Abstract
Cardiac toxicity in cancer therapy and hematopoietic cell transplantation is increasingly appreciated. It is also well described in patients with chronic hemolytic anemias such as sickle cell disease. Traditional chemotherapeutic agents such as anthracyclines have a well-established history of causing cardiac complications. Newer chemotherapeutic agents have less established toxicities but likely place patients at risk for cardiac complications as well. The most frequently seen acute cardiac toxicities in the PICU include ventricular dysfunction, pulmonary hypertension, and pericardial effusions. Prompt recognition and management of these complications are imperative to achieve the best possible outcomes. The optimal screening regimen for cardiac complications has yet to be established, but there are promising new biomarkers and imaging modalities that may aid in more prompt diagnosis and intervention in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiomyopathy
- Systolic dysfunction
- Diastolic dysfunction
- Pulmonary hypertension
- Cardiac toxicity
- Pericardial effusion
Introduction
There are approximately 14.5 million cancer survivors in the USA and approximately 16,000 new diagnoses of cancer in patients less than 20 years of age annually. In pediatric cancer patients, the 3-year survival rate is upward of 80% [1]. Cardiotoxicity is the leading cause of morbidity and mortality in long-term survivors of pediatric cancer. These patients are nine times as likely to have a cerebrovascular event, ten times more likely to have coronary artery disease, and fifteen times more likely to have heart failure than their siblings. Therefore, there is a need for providers with specialized knowledge to care for this unique population both in the acute and chronic setting [2]. Cardiac complications of sickle cell disease are also increasingly recognized [3].
Normal Heart Tissue Anatomy
The heart has three layers of tissue: endocardium, myocardium, and epicardium.
The epicardium is responsible for producing pericardial fluid for lubrication. The endocardium closely resembles the endothelial tissue and lines the inner surface of the heart, forming a vascular network to maintain the function of cardiac myocytes; the endocardium also contains the conduction system fibers. The myocardium is highly vascular and always open to perfusion. It relies on a complex arteriocapillary system as there are no major vessels to perfuse this tissue. Any damage to these myocardial capillaries results in poor perfusion and decreased contractility. The major blood supply to the heart is from the right and left coronary arteries. They originate from the root of the aorta, and the left divides into the left main and the left circumflex arteries.
Heart Failure and Therapy-Related Cardiotoxicity
Heart failure is a clinico-pathological syndrome of structural and functional defects of the heart, rendering it unable to maintain adequate cardiac output to meet the demands of vital organs and peripheral tissues. There are many definitions that describe cardiotoxicity in pediatric oncology patients, but none are comprehensive enough to account for baseline risk or to guide clinical outcomes. The National Cancer Institute (NCI) of the National Institutes of Health (NIH) has published standardized definitions for adverse events (AEs), known as the Common Terminology Criteria for Adverse Events (CTCAE). They define heart failure based on grades. Grade 1 is asymptomatic elevation of biomarkers or abnormalities on imaging. Grades 2 and 3 consist of grade 1 plus mild to moderate symptoms upon exertion. Grade 4 includes life-threatening symptoms requiring hemodynamic support, and Grade 5 involves death [4]. Common pathological causes of heart failure in oncology and hematopoietic cell transplant patients are discussed below.
Pathophysiology of Radiation-Induced Heart Damage (RIHD)
Tissue irradiation leads to fibrosis. Fibrosis is both an acute and late effect of tissue irradiation. Within minutes of radiation exposure, there is vasodilation, increased vascular permeability, and increased expression of adhesion molecules and growth factors. This prompts an acute inflammatory response and an influx of cytokines. The most commonly involved cytokines are tumor necrosis factor (TNF) and interleukins (IL-1, IL-6, and IL-8). After a few hours of radiation exposure, pro-fibrotic cytokines are released. The common ones are platelet-derived growth factor (PDGF), transforming growth factor B (TGF-B), basic fibroblast growth factor (bFGF), insulin-like growth factor (IGF), and connective tissue growth factor (CTGF). The expression of proto-oncogenes, including c-myc and c-jun, promotes fibrotic changes. Resulting endothelial damage triggers the coagulation cascade and results in fibrin deposition. The acute phase of radiation-induced damage can last for several days, after which there is usually a period with no obvious microvascular damage.
There are further pathways leading to fibrosis. Mediators such as chronic hypoxia and chronic oxidative stress can result in free radical production. In turn, this increases inflammatory mediators, proteases, and adhesion molecules and decreases nitric oxide. All of these mechanisms have the potential to further damage the endothelium. Upregulation of nuclear factor kappa B (NF-KB), a transcription factor, is a key link between oxidative stress and inflammatory pathways. Chronic hypoxia leads to upregulation of hypoxia-inducible factor (HIFIa), stimulating TGF-B and leading to fibrosis [5].
The microvasculature is more affected with subendothelial fibrosis than the large arteries, which are usually spared. Coronary arteries can develop endothelial damage, vasculopathy, and ischemia. Progressive fibrosis of myocardium leads to decrease in tissue elasticity and compliance resulting in diastolic and systolic dysfunction. A compensatory upregulation of beta-receptors preserves and stabilizes cardiac output, but as damage progresses, congestive heart failure can develop. Fibrotic valvular changes can also occur, though the exact mechanism of such is not well understood. Fibrosis of the pericardium results in a spectrum of pathologies ranging from mild constrictive physiology, severe restriction resulting in tamponade, to that of global pancarditis. Conduction system abnormalities are likely due to microvascular damage and damage to sinoatrial and atrioventricular nodes, typically presenting as heart block. Fibrosis of the left ventricle can lead to ventricular ectopy, such as premature ventricular contractions (PVCs) and ventricular tachycardia.
RIHD results in significant morbidity and mortality and requires further understanding of the pathophysiology for the development of therapeutic targets to prevent microvascular damage, inflammation and late fibrosis [6].
Pathophysiology of Renin Angiotensin-Aldosterone System (RAAS) Induced Cardiovascular Damage
The renin angiotensin-aldosterone system (RAAS) is an important physiological system that maintains intravascular blood volume and blood pressure. It involves various organ systems such as the central nervous system, kidneys, liver, lungs, and adrenal glands. When there is decreased cardiac output, poor renal perfusion, low blood pressure, or blood volume for any reason, RAAS becomes activated. This causes renin to be released into the bloodstream secondary to various mechanisms:
-
1.
Afferent arteriole dilation (baroreceptors) in response to low blood pressure.
-
2.
Increased sodium reabsorption due to reduced GFR (poor renal perfusion), sensing of low sodium load at the macula densa (chemoreceptors), which in turn release nitric oxide and prostaglandins to cause the release of renin.
-
3.
Carotid bodies (baroreceptors) sensing low blood pressure cause an increased sympathetic outflow which directly stimulates beta-receptors on the juxtaglomerular apparatus and cause renin release.
Renin converts angiotensinogen into angiotensin I, which gets converted by pulmonary capillary endothelium into angiotensin II and inhibition of bradykinin (vasodilator). The overall result is very potent vasoconstriction.
Angiotensin II is subsequently responsible for:
-
1.
Directly stimulating the sympathetic nervous system by decreasing reuptake of norepinephrine at the presynaptic motor endplate
-
2.
Increasing expression of adrenergic receptors on vascular tissues
-
3.
Stimulating receptors on venous and arteriolar surfaces to cause strong vasoconstriction
-
4.
Increasing water and sodium reabsorption by stimulating the adrenal cortex to produce aldosterone, acting on the hypothalamus and the pituitary gland to cause release of ADH and increases thirst by acting on the thirst center
Chemotherapy, radiation, and bone marrow transplant individually or in combination can cause a significant inflammatory state, inciting endothelial damage which results in vasodilation and increased vascular permeability. Chronic inflammation and activation of RAAS leads to progressive pathological, morphological, and geometric changes in the heart. The consequence of this is persistent and chronic activation of the RAAS. Concurrent heart dysfunction promotes chronic sympathetic activation. Chronic pathological activation of the sympathetic system and RAAS causes angiotensin II and aldosterone to act on the myocardial cells and fibroblasts, stimulating proto-oncogenes. As a result, growth factors are produced by the myocardial cells, resulting in pathological hypertrophy and accumulation of excess connective tissue, extracellular matrix, and fibrotic tissue. The pathologic remodeling of the myocardium makes it poorly contractile and non-compliant.
Angiotensin II and aldosterone also act on vascular smooth muscle and cause pathological changes which result in vasculopathy and poor response to treatment. In turn, this leads to progressive hypertension and cardiovascular failure. Therefore, ACE inhibitors, angiotensin receptor blockers, and beta-blockers are key medications to be started preemptively to prevent or improve pathological remodeling and treat hypertension.
Pathophysiology of Chemotherapy-Induced Cardiovascular Damage
Conventional chemotherapy targets rapidly dividing cells, while targeted agents work on tumor specific pathways. Anthracyclines (i.e., doxorubicin, daunorubicin, epirubicin), alkylating agents (i.e., busulfan, cyclophosphamide), antimetabolites (i.e., 5 fluorouracil, cytarabine), anti-microtubule agents (i.e., vinca-alkaloids), targeted agents (i.e., trastuzumab), cisplatin, mitoxantrone, pyrimidine analogues, and several other known and suspected agents can affect the heart. Cancer drugs interact with cardiovascular signaling, particularly during times of increased stress.
Anthracyclines
The mechanism of anthracycline-induced toxicity is not completely understood. The drug enters the cardiac myocyte, intercalates into nuclear DNA, and impairs protein synthesis. Additionally, it forms reactive oxygen species through iron complex formation, which damages the mitochondria. As calcium homeostasis is initially affected, diastolic dysfunction may be the initial presentation of anthracycline-mediated cardiotoxicity. Diastolic dysfunction may be a harbinger of future systolic heart failure. Pathologic investigation has shown that as more anthracycline accumulates in cardiac cells, there is evidence of apoptosis and myocardial necrosis, which likely results in the observed myocardial dysfunction and clinical heart failure syndromes [7].
Anthracyclines and the anthracycline analogues are commonly used agents in many cancer protocols. They are known to be cardiotoxic and, in many instances, require a dose reduction or holding of the medication. There is a reported 2.8% incidence of heart failure in the first 6 years after a cumulative dose of 300 mg/m2. A direct correlation exists between cumulative dosing and incidence of cardiotoxicity [8, 9]. Survivors have reduced LV systolic function and inadequate diastolic filling with larger LV dimension. This pathology is caused by a decrease in number of cardiomyocytes and stem cells capable of generating cardiac tissue [10].
Prolonged QTc intervals, sinus node dysfunction, PVCs, and decreased QRS voltage have been described after anthracycline administration in 10–30% of survivors [11]. Rhythm abnormalities associated with anthracyclines are usually transient and have been reported with a cumulative dose of 120 mg/m2. There is also evidence of ischemic cardiac injury with doxorubicin and another anthracycline, pirarubicin. Genetic predisposition, arterial hypertension, previous or concurrent heart disease, and combination with radiation, alkylating agents, and anti-microtubule therapy increase sensitivity to anthracyclines. Currently, there are no evidence-based guidelines in pediatrics for treatment of heart failure secondary to anthracyclines.
Alkylating Agents
Alkylating agents (i.e., cyclophosphamide, ifosfamide, melphalan) inhibit DNA transcription and affect protein synthesis. Cardiovascular symptoms usually present within the first week to first month of therapy. Low doses are usually not associated with toxicity, but at higher doses, patients can present with severe symptoms.
Other Agents
Monoclonal antibodies may have deleterious effect on cardiovascular function, though the data is retrospective, and the sample size is small [12]. Trastuzumab is a monoclonal antibody that targets HER-2 receptor. In adults, it causes cardiotoxicity in approximately 4–7% of patients when taken alone and 27% when taken with anthracyclines. The effects are usually reversible after stopping treatment. It is hypothesized that the use of monoclonal antibodies inhibits repair processes of the damage caused by the anthracyclines [13]. Vascular endothelial growth factor (VEGF) plays an important role in myocardial and vascular homeostasis. Anti-VEGF medications disrupt myocardial metabolism, impairing myocardial function. Bevacizumab, an anti-VEGF monoclonal antibody, has been shown to decrease LV shortening fraction. As the use of monoclonal antibodies becomes more prevalent, it is crucial to watch for impaired cardiac function during treatment, particularly when treatment is combined with other cardiotoxic agents. Cardioprotective agents, such as dexrazoxane, may have benefit in these patients.
Tyrosine kinases catalyze the transfer of phosphate from ATP to tyrosine residues in polypeptides involved in growth receptor signal transduction. Tyrosine kinase inhibitors (TKIs) are frequently used in cancer therapy and are linked to left ventricular dysfunction, heart failure, and arrhythmias [12]. Imatinib and dasatinib are small molecule TKIs and have been described to cause cardiac failure. They are known to cause serositis. Patients may present with peripheral edema, pleural, and pericardial effusions [14].
Risk Factors for Cardiotoxicity
Treatment and Patient-Related Risk Factors
-
1.
Cumulative anthracycline dose
-
2.
Mantle field radiation
-
3.
Cranial radiation
-
4.
Inflammatory state during therapy
-
5.
Cancer diagnosis
-
6.
Female gender
-
7.
Young children
-
8.
Genetic factors
-
9.
Preexisting cardiovascular disease
-
10.
Traditional risk factors for coronary vascular disease
A high cumulative dose of anthracyclines is the biggest risk factor for cardiotoxicity, but there is no dose of anthracyclines where the risk is absent. Damage has been reported with doses <240 mg/m2 [15]. Continuous infusions to reduce peak serum levels have been suggested but data does not support the practice in children. Patients treated with mantle field radiation are at higher risk for cardiac dysfunction than patients who never received radiation at all or received radiation outside of the mantle field [16]. Cranial radiation is described as a risk factor secondary to pituitary damage and growth hormone deficiency. Incidence of cardiac dysfunction after cranial radiation is 4% which increases to approximately 13% with concomitant use of anthracyclines. The female gender is more susceptible to anthracycline toxicity, most likely due to higher percentage of body fat affecting distribution of the drug [17].
A strong inflammatory response during therapy is felt to cause increased microvascular endothelial damage leading to increased risk of cardiac complications [18]. Cancer itself is a risk factor for cardiovascular damage as children who have not received cardiotoxic chemotherapy or radiation still have an increased risk for cardiovascular abnormalities [18]. Young children are at higher risk for LV wall thinning and dysfunction [17]. Mutations in the HFE gene and C282Y allele (hereditary hemochromatosis) carry a significantly increased risk for cardiovascular injury [19]. Patients with preexisting cardiovascular impairment are at much higher risk for progressive heart failure [18]. Traditional risk factors for coronary vascular disease such as physical inactivity, obesity, insulin resistance, vitamin D deficiency, and smoking increase the likelihood of cardiovascular damage post-therapy.
Clinical Manifestations of Cardiac Toxicity
Cardiac complications in children undergoing cancer-directed therapy or hematopoietic cell transplantation are increasingly appreciated in the ICU. The most common acute manifestations include cardiac dysfunction, pulmonary hypertension, and pericardial effusions. These will be discussed in more detail.
Cardiac Dysfunction
Acute cardiotoxic events happen within the first week of therapy and are usually reversible with dose reduction or in most cases discontinuation of the respective chemotherapy. The reversibility is not 100%, and there can be progression of left ventricular (LV) dysfunction. Early onset cardiotoxicity occurs within weeks to months and can present with restrictive cardiomyopathy and filling defects or dilated cardiomyopathy with LV wall thinning and systolic dysfunction. This is usually progressive. Late onset cardiotoxicity is more common, defined by cardiac dysfunction after 1 year of exposure to chemotherapy. This can be asymptomatic for several years before it is clinically evident, necessitating the need for surveillance echocardiograms. It most commonly manifests as LV wall thinning, dilation, and decreased function and is progressive [20].
Dilated cardiomyopathy can convert to a restrictive cardiomyopathy with diastolic dysfunction, or diastolic dysfunction can be an early marker or future systolic heart failure. The proposed mechanism causing this is that with LV failure, there is a limited number of normal cardiomyocytes and with somatic growth of children, there is inadequate and pathological hypertrophy leading to a restrictive cardiomyopathy [8]. Approximately 60% of children exposed to anthracyclines alone or in combination with other cardiotoxic agents have subclinical changes in their LV structure and function on echocardiography [21].
Cancer therapy-induced arterial hypertension is a well-described entity. This is most commonly associated with angiogenesis inhibitors but can present with other cancer drugs as well.
Hypertension can present at any time during therapy and cause complications such as heart failure, stroke, posterior reversible leukoencephalopathy, proteinuria, and thrombotic microangiopathy. In most cases, the hypertension is reversible and improves as treatment is withheld or stopped but may persist [22].
Cancer is a hypercoagulable state. Many cancer agents (i.e., hormonal therapy, cisplatin, bevacizumab, sunitinib, and sorafenib) have been known to increase the risk of thromboembolic events [23, 24]. Currently, no clear guidelines exist for thromboprophylaxis in these patients, but most cancer patients will benefit from prophylactic anticoagulation. In addition to the cancer-induced hypercoagulable state, coronary artery spasm and rhythm disturbances have been reported with pyrimidine analogues such as 5-fluorouracil. These mechanisms may partially explain the increased risk of early ischemic heart disease in pediatric cancer survivors.
QT prolongation is associated with a number of cancer agents, placing patients at risk for cardiac arrhythmias [25]. Physicians should exercise extreme caution when there is concomitant use of other QT-prolonging drugs (antiemetics, methadone, psychotropic agents) particularly in patients with ongoing electrolyte disturbances.
Pulmonary Hypertension
Pulmonary hypertension (PH) is defined as elevation in the pulmonary arterial pressure. It is rare in children, estimated to occur in <10 cases per 1 million children [26]. The etiology of pediatric pulmonary hypertension in the general population is varied. Over half of pediatric cases are idiopathic or familial, while the remainder are associated with other diseases, most commonly congenital heart disease or chronic lung disease. Endothelial cell damage seems to play a role in the development of PH. The inciting injury may be caused by hypoxia, increased blood flow, or toxins. This injury leads to smooth muscle cell proliferation in small peripheral pulmonary arteries which normally are not muscularized. There also seems to be evidence for a genetic predisposition, with mutations in the bone morphogenetic protein receptor type 2 (BMPR2) and TGF-Β superfamily being associated with PH [26].
Diagnosis of pulmonary hypertension in critically ill children is primarily accomplished noninvasively by echocardiogram, though cardiac catheterization is the gold standard. The ECG may show changes suggesting right atrial enlargement right axis deviation, bundle branch block, or right ventricular hypertrophy in cases of chronic pulmonary hypertension. However, ECG is not sufficient to make a diagnosis. Chest X-ray is useful to look for lung disease as the etiology of the pulmonary hypertension. It may also show an enlarged right heart or pulmonary arteries, though many patients may not have any findings. The echocardiogram is what is most frequently used to make the diagnosis in critically ill oncology patients. Tricuspid valve regurgitant jet velocity can be used to estimate the pulmonary arterial pressure, with the use of the modified Bernoulli equation. However, in the setting of poor right ventricular function, this may be falsely low as a heart pumping without force is not able to generate as much velocity across the valve. Therefore, this number alone should not be the sole determination of the diagnosis. Other echocardiographic findings of pulmonary hypertension include septal wall flattening and right ventricular hypertrophy/dysfunction [26, 27].
Pulmonary hypertension is increasingly recognized as a complication of hematopoietic cell transplant and chemotherapy [28, 29]. It is also a recognized complication in patients with chronic hemolytic anemias such as sickle cell disease [30]. Early diagnosis and intervention are likely to provide improved outcomes. Therefore, it is important for hematologists, oncologists, and intensivists to consider this diagnosis in critically ill hematology, oncology, and stem cell transplant patients.
Pulmonary hypertension is not isolated to the pediatric oncology and hematopoietic cell transplant population. It is also prevalent in patients with sickle cell disease and is increasingly appreciated as an important complication impacting morbidity and mortality [3]. Thirty to forty percent of adults with sickle cell disease have echocardiographic evidence of PH. The incidence in children is variable, depending upon the publication, ranging from 8% to 47%. The risk for development of PH seems to be highest in patients with hemoglobin SS as opposed to other forms of sickle cell disease, those who were 13 or older and more anemic [3]. Other variables associated with PH in sickle cell patients include elevated serum creatinine, brain natriuretic peptide, cardiac troponin levels, history of sleep apnea, prolonged QTc, and short 6-min walk test [3, 30,31,32]. Pulmonary hypertension has also been described in other types of anemia. PH occurring several years after splenectomy has been described in patients with hereditary spherocytosis [33] and in patients with congenital dyserythropoietic anemia, a rare congenital macrocytic anemia [34].
Acute cardiac complications in HCT patients were once thought to be quite rare, reported to occur in less than 1% of pediatric HCT patients [35]. Newer data suggests cardiac abnormalities are more common. Cincinnati Children’s Hospital reports a 30% incidence of abnormalities found on routine screening echocardiograms at day +7 after HCT [28]. In their study 13% of children had elevated right ventricular pressures on day +7 echocardiogram, and this was significantly associated with later development of thrombotic microangiopathy (TMA). Sixty percent of patients with elevated right ventricular pressures were diagnosed with TMA vs. 23% of patients with a normal day +7 echocardiogram (p = 0.0003). The incidence of TMA was even higher in HCT patients who had both a pericardial effusion and elevated right ventricular pressure at day +7. One hundred percent of these patients went on to develop TMA. The team at Cincinnati went on to broaden screening echocardiograms to include any HCT patient with respiratory distress, hypoxia, shock, or TMA on admission to the PICU and every 1–2 weeks thereafter [36]. In this population 50% of patients had echocardiographic findings warranting either intervention or further screening. Elevated right ventricular pressure was the most common abnormality seen in 34% of patients, 20% were deemed to be at risk for pulmonary hypertension, and 14% diagnosed with pulmonary hypertension. All patients diagnosed with pulmonary hypertension required treatment with pulmonary vasodilators. The majority of patients diagnosed with pulmonary hypertension did not have other physical exam or radiographic findings on CXR that would have clued clinicians into the diagnosis.
PH has also been described in specific populations undergoing HCT. Patients with malignant infantile osteopetrosis who undergo HCT appear to be at high risk for development of PH. The European Blood and Marrow Transplantation Group (EBMT) published a case series where 8 of 28 infants transplanted for malignant infantile osteopetrosis developed PH with a mortality rate of 62% [37]. A group from the USA also reported a case series where 5 of 12 infants transplanted for malignant infantile osteopetrosis developed PH with a mortality rate of 80% [38]. The reason for this association remains unclear. However, these patients warrant close monitoring and early intervention for PH.
Pulmonary veno-occlusive disease (PVOD) is a possible etiology for the development of pulmonary hypertension in HCT patients and should be considered in the differential. Patients with PVOD may present as either an early or late complications of HCT. Patients often present with increasing dyspnea. Their CXR may show cardiomegaly and pulmonary edema, while an echocardiogram shows pulmonary hypertension. If a biopsy is performed, characteristic findings are fibrosis of the small pulmonary veins and venules, while the larger pulmonary veins are normal [39, 40]. Lung biopsy had been the gold standard; however, it carries a high risk of complications. Mineo and colleagues looked at the accuracy of chest CT to make the diagnosis. They found that presence of two of three of the following findings, ground glass appearance, septal thickening, and mediastinal lymphadenopathy, was 95.5% sensitive and 89% specific for the diagnosis of PVOD [41]. PVOD is an important diagnosis to consider as these patients may respond to therapy much differently than other patients with PH. Defibrotide is a theoretically useful agent in this population, though there is little published data for use other than in hepatic VOD. Its use was described by the EBMT group in their case series of eight osteopetrosis patients with PH. Defibrotide was used in four of the eight patients for possible PVOD and/or documented liver VOD. Two of the four patients given defibrotide survived, while one of the four patients not given defibrotide survived [37]. The numbers are much too small to make any conclusions other than its use has been described in patients who may have had PVOD. The other potentially important aspect when considering treatment for PH due to PVOD is that traditional pulmonary vasodilators used for PH may cause pulmonary edema in patients with PVOD so should be used with caution [42].
PH has also been described in oncology patients. The majority of case reports have been in patients with neuroblastoma, particularly those undergoing autologous HCT [29, 43]. Patients who have received busulfan/melphalan conditioning may have the highest risk of developing PH [29], but PH has been described in patients who have received other chemotherapy [44]. PVOD was suspected as the cause of PH in two of the case reports of pulmonary hypertension in patients with neuroblastoma [43, 44]. Leukemic infiltrate in the lung has been suspected as a cause of PH in a case report of a patient with juvenile myelomonocytic leukemia [45]. There are case reports of PH as the cause of death in patients with hemophagocytic lymphohistiocytosis and idiopathic myelofibrosis [46, 47].
Treatment of pulmonary hypertension in the pediatric hematology/oncology/HCT population is the same as in other populations, with the caveat that PVOD should be considered in the differential as the cause of the PH, particularly if the patient’s condition worsens with pulmonary artery vasodilator therapy. Acute management when patients present to the PICU acutely ill would include oxygen to maintain saturations at least 95%, inhaled nitric oxide (NO), diuretics if the patient is felt to be volume overloaded, or volume supplementation if the patient has a low right ventricular filling pressure [27]. Intravenous prostanoids such as epoprostenol may be used for their pulmonary vasodilatory effect. Patients need to be monitored closely for hypotension as they also decrease the systemic vascular resistance. Patients with hypotension and poor cardiac output may require inotropic and vasopressor support with milrinone being a particularly good choice for inotropy as it does not increase the heart rate. Vasopressor agents may be necessary to counteract the vasodilator effects of systemic prostanoids. Avoidance of hypotension is important to maintain adequate coronary perfusion. Oral agents such as sildenafil or bosentan can be added to help with weaning off of iNO [27, 48].
Pericardial Effusion (PCEF)
Pericardial effusion (PCEF) is a life-threatening complication among pediatric oncology patients [49, 50]. Reported incidence of PCEF in children with cancer ranges from 0.2% to 17% [51]. Knowledge continues to develop surrounding the incidence, risk factors, and outcomes associated with PCEF in children with oncologic disease. In the interim, it is incumbent upon clinicians to understand potential causal pathways, symptomology, and treatment considerations for PCEF in this population. PCEF in children is more frequently associated with hematopoietic stem cell or bone marrow transplant (HSCT or BMT). However, clinicians report, both in literature and anecdotally, occurrence of PCEF across all various types of pediatric oncological disease.
Risk Factors for PCEF
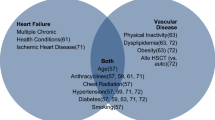
The population at highest risk for PCEF are patients who undergo HCT. Potentiating risk factors associated with incidence of PCEF include donor type, conditioning, infection, toxicity related to immunosuppressives, and graft-versus-host disease (GVHD) [51, 52]. Patients with high-risk disease status prior to HCT (e.g., active leukemia in relapse, active leukemia after induction failure, progressive solid tumor, and/or second allogeneic HSCT), myeloablative conditioning, and calcineurin inhibitor (CNI) (e.g., cyclosporine and tacrolimus) as well as other therapeutic agents (Fig. 12.1) are at relative increased risk for incidence of PCEF [53]. The literature varies on whether age, gender, and other factors are reliable predictors.
Oncological Pathways to PCEF
In normal physiology, the pericardium, a two-layered sac, protects the myocardium via the provision of a mechanical barrier, reducing friction between the heart and structures in close proximity [49]. The pericardial cavity lies between the visceral and parietal layers of the pericardium, containing a minute amount of plasma ultrafiltrate. In an acute pericarditis, the pericardium becomes inflamed, staging an effusive process that can lead to rapid accumulation of fluid in the pericardial cavity. The fluid can be serous, purulent, or sanguineous depending on the cause. Common pathways leading to occurrence of PCEF in pediatric oncology patients listed by frequency of occurrence (Fig. 12.2) [49]:
Subcategories of inflammatory or systemic-mediated occurrence of PCEF include global serositis, thrombotic microangiopathy (TMA), and pervasive neoplastic disease. The vast majority of PCEF in pediatric oncology patients is of uncertain origin. The notion of idiopathic PCEF is typically confirmed in the absence of significant diagnostic findings (i.e., negative cultures and pathology of PCEF fluid, nontoxic clinical presentation, no mechanical confounders, no known trauma history, etc.). Of the microorganisms attributed to infectious etiology of PCEF, the most frequent cause is viral infection (i.e., EBV, CMV, adenovirus, etc.); less common are bacterial, fungal, and parasitic infections [49].
Two specific inflammatory etiologies related to PCEF are worth highlighting. They are serositis and TMA. Serositis is an operational definition of a syndrome involving inflammation of serous tissues in the body, often resulting in fluid collections in the pericardial, pleural, and peritoneal cavities. Serositis is often considered in the absence of antimicrobial pathogenicity and in the setting of concurrent pleural effusions; it may or may not be indicative of underlying GVHD or immunosuppressive toxicity. Patients with TMA may present with pericardial effusions and pleural effusions in addition to thrombocytopenia, hemolytic anemia, acute kidney injury (AKI), altered mental status, severe pulmonary arterial hypertension (PAH), and dysfunction of other organs, leading to multi system organ failure (MSOF) [54].
Symptomology and Diagnosis of PCEF
A pattern of symptoms are often present in patients experiencing PCEF, including but not limited to:
-
Tachycardia, accompanied by S3 (gallop), or pericardial friction rub on auscultation
-
Narrow or decreased pulse pressure
-
Dyspnea with or without oxygen desaturation
-
Chest pain
-
ECG changes, including low-voltage QRS complexes, electrical alternans, ST segment elevation, PR segment depression, and/or T-wave inversions
-
Pulsus paradoxus (quantified as a systolic blood pressure (SBP) reduction of >10 mmHG)
-
Kussmaul sign (i.e., elevation in jugular venous pressure during inspiration)
-
In large PCEF, globular appearance of cardiac silhouette on chest radiography (CXR)
High-risk features such as fever, leukocytosis, elevated troponin, and history of PCEF or pericarditis are associated with poorer outcomes [49].
While awareness of symptomology is helpful, echocardiogram (ECHO) serves as the diagnostic gold standard. Of note, rarely do volumes quantified on ECHO match drained volumes upon intervention [51]. Perhaps counterintuitively, clinicians must be keenly aware that volume of effluent does not directly correlate with clinical presentation or allow for enhanced prognostication. In fact, “relatively small effusions may lead to important hemodynamic compromise,” particularly in the small pericardial cavities of young children [50]. This appears especially true in the pediatric oncology population, where underlying diastolic dysfunction may contribute to hemodynamic instability even without classic markers of tamponade physiology.
Tamponade Physiology
By definition, cardiac tamponade occurs when the chambers of the heart are compressed as a result of increased intrapericardial pressure to the extent that systemic venous return to the right atrium (RA) is impaired; increased intrapericardial pressure decreases myocardial transmural pressure, and the chambers of the heart demonstrate reduced capacity and compliance, consequently reducing cardiac output and blood pressure. Typical ECHO findings in tamponade include diastolic collapse of the RA and right ventricle (RV) as intrapericardial pressure exceeds the intracavitary pressure [49].
In early tamponade, sinus tachycardia preserves cardiac output. Hypertension may also occur as part of a sympathetic response to pericardial annoyance. Atypical symptoms such as chest or shoulder discomfort and nausea may also be present [49]. Late signs of tamponade physiology may manifest as cardiogenic shock, hypotension, and pulsus paradoxus, and narrowing pulse pressure will progress to cardiac collapse without intervention.
Management of PCEF
Acute PCEF management is largely based on individual clinical presentation. Asymptomatic low-risk patients may be managed within a low-acuity setting. Management for stable PCEF should include serial ECHO follow-up, hemodynamic monitoring, and treatment of identifiable underlying causes. However, persistent and enlarging PCEF in the oncology patient may require more aggressive treatment, even despite evidence of hemodynamic compromise or tamponade physiology.
Diuretics are mostly ineffective, though often used. Infectious PCEF may be treated based on suspicion of pathogen. However, serologic testing and culturing of effluent are often low yield. Rituximab may be indicated in EBV-mediated PCEF. Immunosuppressive agents are often not helpful even with inflammatory etiologies. Discontinuation of CNIs has proven to be efficacious in ameliorating PCEF [51]. In TMA-associated PCEF, eculizumab and plasmapheresis have been used with some success [54]. If methotrexate has been used recently, leucovorin rescue may be helpful.
Large PCEF at risk for tamponade physiology, hemodynamic compromise, or rapidly increasing effusions necessitate critical care observation. Volume expansion may be required to optimize cardiac output, specifically in hypotension. Inotropic support is controversial as endogenous sympathetic output is typically maximal in tamponade [49]. Patients with concomitant respiratory distress may require noninvasive or invasive respiratory support with the caveat that positive-pressure ventilation (PPV) may worsen acute tamponade by impeding cardiac filling.
Evacuation of PCEF is achieved by ECHO-guided percutaneous pericardiocentesis, with or without drain placement. Recurrent PCEF may better be addressed by pericardial window or pericardectomy. Pericardiocentesis can be complicated in small children who have very little distance between the myocardium and the rim of the effusion [50]. Additionally, sedation in children with tamponade physiology is fraught with risk for hemodynamic compromise. Preprocedure tachypnea, cardiac tamponade, and hypoxia have proven to be reliable prognosticators for adverse events [55]. The preferred induction agent is ketamine. The transition from spontaneous respiration to positive-pressure ventilation carries risk of hemodynamic compromise and should be done with caution.
Monitoring for Cardiac Toxicity
Currently, there are no established evidence-based guidelines for monitoring cardiovascular structure and function during and posttreatment. It is widely recognized that it is necessary to do so, but there is no consensus on the modalities or their frequency of use.
EKG
There is evidence that prolonged QT interval can predict cardiac disease [25, 56]. Conduction abnormalities and cardiac hypertrophy are all associated with cancer-related therapy, whether drug induced or radiation induced. Therefore, an EKG is highly recommended for assessment and monitoring of cardiac dysfunction. EKG should be performed prior to initiation of therapy to establish baseline, periodically during therapy and after completion for comparison.
ECHO
Echocardiogram is a necessary tool in the evaluation of cardiac dysfunction during cancer therapy. Thorough echocardiographic protocols include assessment of diastolic dysfunction and myocardial strain as these abnormalities are often present before a decrease in systolic function is seen. Increased values of indexed left atrial volume are a direct result of impaired diastolic dysfunction, which is commonly seen in patients with early chemotherapy-induced cardiomyopathy [57]. Figure 12.3 shows a patient with a dilated left atrium, compared to a patient with a normal left atrial size. Specific Doppler velocities that are obtained during diastole can evaluate mitral valve inflow and cardiac wall deformation and have been shown to predict systolic heart failure in those who have received chemotherapy [58]. Figure 12.4 shows an elevated mitral valve inflow velocity, compared with a normal mitral valve inflow velocity. There has also been much attention given to evaluation of myocardial strain parameters, with a decrease in global longitudinal strain most reliably correlated with decreased cardiac function after chemotherapy [59]. Figure 12.5 shows an abnormal global longitudinal strain (GLS) profile, compared to a normal GLS profile. Figure 12.6 shows a patient with a large circumferential pericardial effusion.
Given the studied associations between cardiac toxicity and both anthracycline agents and chest radiotherapy, serial echocardiographic assessment has become an established part of these protocols. However, much is being learned about the acute hemodynamic alterations caused by bone marrow transplantation (BMT), with data suggesting cardiac dysfunction as a direct effect of hematopoietic cell transplantation and the need for serial echocardiography as part of these protocols as well [36].
Cardiac Magnetic Resonance Imaging
Cardiac MRI scans are superior to echocardiography to assess myocardial tissue structure. It is the gold standard for detection of ventricular volumes and function [60]. It provides a better assessment of myocardial edema, inflammation, and fibrosis and may identify cardiomyopathy more reliably than echocardiography. Cardiac MRI therefore has an important role in identification of early and late cardiotoxicity.
Positron Emission Tomography/Magnetic Resonance (PET/MR)
PET/MR is an emerging modality for assessment of cardiomyopathy. Imaging allows for determination of myocardial perfusion, glucose metabolism, and myocardial viability [61].
Biomarkers
Troponins
Cardiac troponins (troponin I and troponin C) present in the serum may indicate acute cardiac damage. Elevated troponins in the first 90 days of cancer therapy have been associated with reduced LV mass and thickness and function 5 years later [18]. Absence of troponin elevation has a high negative predictive value. Elevation has been described to predict diastolic dysfunction in approximately 35% of patients. Elevation of troponin at the completion of therapy is predictive of subsequent left ventricular dysfunction. Cardinale et al. demonstrated a relationship between elevated troponin, anthracycline-induced cardiotoxicity, and patients who would benefit from ACE inhibitors [62].
Natriuretic Peptides
BNP and NT-pro BNP are elevated due to wall stress secondary to pressure or volume overload. These are elevated acutely in cardiac dysfunction but also predict LV remodeling in future years. As they are elevated in cardiac stress and do not necessarily indicate cardiomyocyte damage, they have the potential to detect early subclinical changes.
The elevation of NT-proBNP (natriuretic peptide), MR-pro ANP (mid-regional atrial natriuretic peptide, MR-proADM (mid-regional pro adrenomedullin), copeptin, and high-sensitivity troponin T prior to therapy is associated with higher mortality [23]. Elevation of BNP in the first 90 days is predictive of left ventricular dysfunction within 3, 6, 12 months, and 4 years [63, 64]. NT-proBNP is also shown to correlate with the increased cumulative exposure to anthracyclines.
High-Sensitivity C-Reactive Protein (CRP) and Myeloperoxidase (MPO)
High-sensitivity CRP predicts cardiotoxicity in patients treated with trastuzumab. MPO levels which rise early in therapy persist and are associated with cardiotoxicity [65].
Management
Cardiotoxicity causes a significant amount of morbidity and mortality and is progressive in nature. Therefore, early medical intervention is extremely important. Labs that help monitoring perfusion and guide acute heart failure management include lactic acid and mixed venous saturation. Volume status can be best predicted by pulse pressure variation, stroke volume variation, and IVC dynamics.
Milrinone (Phosphodiesterase 3 Inhibitors)
Milrinone is a commonly used infusion for acute heart failure. It increases the overall performance of the heart and decreases pulmonary vascular resistance. It is an ideal choice for systolic and diastolic dysfunction as per its inotropic, vasodilatory, and lusitropic effects.
As most patients with heart failure secondary to cancer therapy have hypertension, milrinone can decrease afterload while improving the contractility. There are times when other antihypertensives may be combined with milrinone specifically if there is persistent hypertension. Antihypertensives can be administered intermittently or as an infusion (beta-blockers or calcium channel blockers). The goal is to main borderline normal mean arterial pressure according to age.
Other Inotropes and Vasopressors
Low-dose epinephrine and inotropic dose of dopamine can be used alone or in combination with milrinone. This approach is especially beneficial in patients with poor contractility and hypotension. Vasopressin can be considered a vasopressor in addition to the inotropes to elevate the SVRI and improve organ perfusion. In patients with cardiac dysfunction and ongoing distributive shock as seen in sepsis or cytokine release syndrome, a short course of norepinephrine can be considered as the first line therapy. Low-dose epinephrine or dopamine may be added to provide more inotropic support. A calcium infusion may also be used as an inotropic and or vasopressor agent, specially in younger children.
Patients can present with poor contractility and high SVRI. In this case milrinone is the ideal choice. If the SVRI remains high even with titration of milrinone and patient is clinically symptomatic with evidence of poor perfusion, other vasodilators and positive-pressure ventilation should be considered to rest the patient and provide afterload reduction. Continuous measurement of systemic vascular resistance index, cardiac index, or regional oximetry may be useful for more informed titration of infusions in these very complicated and dynamic situations. Further study in this area is warranted.
Positive-Pressure Ventilation
Positive-pressure ventilation lowers the transmural pressure and off-loads the heart. This can be extremely beneficial in decreasing the metabolic demand by decreasing the work of breathing. Positive-pressure ventilation should be strongly considered in any patient with increased work of breathing and moderate to severe heart failure with down trending indicators of perfusion. Extreme caution is indicated when intubating these patients as their poor cardiac output puts them at high risk for decompensation with sedatives and the stress of intubation.
Angiotensin-Converting Enzyme (ACE) Inhibitors and Angiotensin Receptor Blockers (ARB)
ACE inhibitors reduce afterload, myocardial stress and show improvements in heart failure by preventing angiotensin I from converting into angiotensin II, a very potent vasoconstrictor and preventing abnormal remodeling in adults. Their efficacy still needs to be studied further in the pediatric population, particularly cancer survivors. A trial in pediatrics with enalapril showed improved LV function after doxorubicin use, but the improvement effect was transient and was lost after 10 years [66]. ARBs have similar to arguably better effects to that of ACE inhibitors and do not carry the risk for cough, a possible side effect of ACE inhibitors. However, caution is warranted in initiating these medications in the face of acute kidney injury as they lower the glomerular filtration rate by vasodilating the efferent arteriole.
Early initiation of ACE inhibitors may benefit cancer patients treated with cardiotoxic mediations or radiation therapy. This strategy warrants further study in the pediatric population.
Beta-Blockers
Beta-blockers prevent sympathetic stimulation of the heart by blocking beta-receptors. They have shown to be efficacious in adults and children with anthracycline use, as they control heart rate, lower sympathetic output response, and block renin release. Beta-blockers may be especially efficacious in the setting of diastolic dysfunction, which can be benefitted by reduction in heart rate. Improved LV function and ejection fraction were noted in children who received beta-blockers and anthracyclines. Children pretreated with beta-blockers show improved global peak systolic strain, LV shortening fraction, and a decrease in plasma troponin I and lactic dehydrogenase compared to controls [67].
Follow-Up
Proactive post treatment follow-up is of paramount importance. Recently the Children’s Oncology group (COG) has published recommendations for long-term follow-up of childhood cancer survivors. These recommendations give detailed guidelines regarding frequency of monitoring based on anthracycline exposure, cumulative dose, and total dose of radiation received [68].
Future Directions
Prevention is key, and cardioprotective therapy (including dexrazoxane as well as ACE-inhibitors, ARBs, or beta-blockers) in the first 90 days of therapy should be a strong consideration [18]. All children with cancer are at higher risk regardless of the type of therapy, likely secondary to endothelial damage and remodeling. Further trials are needed to assess biomarker-guided therapy.
Current practice is to involve critical care and cardiology only when ventricular dysfunction or heart failure is clinically evident. This approach not only misses the opportunity for early recognition and intervention but also prevents risk assessment and therefore a lack of an individualized treatment plan.
An onco-critical care cardiac team working in unison should assess patients’ risk factors, before and after therapy, and an individual-based strategy should be developed. The strategy may need modification as the clinical course dictates. A team approach is required to provide optimal management. There should be joint conferences, journal clubs, collaborative research, and continuous education for trainees and hospital staff. Adult colleagues should be consulted for uncommon pediatric issues such as early coronary artery disease. Heightened recognition, improved understanding of pathophysiology, and universally accepted definitions of cancer-related cardiotoxicity will allow for further advancement in the field.
References
Ward E, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103.
Moller TR, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J Clin Oncol. 2001;19(13):3173–81.
Lilje C, et al. A modified noninvasive screening protocol for pulmonary hypertension in children with sickle cell disease-Who should be sent for invasive evaluation? Pediatr Blood Cancer. 2017;64(11)
Health, N.I.O. Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events (CTCAE) v. 5.0. 2018 2018]; Available from: https://ctep.cancer.gov/protocol/Development/electronic_applications/CTC.htm
Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97(1):149–61.
Taunk NK, et al. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39.
Nousiainen T, et al. Natriuretic peptides during the development of doxorubicin-induced left ventricular diastolic dysfunction. J Intern Med. 2002;251(3):228–34.
Lipshultz SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(12):2629–36.
Kremer LC, et al. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001;19(1):191–6.
Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28(8):1276–81.
Bagnes C, Panchuk PN, Recondo G. Antineoplastic chemotherapy induced QTc prolongation. Curr Drug Saf. 2010;5(1):93–6.
Pansy J, et al. Add-on-therapy with bevacizumab in children and adolescents with poor prognosis non-CNS solid tumors. Anti-Cancer Drugs. 2013;24(2):198–203.
Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–44.
Quintas-Cardama A, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25(25):3908–14.
Trachtenberg BH, et al. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol. 2011;32(3):342–53.
Mulrooney DA, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606.
Lipshultz SE, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332(26):1738–43.
Lipshultz SE, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012;30(10):1050–7.
Lipshultz SE, et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer. 2013;119(19):3555–62.
Giantris A, et al. Anthracycline-induced cardiotoxicity in children and young adults. Crit Rev Oncol Hematol. 1998;27(1):53–68.
Lipshultz SE, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324(12):808–15.
Maitland ML, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102(9):596–604.
Ay C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–82.
Choueiri TK, et al. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28(13):2280–5.
Nakamae H, et al. QT dispersion correlates with systolic rather than diastolic parameters in patients receiving anthracycline treatment. Intern Med. 2004;43(5):379–87.
McArthur J, Duncan C, Rajapreyar P, Talano J, Tamburro R. Critical illness involving children undergoing hematopoietic cell transplantation. In: Care PC, Fuhrman BZJ, editors. Fuhrman and Zimmerman’s pediatric critical care. 5th ed. Philadelphia: Elsevier; 2017.
Kaestner M, et al. Pulmonary hypertension in the intensive care unit. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart. 2016;102(Suppl 2):ii57–66.
Dandoy CE, et al. Abnormal echocardiography 7 days after stem cell transplantation may be an early indicator of thrombotic microangiopathy. Biol Blood Marrow Transplant. 2015;21(1):113–8.
Desai AV, et al. Toxicities of busulfan/melphalan versus carboplatin/etoposide/melphalan for high-dose chemotherapy with stem cell rescue for high-risk neuroblastoma. Bone Marrow Transplant. 2016;51(9):1204–10.
Ambrusko SJ, et al. Elevation of tricuspid regurgitant jet velocity, a marker for pulmonary hypertension in children with sickle cell disease. Pediatr Blood Cancer. 2006;47(7):907–13.
Hebson C, et al. Elevated tricuspid regurgitant velocity as a marker for pulmonary hypertension in children with sickle cell disease: less prevalent and predictive than previously thought? J Pediatr Hematol Oncol. 2015;37(2):134–9.
Liem RI, et al. Tricuspid regurgitant jet velocity elevation and its relationship to lung function in pediatric sickle cell disease. Pediatr Pulmonol. 2009;44(3):281–9.
Das A, et al. Risk factors for thromboembolism and pulmonary artery hypertension following splenectomy in children with hereditary spherocytosis. Pediatr Blood Cancer. 2014;61(1):29–33.
El-Sheikh AA, et al. Congenital dyserythropoietic anemia type I presenting as persistent pulmonary hypertension with pigeon chest deformity. Pediatr Blood Cancer. 2014;61(8):1460–2.
Murdych T, Weisdorf DJ. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone Marrow Transplant. 2001;28(3):283–7.
Dandoy CE, et al. Team-based approach to identify cardiac toxicity in critically ill hematopoietic stem cell transplant recipients. Pediatr Blood Cancer. 2017;64(10)
Steward CG, et al. Severe pulmonary hypertension: a frequent complication of stem cell transplantation for malignant infantile osteopetrosis. Br J Haematol. 2004;124(1):63–71.
Kasow KA, et al. Malignant infantile osteopetrosis and primary pulmonary hypertension: a new combination? Pediatr Blood Cancer. 2004;42(2):190–4.
Bunte MC, et al. Pulmonary veno-occlusive disease following hematopoietic stem cell transplantation: a rare model of endothelial dysfunction. Bone Marrow Transplant. 2008;41(8):677–86.
Trobaugh-Lotrario AD, et al. Pulmonary veno-occlusive disease after autologous bone marrow transplant in a child with stage IV neuroblastoma: case report and literature review. J Pediatr Hematol Oncol. 2003;25(5):405–9.
Mineo G, et al. Pulmonary veno-occlusive disease: the role of CT. Radiol Med. 2014;119(9):667–73.
Rowan CB, O; McArthur J. Non-infectious pulmonary complications of hematopoietic stem cell transplant. J Pediatr Intensive Care. 2014;3:133–46.
Ozyoruk D, et al. Pulmonary arterial hypertension in a child with stage-IV neuroblastoma after autologous hematopoietic stem cell transplantation and review of the literature. Pediatr Transplant. 2015;19(7):E185–8.
Yildirim ZK, et al. Resolution of pulmonary hypertension with low-molecular-weight heparin, steroid, and prostacyclin analogue therapy: could it be early-phase pulmonary veno-occlusive disease? Pediatr Hematol Oncol. 2011;28(6):529–34.
Alioglu B, et al. Pulmonary hypertension in a child with juvenile myelomonocytic leukemia secondary to pulmonary leukemic cell infiltration. Pediatr Hematol Oncol. 2006;23(8):667–75.
Zeilhofer U, et al. Pulmonary hypertension following haematopoietic stem cell transplantation for primary haemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2013;60(3):521–3.
Shankar S, et al. Pulmonary hypertension complicating bone marrow transplantation for idiopathic myelofibrosis. J Pediatr Hematol Oncol. 2004;26(6):393–7.
Berger RM, et al. FUTURE-2: results from an open-label, long-term safety and tolerability extension study using the pediatric FormUlation of bosenTan in pUlmonary arterial hypeRtEnsion. Int J Cardiol. 2016;202:52–8.
Khandaker MH, et al. Pericardial disease: diagnosis and management. Mayo Clin Proc. 2010;85(6):572–93.
Law MA, et al. Novel, long-axis in-plane ultrasound-guided pericardiocentesis for postoperative pericardial effusion drainage. Pediatr Cardiol. 2016;37(7):1328–33.
Versluys AB, et al. Predictors and outcome of pericardial effusion after hematopoietic stem cell transplantation in children. Pediatr Cardiol. 2018;39(2):236–44.
Neier M, et al. Pericardial effusion post-SCT in pediatric recipients with signs and/or symptoms of cardiac disease. Bone Marrow Transplant. 2011;46(4):529–38.
Galderisi M, et al. Cancer therapy and cardiotoxicity: the need of serial Doppler echocardiography. Cardiovasc Ultrasound. 2007;5:4.
Dhakal P, Bhatt VR. Is complement blockade an acceptable therapeutic strategy for hematopoietic cell transplant-associated thrombotic microangiopathy? Bone Marrow Transplant. 2017;52(3):352–6.
Rawlinson E, Bagshaw O. Anesthesia for children with pericardial effusion: a case series. Paediatr Anaesth. 2012;22(11):1124–31.
Markman TM, et al. Electrophysiological effects of anthracyclines in adult survivors of pediatric malignancy. Pediatr Blood Cancer. 2017;64(11)
Nagueh SF, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–60.
Nagiub M, Nixon JV, Kontos MC. Ability of nonstrain diastolic parameters to predict doxorubicin-induced cardiomyopathy: a systematic review with meta-analysis. Cardiol Rev. 2018;26(1):29–34.
Hare JL, et al. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J. 2009;158(2):294–301.
Thavendiranathan P, et al. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: a systematic review. Circ Cardiovasc Imaging. 2013;6(6):1080–91.
Catana C, Guimaraes AR, Rosen BR. PET and MR imaging: the odd couple or a match made in heaven? J Nucl Med. 2013;54(5):815–24.
Cardinale D, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749–54.
Mackay B, et al. Assessment of anthracycline cardiomyopathy by endomyocardial biopsy. Ultrastruct Pathol. 1994;18(1–2):203–11.
Mitani I, et al. Doxorubicin cardiotoxicity: prevention of congestive heart failure with serial cardiac function monitoring with equilibrium radionuclide angiocardiography in the current era. J Nucl Cardiol. 2003;10(2):132–9.
Wassmuth R, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am Heart J. 2001;141(6):1007–13.
Lipshultz SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20(23):4517–22.
El-Shitany NA, et al. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J Card Fail. 2012;18(8):607–13.
Hudson MM, et al. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers version 3.0. 2008. Available from: http://www.survivorshipguidelines.org/pdf/LTFUGuidelines.pdf
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing
About this chapter
Cite this chapter
Ghafoor, S., James, M., Goldberg, J., McArthur, J.A. (2019). Cardiac Dysfunction in Hematology Oncology and Hematopoietic Cell Transplant Patients. In: Duncan, C., Talano, JA., McArthur, J. (eds) Critical Care of the Pediatric Immunocompromised Hematology/Oncology Patient. Springer, Cham. https://doi.org/10.1007/978-3-030-01322-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-01322-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-01321-9
Online ISBN: 978-3-030-01322-6
eBook Packages: MedicineMedicine (R0)