsed degenerated L4–L5 disc spaces with degenerative hyperintenisty zone sign and degenerated L5–S1 disc space with Modic changes. Patient sagittal profile showed lost lower lumbar lordosis. Axial MRI show good facet state and can help to assess vascular topography of the patient (Fig. 14.4a, b).
(a) 46 years old female with LBP plain X ray Ap/Lateral show degenarative narrow disc at L4–5–S1 MRI show degenerated disc with modic changes at L5–S1 and Hyperintenisty zone at L4–5. (b) Axial cut at L4–5–S1 disc level show good facet state, show vascular topography at L4–5–S1. (c) Postoperative plain X ray showing double level SB Chareté III Lumbar disc prosthesis in place and mobile in dynamic view. (d) Intraoperative image intensifier to assess proper centralization of the disc prosthesis in AP view and as posterior as possible in lateral view
L4–5, L5–S1 lumbar disc arthroplasty was the decision taken by the author to remove the degenerated disc and restore the mobility at the index levels (Fig. 14.4c). The authors prefer this method of surgical treatment for her disabling LBP because the patient is young with stable spine and the posterior facets are not degenerated.
The surgery was uneventful done through an open retroperitoneal approach. At 6 years of follow-up, the patient was free of back pain and visual analog scale and Oswestry Disability Index score are improved significantly. She required no pain medication and was able to return to her household activity.
14.4.1 Surgical Technique
Under general anesthesia, the patient is positioned supine, urinary catheter was applied. It is important to allow hyperextension of the lumbar region using the bed controls. Also, the plane of the anterior superior iliac spines must be horizontally kept parallel to the floor; the patient must be centralized properly and this is checked by image intensifier prior to sterilization and draping. The pressure points should be properly protected. Both lower limbs should be kept in neutral position, with mild knee flexion. We used to have the help of access surgeon to help us while manipulating great vessels; a paramedial, left-sided retroperitoneal approach is preferred.
At lower abdomen 12-cm left-sided paramedical vertical incision is made. After the skin incision, subcutaneous tissue is dissected by electrocautery to expose the anterior rectus sheath. Identification of the midline fascial raphe of the rectus, and the left rectus is mobilized to the left side with careful attention to avoid injury to the inferior epigastric vessels. Blunt finger dissection is then used to develop the retroperitoneal plane and release of the arcuate ligament. The peritoneum is bluntly dissected and retracted off the abdominal wall. The ureter should be identified and retracted with the peritoneum. The genitofemoral nerve can usually be identified on the surface of the psoas muscle. It is mandatory to identify and ligate the iliolumbar vein, to allow mobilization of the great vessels to the right side, when operating L4–5 level. We usually start by operating L5–S1 level, it is approached between the bifurcation of the great vessels. Care should be taken while dissecting the anterior surface of L5–S1 to avoid injury of the superior hypogastric plexus injury. It is recommended to use blunt dissection at that level and no use of monopolar electrocautery to avoid hypogastric plexus injury. Identification and transection after ligation of median sacral artery is necessary. Median sacral artery helps to identify midline. Table-held retractors can be used on each side of the spine to create a safe corridor to approach L5–S1 disc. L5–S1 disc midline is confirmed by image intensifier.
An anterior annulotomy is completed using a knife, centered on the midline mark. Symmetrical complete discectomy and cartilaginous endplate removal are carried out. For lumbar spinal arthroplasty, it is mandatory to fully restore the disc height to recreate segmental mobility.
Special intervertebral distractor used to aid in remobilization of the degenerated segment, and release of the posterior longitudinal ligament may be needed in collapsed segments.
Trial implants can be inserted into the disc space. The trial implants should be centered on the previously marked midline. The operating table break at the level of the operated disc space can aid in placing the trial implant by putting the patient torso into extension and thereby opening the anterior disk space. Once a trial implant of the appropriate size is placed, an AP image is taken to verify the central midline position and neutral rotation of the implant in the midline. The SB Charité III device was applied at L5–S1 then at L4–5 disc. It has spikes on the endplate surface for initial stabilization. Anteroposterior and Lateral fluoroscopic images should be made frequently to verify the trajectory angle and the proper location of the prosthesis (Fig. 14.4d). The device should be inserted exactly in the midline in AP view, and as far posterior as possible in lateral view.
14.5 Discussion
The degenerative process in the functional spine unit usually starts from the intervertebral discs leading to pathological changes in the surrounding ligaments, vertebral bodies, and posterior bulging of posterior disc surface, narrowing of the central spinal canal, decrease disc height, buckling of the ligamentum flavum, osteophyte formation, and sliding of vertebral bodies [1].
This degenerative cascade leads to different clinical symptoms like lower back pain (LBP), radicular pain, and neurogenic intermittent claudication. There are many conservative treatment options, including physiotherapy and steroid local injections. Where conservative management fails, surgery may be indicated. Currently, surgical management involves decompression only, which may potentially destabilize the spine, or decompression followed by fusion to prevent further destabilization [2].
Spinal functional unit fusion is the mainstay to treat degenerative disc conditions, many spinal fusion techniques were described in the literatures. Posterolateral intertransverse fusion (PLF) is a useful procedure with good fusion rates for most degenerative disc conditions. Interbody fusion techniques involve placement of an implant (cage, spacer, or structural graft) within the intervertebral space after complete discectomy and endplate preparation; interbody fusion can be done either anterior lumbar interbody fusion (ALIF) or posterior lumbar interbody fusion, (PLIF) approaches were described to restore the structural integrity of degenerated functional spine unit or degenerated unstable discs. There is no solid evidence showing that the functional outcome scores are better after anterior column support than other posterior fusion techniques [3, 4].
In 1982, Harms from Germany introduced transforaminal lumbar interbody fusion (TLIF) to avoid neural structures, dural manipulation, and subsequent epidural fibrosis [3].
To have 360-degree solid fusion, PLF can be combined with posterior interbody fusion techniques to circumferentially stabilize the relevant unstable segment, but it is unclear whether this improves the fusion rates [4, 5].
Different techniques are described in the literature: posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF), minimally invasive TLIF (MI-TLIF), oblique lumbar interbody fusion/anterior to psoas (OLIF/ATP), anterior lumbar interbody fusion (ALIF), lateral lumbar interbody fusion (LLIF), and extreme lateral interbody fusion (X-LIF). There is no clear definitive evidence for one approach being superior to another in terms of fusion or clinical outcomes. Minimally invasive surgical techniques (MIS) mean to achieve the target with less collateral damage. MIS has the advantage of less hospital stay, less blood loss, and early reintegration in the daily life activity [6].
The aims for all types of lumbar fusion are the same: reduction of the back pain with or without radicular symptoms by neural decompression, restabilizing the degenerated segment, and the restoring intervertebral disc height (in interbody fusion techniques) [7].
Postoperative we examine patient radiologically for spinal fusion criteria were based on presence of bonebridge between the endplates of both adjacent vertebral bodies inside and/or outside the cage, no osteolysis around the implant or loosening, no angular motion in lateral flexion-extension radiographs more than 3°, and no cage migration [8, 9].
In recently published meta-analysis to compare posterolateral fusion versus interbody fusion for degenerative spondylolisthesis, they concluded that, according to the available literature there was no statistically significant difference in functional and operative outcomes following fusion alone versus with interbody [10].
There is a large volume of literature detailing clinical and radiological outcomes following specific interbody fusion techniques; however, little are class 1 data comparing the various available techniques. Surgeons who have been trained in one specific fusion technique will favor that technique; most of the literature uniformly supports the concept of interbody fusion techniques over on-lay posterior spinal fusion for sagittal and coronal plane deformities corrections [11].
When comparing clinical outcomes, of anterior and posterior surgeries, most studies showed that clinical outcomes in ALIF were similar to TLIF [12].
Available data in the literature suggests that anterior fusion techniques are superior to posterior in terms of disc space height restoration, lumbar lordosis, and spinal deformity correction, and that clinical, functional outcome, and fusion rates were similar to those in posterior fusion techniques [13].
The advent of total disc replacement (TDR) offered a new alternative that aims to restore and maintain mobility and stability at the diseased functional spine unit. The history of lumbar disc arthroplasty began in the 1950s with insertion of stainless steel metal spheres into the disc space after discectomy by Fernström [14].
The lumbar disc replacement prosthesis continued to evolve during the 1970s as a partial replacement by nuclear implant, going from a metal sphere to a silicone rubber prosthesis to a polyurethane injectant. It was in 1984 that the modern lumbar disc prosthesis began to be developed. In Charité hospital in Berlin, Dr. Büttner-Janz and Dr. Schellnack designed a modular three-piece TDR device known as the SB Charité, and it was implanted in September 1984. The prosthesis was evolved and finally SB Charité III (DePuy Spine, Raynham, MA) came to use worldwide in 1987. Many prosthesis are now available in the market with different designs.
TDR is done through anterior approach, it is contraindicated in active systemic infection or infection localized to the site of implantation, osteopenia or osteoporosis, bony lumbar spinal stenosis, allergy or sensitivity to implant materials, isolated radicular compression syndromes, especially due to disc herniation, pars defect, and spondylolisthesis.
There is reported long-term follow-up data for lumbar spine arthroplasty with greater than an 80% excellent or good long-term (more than 10 years) clinical outcomes. TDR was done using the SB Charité lumbar disc prosthesis. This study concluded that 90% of prostheses were still mobile at a mean of 13.2 years of follow-up. The reoperation rate was 7.5%, and the rate of adjacent-level degenerative pathology was found to be 2.8%. Almost 90% of the patients returned to their work after the procedure. As for complications, David reported a 4.6% rate of posterior facet joint arthrosis, a 2.8% rate of implant subsidence, and less than a 2% rate of core subluxation [15].
According to the FDA IDE prospective randomized studies for TDR, comparing lumbar spine arthroplasty to fusion, TDR-produced clinical and functional outcomes are at least similar or non-inferior to those of fusion and superior to fusion results on some measures [16, 17].
14.6 Conclusion
Different treatment modalities are available to manage patients with disabling low back pain due to degenerative disc disease. It should be stressed that appropriate patient selection and meticulous surgical technique are paramount in obtaining successful clinical outcomes.
Surgeon experience and following good level of evidence help to improve patient’s health-related quality of life.
References
Leone A, Guglielmi G, Cassar-Pullicino VN, Bonomo L. Lumbar intervertebral instability: a review. Radiology. 2007;245:62–77.
Koreckij TD, Fischgrund JS. Degenerative spondylolisthesis. J Spinal Disord Tech. 2015;28:236–41. https://doi.org/10.1097/BSD.0000000000000298.
Humphreys SC, Hodges SD, Patwardhan AG, Eck JC, Murphy RB, Covington LA. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976). 2001;26(5):567–71.
Christensen FB, Hansen ES, Eiskjaer SP, et al. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine (Phila Pa 1976). 2002;27(23):2674–83.
Fritzell P, Hagg O, Wessberg P, Nordwall A, Swedish Lumbar Spine Study Group. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine (Phila Pa 1976). 2002;27(11):1131–41.
Mobbs RJ, Sivabalan P, Li J. Minimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologies. J Clin Neurosci. 2012;19:829–35.
Harms JG, Jeszenszky D. Die posteriore, lumbale, interkorporelle fusion in unilateraler transforaminaler technik. Oper Orthop Traumatol. 1998;10:90–102.
Abbushi A, Cabraja M, Thomale UW, Woiciechowsky C, Kroppenstedt SN. The influence of cage positioning and cage type on cage migration and fusion rates in patients with monosegmental posterior lumbar interbody fusion and posterior fixation. Eur Spine J. 2009;18:1621–8.
McAfee PC, DeVine JG, Chaput CD, Prybis BG, Fedder IL, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976). 2005;30:S60–5.
Cambpell RC, Mobbs RJ, Lu VM, Xu J, Rao PJ, Phan K. Posterolateral fusion versus interbody fusion for degenerative spondylolisthesis systematic review and metaanalysis. Global Spine J. 2017;7(5):482–90.
Kowalski RJ, Ferrara LA, Benzel EC. Biomechanics of bone fusion. Neurosurg Focus. 2001;10:E2.
Kim JS, Kang BU, Lee SH, et al. Mini-transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion augmented by percutaneous pedicle screw fixation: a comparison of surgical outcomes in adult low-grade isthmic spondylolisthesis. J Spinal Disord Tech. 2009;22:114–21.
Faundez AA, Schwender JD, Safriel Y, et al. Clinical and radiological outcome of anterior-posterior fusion versus transforaminal lumbar interbody fusion for symptomatic disc degeneration: a retrospective comparative study of 133 patients. Eur Spine J. 2009;18:203–11.
Fernström U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand Suppl. 1966;357:154–9.
David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the Charité artificial disc in 106 patients. Spine. 2007;32:661–6.
Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion. Part I: evaluation of clinical outcomes. Spine. 2005;30:1565–75.
Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32:1155–62.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Elhawary, Y., Khattab, M.F. (2020). Surgical Disc Replacement and Fusion Techniques. In: Manfrè, L., Van Goethem, J. (eds) The Disc and Degenerative Disc Disease. New Procedures in Spinal Interventional Neuroradiology. Springer, Cham. https://doi.org/10.1007/978-3-030-03715-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-03715-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-03714-7
Online ISBN: 978-3-030-03715-4
eBook Packages: MedicineMedicine (R0)