Abstract
The Northwest of Argentina has numerous sugar industries, and about 99.5% of the total sugar production is concentrated in the provinces of Tucumán, Salta, and Jujuy. Integrated sugarcane factories (sugar mills coupled to a bioethanol distillery) are common in our country. The liquid fraction generated from rectification and distillation operations of bioethanol, known as vinasse, is not itself a hazardous waste, but because of its complex composition, it is considered potentially dangerous.
The province of Tucumán has achieved a substantial improvement with regard to the vinasse spills onto watercourses near sugar-alcohol industries. However, millions of liters of effluent are annually accumulated in open-pit pools to the limit of their capacity, threatening the sustainability of the ecosystem.
A variety of physicochemical and microbiological technologies is continually evaluated to mitigate the environmental impact of vinasse. However, microbiological conditioning of distillery effluents has been reported as effective and eco-friendly. Particularly, fungal technology has made great contributions to the treatment of vinasse since fungi possess an extraordinary ability to digest complex waste materials. Additionally, fungus-based processes offer the possibility to obtain value-added products from waste materials. The present chapter provides an overview of the current scope of fungal technology applied for treatment of vinasse. Additionally, the first advances on the potential of an autochthonous fungal strain to degrade a local sugarcane vinasse sample are discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Sugarcane Vinasse: Physicochemical Characteristics and Conventional Management Practices
The Northwest of Argentina has numerous sugar industries, and about 99.5% of the total sugar production is concentrated in the provinces of Tucumán, Salta, and Jujuy. Most of the sugar mills coexist with distilleries (integrated sugarcane factories) and utilize the molasses or cane juice from cane sugar manufacturing as the starting material for bioethanol production. The liquid fraction generated from rectification and distillation operations of bioethanol, known as vinasse, is a dark-colored acid effluent with an unpleasant odor. Vinasse itself is not a hazardous waste (EPA 2016), but because of its complex composition, it is considered potentially dangerous. Certainly, distilleries are one of the most polluting industries since more than 80% of its raw materials are converted into waste and discharged into the water bodies and soils, which induce undesirable physical, chemical, and biological changes to the environment (Colin et al. 2018).
Vinasse composition depends on the feedstock used, e.g., sugarcane in tropical areas (Argentina, Brazil, Colombia) and corn in areas such as the United States, the European Union, and China. The fermentation/distillation conditions also affect the final effluent composition (Vohra et al. 2014; Moran-Salazar et al. 2016). However, all vinasses share some similar properties such as a low pH (3.5–5.0), high concentrations of salts, and a high chemical oxygen demand (COD) and biochemical oxygen demand (BOD) (Rodrigues Reis and Hu 2017). According to available literature, lactic acid, acetic acid, glycerol, and ethanol are the major organic components of sugarcane vinasse (España-Gamboa et al. 2011). Potassium and recalcitrant colored organic compounds (phenolics from the feedstock and melanoidins) are also predominant constituents (España-Gamboa et al. 2011; Rajasundari and Murugesan 2011).
A traditional plant generates between 10 and 15 L of vinasse for each liter of alcohol produced (Cavalett et al. 2012). It has been projected a bioethanol production of 1.12 billion liters in Argentina for 2018 (Kenneth 2017), which is equivalent to an annual production of several billions of liters of vinasse. Although our province (Tucumán, Argentine) has achieved a substantial improvement with regard to the vinasse spills onto watercourses, several liters of effluent are accumulated in open-pit pools to the limit of their capacity. Hence, the accumulation of large volumes of vinasse remains a key bottleneck for production of bioethanol. Table 9.1 summarizes some potential effects of the distillery wastes on the environment, according to EPA Guidelines (2004).
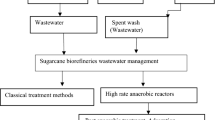
A variety of physicochemical and microbiological technologies is continually evaluated to mitigate the environmental impact of vinasse. A schematic view of the conventional practices for vinasse management applied in the Northwest of Argentina is shown in Fig. 9.1. Agricultural use of vinasse is a frequent practice especially in Latin American countries since this residue contains all essential elements for crop growth (Fig. 9.1a). In order to avoid detrimental effects on the soil, this practice requires the previous dilution of the effluent, which has as the main disadvantage the consumption of water. However, the use of vinasse in a crude form has been documented as a promising practice for the recovery of unproductive saline soils (Mornadini and Quaia 2013). Alternatively, there exist different concentration methods (natural, thermal or by using membranes) to reduce the vinasse volume before effluent can be used as fertilizer (Fig. 9.1b).
Biological conditioning of vinasse has been reported as a most eco-friendly practice (Colin et al. 2016; Pires et al. 2016; Nair and Taherzadeh 2016). Certain studies have highlighted a possible recycling of the treated microbiologically effluent as irrigation water or fertilizer (Nitayavardhana et al. 2013; Colin et al. 2018) (Fig. 9.1c). Because of its high organic load, vinasse can also constitute a cheap feedstock to obtain a variety of bioproducts by microbial fermentation (Fig. 9.1c).
Among microbial technologies, fungal technology has made great contributions to the treatment of the distillery effluents since fungi possess an extraordinary ability to digest complex waste materials. In fact, it have been documented a diversity of fungal strains with the ability to improve the vinasse quality (Romanholo Ferreira et al. 2011; Baldiris et al. 2012; Tapia-Tusell et al. 2015; España-Gamboa et al. 2015, 2017; Vilar et al. 2018).
The next sections provide an overview of the biological treatments of sugarcane vinasse carried out by filamentous fungi. In addition, the first advances of the potential of an indigenous fungus strain to degrade a local sugar cane vinasse sample are reported.
9.2 Fungal Technology Applied to the Treatment of Distillery Effluents: Basic Principles
Biological treatment of distillery waste often faces problems in meeting wastewater discharge standards. Hence, a variety of processes, both aerobic and anaerobic, has been examined in order to improve the quality of these effluents. Whether a treatment for the valorization of an effluent by microbial pathways is the most appropriate not only depends on the possibility to reduce its toxicity but also on the possibility to obtain valuable bioproducts with market acceptance (Colin et al. 2016). For example, anaerobic digestion of vinasse has been described as an effective and economical option as it eliminates the COD and it converts vinasse into methane (biogas), a green and renewable fuel readily usable in ethanol facilities. This process is carried out in four stages (hydrolysis, acidogenesis, acetogenesis, and methanogenesis), and each stage involves different bacterial genera (Kondusamy and Kalamdhad 2014). However, the presence of recalcitrant compounds in vinasse (phenols, melanoidins, sugar decomposition products, etc.) can be toxic to many anaerobic microorganisms and inhibit degradation processes.
Fungus-based aerobic processes have made great contributions to the development of efficient systems for treatment of complex waste products. Fungi possess the ability to produce high amounts of non-specific enzymes, which allows degradation of recalcitrant pollutants. They are also able to survive under acidic conditions that enable treatment of effluents such as vinasse. Filamentous fungi are easy to culture and handle at laboratory scale. Additionally, processes involving fungi can be easily scaled up and require less complicated processes for biomass separation than bacterial processes (Ferreira 2015). Unlike conventional treatments with bacteria that generate large amounts of low-value biomass, fungal biomass has a great biotechnological potential since it has a relatively high protein content (around 50% of dry weight). Therefore, the purpose of many fungal processes is to produce biomass for food/feed applications. In respect to this, Sartori et al. (2015) reported on four fungal species (Pleurotus sajor-caju, Pleurotus albidus, Pleurotus ostreatus, and Pleurotus flabellatus), whose dry mycelia obtained from vinasse are a possible dietary supplement in the fish industry. Similarly, Nair and Taherzadeh (2016) state that the use of fungal biomass cultured in vinasse can produce additional revenues to ethanol plants, because they provide feed and feed supplements for the production of livestock.
In addition to the biomass itself, certain high value-added fungal products could be recovered from vinasse-based supernatants. For example, Aguiar et al. (2010) reported the production of lignocellulolytic enzymes (endoglucanase, exoglucanase, laccase, manganese peroxidase, and peroxidase) by fungal strains such as Pleurotus sajor-caju CCB020, Pleurotus ostreatus, and Trichoderma reesei, grown on sugarcane bagasse combined with vinasse. These enzymes can be purified and used in diverse biotechnological applications, e.g., in the degradation of lignocellulosic waste materials, the most abundant biomass on the earth’s surface. Oliveira et al. (2012) demonstrated the feasibility of Aspergillus CCT 4355 to produce organic acids using sugarcane bagasse impregnated with vinasse, as an alternative method to conventional submerged processes. More recently, Dorla et al. (2014) confirmed the production of carotenoid pigments by six different fungal strains (Phycomyces blakesleeanus, Mucor circinelloides, Gibberella fujikuroi, Neurospora crassa, Aspergillus carbonarius, and Aspergillus giganteus) cultured on vinasse agar. These natural pigments are found in fruits and vegetables, and they are known for their antioxidant and pro-vitamin A activity, properties that can be used to fight certain diseases and/or to delay their appearance.
Despite these advantages, the morphological complexity of filamentous fungi is a limiting factor in fungal fermentations. When fungi give rise to dispersed mycelial growth, common operational difficulties such as an increase in the medium viscosity, a decrease in oxygen supply, or wrapping of the dispersed filaments around agitators can occur. The morphological factor is a common bottleneck in industrial processes. Fungal development in pelleted form often eliminates these problems and facilitates downstream processing (Colin et al. 2013). Fungal pellets are spherical, ellipsoidal, or oval masses of intertwined hyphae whose size usually range from several hundreds of μm to several mm (Fig. 9.2). The characteristics of these pellets, including form, size, and fluffiness, depend on each particular strain as well as the culture conditions (Colin et al. 2013; Espinosa-Ortiz et al. 2016).
Certain pollutants have demonstrated higher removal efficiency with fungal pellets instead of other forms of fungal growth (Espinosa-Ortiz et al. 2016). However, one of the main issues in reactors operated with fungal pellets consists of maintaining the viability of these structures during long-term reactor processes. According to their size or fluffiness, large pellet structures can suffer diffusional limitation of oxygen and other nutrients and induce autolytic processes within big pellets. Therefore, small fluffy pellets are often assumed to be more suitable for high-performance fermentations (Colin et al. 2013). Despite these limitations, there exist a considerable number of studies in the literature concerning the treatment of sugarcane vinasse using filamentous fungi as biological agents. Some of the most valuable reports are considered in the following section.
9.3 Fungus-Treated Sugarcane Vinasse
Biodegradation studies conducted by Baldiris et al. (2012) evaluated the physicochemical changes in a vinasse sample after inoculation with Trichoderma viride or Schizophyllum commune, strains isolated at the Universidad Santiago de Cali (Colombia) from contaminated trees. Bioprocesses were conducted by inoculating 8 g of the mycelium of each strain in 100 mL of 5% (v/v) vinasse. Samples were incubated at 25 °C either under static conditions during 120 days or on an orbital shaker (150 rpm) for 20 days. In each case, vinasse samples without inoculation were used as abiotic controls. At the end of the incubation periods, physicochemical parameters of importance were determined in biologically treated supernatants and in abiotic controls. Microbial inoculations demonstrated neutralization of the vinasse, with an average pH of 7.2 ± 0.5. In addition, COD and BOD removal of the treated effluent ranged from 70–75% to 71–78%, respectively. Interestingly, the authors remarked that parameters such as phenol, potassium, and total nitrogen content showed higher removal percentages under static conditions than under shaking conditions, regardless of the strain assayed.
Tapia-Tussell et al. (2015) evaluated the expression of the laccase gene in the presence of phenolic compounds (guaiacol, ferulic acid, and vanillic acid) by Trametes hirsuta Bm-2, isolated in Yucatán, Mexico. The authors demonstrated that all compounds assayed had an inductive effect on the level of laccase activity compared with the control, and guaiacol showed the highest induction. Additionally, the effectiveness of T. hirsuta Bm-2 to remove phenolic compounds from different sugarcane vinasse concentrations (5%, 10%, 15%, and 20%) was examined using a final working volume of 100 mL. The total phenolic content and the percentage of discoloration were measured during 196 h of incubation on an orbital shaker (130 rpm) at 28 ± 2 °C. The highest discoloration (72.23%) was obtained in 10% (v/v) vinasse after 192 h of cultivation; higher vinasse concentrations showed less discoloration power for the strain. An increase in removal of phenol compounds coincided with a higher laccase activity, suggesting the potential of this strain to decolorize effluents.
España-Gamboa et al. (2015) assayed phenol and color removal of vinasse in an air-pulsed bioreactor using Trametes versicolor, a strain collection, provided by the Department of Chemical Engineering (Universidad Autónoma de Barcelona, Barcelona, Spain). This white-rot fungus is an excellent laccase producer, and it is, therefore, used in the degradation of a wide variety of recalcitrant pollutants, including textile dyes and phenols. T. versicolor pellets with a diameter of approximately 3 mm obtained in a 2% (w/v) malt extract medium were inoculated in vinasse diluted with distilled water at a 1:10 ratio (500 mL final working volume). The bioreactor was subsequently operated in the continuous mode for a period of 25 days, removing 80% of total phenols, 17% of the color, and until 60% of the COD. The highest laccase activity was recorded at the fifth day of the batch experiment (428 U/L), and a significant decrease was observed on day 7. Vinasse neutralization was detected because of the metabolism of this strain, with a pH value that ranged from 4.5 to 6.9. Recently, the authors provided new information regarding treatment of vinasse by T. versicolor, using a fluidized bed bioreactor (FBR) coupled to a UASB (upflow anaerobic sludge blanket) reactor under non-sterile conditions (España-Gamboa et al. 2017). Continuous operation of the FBR alone was carried out for 26 days, with a successful reduction in phenolic compounds (67%) and COD (38%). However, when they coupled the FBR to a UASB reactor, the authors observed a better effluent quality. They also reported methane content of 74% with a yield of 0.18 m3 CH4/kg COD removed. The authors concluded that coupling of an FBR to a UASB reactor could be a promising environmental technology for the treatment of vinasse. However, cost-benefit analysis is required in order to determine the feasibility of this process at full scale.
Diverse organisms can be used to predict the effectiveness of biological treatments applied to industrial waste materials. Romanholo Ferreira et al. (2011), for example, predicted the potential effects of sugarcane vinasse treated with a lignocellulolytic fungus, Pleurotus sajor-caju CCB020, using aquatic organisms as toxicological indicators. Firstly, Erlenmeyer flasks containing 100 mL of vinasse with an adjusted pH of 6.0 were inoculated with a standardized amount of fungal mycelium. Then, flasks were incubated for 15 days on an orbital shaker (180 rpm) at 28 ± 2 °C under no light conditions. A vinasse sample without inoculation was used as an abiotic control. Besides, a vinasse sample inoculated with the same amount of heat-killed biomass was used to rule out effluent discoloration produced by adsorption processes. At the end of the incubation period, physicochemical parameters were analyzed both in raw and microbiologically treated vinasse. Analysis of the effluent incubated with P. sajor-caju CCB020 revealed a significant reduction in parameters such as phenols (98.2%), total suspended solids (97.6%), phosphate (85.5%), calcium (69.5%), COD (82.7%), and BOD (75.3%). Other parameters analyzed showed less reduction compared to the crude effluent: reducing sugars (34.2%), magnesium (24.2%), sulfate (19.3%), conductivity (18.1%), total dissolved solids (9.0%), and potassium (7.9%). Secondly, populations of four aquatic organisms (Pseudokirchneriella subcapitata, Daphnia magna, Daphnia similis, and Hydra attenuata) were exposed to different concentrations of crude and treated vinasse in order to determine the following parameters: growth inhibition concentration (IC50), lethal concentration (LC50), and effective concentration (EC50), all of which correspond to the concentration that causes effect in 50% of the organisms. As expected, the toxicity levels largely depended on the vinasse concentration assayed, and D. magna was the least sensitive organism. Only treated vinasse without dilution was extremely toxic to the organisms assayed. However, crude vinasse at concentrations of 12.5%, 25%, and 50% were also highly toxic to the indicator organisms. The authors concluded that P. sajor-caju CCB020 could be applied to bioprocessing of vinasse treatment, displaying an effective reduction in the effluent toxicity and improvement in the vinasse physicochemical properties.
More recently, Vilar et al. (2018) evaluated the effectiveness of a combined process for sugarcane vinasse degradation, consisting of a biological treatment with P. sajor-caju CCB020 followed by electrochemical oxidation (EO). After 15 days of fermentation, the biological treatment displayed an efficient decrease in color (97%), COD (50.6%), and the reduction in the total organic matter was 57.3%. However, the authors observed that biological treatment followed by EO, using a Ti/(RuO2) 0.7 (IrO2)0.1(Sb2O3) 0.2 anode, increased removal of color, and COD until 100%, and 61%, respectively. In order to evaluate the effectiveness of the biological and combined treatment for vinasse degradation, the authors carried out toxicity assays using lettuce seeds (Lactuca sativa) and Raphidocelis subcapitata microalgae as bioindicators. After exposure of the toxicological indicators to the different concentrations of treated and untreated vinasse, root relative growth (RRG), germination index (GI), and EC50 were determined for L. sativa, and IC50 was calculated for R. subcapitata. Based on this assay, they concluded that R. subcapitata was more susceptible than L. sativa to the toxic effect of vinasse. However, application of the combined treatment allowed a significant reduction in vinasse toxicity, with 0% lethality for either bioindicator.
9.4 Autochthonous Vinasse-Degrading Fungus
Previously, our work team conducted experiments using native actinobacteria from our collection of cultures for vinasse treatment (Colin et al. 2016; Aparicio et al. 2017). Four actinobacteria tested (Streptomyces sp. MC1, Streptomyces sp. A5, Streptomyces sp. M7, and Amycolatopsis tucumanensis DSM 45259T) were effective in terms of BOD removal from sugarcane vinasse samples. However, bioprocesses required a prior vinasse neutralization to start the bacterial development.
Recently, our research group has isolated an autochthonous fungal strain from a soil from the Northwest of Argentina, named strain V1, which was able to grow under acid conditions (Del Gobbo et al. 2017). Determination of the vinasse-degrading potential of this strain could be an essential step to predict the efficiency of an eventual biological treatment of distillery effluents. Thereby, the current section presents the first advances in the treatment of a sugarcane vinasse sample, by using this autochthonous fungus.
9.4.1 Experimental Procedures
An economical solid medium consisting of vinasse only supplemented with 2% agar (agar-vinasse medium, AVM) was successfully used for the production of spores (Fig. 9.3). After 5–7 days of incubation at 30 °C, spores harvested from AVM were inoculated in Erlenmeyer flasks containing 30% (v/v) vinasse without the addition of exogenous nutrients.
To optimize the biodegradation process, two experimental factors, spore concentration (A) and incubation time (B), were evaluated using two levels for each factor (Table 9.2). Consequently, four different bioprocess experiments were conducted in orbital shakers (150 rpm at 30 °C) at a final working volume of 100 mL and without pH control. Non-inoculated vinasse samples were used as abiotic controls.
At the end of the incubation periods, biomass was harvested by centrifugation (10,000 g for 10 min at 4 °C) and washed with distilled water. Then, dry weight was determined at 80 °C in aluminum foil cups until constant weight (Colin et al. 2017). pH and BOD values of the supernatants inoculated with the strain V1 were determined according to the Standard Methods for the Examination of Water and Wastewater (A.P.H.A. AWWA et al. 2012) and compared with the respective abiotic controls. A two-factor full factorial experiment was designed in order to evaluate the main effects of the A and B factors and their interactions on biomass, pH, and BOD removal.
9.4.2 Data Interpretation and Statistical Approaches
Although the addition of carbon or nitrogen sources to vinasse is almost always necessary to promote microbial growth, the strain V1 was able to develop without the addition of exogenous nutrients. Fungal growth in the four bioprocesses was consistent with pelleted development, forming very small spherical masses that showed an approximate diameter of 100 μm (Fig. 9.4).
Experimental results are shown in Table 9.3. Biomass was higher than 1 g/L under all assay conditions. However, regardless of the initial inoculum concentration, processes conducted for 144 h practically doubled the biomass concentration compared to that for 72 h.
At the end of each experiment, the pH of the abiotic controls remained unchanged compared to the initial value (Table 9.3). Bioprocesses conducted for 72 h did not show any significant increase in the pH with respect to the abiotic controls. However, the pH significantly increased in bioprocesses conducted for 144 h, with values close to neutral (Table 9.3). Tiso and Schechte (2015) reported that aerobic neutralization of the pH is caused by the formation of ammonia in a process known as ammonification or mineralization. This process involves protein deamination and decomposition of other nitrogen compounds.
BOD removal mediated by strain V1 was also analyzed. Determinations after 72 h of incubation revealed a removal close to 50%, regardless of the initial spore concentration (Table 9.3). However, bioprocesses conducted for 144 h exhibited a much higher BOD removal, close to 80% (Table 9.3). It is key to remark that the application of Pearson’s correlation coefficient confirmed a positive linear association between the biomass concentration and BOD removal (r = 0.97; p = 0.0001). From these results, it can be inferred that BOD removal would be associated with the growth of the strain V1.
The estimated effects analysis of the factor A and B for biomass, pH, and BOD removal is given in Table 9.4. From this analysis, it can be concluded that the biomass concentration was only affected by the incubation time (factor B). Consequently, spore concentration (factor A) and AB interaction had no significant effect on the growth of the strain V1 (p > 0.05). Similarly, the ability of the strain to neutralize vinasse and diminish the BOD was associated with factor B, while factor A and the AB interaction only had negligible effects on these parameters (p > 0.05).
9.5 Concluding Remarks
As mentioned throughout this chapter, fungus-based aerobic processes not only offer a possibility to reduce vinasse toxicity but also to obtain value-added bioproducts. Accordingly, distillery effluents could be considered valuable industrial by-products that can substitute expensive synthetic substrates conventionally used in microbial fermentations.
The present chapter also provides the first advances on the potential of an autochthonous fungus to degrade sugarcane vinasse, at laboratory scale. All conditions assayed presented a satisfactory biomass yield, which could have a valuable biotechnological potential for food purposes. In addition, it has been demonstrated the ability of this strain to neutralize vinasse and remove close to 80% of the BOD after only 144 h of treatment. Currently, complementary studies are being conducted in order to validate our findings at the pilot scale.
References
Aguiar MM, Romanholo Ferreira LF, Rosim Monteriro RT (2010) Use of vinasse and sugarcane bagasse for the production of enzymes by lignocellulolytic fungi. Braz Arch Biol Technol 53(5):1245–1254
Aparicio JD, Benimeli CS, Almeida CA, Polti MA, Colin VL (2017) Integral use of sugarcane vinasse for biomass production of Actinobacteria and their potential application in soil remediation technologies. Chemosphere 181:478–484
APHA, AWWA, WEF (2012) Standard methods for examination of water and wastewater, 22nd edn. American Public Health Association, 1360 pp, Washington, DC. isbn:978-087553-013-0
Baldiris LF, López E, Castillo J, Caicedo LD (2012) Biodegradation of sugarcane vinasse with strains of the fungus Schyzophyllum commune and Trichoderma viride. Ingenium 6(14):39–46
Cavalett O, Junqueira TL, Dias MOS, Jesus CDF, Mantelatto PE, Cunha MP (2012) Environmental and economic assessment of sugarcane first generation biorefineries in Brazil. Agric Ecosyst Environ 14:399–410
Colin VL, Baigorí MD, Pera LM (2013) Tailoring fungal morphology of Aspergillus niger MYA 135 by altering the hyphal morphology and the conidia adhesion capacity: biotechnological applications. AMB Express 3:1–13
Colin VL, Juárez Cortes AA, Aparicio JD, Amoroso MJ (2016) Potential application of a bioemulsifier-producing actinobacterium for treatment of vinasse. Chemosphere 144:842–847
Colin VL, Bourguignon N, Gómez JS, Gianni K, Ferrero MA, Amoroso MJ (2017) Production of surface-active compounds by a hydrocarbon-degrading actinobacterium: presumptive relationship with lipase activity. Water Air Soil Pollut 228:454
Colin VL, Rulli MM, Del Gobbo LM, Amoroso MJ (2018) Potential application of an indigenous actinobacterium to remove heavy metals from sugarcane vinasse. In: Donati RE (ed) Heavy metals in the environments: microorganisms and bioremediation. CRC Press Taylor & Francis Group, Boca Raton, pp 47–58
Del Gobbo LM, Rulli MM, Cuozzo SA, Colin VL (2017) Selection of vinasse-degrading microorganisms. Congreso Argentino de Microbiologia General, SAMIGE-2017
Dorla E, Caro Y, Fouillaud M, Dufossé L, Laurent P (2014) Valorization of vinasse, a rum distillery effluent, by the production of carotenoid pigments using filamentous fungi. Conference: 7th International Congress of Pigments in Food – New technologies towards health, through colors, Novara, Italy, June 18–21 2013, At Novara, Italy
EPA (2016) Resource Conservation and Recovery Act (RCRA) regulations. http://www.epa.gov/rcra/resource-conservation-and-recovery-act
EPA Guidelines for Wineries and Distilleries (2004) South Australian wine industry association environment committee. ISBN 1-876562-66-8
España-Gamboa E, Mijangos-Cortes J, Barahona-Perez L, Dominguez-Maldonado J, Hernandez-Zarate G, Alzate-Gaviria L (2011) Vinasse: characterization and treatments. Waste Manag Res 29(12):1235–1250
España-Gamboa E, Vicent T, Font X, Mijangos-Cortés J, Canto-Canché B, Alzate-Gaviria L (2015) Penhol and color removal in hydrous ethanol vinasse in an air-pulsed bioreactor using Trametes versicolor. J Biochem Technol 6(3):982–986
España-Gamboa E, Vicent T, Font X, Dominguez-Maldonado J, Canto-Canché B, Alzate-Gaviria L (2017) Pretreatment of vinasse from the sugar reninery industry under non-sterile conditions by Trametes versicolor in a fluidized bed bioreactor and its effect when coupled to an UASB reactor. J Biol Eng 11:6
Espinosa-Ortiz EJ, Rene ER, Pakshirajan K, van Hullebusch ED, Lens PNL (2016) Fungal pellets reactor in wastewater treatment: applications and perspectives. Chem Eng J 283:553–571
Ferreira JA (2015) Integration of filamentous fungi in ethanol dry-mill biorefinery. Ph.D. thesis, Swedish Centre for Resource Recovery, University of Borås, Borås, Sweden
Kenneth J (2017) Global agricultural information network. USDA United States Department of Agriculture Foreign Agricultural Service. pp 1–18
Kharayat Y (2012) Distillery wastewater: bioremediation approaches. J Integr Environ Sci 9(2):69–91
Kondusamy D, Kalamdhad A (2014) Pre-treatment and anaerobic digestion of food waste for high rate methane production – a review. J Environ Chem Eng 2:1821–1830
Moran-Salazar RG, Sanchez-Lizarraga AL, Rodriguez-Campos J, Davila-Vazquez G, Marino-Marmolejo EN, Dendooven L, Contreras-Ramos SM (2016) Utilization of vinasses as soil amendment: consequences and perspectives. SpringerPlus 5(1):1007
Mornadini M, Quaia E (2013) Alternativas para el aprovechamiento de la vinaza como subproducto de la actividad sucroalcoholera. Avanc Agroindustrial 34(2). EEAO-DOSSIER, pp 40–43
Nair RB, Taherzadeh MJ (2016) Valorization of sugar-to-ethanol process waste vinasse: a novel biorefinery approach using edible ascomycetes filamentous fungi. Bioresour Technol 221:469–476
Nitayavardhana S, Issarapayup K, Pavasant P, Khanal SK (2013) Production of protein-rich fungi biomass in an airlift bioreactor using vinasse as substrate. Bioresour Technol 113:301–306
Oliveira AF, Matos VC, Bastos RG (2012) Cultivation of Aspergillus niger on sugarcane bagasse with vinasse. Biosci J 28(6):889–894
Pires JF, Ferreira GMR, Reis KC, Schwan RF, Silva CF (2016) Mixed yeasts inocula for simultaneous production of SCP and treatment of vinasse to reduce soil and fresh water pollution. J Environ Manag 182:455–463
Rajasundari K, Murugesan R (2011) Decolourization of distillery waste water – role of microbes and their potential oxidative enzymes (review). J Appl Environ Biol Sci 1:54–68
Rodrigues Reis CE, Hu B (2017) Vinasse from sugarcane ethanol production: better treatment or better utilization? Front Energy Res 5:7
Romanholo Ferreira LF, Aguiar MM, Mesias TG, Pompeu GB, Queijeiro Lopez AM, Silva DP, Monteiro RT (2011) Evaluation of sugar-cane vinasse treated with Pleurotus sajor-caju utilizing aquatic organisms as toxicological indicators. Ecotoxicol Environ Saf 74:132–137
Sartori SB, Ferreira LFR, Messias TG, Souza G, Pompeu GB, Monteiro RTR (2015) Pleurotus biomass production on vinasse and its potential use for aquaculture feed. Mycology 6(1):28–34
Tapia-Tusell R, Pérez-Brito D, Torres-Calzada C, Cortéz-Velázquez A, Alzate-Gaviria L, Chablé-Villacís R, Solís-Pereira S (2015) Laccase gene expression and vinasse biodegradation by Trametes hirsuta strain Bm-2. Molecules 20:15147–15157
Tiso M, Schechter AN (2015) Nitrate reduction to nitrite, nitric oxide and amonia by gut bacteria under physiological conditions. PLoS One 10(3):e0119712
Vilar DS, Carvalho GO, Pupo MMS, Romanholo Ferreira LF (2018) Vinasse degradation using Pleurotus sajor-caju in a combined biological-electrochemical oxidation treatment. Sep Purif Technol 192:287–296
Vohra M, Manwar J, Manmode R, Padgilwar S, Patil S (2014) Bioethanol production: feedstock and current technologies. J Environ Chem Eng 2(1):573–584
Acknowledgments
The present study has been supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 2015 N° 0292) and CONICET.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Del Gobbo, L.M., Colin, V.L. (2018). Fungal Technology Applied to Distillery Effluent Treatment. In: Prasad, R., Aranda, E. (eds) Approaches in Bioremediation. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-02369-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-02369-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-02368-3
Online ISBN: 978-3-030-02369-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)