Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by obstruction of major pulmonary arteries due to unresolved or recurrent pulmonary emboli leading to pulmonary hypertension (Moser et al., Circulation 32(3):377–385, 1965; McLaughlin et al., Chest 143(2):324–332, 2013). Pulmonary thromboendarterectomy is an endarterectomy of the proximal pulmonary vascular tree and is the treatment of choice for chronic thromboembolic pulmonary hypertension. Each year, there are between 500 and 2500 patients diagnosed with CTEPH, an estimated 0.1–0.5% of patients who survive pulmonary embolism (Moser et al., Circulation 81(6):1735–1743, 1990; Jamieson and Kapelanski, Curr Probl Surg 37(3):165–252, 2000)
The most common presenting symptom of chronic thromboembolic pulmonary hypertension is exertional dyspnea. The diagnosis is confirmed with echocardiography, right-sided cardiac catheterization, and pulmonary angiogram. Patients with CTEPH, when left untreated, develop a small vessel vasculopathy that mimics idiopathic pulmonary hypertension. Perioperative monitoring includes femoral and radial arterial pressures, processed EEG, pulmonary artery pressures, and transesophageal echocardiography. Anesthetic induction and maintenance are tailored to hemodynamic stability, right ventricular coronary perfusion pressure, and right ventricular support. A multidisciplinary approach is important for the success of this operation involving the specialties of surgery, pulmonary medicine, critical care, cardiology, anesthesiology, and radiology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
Chronic thromboembolic pulmonary hypertension (CTEPH) results from recurrent or residual intraluminal organized fibrotic clot leading to increased pulmonary vascular resistance (PVR), severe PH, and eventually right heart failure (RHF).
-
Incidence of thromboembolic disease is difficult to estimate because of the nonspecific nature of the presenting symptoms and the lack of public awareness of the disorder.
-
Pulmonary thromboendarterectomy is an endarterectomy of the proximal pulmonary vascular tree and is the preferred treatment for chronic thromboembolic pulmonary hypertension.
-
The most common presenting symptom of chronic thromboembolic pulmonary hypertension is exertional dyspnea. The diagnosis is confirmed with echocardiography, right-sided cardiac catheterization, and pulmonary angiogram.
-
Most common complications of PTE procedure are reperfusion pulmonary edema (RPE), pulmonary hemorrhage, and persistent pulmonary hypertension
-
Riociguat is the first FDA-approved medication for treating certain patient subgroups with chronic thromboembolic pulmonary hypertension (CTEPH).
-
Balloon pulmonary angioplasty is an alternative approach to thromboendarterectomy surgery in patients with surgically inaccessible chronic thromboembolic disease.
Introduction
Pulmonary thromboendarterectomy (PTE), a complete endarterectomy of the pulmonary vascular tree, is the definitive treatment for chronic thromboembolic pulmonary hypertension (CTEPH). Pulmonary embolism (PE) is a relatively common cardiovascular event, and in a small percentage of cases, it leads to a chronic condition in which repeated microemboli as well as ongoing inflammatory response lead to accumulation of connective and elastic tissue on the endovascular surface of the pulmonary vessels [1, 2].
Pulmonary thromboembolism is a significant cause of morbidity and mortality worldwide. Acute PE has been estimated to occur in approximately 63 per 100,000 patients per year in the United States with in-hospital mortality occurring in 11.1% [3,4,5]. These statistics probably represent underestimates; however, since in 70–80% of patients in whom the primary cause of death was PE, the diagnosis was unsuspected premortem [6].
If left untreated, the prognosis for patients with CTEPH is poor. In fact, once the mean pulmonary pressure in patients with CTEPH reaches 50 mmHg or more, the 3-year mortality approaches 90% [7]. Although medical management can provide temporary symptomatic relief, it is noncurative and generally ineffective. The only potentially curative options are lung transplantation and PTE, with PTE preferred because of its favorable long-term morbidity and mortality profile.
This chapter, based in large part on the experience at UCSD, provides a review of the natural history of CTEPH, a description of PTE, a discussion of anesthetic factors unique to PTE and CTEPH, and a case discussion on managing massive pulmonary hemorrhage, one of the feared complications of the operation.
Classification of Pulmonary Hypertension
Pulmonary hypertension (PH) is classified by the World Health Organization into five types known as the Evian classification [8]: (1) pulmonary arterial hypertension (PAH); (2) pulmonary venous hypertension typically from left heart disease; (3) PH due to respiratory disease such as chronic bronchitis, emphysema, and hypoxemia; (4) pulmonary hypertension due to embolic disease (CTEPH); and (5) PH caused by diseases affecting the pulmonary vasculature.
Further classification of pulmonary hypertension by Galie et al. [9, 10] is defined by the presence of precapillary and postcapillary PH. Precapillary PH as assessed by right heart catheterization is characterized by mean pulmonary artery pressure (mPAP) >25 mmHg; normal pulmonary capillary wedge pressure (PCWP), i.e., <15 mmHg; and an elevated pulmonary vascular resistance (PVR) more than 300 dynes·s·cm−5. Postcapillary PH, often due to left heart disease, is the most frequent form of PH and is characterized by mPAP >25 mmHg, PCWP >15 mmHg, and normal PVR [11].
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
Most cases of acute PE resolve within weeks and the patient recovers to their previous level of function. However, for unknown reasons, embolic resolution is sometimes incomplete. If the acute emboli are not lysed in 1–2 weeks, the embolic material becomes attached to the pulmonary arterial and arteriolar walls [12]. With time, the embolic material progressively becomes converted to connective and elastic tissue [13]. This chronic obstructive disease may lead to a small vessel arteriolar vasculopathy characterized by excessive smooth muscle cell proliferation in pulmonary arterioles. This vasculopathy is seen in the remaining open vessels, which are subjected to long exposure to high flow and pressure. Pulmonary hypertension results from both mechanical obstruction and from small vessel vasculopathy. Once pulmonary hypertension has developed, patients require expeditious treatment. CTEPH patients generally do not respond well to medical management, which is reserved for patients who are not surgical candidates [14,15,16]. The only curative option is to proceed with surgical removal of the thromboembolic material by means of endarterectomy.
Incidence
The incidence of pulmonary hypertension caused by PE remains unknown. It has been estimated that there are more than 500,000 survivors of symptomatic episodes of acute PE per year [17]. One recent prospective study indicates that thromboembolic disease develops in as many as 3.8% of patients with acute PE [18]. Thus, a conservative estimate is that 19,000 individuals progress to CTEPH in the United States each year. Considering that only 200–300 PTEs are performed annually worldwide, it is clear that acute PE and CTEPH are under-diagnosed, and PTE is underutilized.
Etiologic Factors
No clear etiology has been defined for the development of CTEPH, although hypercoagulability is certainly a risk. Lupus anticoagulant may be detected in approximately 10% of chronic thromboembolic patients, and 20% carry anticardiolipin antibodies, lupus anticoagulant, or both [19]. A recent study has demonstrated that the plasma level of factor VIII, a protein that is associated with both primary and recurrent venous thromboembolism, is elevated in 39% of patients with CTEPH. Analyses of plasma proteins in patients with chronic thromboembolic disease have shown that fibrin from these patients is resistant to thrombolysis in vitro. In this study, the fibrin β chain N-terminus was particularly resistant to thrombolysis, suggesting that it could be responsible for thrombus nonresolution [20].
Case reports and anecdotal experience have suggested links between chronic thromboembolism and previous splenectomy, permanent intravenous catheters, and ventriculoatrial shunts for the treatment of hydrocephalus or chronic inflammatory conditions. In addition to these observations, associations with sickle cell disease, hereditary stomatocytosis, and the Klippel-Trenaunay syndrome have been described [21]. However, the vast majority of cases of CTEPH cannot be traced to a specific known coagulation defect or underlying medical condition.
Pathology and Pathogenesis
Although most individuals with CTEPH are unaware of a past thromboembolic or deep venous thrombosis, CTEPH likely stems from acute embolic episodes that do not completely resolve. Why some patients fail to resolve their emboli is unclear, but a variety of factors may play a role. The volume of acute embolic material may simply overwhelm the lytic mechanisms. The total occlusion of a major arterial branch may prevent lytic material from reaching, and therefore dissolving, the embolus completely. The emboli may be made of substances that cannot be lysed by normal mechanisms. These may include organized fibrous thrombus, fat, or tumor emboli, from stomach, breast, kidney, and right atrial (myxoma) origin. The lytic mechanisms themselves may be abnormal, or some patients may have a hypercoaguable state. Hypercoagulability may result in spontaneous thrombosis within the pulmonary vascular bed, embolization, or lead to proximal propagation of embolic material. With time, the increased pressure and flow of redirected pulmonary blood flow in the previously normal pulmonary vascular bed can create a vasculopathy in the arterioles, similar to that of the Eisenmenger syndrome. This, as well as resulting right-sided heart failure, can lead to an inoperable, lethal situation, so early surgical intervention is recommended.
Clinical Presentation
The most common symptom of CTEPH, as with pulmonary hypertension in general, is exertional dyspnea. This dyspnea is out of proportion to abnormalities found on clinical examination. Syncope is another common symptom of pulmonary hypertension, particularly in patients with advanced disease. Other common findings include chest tightness, hemoptysis, peripheral edema, and early satiety.
The physical signs of pulmonary hypertension are the same regardless of the underlying pathophysiology. Jugular venous distension is common, with prominent V waves. The right ventricle is usually palpable near the lower left sternal border, and pulmonary valve closure may be audible in the second intercostal space. Patients with advanced disease may be cyanotic. A systolic murmur characteristic of tricuspid regurgitation is common, and murmurs over the lung fields resulting from turbulent flow in the pulmonary vessels may also be appreciated.
Workup may include chest radiograph (CXR), pulmonary function testing, right heart catheterization with pulmonary angiography, high-resolution magnetic resonance imaging, arterial blood gas analysis, ventilation/perfusion scanning, and echocardiography. CXR may show lung opacities suggestive of previous scarring, hyperlucent areas suggestive of regional decreased blood flow, right-sided cardiomegaly, and dilatation of the pulmonary vessels (Fig. 49.1). Diffusing capacity (DLCO) is often reduced and may be the only abnormality on pulmonary function testing. Pulmonary arterial pressure is elevated, sometimes being supra-systemic. Resting cardiac output is often low, with reduced pulmonary arterial oxygen saturation. Many patients exhibit hypoxia, particularly with exercise; room air arterial oxygen tension ranges between 50 and 83 torr, the average being 65 torr [22]. CO2 tension is often slightly reduced, although dead space ventilation is increased. Ventilation-perfusion studies show moderate mismatch but correlate poorly with the degree of pulmonary vascular obstruction [23].
Transthoracic echocardiography is often the first study to provide clear evidence of pulmonary hypertension. An estimate of pulmonary artery systolic pressure is often provided by Doppler of the tricuspid regurgitant envelope. Echocardiographic findings vary depending on the stage of the disease and include right ventricular enlargement, leftward displacement of the interventricular septum, and encroachment of the enlarged right ventricle on the left ventricular cavity with abnormal systolic and diastolic function of the left ventricle. Thankfully, many of these abnormalities resolve after successful PTE [24]. Contrast echocardiography may demonstrate a persistent foramen ovale, the result of high right atrial pressures opening the previously closed intra-atrial communication.
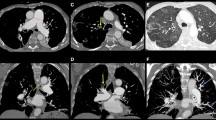
Pulmonary angiography is the gold standard for defining pulmonary vascular anatomy and is performed to confirm the diagnosis and to determine the location and surgical accessibility of thromboembolic disease. In angiographic imaging, thrombi appear as unusual filling defects, pouches, webs, or bands, or completely thrombosed vessels that may resemble congenital absence of a vessel (Fig. 49.2). More recently, high-resolution computed tomography scanning [25], SPECT-CT fusion imaging [26], and magnetic resonance angiography [27] have been used successfully to screen patients with suspected thromboembolic disease.
In approximately 10% of cases, the differential diagnosis between primary pulmonary hypertension and distal and small vessel pulmonary thromboembolic disease remains unclear and difficult to establish. In these patients, pulmonary angioscopy is often helpful. The pulmonary angioscope is a fiberoptic scope that is placed through a central line into the pulmonary artery. The tip contains a balloon that is then filled with saline and pushed against the vessel wall. A bloodless field can thus be obtained to view the pulmonary artery wall. The classic appearance of chronic pulmonary thromboembolic disease by angioscopy consists of intimal thickening, with intimal irregularity and scarring, and webs across small vessels. The presence of embolic disease, occlusion of vessels, or the presence of thrombotic material is diagnostic.
Many CTEPH patients have longstanding pulmonary hypertension; as many as 37% of them receive medical pulmonary vasodilator therapy [28]. This therapy may consist of phosphodiesterase 5 inhibition (e.g., sildenafil) [29], endothelin-1 inhibition (e.g., bosentan) [30, 31], and prostacyclin analogs (e.g., iloprost, flolan, remodulin) [32,33,34]. It is prudent to continue these medications preoperatively and to consider their use postoperatively if the surgical result is suboptimal. Abrupt cessation of a prostacyclin analog can result in potentially catastrophic rebound pulmonary hypertension [35]. If a patient presents with an epoprostenol (Flolan™) infusion, one approach is to continue this infusion throughout the pre-CPB period, discontinue it during CPB, and restart it after CPB if the surgical result is suboptimal. If the surgical result is good, keep it available to be restarted if pulmonary hypertension develops postoperatively.
The Surgical Procedure
Surgical Approach and Technique
PTE, being an endarterectomy of the proximal pulmonary vascular tree, is performed through a midline sternotomy and requires cardiopulmonary bypass (CPB) with deep hypothermic circulatory arrest (DHCA). Although used in the past, lateral thoracotomy is suboptimal [36]. Median sternotomy allows treatment of both pulmonary arteries, which is necessary in almost all cases [36, 37]. The use of CPB with periods of complete circulatory arrest provides the bloodless operative field necessary for complete meticulous lobar and segmental dissections [38].
The procedure follows four basic but important principles. (1) The endarterectomy must be bilateral; therefore the approach is through a median sternotomy. (2) Identification of the correct dissection plane is crucial, and at times the plane of dissection has to be identified in each of the segmental and subsegmental branches. (3) Perfect visualization is essential, and a thorough distal endarterectomy cannot be performed without the use of circulatory arrest. Circulatory arrest is usually limited to 20 min at a time, and supported by cooling to 18 °C. (4) A complete endarterectomy all the way to the distal ends of the smallest vessels is essential.
Following median sternotomy, CPB is established with cannulation of the ascending aorta and the inferior and superior vena cava. Cooling is instituted immediately. A gradient of not more than 10 °C is maintained between the arterial blood and the bladder/rectal temperature. This allows an even distribution of cooling and warming, as well as helping to prevent release of gas bubbles into the circulation upon rewarming. Pulmonary artery and pulmonary venous vents are inserted. During the cooling phase venous oxygen saturation increases, with a saturation of 80% typical at 25 °C, and 90% at 20 °C. Hemodilution to a hematocrit of 18–25% is utilized to decrease blood viscosity, optimize capillary blood flow, and promote uniform cooling. Complete cooling typically requires 45–60 min, depending on the size and perfusion characteristics of the patient.
As core temperature approaches 20 °C and tympanic membrane temperature approaches 16–18 °C, the aorta is cross-clamped. Immediately after aortic cross-clamping, cardioplegia solution is administered into the aortic root. Additional myocardial protection is afforded by a circulating cold water cooling jacket around the heart. An incision is made in the right pulmonary artery with the surgeon standing on the patient’s left. The right pulmonary artery endarterectomy plane is established and dissection continues until bronchial artery flow impairs good visualization. At this point circulatory arrest is imperative. Bronchial flow in these patients is frequently substantial and without circulatory arrest complete endarterectomy cannot be accomplished.
Circulatory arrest is limited to 20-min epochs. An experienced surgeon can usually accomplish the entire unilateral endarterectomy within this time period. If additional arrest time is necessary, reperfusion is carried out at 18 °C core temperature for a minimum of 10 min. At the completion of the endarterectomy, perfusion is reestablished, while the pulmonary artery incision is closed.
Following a 10-min period of hypothermic perfusion, the left pulmonary artery is incised, and an endarterectomy is performed. Following completion of the left endarterectomy, a patent foramen ovale (PFO), if present, is repaired. Any additional procedures such as coronary artery bypass grafting or valve replacement can be performed during the rewarming period.
Surgical Subtypes
There are five categories of pulmonary occlusive disease related to disease extent that can be appreciated. The UCSD classification system describes these different levels based on the thromboembolic specimen and corresponds to the degree of difficulty of the endarterectomy [39] (Table 49.1).
Level 0 represents no evidence of chronic thromboembolic disease present; in other words, there has been a misdiagnosis, or perhaps one lung is completely unaffected by thromboembolic disease, both of which are rare. In this entity there is intrinsic small vessel disease, although secondary thrombus may occur as a result of stasis. Small vessel disease may be unrelated to thromboembolic events (“primary” pulmonary hypertension) or occurs in relation to thromboembolic hypertension as a result of a high-flow or high-pressure state in previously unaffected vessels similar to the generation of Eisenmenger’s syndrome. We believe that there may also be sympathetic “cross-talk” from an affected contralateral side or stenotic areas in the same lung. Level I (Fig. 49.3) disease refers to the situation in which thromboembolic material is present and is readily visible on the opening of the main left and right pulmonary arteries. A subset of level I disease, level Ic, is complete occlusion of either the left or right pulmonary artery and non-perfusion of that lung. Complete occlusion may present an entirely different disease, especially when it is unilateral and on the left side. In level II (Fig. 49.4), the disease starts at the lobar or intermediate level arteries and the main pulmonary arteries are unaffected. Level III (Fig. 49.5) disease is limited to thromboembolic disease originating in the segmental vessels only. Level IV (Fig. 49.6) is disease of the subsegmental vessels, with no other disease appreciated at more proximal levels. Level III and level IV disease present the most challenging surgical situation. The disease is very distal and confined to the segmental and subsegmental branches.
Anesthetic Management
Setup and Preparation
A typical “setup” for a pulmonary endarterectomy includes preparation for transesophageal echocardiography (TEE), pulmonary artery catheterization, hemodynamic support, cerebral function monitoring, and a cooling device for head cooling. On the day of surgery, a large bore peripheral intravenous catheter and a radial arterial catheter are inserted. The patient may then be given light sedation (with caution) and brought to the operating room. Even small amounts of sedation may cause respiratory depression, leading to a catastrophic rise in pulmonary vascular resistance. Supplemental oxygen should be considered in the preoperative area, particularly if sedation is administered.
Anesthetic Induction and Pre-CPB Management
After thorough preoxygenation and ventilation encouragement, anesthetic induction can be accomplished with midazolam, fentanyl, and a muscle relaxant. Myocardial depressants such as propofol should be used with extreme caution, if at all. In cases of tenuous hemodynamics, etomidate may be useful because of its relative lack of cardiovascular depression. A pulmonary artery catheter is generally placed after induction rather than before, since the hemodynamic status and goals are usually known by the time the patient reaches the operating room. Also, lying awake in the supine or Trendelenburg position may be stressful for patients with advanced disease, occasionally leading to cardiorespiratory instability. If preoperative transthoracic echocardiography shows evidence of right atrial or right ventricular thrombi, TEE is performed immediately after induction, prior to placement of a pulmonary artery catheter.
Although some patients with CTEPH presenting for pulmonary endarterectomy have associated left ventricular pathology, most do not. Hemodynamic management is thus centered on right ventricular function. The right ventricle is usually hypertrophic and dilated, as is the right atrium. Because of the high right-sided pressures, the coronary blood supply to right ventricle is at risk. Maintenance of adequate systemic vascular resistance (SVR), inotropic state, and normal sinus rhythm serve to preserve systemic hemodynamics as well as right ventricular coronary perfusion. The preoperative cardiac catheterization data, including cardiac output, pulmonary vascular resistance (PVR), patency of coronary arteries, and right ventricular end-diastolic pressure (RVEDP) are useful in planning the induction sequence. Elevated RVEDP (>14 mmHg), severe tricuspid regurgitation, and preoperative PVR > 1000 dyne-s-cm−5 are signs of impending decompensation. In such cases inotropic support (e.g., dopamine or epinephrine), as well as vasopressor support (e.g., phenylephrine or vasopressin), should be considered for the induction and pre-CPB period. Generally, patients with CTEPH have fixed PVR because of mechanical obstruction. However, high PVR can still be exacerbated by factors that increase PVR (e.g., hypoxia, hypercarbia, acidosis, pain, and anxiety). Thus, these stressors should be minimized during induction and immediate pre-CPB period. Attempts to lower the PVR pharmacologically (e.g., nitroglycerin, nitroprusside) should be avoided as they have minimal efficacy in treating CTEPH and can dangerously jeopardize the coronary perfusion pressure to the right ventricular myocardium. This can rapidly lead to hypotension and cardiovascular collapse. Direct pulmonary vasodilators such as nitric oxide and prostaglandins, which may be useful in the medical management of patients with other types of pulmonary hypertension, generally show limited benefit for pulmonary endarterectomy patients in the perioperative period. The effects of phenylephrine on right ventricular performance in pulmonary hypertension has been studied by Rich et al. [40] They documented improved right ventricular performance (increased MAP, coronary artery perfusion pressure, maintained cardiac output) with phenylephrine administration. Since hemodynamic collapse can occur very rapidly in these patients, it is particularly important to treat decreases in blood pressure and heart rate rapidly and aggressively. The muscle relaxant is chosen according to airway issues and desired hemodynamic response. Pancuronium, rocuronium, and vecuronium have all been used successfully in these patients.
If the superior vena cava is patent, an internal jugular introducer and pulmonary artery catheter are inserted. Placement of the pulmonary artery catheter may be difficult because of right atrial and right ventricular dilatation, as well as tricuspid regurgitation and pulmonary artery pathology. Transesophageal echocardiography has been shown to be helpful in the live guidance of pulmonary arterial catheter placement in CTEPH patients [41].
Next, a femoral arterial catheter is placed. This is because, in cases involving prolonged hypothermic CPB, the systemic arterial pressure is significantly underestimated by the radial artery catheter in the post-CPB period [42]. This phenomenon has been noticed by others [43] and appears to be accentuated by prolonged periods of profound hypothermia. It is not uncommon for a mean arterial pressure (MAP) gradient of as much as 20 mmHg to develop after CPB. The mechanism is unclear; causes involving peripheral vasoconstriction and vasodilatation have been proposed [44]. Although the time course for recovery of the radial arterial wave is variable, typically the radial and femoral pressure measurements show reasonable agreement by the morning following surgery [42].
TEE is valuable in monitoring and assessing cardiac function and filling during PTE. The most useful views include the transgastric mid-papillary short-axis view to assess left ventricular size and septal motion (Fig. 49.7; Video 49.1); the midesophageal four-chamber view for relative chamber sizes, intracardiac thrombus, and tricuspid valve assessment (Fig. 49.8; Video 49.2); the midesophageal bicaval view (interatrial septal integrity, thrombosis of the great veins); and the midesophageal ascending aortic short-axis view for size of the pulmonary artery (PA) and detecting PA thrombus. It is not uncommon to find substantial dilatation of the PA, as well as thromboembolic material (Fig. 49.9; Video 49.3). The integrity of the interatrial septum is investigated with the use of an agitated saline test. PFO is present in 25–35% of PTE patients [45]. If a PFO is present, it is repaired, since, postoperatively some patients may experience high right-sided pressures. Such pressures, in the presence of a PFO, could lead to right-to-left shunt and hypoxemia.
Processed electroencephalogram (EEG) is monitored throughout the procedure. This allows confirmation of minimal oxygen utilization of the brain prior to circulatory arrest (isoelectric EEG), as well as monitoring of level of consciousness during normothermia. In our institution the SedLine monitor (Masimo, Irvine, CA), a four-channel processed electroencephalograph monitor, provides monitoring of the isoelectric electroencephalogram and confirmation of minimal oxygen utilization of the brain before circulatory arrest. Temperature monitoring is accomplished with a urinary catheter with temperature monitoring capabilities, a rectal probe, and tympanic membrane probe, which provides an estimation of brain temperature [46]. The rectal and bladder probes estimate core temperature, and the PA catheter measures blood temperature, allowing quantification of thermal gradients.
During the precardiopulmonary bypass period, the head is wrapped in a circulating cold water blanket. The water, maintained at 4 °C, is circulated through the blanket by an electric pump. This system (Polar Care, Breg, Inc., Vista, CA), originally designed as a “knee wrap” for orthopedic and physical medicine purposes, is easily applied to the head. It contains a thermometer within the fluid circulation system for confirmation of adequate blanket cooling, as well as a flow control dial. This head wrap is used in all PTEs at UCSD, with no complications. It is our belief that the blanket provides better cooling to the surface of the cranium, particularly posterior regions, than application of ice bags, and is easier to apply.
If the hematocrit and hemodynamics permit, 1–2 units of autologous blood are harvested for reinfusion after CPB. Another consideration is prior exposure and response to heparin. Because of prior exposure, some patients develop heparin-induced antiplatelet antibodies, causing a propensity to heparin-induced thrombocytopenia. Anticoagulation for these patients has been managed with preheparin administration of iloprost (a prostacyclin analog), heparinoid [37, 38], hirudin, and bivalirudin [47, 48]. Most recently, we have had success using the platelet-inhibitor tirofiban [49, 50].
Management of Deep Hypothermic Circulatory Arrest (DHCA)
Prior to DHCA mannitol (12.5 g), methylprednisolone sodium succinate (30 mg/kg; maximum dose of 3 g), and phenytoin sodium (15 mg/kg) are administered. Mannitol is used to promote an osmotic diuresis, minimize cellular edema, and for free radical scavenging. Methylprednisolone theoretically functions as a cell-membrane stabilizer and anti-inflammatory agent. Phenytoin may provide some protection against postoperative seizures. Historically sodium thiopental (6 mg/kg) was administered as a cerebral protection agent. Due to sodium thiopental’s lack of commercial availability, propofol (2.5 mg/kg) is utilized to ensure complete isoelectricity immediately prior to instituting deep hypothermic circulatory arrest. While there is no clear clinical evidence supporting added benefit of propofol or barbiturate administration for DHCA, we give propofol for three reasons: (1) brain cooling may be uneven or incomplete, (2) cerebral emboli may occur during rewarming (pulmonary endarterectomy is an “open-chamber” procedure), and (3) even at 18 °C we often notice sparse EEG activity which is then abolished with administration of propofol.
After assurance of an isoelectric EEG, tympanic membrane temperature 18 °C or less, and a bladder or rectal temperature of 20 °C or less, circulatory arrest is instituted. At this time, all monitoring lines are turned off to the patient, decreasing the risk of entraining air into the vasculature during exsanguination. The duration of DHCA is limited to 20 min epochs, typically one epoch per left and right pulmonary endarterectomy, respectively. If additional time is needed on either side, hypothermic circulation is reestablished for 10 min prior to additional periods of DHCA.
Monitoring jugular venous bulb oxygen saturation may be useful in detecting adverse cerebral effects of rapid warming [51] or for prognosticating postoperative neurologic function [52]. However, since our warming rate is slow, and our neurologic results are good, we choose not to expose our patients to the added risks of jugular venous bulb catheterization. Surface cerebral oximetry is a noninvasive technique applying near-infrared spectroscopy to measure hemoglobin oxygen saturation in the brain underlying the sensor. The number reported by the monitor is rSO2, which is a measure of the mixed arterial and venous blood in the brain. Since venous blood volume accounts for 70–90% of total cerebral blood volume, rSO2 reflects oxygen saturation of venous blood and thus the relationship between cerebral oxygen metabolism (demand) and cerebral blood flow (supply). In healthy volunteers, rSO2 has been found to correlate with jugular venous saturation [53, 54], although, during cardiac surgery, the correlation between the two monitors is not always close [55]. Ongoing research in this area and additional neuropsychiatric outcome studies may prove this monitor useful during the conduct of DHCA.
Post-DHCA Rewarming
A 10° gradient between blood and bladder/rectal temperature is not exceeded during rewarming, and the perfusate temperature is never greater than 37.5°. Warming too quickly promotes systemic gas bubble formation, cerebral oxygen desaturation, and uneven warming. Rewarming times are variably related to the patient’s weight and systemic perfusion; 90–120 min is usually required to achieve a core temperature of 36.5 °C. Adequate and even rewarming aims to prevent “after-drop”, whereby uneven rewarming redistributes post-CPB leading to a drop in temperature with the attendant risks of hypothermia.
Separation from CPB
With the following few exceptions, the process of separation from CPB is similar to other surgeries involving CPB. End-tidal carbon dioxide (ETCO2) is a poor measure of ventilation adequacy in these patients both pre- and post-CPB, since dead space ventilation is an integral part of the disease process. After successful surgery, the arterial-ETCO2 gradient may be decreased compared to preoperative values, but the time course for this improvement is variable often with weeks to months for complete resolution. Aggressive hyperventilation is utilized due to the metabolic acidosis that may occur from cardiopulmonary bypass, hypothermia, and deep hypothermic circulatory arrest. The anesthesiologist checks the endotracheal tube for frothy sputum or bleeding because reperfusion pulmonary edema and airway bleeding, two of the most dreaded complications of the procedure, may manifest at this time [56]. Suction of the endotracheal tube during rewarming (while the surgical PA vent remains in place) allows the early identification of bleeding prior to pressurization of the pulmonary arterial circuit. While still on CPB, the TEE is used to detect intracavitary air as well as to evaluate left and right ventricular function.
For separation from CPB, modest inotropic support (e.g., dopamine, 3–7 μg/kg/min) is often necessary because of the long hypothermic period and long aortic cross-clamp time. In patients with particularly poor ventricular function epinephrine 0.04–0.15 μg/kg/min is added. Discussion with the surgical team regarding CTEPH classification and the success of the endarterectomy should occur prior to separation. If the surgery has only been partially successful because of small vessel disease, pulmonary vasodilators such as milrinone, intravenous prostacyclin, and nitric oxide are considered. If the surgery has been successful, the TEE reveals immediate improvements in the left- and right-sided geometry [57, 58]. The distention of the right atrium and right ventricle is greatly decreased, resulting in improvement of function of both ventricles (Fig. 49.10a, b; Videos 49.4 and 49.5). Tricuspid regurgitation, if it was present before the endarterectomy, has greatly decreased or resolved. Significant improvement in hemodynamic status is usually noted, including a doubling of the cardiac index, dramatic decrease in PA pressures, and a drop in the PVR to 25% of the preoperative value [4].
(a) Midesophageal four-chamber view in a patient with CTEPH prior to pulmonary thromboendarterectomy. Note the dilated right heart, deviated septums, and underfilled left heart. RA right atrium, RV right ventricle, LV left ventricle. (b) Midesophageal four-chamber view of the same patient status post-pulmonary thromboendarterectomy and tricuspid valve repair. Note the decompression of the RA and RV with increased left heart size. RA right atrium, RV right ventricle, LV left ventricle
Post-CPB Management
After heparin reversal, bleeding diathesis is rare, and transfusion requirements are usually minimal [4]. Antifibrinolytic agents such as ε-amino-caproic acid are not routinely used for pulmonary endarterectomy in our institution. Two procedure-related complications may potentially present themselves immediately upon separation from CPB. Pink frothy sputum, if present, likely indicates the onset of reperfusion pulmonary edema. In this case, the endotracheal tube is suctioned, and increasing amounts of positive end-expiratory pressure (PEEP) are applied beginning with 5 cmH2O escalating to 8 and 10 cmH2O. The volume of pulmonary edema can be profound requiring frequent anesthesia circuit changes and maintenance of high levels of PEEP. If oxygenation and ventilation are significantly impaired, the resultant hypoxia and hypercarbia can lead to worsening RV dysfunction. Venovenous extracorporeal membrane oxygenation may be considered to improve the hypoxia and hypercarbia.
Secondly, if frank blood is emanating from the endotracheal tube, disruption of the blood-airway barrier has likely occurred secondary to surgical injury. The approach to this clinical scenario often begins with the surgeon’s high index of suspicion of adventitial disruption. Prior to pressurizing the pulmonary arterial circuit via reduction of the PA vent, the anesthesiologist should pass an airway suction catheter. Identification of blood in the endotracheal tube warrants a fiberoptic bronchoscopy. If identification of pulmonary hemorrhage occurs after pressurization of the pulmonary arteries during cardiac ejection, localization of the culprit segment may be difficult. With the assistance of the surgical team and perfusionist, a slow reinstitution of cardiac ejection while concurrently visualizing via bronchoscopy can identify the specific lesion location. Management subsequently includes lung or lobar isolation via bronchial blocker placement, PEEP, separation from CPB, reversing anticoagulation, and treatment of coagulopathy. Assuming adequate oxygenation, ventilation, and coagulation, bronchial blocker balloon deflation may occur under direct fiberoptic visualization [59].
Significant pulmonary hemorrhage may lead to inadequate oxygenation, ventilation, and potential subsequent cardiac dysfunction prompting the use of various methods of extracorporeal life support. The choice of support depends upon the clinical scenario as outlined in the algorithm in Fig. 49.11. With preserved cardiac function, oxygenation and ventilation may be assisted via venovenous extracorporeal membrane oxygenation (ECMO). Utilization of the Avalon Elite Bicaval Dual-Lumen Catheter (Maquet, Rastatt, Germany) via the right internal jugular vein allows the institution of venovenous ECMO with minimal or no anticoagulation, an advantage in the setting of massive pulmonary hemorrhage [59]. As RV dysfunction develops, ECMO may be employed with right atrial inflow and pulmonary arterial outflow. Lastly if biventricular failure ensues, venoarterial ECMO may be employed.
Algorithm for the management of post-pulmonary thromboendarterectomy pulmonary hemorrhage. CPB cardiopulmonary bypass, FOB fiberoptic bronchoscopy, ECMO extracorporeal membrane oxygenation, LV left ventricular, PA pulmonary artery, RA right atrial, RV right ventricular, va venoarterial, vv venovenous. (Reprinted with permission from Cronin et al. [59])
Postoperative Management
Intensive care unit management is similar to other post-cardiac surgical patients with a few exceptions. Two major postoperative complications unique to PTE that can present in the ICU are reperfusion pulmonary edema and pulmonary arterial steal. Reperfusion pulmonary edema is a localized form of high-permeability (noncardiogenic) lung injury, a form of adult respiratory distress syndrome, localized to the area of lung having received the endarterectomy. It usually occurs within the first 24 h but may appear up to 72 h following PTE [60]. In most cases it is mild; reperfusion edema resulting in clinically significant morbidity occurs in only 10% of cases. In its most severe form, it begins immediately post-CPB, in the operating room as described above. These patients are often extremely ill, requiring aggressive intensive care and ventilator management. Pressure control, PEEP, and inverse ratio ventilation are used judicially in an effort to improve V/Q matching and minimize further pulmonary injury. Occasionally extracorporeal support is required [61, 62]. Pulmonary arterial steal represents a postoperative redistribution of pulmonary arterial blood away from the previously well-perfused segments into the newly endarterectomized segments [63]. Whether the cause is failure of autoregulation in the newly endarterectomized segments or secondary small vessel, changes in the previously open segments have not been clarified. However, long-term follow-up has documented a decrease in pulmonary vascular steal in the majority of patients, suggesting a remodeling process in the pulmonary vascular bed [64].
Other postoperative complications are rare but can include pulmonary hemorrhage (0.4%), neurologic sequelae (0.4%), mediastinal bleeding (3.5%), GI bleeding (1.6%), atrial fibrillation (2.6%), renal failure requiring renal replacement therapy (1%), and sepsis (1.2%) [65].
PTE patients usually awaken within 1–2 h after surgery, and a brief neurologic examination is performed. The patient is then sedated with a propofol infusion and analgesics. They remain intubated overnight, since the onset of reperfusion pulmonary edema may be delayed. If pulmonary, cardiac, and neurologic function is good, and there is no bleeding diathesis, extubation occurs the following morning. Discharge from the intensive care unit typically occurs on the second or third postoperative day, and the patients are usually discharged from the hospital 1 week after the operation.
Outcome After PTE
There has been steady improvement in mortality rate at UCSD since 1980, with current perioperative mortality rate being less than 3% (Fig. 49.12). We believe these result from improvements in preoperative preparation, surgical technique, anesthetic care, perfusion technique, and postoperative management. The positive effect of experience, in the form of case volume, on outcome has been well documented for other types of complicated surgery, such as liver transplantation [66]. In addition, we have developed close collaboration between the Pulmonary Medicine, Cardiac Surgery, and Anesthesiology departments. This “team approach,” we believe, is absolutely essential to a successful PTE program.
With this operation, a reduction in pulmonary pressures and resistance to normal levels and corresponding improvement in pulmonary blood flow and cardiac output are generally immediate and sustained [65, 67, 68]. Mortality rate and improvements in hemodynamics depend heavily on surgical subtype, with CTEPH Type 1 and 2 fairing better than Types 3 and 4. Type 0, not being CTEPH but rather small vessel disease, is associated with poor outcome [65]. There is a trend of patients presenting with more segmental and subsegmental disease as identified by Madani et al [69]. Despite a trend of patients presenting with more distal disease with its attendant increased surgical complexity, our experience continues to demonstrate a dramatic reduction in pulmonary arterial pressures and pulmonary vascular resistance (Table 49.2). Patients who have undergone a successful PTE enjoy long-term benefit. Typically patients preoperatively present as New York Heart Association (NYHA) class III or IV and often maintain NYHA I and II function indefinitely following the operation [70].
Future
While surgical management of CTEPH continues to be the proven mainstay of treatment, there are continued advances both in medical management and percutaneous treatments of these patients. Adempas® (Riociguat) is an FDA-approved drug which acts via guanylate cyclase and nitric oxide; the drug is a pulmonary vasodilator specifically indicated for the treatment of residual or recurrent pulmonary hypertension after PTE or those with inoperable CTEPH. Its role and the role of other pulmonary vasodilators (i.e., phosphodiesterase inhibitors, endothelin antagonists, prostaglandins, etc.) in the treatment are continuing to be elucidated. The exact role of medical management prior to surgical PTE still remains unknown.
The experience with percutaneous balloon pulmonary angioplasty (BPA) has continued to grow either as an alternative to those patients with surgically inaccessible CTEPH or those with high perioperative risk due to comorbidities. Reperfusion pulmonary edema and pulmonary vascular injury remain concerns with this percutaneous technique as well. The exact role of BPA in the management of CTEPH patients requires further study.
Research to determine the etiology of CTEPH, as well as the mechanisms and factors leading to reperfusion pulmonary edema, vascular steal, and ischemic neurologic injury continues. Understanding these processes will most likely lead to improved prophylaxis and treatment. Anesthesiologists, in particular, will be an integral part of future research on the immediate perioperative period. This will include efforts to improve the management of residual “small vessel disease,” right ventricular failure, cerebral function and oxygenation monitoring, postoperative pulmonary edema, pulmonary bleeding, and organ protection.
References
Moser KM, Houk VN, Jones RC, Hufnagel CC. Chronic, massive thrombotic obstruction of the pulmonary arteries: analysis of four operated cases. Circulation. 1965;32(3):377–85.
McLaughlin VV, Langer A, Tan M, et al. Contemporary trends in the diagnosis and management of pulmonary arterial hypertension. Chest. 2013;143(2):324–32.
Moser KM, Auger WR, Fedullo PF. Chronic major-vessel thromboembolic pulmonary hypertension. Circulation. 1990;81(6):1735–43.
Jamieson SW, Kapelanski DP. Pulmonary endarterectomy. Curr Probl Surg. 2000;37(3):165–252.
DeMonaco NA, Dang Q, Kapoor WN, Ragni MV. Pulmonary embolism incidence is increasing with use of spiral computed tomography. Am J Med. 2008;121(7):611–7.
Lindblad B, Eriksson A, Bergqvist D. Autopsy-verified pulmonary embolism in a surgical department: analysis of the period from 1951 to 1988. Br J Surg. 1991;78(7):849–52.
Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism. Chest. 1982;81(2):151–8.
Rich S Rubin L, Abenhail L, et al. Executive summary from the World Symposium on Primary Pulmonary Hypertension. Paper presented at: World Symposium on Primary Pulmonary Pulmonary Hypertension; September 6–10, 1998, Evian; 1998.
Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34(6):1219–63.
Dadfarmay S, Berkowitz R, Kim B, Manchikalapudi RB. Differentiating pulmonary arterial and pulmonary venous hypertension and the implications for therapy. Congest Heart Fail. 2010;16(6):287–91.
Bossone E, D’Andrea A, D’Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr. 2013;26(1):1–14.
Bernard J, Yi ES. Pulmonary thromboendarterectomy: a clinicopathologic study of 200 consecutive pulmonary thromboendarterectomy cases in one institution. Hum Pathol. 2007;38(6):871–7.
Guillinta P, Peterson KL, Ben-Yehuda O. Cardiac catheterization techniques in pulmonary hypertension. Cardiol Clin. 2004;22(3):401–15.
Post MC, Plokker HWM, Kelder JC, Snijder RJ. Long-term efficacy of bosentan in inoperable chronic thromboembolic pulmonary hypertension. Neth Hear J. 2009;17(9):329–33.
Vassallo FG, Kodric M, Scarduelli C, et al. Bosentan for patients with chronic thromboembolic pulmonary hypertension. Eur J Intern Med. 2009;20(1):24–9.
Jaïs X, D’Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2008;52(25):2127–34.
Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975;17(4):259–70.
Pengo V, Lensing AWA, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–64.
Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345(20):1465–72.
Bonderman D, Turecek PL, Jakowitsch J, et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2003;90:372–6.
Lang IM. Chronic thromboembolic pulmonary hypertension — not so rare after all. N Engl J Med. 2004;350(22):2236–8.
Kapitän KS, Buchbinder M, Wagner PD, Moser KM. Mechanisms of hypoxemia in chronic thromboembolic pulmonary hypertension. Am Rev Respir Dis. 1989;139(5):1149–54.
Moser KM. Thromboendarterectomy for chronic, major-vessel thromboembolic pulmonary hypertension. Ann Intern Med. 1987;107(4):560.
D’Armini AM, Zanotti G, Ghio S, et al. Reverse right ventricular remodeling after pulmonary endarterectomy. J Thorac Cardiovasc Surg. 2007;133(1):162–8.
Reichelt A, Hoeper MM, Galanski M, Keberle M. Chronic thromboembolic pulmonary hypertension: evaluation with 64-detector row CT versus digital substraction angiography. Eur J Radiol. 2009;71(1):49–54.
Suga K, Kawakami Y, Iwanaga H, Hayashi N, Seto A, Matsunaga N. Comprehensive assessment of lung CT attenuation alteration at perfusion defects of acute pulmonary thromboembolism with breath-hold SPECT-CT fusion images. J Comput Assist Tomogr. 2006;30(1):83–91.
Nikolaou K, Schoenberg SO, Attenberger U, et al. Pulmonary arterial hypertension: diagnosis with fast perfusion MR imaging and high-spatial-resolution MR angiography—preliminary experience. Radiology. 2005;236(2):694–703.
Jensen KW, Kerr KM, Fedullo PF, et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation. 2009;120(13):1248–54.
Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361(19):1864–71.
Rubin LJ, Badesch DB, Barst RJ. Bosentan therapy for pulmonary arterial hypertension. ACC Curr J Rev. 2002;11(5):30.
Confalonieri M, Kodric M, Longo C, Vassallo FG. Bosentan for chronic thromboembolic pulmonary hypertension. Expert Rev Cardiovasc Ther. 2009;7(12):1503–12.
Nagaya N, Sasaki N, Ando M, et al. Prostacyclin therapy before pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension*. Chest. 2003;123(2):338–43.
Ono F, Nagaya N, Okumura H, et al. Effect of orally active prostacyclin analogue on survival in patients with chronic thromboembolic pulmonary hypertension without major vessel obstruction. Chest. 2003;123(5):1583–8.
Vizza CD, Badagliacca R, Sciomer S, et al. Mid-term efficacy of Beraprost, an Oral prostacyclin analog, in the treatment of distal CTEPH: a case control study. Cardiology. 2006;106(3):168–73.
Augoustides JG, Culp K, Smith S. Rebound pulmonary hypertension and cardiogenic shock after withdrawal of inhaled prostacyclin. Anesthesiology. 2004;100(4):1023–5.
Jamieson SW. Pulmonary thromboendarterectomy. Heart. 1998;79(2):118–20.
Fedullo PF, Auger WR, Channick RN, Moser KM, Jamieson SW. Chronic thromboembolic pulmonary hypertension. Clin Chest Med. 1995;16(2):353–74.
Jamieson SW, Auger WR, Fedullo PF, et al. Experience and results with 150 pulmonary thromboendarterectomy operations over a 29-month period. J Thorac Cardiovasc Surg. 1993;106(1):116–26; discussion 126–7.
Madani M. Surgical Treatment of Chronic Thromboembolic Pulmonary Hypertension: Pulmonary Thromboendarterectomy. Methodist Debakey Cardiovasc J. 2016; 12(4): 213–218.
Rich S, Gubin S, Hart K. The effects of phenylephrine on right ventricular performance in patients with pulmonary hypertension. Chest. 1990;98(5):1102–6.
Cronin B, Robbins R, Maus T. Pulmonary artery catheter placement using transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2017;31(1):178–83.
Manecke GR, Parimucha M, Stratmann G, et al. Deep hypothermic circulatory arrest and the femoral-to-radial arterial pressure gradient. J Cardiothorac Vasc Anesth. 2004;18(2):175–9.
Mohr R, Lavee J, Goor D. Inaccuracy of radial artery pressure measurement after cardiac operations. Surv Anesthesiol. 1988;32(1):1.
Urzua J. Aortic-to-radial arterial pressure gradient after bypass. Anesthesiology. 1990;73(1):191.
Dittrich HC, McConn HA, Wilson WC. Identification of interatrial communication in patients with elevated right atrial pressure using surface and transesophageal contrast echocardiography. J Am Coll Cardiol. 1993;21(Suppl):135A.
Schuhmann MU, Suhr DF, v Gösseln HH, Bräuer A, Jantzen J-P, Samii M. Local brain surface temperature compared to temperatures measured at standard extracranial monitoring sites during. J Neurosurg Anesthesiol. 1999;11(2):90–5.
Riess F-C, Löwer C, Seelig C, et al. Recombinant hirudin as a new anticoagulant during cardiac operations instead of heparin: successful for aortic valve replacement in man. J Thorac Cardiovasc Surg. 1995;110(1):265–7.
Pötzsch B, Hund S, Madlener K, Unkrig C, Müller-Berghaus G. Monitoring of recombinant hirudin: assessment of a plasma-based ecarin clotting time assay. Thromb Res. 1997;86(5):373–83.
von Segesser LK, Mueller X, Marty B, Horisberger J, Corno A. Alternatives to unfractioned heparin for anticoagulation in cardiopulmonary bypass. Perfusion. 2001;16(5):411–6.
Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76(2):638–48.
Vanderlinden J, Ekroth R, Lincoln C, Pugsley W, Scallan M, Tyden H. Is cerebral blood flow/metabolic mismatch during rewarming a risk factor after profound hypothermic procedures in small children? Eur J Cardiothorac Surg. 1989;3(3):209–15.
Yoshitani K, Kawaguchi M, Sugiyama N, et al. The association of high jugular bulb venous oxygen saturation with cognitive decline after hypothermic cardiopulmonary bypass. Anesth Analg. 2001;92:1370–6.
Henson LC, Calalang C, Temp JA, Ward DS. Accuracy of a cerebral oximeter in healthy volunteers under conditions of Isocapnic hypoxia. Anesthesiology. 1998;88(1):58–65.
Daubeney PEF, Pilkington SN, Janke E, Charlton GA, Smith DC, Webber SA. Cerebral oxygenation measured by near-infrared spectroscopy: comparison with jugular bulb oximetry. Ann Thorac Surg. 1996;61(3):930–4.
Chen CSLN, Liu K. Detection of cerebral desaturation during cardiopulmonary bypass by cerebral oximetry. Acta Anaesthesiol Sin. 1997;35(1):59.
Manecke GR, Kotzur A, Atkins G, et al. Massive pulmonary hemorrhage after pulmonary thromboendarterectomy. Anesth Analg. 2004;99(3):672–5.
Dittrich HC, Nicod PH, Chow LC, Chappuis FP, Moser KM, Peterson KL. Early changes of right heart geometry after pulmonary thromboendarterectomy. J Am Coll Cardiol. 1988;11(5):937–43.
Dittrich HC, Chow LC, Nicod PH. Early improvement in left ventricular diastolic function after relief of chronic right ventricular pressure overload. Circulation. 1989;80(4):823–30.
Cronin B, Maus T, Pretorius V, et al. Case 13 – 2014: management of pulmonary hemorrhage after pulmonary endarterectomy with venovenous extracorporeal membrane oxygenation without systemic anticoagulation. J Cardiothorac Vasc Anesth. 2014;28(6):1667–76.
Levinson RM, Shure D, Moser KM. Reperfusion pulmonary edema after pulmonary artery thromboendarterectomy. Am Rev Respir Dis. 1986;134(6):1241–5.
Thistlethwaite PA, Madani MM, Kemp AD, Hartley M, Auger WR, Jamieson SW. Venovenous extracorporeal life support after pulmonary endarterectomy: indications, techniques, and outcomes. Ann Thorac Surg. 2006;82(6):2139–45.
Berman M, Tsui S, Vuylsteke A, et al. Successful extracorporeal membrane oxygenation support after pulmonary thromboendarterectomy. Ann Thorac Surg. 2008;86(4):1261–7.
Olman MA, Auger WR, Fedullo PF, Moser KM. Pulmonary vascular steal in chronic thromboembolic pulmonary hypertension. Chest. 1990;98(6):1430–4.
Moser KM, Metersky ML, Auger WR, Fedullo PF. Resolution of vascular steal after pulmonary Thromboendarterectomy. Chest. 1993;104(5):1441–4.
Thistlethwaite PA, Kaneko K, Madani MM, Jamieson SW. Technique and outcomes of pulmonary endarterectomy surgery. Ann Thorac Cardiovasc Surg. 2008;14(5):274–82.
Edwards EB, Roberts JP, McBride MA, Schulak JA, Hunsicker LG. The effect of the volume of procedures at transplantation centers on mortality after liver transplantation. N Engl J Med. 1999;341(27):2049–53.
Menzel T, Kramm T, Mohr-Kahaly S, Mayer E, Oelert H, Meyer J. Assessment of cardiac performance using Tei indices in patients undergoing pulmonary thromboendarterectomy. Ann Thorac Surg. 2002;73(3):762–6.
Thistlethwaite PA, Madani M, Jamieson SW. Outcomes of pulmonary endarterectomy surgery. Semin Thorac Cardiovasc Surg. 2006;18(3):257–64.
Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg. 2012;94(1):97–103. discussion 103
Corsico AG, D’Armini AM, Cerveri I, et al. Long-term outcome after pulmonary endarterectomy. Am J Respir Crit Care Med. 2008;178(4):419–24.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Material
Transgastric short-axis view demonstrated severe right ventricular enlargement and a deviated interventricular septum (MP4 2592 kb) (MP4 728 kb)
Midesophageal four-chamber view demonstrating a dilated right heart, deviated septum, and underfilled left heart (MP4 625 kb)
Midesophageal ascending aortic short-axis view demonstrating thromboembolic material at the origin of the right pulmonary artery (MP4 2592 kb)
Midesophageal four-chamber view in a patient with CTEPH prior to pulmonary thromboendarterectomy. Note the dilated right heart, deviated septums, and underfilled left heart (MP4 630 kb)
Midesophageal four-chamber view of the same patient status post-pulmonary thromboendarterectomy and tricuspid valve repair. Note the decompression of the RA and RV with increased left heart size (MP4 313 kb)
Clinical Case Discussion
Case: A 68-year-old woman with CTEPH underwent a PTE and has just been separated from CPB. The surgeon tells you that the endarterectomy was difficult because it was Type 3 disease and the thromboembolic material was particularly “sticky.” You suspected such because the surgeon required two circulatory arrests on the right side, and he usually requires only one on each side. Large amounts of dark blood appear in the endotracheal tube as you begin ventilating.
Questions
-
What is the most likely cause of this bleeding?
-
What diagnostic maneuvers can be performed to determine the cause and location of the bleeding?
-
What are the therapeutic options, and how will they be chosen?
The most likely cause is surgical trauma, puncture of the distal pulmonary arteries resulting from aggressive endarterectomy. Other possibilities include nonsurgical PA rupture (high pressure, PA catheter trauma). Initial maneuvers include reinstitution of CPB including decompression of the pulmonary arterial tree with a PA vent, thereby temporarily reducing the amount of airway bleeding. Fiberoptic bronchoscopy can assist in localizing the site of the bleeding. Smaller bleeds may be managed with lung isolation, separation from CPB, reversal of heparin, as well as correction of coagulopathies. Lung isolation techniques include double-lumen tubes and bronchial blockers. A preferred technique is to exchange the endotracheal tube for a larger size (i.e., 9.0 mm ETT) to allow a bronchial blocker and a larger adult-sized bronchoscope simultaneously. The use of a pediatric size scope yields a smaller suction channel. Attempts to place the bronchial blocker in a subsegment if possible should be sought to maximize the amount of salvaged lung and prevent spillage of blood into the remaining segments. Larger pulmonary hemorrhage events or those associated with worsening hypoxia and hypercarbia may require ECMO. The decision for the method of ECMO rests on the hemodynamic status of the patient with TEE evidence of ventricular dysfunction playing a key role. Assuming biventricular function is intact, venovenous ECMO may be instituted via a single cannula placed percutaneously through the right internal jugular vein. This approach allows for ECMO support with minimal anticoagulation [59]. An algorithm for management of post-CPB hemorrhage is presented in Fig. 49.11.
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Maus, T.M., Banks, D. (2019). Anesthesia for Pulmonary Thromboendarterectomy. In: Slinger, P. (eds) Principles and Practice of Anesthesia for Thoracic Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-00859-8_49
Download citation
DOI: https://doi.org/10.1007/978-3-030-00859-8_49
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-00858-1
Online ISBN: 978-3-030-00859-8
eBook Packages: MedicineMedicine (R0)