Abstract
Repair of a thoracoabdominal aortic aneurysm (TAAA) is among the most challenging cases for an anesthesiologist. The anesthetic technique is demanding and requires a high level of expertise, including proficiencies in managing one-lung ventilation, massive blood loss, coagulopathy, and cerebrospinal fluid drains. Specialized care from all members of the perioperative team has been shown to have a significant impact on outcomes.
The main complications include respiratory failure, renal failure, paraplegia, stroke, and major cardiac complications. To prevent spinal cord ischemia, different techniques may be used to achieve the same physiological goal of maintaining spinal cord perfusion pressure. Techniques to prevent and minimize spinal cord ischemia primarily focus on maximizing collateral flow by optimizing the spinal cord perfusion pressure while prolonging ischemic tolerance and reducing reperfusion injury with hypothermia and pharmacotherapy. The mainstay interventions to prevent renal injury include avoidance of nephrotoxic insults and selective cold renal perfusion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Thoracoabdominal aortic aneurysm
- Left heart bypass
- Spinal cord injury
- Cerebrospinal fluid drainage
- Renal protection
-
Open thoracoabdominal aortic surgery is high risk, with placement of an aortic cross-clamp resulting in significant physiological derangements. The main complications include respiratory failure, renal failure, paraplegia, stroke, and major cardiac complications.
-
The anesthetic technique is demanding and requires a high level of expertise, including proficiencies in managing one-lung ventilation, massive blood loss, coagulopathy, and cerebrospinal fluid drains.
-
To prevent spinal cord ischemia, different techniques may be used to achieve the same physiological goal of maintaining spinal cord perfusion pressure. Techniques to prevent and minimize spinal cord ischemia primarily focus on maximizing collateral flow by supporting mean arterial pressure and reducing cerebrospinal fluid pressure while prolonging ischemic tolerance and reducing reperfusion injury with hypothermia and pharmacotherapy.
-
The mainstay interventions to prevent renal injury include avoidance of nephrotoxic insults and selective cold renal perfusion.
-
There is an increasing shift to recognize the importance of consensus definitions, standards for reporting outcomes, and coordinated multicentered trials in order to improve the quality of evidence necessary for guideline development.
Introduction
The aorta and arterial system is an essential component of the cardiovascular system, providing oxygen and other essential elements to tissues and cells for aerobic metabolism. This is critical to preserving end-organ function and preventing ischemic injury. Repair of a thoracoabdominal aortic aneurysm (TAAA) is among the most challenging cases for an anesthesiologist. A demanding anesthetic plan comprises of many elements to contend with the significant physiological derangements that occur during the case. Additionally, despite improvements in surgical technique, the potential for significant morbidity and mortality remains [1]. A high level of expertise and an exacting level of care from all members of the perioperative team have been shown to have a significant impact on outcomes [2, 3].
Diseases of the aorta are many and varied [4, 5]: from acute aortic syndromes including aortic dissection or traumatic injuries that are an immediate threat to life requiring emergent repair to progressive, often asymptomatic aneurysmal disease that prompts elective repair to prevent progression to acute rupture. Disease not only develops in the elderly due to degenerative and atherosclerotic disease related to smoking and hypertension risk factors but also the young with various genetic aortopathies such as Marfan syndrome. Clearly the associated comorbidities anticipated in these different subgroups are unique and the management plan should reflect this variability. A full discussion of the diagnostic guidelines and indications for surgical correction of various types of aortic disease is beyond the scope of this chapter. Additionally, this chapter outlines the management for elective TAAA surgery. While many details discussed are equally relevant to the emergent case, the feasibility of implementation will need to be considered.
Clinicians have recognized the risk of spinal cord injury (SCI) since the inception of surgical repair of TAAAs in the 1950s [6]. Lower extremity neurological deficit may be temporary or permanent and range from minor deficits to paralysis. The reported incidence of SCI after open TAAA procedures varies widely from 0% to 44% [7, 8]. A general decline in rates to 6–8% has been described since the turn of the century [5], potentially because of more routine employment of protective adjuncts [7, 9]. More recently, a permanent paraplegia and paraparesis rate of 2.9% and 2.4%, respectively, was reported in the largest published single-center experience of open TAAA repair [10]. All the same, when permanent paralysis does occur, it has a devastating impact on a patient’s quality of life [11] and long-term prognosis, with a higher complication and mortality rate and longer intensive care and hospital stay [12,13,14,15,16,17,18]. As with all major thoracic and vascular surgery, the risk of other complications is also significant and is associated with a substantial potential for morbidity and mortality. The risk of myocardial infarction, heart failure, ventricular arrhythmia, infectious complications, and reoperation for bleeding is quoted in the range of 1–5%, neurocognitive deficits and renal failure requiring hemodialysis up to 10%, and respiratory failure, the most common postoperative complication, between 5% and 15% [4].
Robust clinical evidence validating optimal anesthetic management to achieve improved clinical outcomes in TAAA is lacking. This is largely due to a dependence on single-center observational studies, with substantial heterogeneity in technical details, a lack of universal definitions, and inconsistent standards in reporting outcomes. While research groups and national and international registries are beginning to address these issues in aortic arch and other types of cardiothoracic and vascular surgery [19,20,21,22], further work is to be done before substantive evidence-based guidelines may be produced. The purpose of this chapter is to review the current literature and provide a rationale for a structured approach for the patient presenting for thoracoabdominal aortic surgery. Some content is complemented by other chapters in this textbook, so particular attention is paid to the requirements unique to TAAA surgery, particularly spinal cord and renal protection measures [23].
Anatomy and Physiology
Crawford Classification of TAAA Repair
TAAAs are commonly categorized by the classification devised by Crawford and colleagues, which divides TAAAs into four anatomic categories based on the extent of the repair required [24] (see Fig. 41.1). Type I extends from proximal to the sixth rib, typically at the origin of the left subclavian artery, to the suprarenal abdominal aorta and typically includes a beveled distal anastomosis at the visceral segment. Type II is the most extensive and extends from the left subclavian artery to the aortoiliac bifurcation. Type III extends from the thoracic aorta distal to the sixth rib to the aortoiliac bifurcation. Type IV extends from below the diaphragm and involves the entire visceral aortic segment and typically most of the abdominal aorta.
In addition to providing a uniform description of the anatomic extent of repair, the Crawford classification also allows the clinical team to estimate the operative risk. In particular, type II TAAA repairs have the greatest potential for morbidity and mortality, specifically the highest incidence of spinal cord ischemia and renal dysfunction.
Pathophysiology of Aortic Clamp
The application and release of a thoracic aortic clamp has major mechanical, humoral, inflammatory, and metabolic effects. Our understanding of these effects is incomplete, largely limited to historical animal studies that have not lead to any therapeutic interventions with proven clinical benefit to date. This is largely due to the complexity of the physiology involved, with an interplay of multiple factors that vary depending on the type of pre-existing disease, location, and duration of the aortic cross-clamp and that not only affect multiple end targets with unique sequential effects but macro- as well as microvascular structures in a dynamic system that is simultaneously responding to the effects of bleeding, anesthetic medications, and fluid treatment [23].
After application of a thoracic aortic clamp, systemic vascular resistance suddenly increases, arterial blood pressure proximal to the clamp increasing up to 40%, with limited changes in heart rate. Distal to the aortic clamp, end-organ perfusion pressures are reduced to 10–20% of baseline. Pre-existing occlusive disease that has stimulated collateral blood vessel development or more distal placement of the clamp reduces the severity of these changes with greater potential for collateral runoff. Animal models have substantiated that the increase in preload and blood pressure proximal to the aortic clamp is due to a shift in blood flow from the splanchnic vasculature into the vena cava, by various potential mechanical and/or humoral mechanisms [25, 26]. On assessment with transesophageal echocardiogram, the left ventricle filling pressures can increase by 40%, the end-diastolic area by 28%, and end-systolic areas by 70% [27]. The ability to compensate for the left ventricular dilation secondary to a significant increase in afterload and to a lesser extent preload is largely dependent on the reserve ionotropic function of the heart. This can be severely limited in the context of coronary artery disease and subendocardial ischemia or with prolonged clamp placement and increasing concentrations of humoral and inflammatory products.
Upon removal of the aortic clamp, the arterial blood pressure suddenly drops with a sudden reduction in systemic vascular resistance due to both mechanical and complex ischemic dilating humoral/metabolic mechanisms. Cardiac output should increase in response but is variable depending on the patient’s cardiac reserve and the effect of returning cold, hyperkalemic, acidotic, ischemic metabolites, as well as other dynamic factors such as the degree of acute bleeding.
Pathophysiology of Spinal Cord Injury
In the last 10 years, significant advances in our understanding of spinal cord blood supply have emphasized the need for a broader, more physiological approach to SCI [28].
Blood Supply of the Spinal Cord
Adamkiewicz and Kady provided the first accurate description of spinal cord blood supply in 1881 [29]. Their classical model was based on two posterolateral and one anterior spinal artery (ASA), the ASA being reinforced at multiple levels by segmental arteries originating from the aorta via the intercostal and lumbar arteries. Significant inter-individual variation within this anatomical model is recognized. During fetal development, an original 31 bilateral segmental arteries variably regress to 6 on average, with a broad range of 2 to 14 [29]. The largest segmental artery (“the artery of Adamkiewicz”) arises most commonly from a left intercostal artery, between T9 and T12 in 75% of patients but as high as T5–T8 in 15% or as low as L1–L2 in 10% [30, 31]. Functional studies using motor-evoked potentials (MEP) also indicate acquired aneurysmal and atherosclerotic disease of the aorta results in significant anatomical variability [32].
In the last decade, evidence of a spinal collateral arterial network (SCAN) has supplanted the classical model of an anatomically defined single important blood supply, whereby a substantial arterial plexus along the entire length of the spinal cord supports its blood supply [9]. The intercostal and lumbar segmental arteries have multiple degrees of connection longitudinally and transversally to arterial networks in the spinal canal (consisting of the ASA and epidural arcades), paravertebral tissues, and multiple paraspinous muscles, the latter being the largest and most extensive of the three [9, 29] (see Fig. 41.2). The importance of additional inflow vessels is emphasized in this model, including the subclavian artery through the vertebral artery, the thyrocervical and costocervical trunks, the internal thoracic arteries through the intercostal arteries, and the hypogastric arteries through the lateral sacral and iliolumbar arteries [9, 29, 33]. There is also evidence of significant inter-individual variation with remodeling potential in porcine studies [9, 34].
Schematic diagram of the blood supply of the spinal cord. (Courtesy of Etz et al. [9])
Considering the potential for inter-individual variation in both conceptual models, advances in imaging the spinal cord blood supply offer potential benefits in defining and modifying an individual’s risk [29]. However, options are currently limited. Invasive intra-arterial catheter angiography provides the best image quality when successful; however, its reported sensitivity is variable, with a risk of renal impairment or iatrogenic paraplegia. Magnetic resonance angiogram (MRA) technology has advanced significantly to reliably image the artery of Adamkiewicz and other larger segmental arteries but is still limited when it comes to the smaller caliber collateral vessels. When collateral arteries are distal to the planned aortic clamp on MRA, intraoperative decline of MEPs has a high negative (97%) but poor positive predictive value (37%) [35].
A Physiological Approach to SCI
While anatomical knowledge has advanced, understanding of circulatory physiology at the level of SCAN remains limited and is central to why the development and management of SCI remains poorly understood.
Simplistically, ischemia occurs when the supply of nutrients required for cellular metabolism does not met the demands for tissue viability. Absolute interruption of spinal cord blood supply for a sufficient period of time will cause irreversible ischemic necrosis. On the basis of early studies using a “clamp and sew” technique, this risk is significantly increased after 30 min of aortic cross-clamp and almost certain after 60 min [36, 37]. Alternatively, perfusion may be permanently marginal and become inadequate for viable cord function at a certain “tipping point” [38], explaining how cases of delayed SCI can present up to 27 days after intervention [39]. Added complexity lies in how variable this can be between individuals, for instance, chronic hypertension appears to shift the tipping point [34], while therapeutic interventions can improve ischemic tolerance. This concept is highlighted in studies where complete segmental arterial supply sacrifice consistently reduced spinal cord perfusion pressure (SCPP), but only lead to SCI in approximately half the cases [40]. Furthermore, while staging the arterial sacrifice minimized the drop in SCPP and improved functional outcomes overall, the cohort that could have tolerated the drop in SCPP without staging was unpredictable [34]. This is important to consider when interpreting the variability in published results.
Additionally, SCPP may be compromised by multiple mechanisms [33, 39, 41]. Spinal cord blood flow is reduced by direct arterial occlusion with placement of an aortic clamp or replacing aortic tissue with a graft. The primary aortic disease process (atherosclerosis, dissection) and embolic phenomena can also compromise the anastomotic capacity of the SCAN [29, 42]. Increased cerebrospinal fluid (CSF) pressure as occurs with placement of an aortic cross-clamp or edema secondary to an ischemic-reperfusion injury can create a “compartment syndrome” effect [43,44,45]. Systemic hypotension or steal phenomenon will further exacerbate suboptimal perfusion [39]. The potential interconnectedness of these various factors adds further complexity, where ischemia can exacerbate edema that further exacerbates ischemia in a spiral-like effect [8, 29, 42, 46].
Ultimately, the adequacy of SCPP is determined by an interplay of multiple factors that not only affects an individual’s SCPP but also determines the SCPP required to prevent SCI (see Fig. 41.3). This creates a unique risk balance in each individual patient and demands a multimodal approach to prevent SCI following TAA intervention.
Surgical Technique
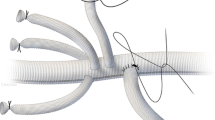
In order to surgically repair aortic disease, a clamp is placed above and below the lesion, the aorta opened, and the diseased segment replaced with a graft (see Fig. 41.4). The thoracic aorta is accessed via a left thoracotomy, which is extended to a paramedian laparotomy when access to the abdominal aorta is also required.
Established surgical methods for TAAA surgery include the “clamp and sew” technique, left heart bypass [47], partial cardiopulmonary bypass [48], and deep hypothermic arrest [49]. There are no established criteria to justify one technique over another and current guidelines recognizing that institutional experience is an important factor in technique selection [4]. The simple “clamp and sew” technique is no longer used in most institutions and is not advisable when the cross-clamp duration is expected to exceed 30 min because of the significant risk of postoperative neurological deficit and mesenteric and renal ischemia.
Left heart bypass provides distal aortic perfusion during aortic clamping by means of a centrifugal pump, with the potential for additional selective perfusion of mesenteric visceral and renal arteries. Proximal decompression is provided by draining oxygenated blood through a left pulmonary vein cannula. The left atrial appendage may alternatively be used, but the positioning is often more awkward with a greater risk of air embolism. Blood is returned to provide distal perfusion through a distal aorta or femoral artery cannula. Close communication between the anesthesiologist and perfusionist is required to ensure that the balance of preload and the pump speed are optimal. Partial bypass is similar in that it ensures distal perfusion by cannulation of the femoral artery and vein while maintaining enough preload in the heart to support the cardiac and cerebral circulations but contrastingly achieves this by the use of full extracorporeal cardiopulmonary bypass. This can achieve higher flow rates that are advantageous in patients with poor left ventricular function or during a prolonged aortic cross-clamp time; however, it requires full heparinization, which increases the risk of increased blood loss and coagulopathy.
Deep hypothermic circulatory arrest (DHCA) is indicated when a proximal aortic clamp site is unable to be safely secured, often when the arch is dissected and/or aneurysmal. The benefits of use are in its simplicity, with a bloodless field and no need for additional cannula or aortic manipulation. Its main limitation is time on cardiopulmonary bypass. On the basis of advanced physiological and clinical testing, the upper safe limit of profound to deep HCA (12–20 °C) is 25–30 min [50, 51]. In a landmark clinical study by Svensson et al., >40 min of profound HCA increased the risk of neurological injury, and >65 min increased the risk in mortality [52]. In a more recent study of 490 patients, safe use of DHCA (18–20 °C) was reported for up to 50 min in terms of stroke risk, but only 61 patients had arrest times >40 min [53]. While it is established that organ metabolism is reduced proportionally to the degree of hypothermia, there is increasing recognition of the potential harm associated with profound and deep hypothermia (<14–20 °C). Various experimental studies raise concerns about the risks of coagulopathy, a proinflammatory response, and end-organ dysfunction, but these concerns are yet to be substantiated in clinical studies [19, 54]. Inconsistent standards in the reporting of outcomes related to end-organ ischemia significantly limits assessment of safe temperature and time parameters for these techniques [19, 54]. Additionally, the impact of relevant patient or interventional modifiers on these parameters is poorly understood [55,56,57].
Selective cerebral perfusion is not commonly used and more difficult to implement for TAA surgery. The specifics of this are covered in more detail in a previous review [58]. If renal ischemia exceeds 30 min, some guidelines recommend selective cold crystalloid renal perfusion, as discussed later in section “Renal Protection” [5] (see Fig. 41.5).
Preoperative Evaluation
As already recognized, the variability in presentation of aortic disease is extensive and will have a major impact in guiding appropriate preoperative assessment and planning. A detailed description of these nuances is beyond the scope of this chapter, and we recommend review of current guidelines as appropriate [4, 5]. From an anesthetic perspective, recognizing certain distinctions is important to assess for likely associated disease, the required urgency, and risk benefits of surgical management.
In addition to a complete anesthetic, medical history and examination, a systematic approach should be taken in considering the need for additional investigations. Review of the content in Chap. 2 related to the preoperative assessment and optimization of the thoracotomy patient is relevant to TAAA surgery. Considering TAAA surgery qualifies as major surgery by entering both the thoracic and abdominal cavities, specific considerations include:
-
Blood assays. Electrolytes and creatinine to assess renal and endocrine function, for side effects of pharmacotherapy, and potential avenues for optimization in the event of arrhythmia. Complete blood count to assess for myelosuppression and anemia. In the event of anemia, iron studies, CRP, vitamin B12, folate, and thyroid function studies should be reviewed to assess for reversible causes. Coagulation studies to assess for bleeding dyscrasias. Liver function studies should be completed if there is suggestion or risk factors for liver disease. Albumin is also a potential marker of nutritional status and frailty.
-
A 12-lead electrocardiogram to provide a baseline measure and assess for evidence of arrhythmic or structural cardiac disease. 24 hour Holter monitoring may be considered for those with a history of intermittent symptoms of palpitations or pre-syncope.
-
A resting echocardiogram if there are any risk factors or evidence of cardiac disease, including age > 40 years in some guidelines [4].
-
A BNP level is recommended by the current Canadian Cardiovascular Society guidelines [59]. Dynamic cardiac testing (including a dobutamine stress echocardiogram or myocardial perfusion scan) is indicated according to the current American Heart Association guidelines if the patient’s functional capacity is poor or cannot be assessed [60]. Current expert opinion is that patients with unstable coronary syndromes and significant coronary artery disease (i.e., left main stenosis or three-vessel disease) should undergo revascularization prior to or at the time of thoracic surgery, while the benefits for those with clinically stable flow-limiting coronary disease remain unclear [4].
-
Neurocognitive testing, a carotid artery duplex scan, brachiocephalic angiography, and brain imaging should be considered if there are risk factors or evidence of cerebrovascular disease. While they are considered reasonable investigations in some guidelines to help assess a patient’s risk profile [4], they are not routine, and their utility is uncertain.
-
A recent chest x-ray or computed tomography (CT) is required to assess for cardiopulmonary disease including distortion of the trachea or left main bronchus by the aortic disease and to assist in sizing the double-lumen tube (DLT).
-
The utility of an arterial blood gas on air, spirometry, pulmonary function testing, and cardiopulmonary testing are discussed at length in Chap. 2.
To complement this assessment, communication with the multidisciplinary team is important to insure optimal patient care. The surgical team will provide important insights into the indications for surgical management and the specific requirements of the surgical plan, including planned clamp positions, the need for additional shunt, bypass support, or selective organ perfusion techniques. Liaison with intensive care services is important to ensure suitability for a period of postoperative support. In the patient with significant comorbidity, referral for assessment or liaison with their treating medical subspecialist may be required. This can be to insure maximal optimization and/or provide an opinion as to the patient’s long-term survival prognosis with respect to their comorbidity.
Putting all of this together, the anesthesiologist needs to provide the patient and surgeon with an individualized summative assessment of the risks of not proceeding with surgery, weighed against the perioperative risk. A perioperative plan should make provision for preoperative optimization of all elements of the patient’s health.
Current international guidelines provide the best evidential support to optimize medical management of cardiac risk [59, 60]. In addition to specifically withhold angiotensin-converting enzyme inhibitors/angiotensin receptor blockers for 24 h prior to surgery to prevent the risk of death and vascular complications [61], other sources recommend withholding all antihypertensive agents the morning of surgery to help support compromised distal perfusion pressures [13, 34]. Counselling to support smoking cessation should be provided. In those with a reversible airways disease component, inhaler therapy should be optimized. Reversible causes of anemia and kidney injury should be treated if the urgency of surgery allows. The intra- and postoperative plan should outline the monitoring and interventions needed to minimize the risk of complication, in particular protective strategies to reduce the risk of spinal cord and renal injury, as discussed later in sections “Spinal Cord Protection Strategies” and “Renal Protection Strategies.”
Although guidelines suggest identifying the “high risk” for implementation of spinal cord protection techniques, there are no recognized criteria to define “high risk” in this population [4]. The extent of the intended aortic repair has long been recognized as an important determinant since Crawford’s classification of TAAA disease [24]; however, many institutions classify all open TAAA repairs as high risk. Although many other patient and procedural factors have been proposed as risk factors for postoperative SCI, none have been consistently validated. The rationale for instituting spinal cord protective strategies is determined on a surgeon and patient-case basis. The following is a summary of independent predictors of SCI with open repair identified by logistic regression modelling that could be considered:
-
Extent of disease, including type II only [62, 63], I and II [15], and I, II, and III [64] Crawford TAA aneurysm classification.
-
Previous thoracic or TAA surgery [65]. In another study thoracic aneurysm repair was protective [62].
-
Presence of aortic rupture [15].
-
Total aortic clamp time [15]. In a later study by the same group distal aortic perfusion eliminated this as a risk factor on repeat analysis [63].
-
Diabetes [62].
-
History of preoperative renal dysfunction [15], renal dysfunction (Cr >2.0 mg/dL, a previous history of renal failure or insufficiency or being on active dialysis) [63].
In the most recent and largest publication of a single-center experience to date, Coselli et al. identified that the independent risk factors for permanent paraplegia varied depending on the extent of TAAA. For extent II repairs, coronary artery disease and chronic aortic symptoms increased risk, while having a genetically triggered disorder was protective. For extent III repairs, cerebrovascular disease, emergency repair, and selective visceral perfusion increased risk of SCI [10].
Intraoperative Management
Monitoring
In addition to ASA standard monitoring [66], extra monitoring of cardiac and neurological function is required for the early detection and minimization of complications. Again, much of what is discussed with regard to monitoring in Chap. 20 should be reviewed.
Five-Lead Electrocardiogram (ECG) and Defibrillation Pads
While lead V provides assessment of the left ventricle lateral wall and provides the greatest sensitivity for detecting intraoperative ischemia, the lead position is directly in the surgical field. Defibrillation pads should also be anteroposteriorly placed prior to induction.
Invasive Blood Pressure
Right radial or brachial arterial line placement provides information about pressures proximal to the aortic clamp, including the cerebral circulation even if the left subclavian arterial flow is compromised by the clamp. If distal perfusion techniques are used, a right femoral artery line may be placed, reserving the left femoral artery for bypass cannulation. If distal perfusion pressure monitoring is not required, we recommend using a second line in the left radial or brachial artery. This is dedicated to blood sampling in order to prevent interruption in pressure monitoring or compromise of the right line from frequent blood sampling.
Pulmonary Artery Catheter (PAC)
While recognizing there is no support for improved clinical outcomes [67], we routinely place a sheath and PAC in the right internal jugular for these cases. Recognizing the standard limitations in its use, including the likely overestimation of the left ventricular end-diastolic pressure with isolated right-lung ventilation, we find the additional information about left ventricular function in terms of filling pressures and cardiac output useful in optimizing hemodynamic management.
Transesophageal Echocardiogram (TEE)
In the absence of an absolute contraindication, placement of a TEE probe is considered reasonable [4] and routine in our institutional practice. In addition to confirming the diagnosis in the acutely unstable patient and assessing for aortic valve involvement, echocardiographic assessment provides dynamic information about filling pressures, volume status, cardiac output, afterload, and ischemia with regional wall motion abnormalities. However, the need for specialized training and resource intensity will not be serviceable in all centers.
Spinal Cord Monitoring
Early detection of inadequate spinal cord perfusion is an essential component in the perioperative care of patients having TAAA surgery, so interventions to improve perfusion may be implemented before compromised spinal tissue is irreversibly infarcted. Early emergence from general anesthesia and regular neurological assessment of the awake patient are key interventions emphasized in postoperative care protocols [41, 68].
Somatosensory- (SSEP) and transcortical motor-evoked potentials (tcMEP) have been used to indirectly assess spinal cord perfusion in the anesthetized patient, to inform surgical and anesthetic management. Consideration for use is recommended in institutions with sufficient resource and experience, although several large volume centers do not use neuromonitoring techniques intraoperatively [4]. While the utility of SSEP monitoring is limited by delayed ischemia detection and a high rate of false-positive results [69], the use of highly sensitive MEP monitoring has been shown to reduce the rate of SCI in the open repair of type I/II TAAA [44]. MEP monitoring restricts neuromuscular blockade and volatile anesthetic agent use, with theoretical risks of seizure, arrhythmic, or neuromuscular complications [70].
Near-infrared spectroscopy (NIRS) monitoring of hemoglobin oxygen saturation in the paraspinous muscles is a new continuous noninvasive technique to assess the adequacy of collateral network perfusion. Feasibility studies have shown that its use reliably predicts patients who develop SCI [71] and correlates well with MEP results [72]. Further research is required to prove reproducibility, determine optimal optode positioning, and indicate whether its use can improve clinical SCI outcomes.
CSF pressure and drain volume monitoring is critical intraoperatively. This is not only to insure spinal perfusion pressure is optimized but to prevent complications from excess CSF drainage, as covered in more detail in section “Technical Specifics of Cerebrospinal Fluid Drainage.”
Cerebral Monitoring
Although various cerebral monitoring techniques are available, there is no substantive evidence that the use of any of them reduces the risk of neurological injury. Their primary current role is to help assess the adequacy and hemispheric symmetry of cerebral perfusion techniques, being poorly predictive of neurological dysfunction secondary to embolic phenomenon and malperfusion in acute aortic dissection [73]. The fact that assessment of cerebral circulation is limited to the frontal lobes is also potentially significant. The threshold values and sensitivity of NIRS to detect insufficiency remain uncertain [73, 74], and most modern studies describe the use of a multimodal monitoring strategy in order to increase the sensitivity of detecting relevant ischemic cerebral dysfunction [75].
Despite the popularity of NIRS and a large body of literature, a systematic review of its use in cardiac surgery found only low-level support for its use in terms of neurological outcomes [74]. A more recent systemic review of NIRS in cardiac surgery recommended strong consideration for use in general cardiac surgery on the basis of improvement in global outcomes such as hospital length of stay and multi-organ morbidity, with no specific assessment of neurological outcomes [76]. While some studies have shown an association between low cerebral oximetry values and various worse neurological outcomes, whether NIRS can inform interventions to modify neurological risk remains unclear [74, 77]. One randomized trial (n = 100) has shown that use of an interventional algorithm to treat cerebral saturations <85% of baseline during CPB significantly improved cognitive testing results at 1 week and month postoperatively [78]. A recent feasibility trial will hopefully herald a large trial with definitive answers in years to come [79].
Routine use of other techniques is largely limited due to technical and logistical factors, with a lack of evidence to substantiate their efficacy. Bilateral transcranial Doppler (TCD) can be used to assess symmetry of middle cerebral artery flow and embolic phenomena; however, this only provides assessment of the anterior circulation, and adequate signals may only be found in three out of four patients [75, 77]. Auditory- and somatosensory-evoked potentials and multichannel electroencephalography can be used to identify intraoperative cerebral events and the adequacy of cerebral metabolic suppression [75]. However, there is no evidence to indicate that use of these techniques has any clinical benefit in addition to standard care [77]. Jugular venous bulb saturation has high specificity in the detection of cerebral ischemia and can indicate maximal suppression of cerebral metabolism but has poor sensitivity and is invasive [75, 77].
Temperature
In addition to a standard nasopharynx temperature, a core rectal or bladder temperature should be recorded in the event active or passive cooling techniques are implemented [4].
Vascular Access
In recognition of the potential for rapid blood loss and the need for massive blood transfusion, we place two large intravenous access lines. We recommend connecting the Belmont rapid infuser device to a 7Fr PAC sheath in the internal jugular or subclavian vein, with left-sided placement allowing for easier access when the patient is in the right lateral position. A 14ga peripheral intravenous line should be secured as a secondary line. It should be directly connected to a fluid infusion set, as needle-free valves and injection ports can significantly reduce the maximal achievable flow rate [80]. A quad-lumen central venous line is also routinely inserted.
Other Tubes
An indwelling urinary catheter is placed at the start of the case. This is not only for postoperative management purposes but also prudent considering the potential for large volumes of fluid and mannitol administered intraoperatively. Urine output has been shown to be an inaccurate marker of renal function or overall fluid status intraoperatively and does not predict the risk of postoperative kidney injury [81,82,83,84,85]. Rather, vigilance is required to prevent urinary retention, with a global assessment of the patient’s clinical state to achieve euvolemia.
A nasogastric tube is likely to be indicated for postoperative management, especially if there has been manipulation of mesenteric blood supply. If a TEE probe is placed, we recommend this be inserted at the end of the case.
Anesthetic Technique
Recognizing the many demands for TAA surgery, the anesthetic technique will need to be tailored to specific patient needs and institutional practices. In our institutional practice, fentanyl/sufentanil, IV lidocaine, with an adequate dose of propofol and non-depolarizing relaxant is used for smooth induction and intubation. For maintenance of anesthesia, we avoid the use of inhalational agents (MAC <0.3) and additional muscle relaxation to allow for SSEP and MEP monitoring. Rather total intravenous anesthesia with remifentanil (0.1–0.2 mcg/kg/min or effect site concentration of 4–7 ng/ml) and propofol (75–125 mcg/kg/min or effect site concentration of 3–6 mcg/ml) is used, guided by a depth of anesthesia monitor and the patient’s hemodynamics.
Positioning
For open repair of TAAAs, the patient is positioned in right lateral decubitus after induction of anesthesia and placement of all vascular lines. The left hip, and to a lesser degree the left shoulder, is rolled back slightly toward the horizontal to allow surgical access to the femoral vessels. The physiological implications of lateral decubitus positioning and one-lung ventilation are comprehensively covered in Chap. 5. As highlighted in Chap. 19, pressure point protection and patient access are also important considerations, and we recommend a systematic approach to insure optimal care. All monitoring and access lines should be reviewed to insure they are secure, away from the surgical field, and not a potential source of pressure injury. We routinely use a beanbag positioning device to stabilize the patient in position. Alternatively, the patient can be secured with a stretch of wide tape or a table strap fasted to the table and applied across the left hip, over the soft tissue between the iliac crest and the femoral head to avoid forming a pressure area. Loose-fitting tape or Velcro straps can be used to help secure the arms on the arm boards. They should be secure, but not so tight as to cause compartment syndrome.
In recognition of the multiple pieces of monitoring, lines, and equipment, it is important to create an ordered, ergonomic, and functional working space. The routine arrangement used at our institution for thoracoabdominal aortic surgery is shown in Fig. 41.6.
One-Lung Ventilation
Lung isolation techniques with collapse of the left lung is important for TAAA repair, as it improves surgical exposure, reduces the time of surgery, and minimizes lung manipulation and trauma. A left-sided double-lumen tube (DLT) should be placed unless the aortic disease distorts the trachea and left main bronchus. Alternatively, a right-sided DLT or a left-sided bronchial blocker can be used. The techniques and important considerations for doing this are covered in Chaps. 16 and 17.
Exchange of the double-lumen for a single-lumen tube (SLT) should be considered at the end of the case, in order to minimize the risk of airway trauma, suboptimal ventilation, and airway toileting from a suboptimally positioned DLT in the cardiovascular intensive care unit (CVICU). However, this risk must be considered in the context of how easy it was to bag-mask ventilate and intubate the patient preoperatively and weighed against the risk of completely losing the airway in the context of significant airway edema [4]. In our practice, unless there is gross facial edema, we routinely inspect the glottis with a videolaryngoscope in the operating room at the end of the case. If there is no significant edema, the DLT will be exchanged for a SLT under direct vision using an airway exchange catheter. In the presence of edema, serial assessments will be made in the CVICU every 24 h and tube exchange delayed until the edema is resolved.
Hemodynamic Management
A key priority for major vascular surgery of this nature is tight hemodynamic control throughout the perioperative period. Minimizing acute extremes in blood pressure and heart rate is important in order to limit changes in wall shear stress across the diseased aorta. In the context of using aortic clamps and massive bleeding, this can be demanding. There are many ways of achieving this same outcome, and the treating anesthesiologist should consider agents with the appropriate pharmacological properties that they are familiar with in order to achieve the best results.
With placement of the proximal aortic clamp, it is important to avoid an excessively high left ventricular filling pressure and heart rate that will increase myocardial oxygen consumption while reducing coronary perfusion. In patients with a degree of coronary artery disease this will result in subendocardial ischemia. Equally, at least a “normal” (i.e., relative to the patient’s awake baseline blood pressure) proximal aortic pressure will need to be maintained if end-organ perfusion distal to the aortic clamp is dependent on collateral flow. While euvolemia should be maintained preoperatively to avoid pre-renal injury, excessive fluid resuscitation should be avoided prior to aortic clamp placement. There is a small body of evidence to support nitroglycerin as more effective that nitroprusside for reducing preload and filling pressures once the clamp is placed, with superior preservation of collateral coronary, renal, and spinal blood flow [86,87,88]. Using β-blockers such as labetalol with its alpha- and beta-effects or esmolol with its short-acting, β1-selective effects could be considered as second-line agents, but the negative inotropy will need to be monitored closely. Nicardipine is a dihydropyridine calcium channel antagonist with a relatively rapid onset and offset of effect that predominately relates to arterial dilation. Its use may be more favorable to β-blockers in the context of bradycardia or reactive airways disease.
Acute hypotension following release of the aortic clamp should be limited by preemptively bolusing fluid and instituting vasopressor/inotropic support approximately 10 min prior. In the event of refractory or severe hypotension, reapplication of the aortic clamp may be required for 1–2 min. Again, while supporting adequate organ perfusion post-clamp release, excess blood pressure that will stress the suture lines of the graft and increase the risk of bleeding must be avoided.
Fluid Management with Massive Blood Loss
A full discussion of the management of massive transfusion and coagulopathy is beyond the scope of this chapter. Rather we would refer to current international guidelines [89, 90]. Close communication with blood bank and operating room staff such as the nurses, anesthetic assistants, and attendants can help meet the high level of resource intensity potentially required.
Targeted transfusion using point-of-care viscoelastic assay (e.g., thromboelastography (TEG) or rotational thromboelastometry (ROTEM))-guided algorithms have been shown to reduce blood transfusion requirements [90]. No single algorithm can be recommended at this time; rather this should be developed at an institutional level with multi-departmental input and regular audit. Figure 41.7 provides an example of the validated algorithm used for cardiac surgery with bypass support at our institution [91]. If available, a massive transfusion protocol may need to be used as a strategy to optimize the delivery of blood products to massively bleeding patients.
ROTEM-guided algorithm for targeted transfusion. (Courtesy of Karkouti et al. [91])
Prophylactic use of antifibrinolytic therapy, in particular tranexamic acid, has been shown to reduce perioperative blood loss and reduce transfusion requirements. The evidence is most substantive for use in cardiac surgery and is appropriate for TAAA surgery in light of the risk for excessive bleeding. In multiple randomized trials, including the largest and most recent ATACAS trial, there is no evidence of an increased risk of thrombotic complications; however, there is a recognized dose-dependent risk of postoperative seizures [90, 92].
Spinal Cord Protection Strategies
To prevent spinal ischemia, different techniques can be used to achieve the same outcomes, as long as the physiological principles of maintaining perfusion pressure to prevent paraplegia are accounted for. Techniques to prevent and minimize SCI primarily focus on maximizing collateral flow by supporting mean arterial pressure and reducing CSF pressure while prolonging ischemic tolerance and reducing reperfusion injury with hypothermia and pharmacotherapy. Despite a wealth of options, interpretation of the large volume of conflicting literature allows for few conclusive recommendations. Except for one randomized controlled trial on cerebrospinal fluid drainage (CSFD), the vast majority of evidence is level B with single-center cohort studies of a few hundred patients [4, 5]. Besides the inherent variability in pathophysiology, comparability of results between studies is limited by the heterogeneity in definitions, inclusion/exclusion criteria, and therapy combinations [8, 31]. The risk of generalizing results from single centers of excellence must also be considered [2, 4], as a surgeon’s experience, treatment volume of an institution [2], and implementation of a protocoled care package [41] have been shown to have a significant effect on outcomes.
Cerebrospinal Fluid Drainage (CSFD)
Evidence from animal studies in the 1960s showing that reduced CSF pressures improved SCPP provided the initial justification for trialing CSFD [8, 30, 31]. Cases of CSFD reversing SCI symptoms [8, 93] and Coselli et al.’s landmark randomized controlled trial [94] validate their potential benefit in humans. Conversely there are cases of SCI developing despite prophylactic CSFD and recovering without it [95], with conflicting findings in the literature as highlighted in the systematic [8] and Cochrane review [31]. While recognizing the weaknesses in the current data, both of these reviews concluded there is sufficient evidence to support the role of CSFD in high-risk patients as part of a multimodal proactive approach [8, 31]. The 2010 ACCF and 2014 ESC guidelines made similar recommendations for use as a protective strategy in high-risk open thoracic aortic repair procedures [4, 5]. However, the clarity of this statement is misleading, with no criteria to identify the “high-risk” patient. While the authors of the Cochrane review caution against extrapolating benefit beyond the selection criteria in the Coselli trial (i.e., open repair of Crawford type I–II TAA in a center of excellence) [31], the other systematic review specified “high risk” as open repair of Crawford types I–III with and without dissection [8]. Given that the risk of SCI is unpredictable, many centers use CSFD in all patients, including those undergoing extent IV repair.
Increasingly the risks of CSFD are recognized, although in a large portion of the literature systemic evaluation of the rate and risks of CSFD complications are poorly reported [96]. Intracranial hemorrhage is the most significant in terms of the potential morbidity and mortality, with an incidence 0.45–7.8%, of whom 10–50% develop significant neurological deficit or die [68, 97,98,99,100,101,102,103]. CSF leak with post-dural puncture headache (incidence 0.74–9.7% [12, 98, 99, 102, 103]), neuraxial hematoma (incidence 0–3% [68, 98, 103,104,105]), catheter fracture, meningitis, para-lumbar infection, and abducens nerve palsy (incidence <1% [68, 103, 106]) is also reported in the literature.
Even more importantly, there is a paucity of literature to substantiate important management standards for CSFD to minimize complications with use. This includes specifics about pressure thresholds and drainage limits, duration of use, and infrastructure requirements. Drainage of larger volumes of CSF is the most consistent independent predictor of hemorrhagic complication [97, 102], with high CVP at the time of cross-clamping also being noted by one group [102]. Maintenance of CSFP >7–10 mmHg with continuous monitoring, limiting drainage to <15–25 mL/h, maintenance of a recumbent position (<30 degrees reverse Trendelenburg), early removal within 48–72 h postoperatively, capping the drain 24 h prior to removal to allow CSFP to normalize, and cautious use of anticoagulation therapy even after drain removal are the most common expert recommendations for safe CSFD in the literature [95, 97, 98, 102, 106], with various “care bundles” described in the literature [98, 107,108,109,110]. However, this is far from universal, with one group advocating a more flexible approach, limiting drainage volumes to the upper limits of CSF circulating volume (140–165 mL) intra- and postoperatively [102], while others individualize the baseline pressure target based on the preoperative “opening pressure” at the time of drain placement [42]. While drainage of bloody CSF is only associated with radiological evidence of intracranial hemorrhage in 50% of cases, it is widely recognized as a sensitive indicator of increased risk for ICH requiring the immediate attention to limit significant morbidity and mortality [97, 98, 102], as covered in section “Technical Specifics of Cerebrospinal Fluid Drainage.” Irrespective of the value of each component, a recent study that protocoled CSFD management achieved a substantial reduction in their institution’s complication rate (from 24.1% to 5.7%, p = 0.067) [68].
Supporting SCPP
Increasingly the importance of supporting systemic blood pressure (with mean arterial pressures 80–100 mmHg) and cardiac output to maintain SCPP is recognized for at least 48 h postoperatively until SCAN has sufficiently remodeled to compensate for the loss of segmental arterial supply [41, 42, 68, 106]. While direct measurement of SCPP [40, 111], functional MEP testing [44, 109], and risk factor analysis [99, 112, 113] have supported this, the role of blood pressure augmentation in reversing symptomatic SCI is most compelling [95, 114]. Consideration of the patient’s preoperative baseline arterial pressure and withholding antihypertensive preoperatively is recommended [13, 34]. Optimization of oxygen delivery with aggressive blood transfusion targets also features in recent institutional protocols [41, 68].
Intraoperatively the established benefits of distal aortic perfusion by cardiopulmonary bypass (CPB) or left heart bypass are recognized for open repair of type I–II TAAA in centers with significant experience [63, 115]. Guidelines recommend a proximal mean arterial pressure of 90–100 mmHg and distal arterial pressure of 60 mmHg to ensure adequate spinal cord perfusion [4]. There is also evidence that non-pulsatile perfusion is inferior to pulsatile perfusion at the same mean pressure [34]. Further technical aspects of these techniques are covered above in section “Surgical Technique.”
Other surgical techniques to optimize collateral blood flow to the spinal are largely controversial. Historically, re-implantation of segmental arterial supply via the intercostal and lumbar arteries has been a dominant protective strategy in surgical TAAA repair. However, by prolonging the aortic cross-clamp time, this intervention is not without risk. Techniques may be indiscriminate or selective based on clinical assessment of back-bleeding, intraoperative MEP, or preoperative imaging [8, 116]. Some retrospective analyses have shown a tenfold increase in SCI risk when critical zone (T9-L1) intercostal vessels were oversewn in type I/II open repair [117, 118], and selective re-implantation has been associated with a significant protective effect when used as part of a multimodal strategy [46, 116, 119, 120]. However, the work of Griepp et al. (n = 95) and later Etz et al. (n = 100) challenged this doctrine by extensively sacrificing segmental arteries under EP monitoring without SCI [121, 122]. While recognizing the limitations of these observational studies, the authors contested re-implantation was not only unnecessary when other means are used to support the SCAN, but it is potentially harmful by prolonging surgery and causing steal phenomenon. In reality, both arguments are likely to hold merit, and while not necessary in the majority, it may be critical in a few, justifying a balanced approach with selective re-implantation in current guidelines [4, 42, 46].
The importance of patency and revascularization of independent arterial beds contributing to the SCAN is equally controversial. While some studies have identified left subclavian artery coverage as a risk factor for SCI [13, 123], it is not a consistent finding [124,125,126,127]. Additionally, prophylactic revascularization may be justified in order to reduce the risk of stroke or left arm ischemia [128, 129].
Staged repair is a relatively new concept based on supporting collateral flow through SCAN remodeling. While the physical effects of staging on SCPP are compelling [34], the complexity of how these impact clinical outcomes, especially for an individual patient, is yet to be fully understood. This is not limited to open repair, with various hybrid and endovascular staging methods described that are beyond the scope of this chapter, and we refer to a previous review for further detail on this [58].
Hypothermia
The protective benefits of hypothermia have been long recognized [130]. Various methods including local epidural cooling [117, 131], profound (<20–22 °C) hypothermia with CPB with or without circulatory arrest [132, 133], moderate (30–34 °C) hypothermia with partial bypass (aortofemoral or atriofemoral) [134, 135], and mild permissive hypothermia [136] have been advocated, with a protective effect in high-risk cases when used in institutions with significant experience.
However, as already noted above in section “Surgical Technique,” the association between hypothermia (<34.5 °C) and an increase in postoperative organ dysfunction and mortality in patients having aortic surgery [137] cautions against routine use, with specific concerns about an increased risk of coagulopathy, pulmonary dysfunction [132, 138], and arrhythmias [134] in open TAAA repair studies. Currently moderate systemic hypothermia is considered a reasonable strategy to protect the spinal cord during open surgical repair of the descending thoracic aorta, and epidural irrigation with a hypothermic solution may be considered [4].
Drugs
While the desire for a pharmacologically protective agent has been exhaustive, the evidence base remains weak [106]. While intravenous naloxone [139, 140] and intrathecal papaverine [135, 141] have been shown to have protective benefit in human trials, the benefits of thiopentone [142], systemic steroids [143], lidocaine [144], intrathecal methylprednisolone [145], deferoxamine [146], superoxide dismutase [146, 147], minocycline [148] and erythropoietin [149], testosterone [150], and dexmedetomidine [151] are limited to animal studies.
The 2010 ACCF guidelines recommend that use of high-dose systemic glucocorticoids, mannitol for osmotic diuresis, intrathecal papaverine, and various anesthetic agents to suppress metabolic suppression may be considered [4].
Hyperbaric Oxygen
In our institution we have had positive experience with early institution of hyperbaric oxygen therapy in three patients, all of whom recovered full neurological function. While the evidence base is currently limited to case reports, this is an emerging field of research.
Technical Specifics of Cerebrospinal Fluid Drainage
Use and familiarly with the manufacture’s recommendations of a dedicated lumbar drainage kit that includes a 14ga introducer needle, specialized multi-orificed silastic drainage catheter, and external drainage and monitoring system is required. Once identifying that a CSFD is indicated, important contraindications should be considered. Current coagulation guidelines for neuraxial techniques should be followed [152]. The presence of intracranial disease that could predispose to neuraxial or intracranial bleeding complications in the context of iatrogenic intracranial hypotension should be considered in liaison with the surgeon, including cerebral aneurysms, arteriovenous malformations, cerebral atrophy, cranial vault abnormalities, chronic subdural hematomas, or a history of recent head trauma [153]. An assessment of risk for infectious complications should also be made as per current guidelines for neuraxial techniques, and while a bacteremia or epidural abscess would be grounds to delay surgery altogether, the drain should also not be placed over a localized area of infection [154]. Other preparations in terms of fully informed consent, strict asepsis, and monitoring are also as per local/national requirements for any neuraxial technique [155, 156].
CSFDs are usually inserted the day prior to surgery in our institution, in an awake patient, in the lateral position, and in a low lumbar intervertebral space [107]; however, variations on this with good clinical outcomes are described [98]. Placement the day prior to surgery allows for contingency planning in the event of a traumatic/bloody tap, including delaying surgery and systemic anticoagulation for 24 h [4, 152]. However, this can be resource intensive with the need to observe the patient in the CVICU overnight to insure the drain doesn’t become blocked due to kinking or clot formation. Placing the patient in the lateral position is intended to decrease the hydrostatic pressure of the column of CSF and limit the volume of CSF uncontrollably drained during insertion of the drain. Puncture of the dura below the level of the conus medullaris is theoretically less likely to transfix the filum terminale. With the patient awake, redirection of the needle and drain in the event of paresthesia may also limit the potential for nerve injury.
Published techniques variably describe feeding 8–20 cm of catheter into the subarachnoid space beyond the epidural tip [107]. While ensuring that a sufficient length is inserted to avoid the accidental displacement of the catheter with patient movement, the length of catheter that can be inserted is often limited by mechanical difficulties feeding the catheter, a desire to limit uncontrolled drainage of CSF, or patient complaints of paresthesia. If adjustment of the catheter position is required, particular care should be taken when withdrawing the catheter back through the needle due to the risk of shearing it against the needle tip. If any slight resistance is felt, the whole needle and catheter must be withdrawn as one unit and the procedure started again.
Once the drain is inserted and the introducer needle removed, the drain should be secured with care to minimize the risk of kinking and connected to the pressure transducer and drainage bag while maintaining strict sterility (see Fig. 41.8). The level of the transducer should be set and zeroed at the level of the right atrium and ideally fixed to the patient and/or bed. When the drain is open, the level of the transducer and drain should be closely monitored and the patient kept at less than 30 degrees reverse Trendelenburg, with a high level of vigilance to avoid accidental excess CSF drainage. Development of an automated device for pressure- or volume-controlled CSF drainage with simultaneous measurement is described in the literature (LiquoGuard®, Moller Medical GmbH, Fulda, Germany), and while potentially having safety benefits over standard dripping chambers, this is yet to be substantiated [157].
If placed the day before surgery, the drain should be left off overnight and opened once an hour to drain 1–2 drops to ensure patency. Once the patient is in the operating room and positioned for surgery, the transducer should be carefully checked and the drain opened to drain to a CSF pressure of 10 mmHg prior to aortic clamp placement, limited to 10–20 ml/h (or as per the agreed institutional practice, the rationale for which was explored in section “Spinal Cord Protection Strategies” above). At least hourly review and documentation of the drainage pressure (absolute number and waveform), drainage volumes, and the presence of any blood are crucial for safe use. Similarly, at the end of the case, the drain should be clamped before repositioning the patient and only resumed once the patient is finally positioned on their CVICU bed and close monitoring is possible.
In the context of intra- or postoperative evidence of neurological deficit, spinal cord perfusion should be optimized with an increase in mean arterial pressure targets, review of hemoglobin targets, and consideration to further decrease CSF pressure and increase drainage targets. In the postoperative period, the need for imaging (ideally magnetic resonance imaging (MRI) but not always feasible) to assess whether spinal ischemia or an epidural hematoma is the precipitant cause of deficit and the role of hyperbaric therapy can also be considered following optimization of CSF hemodynamics. In the event of frankly bloody CSF drainage, a cessation or reduction in the drainage volume limit should be considered, coagulation function optimized, with imaging to assess for intracranial hemorrhage as soon as the patient is stable enough, with neurosurgical consultation as appropriate.
The CSFD should be removed as soon as possible to reduce infectious complications. Some guidelines recommend drainage for at least 48 h and up to 72 h postoperatively to prevent delayed onset paraplegia [5, 31]. Alternatively, once the patient is neurologically accessible with normal lower limb findings and hemodynamically stable, the CSFD can be clamped and removed if the neurological status remains stable for a further 24 h. Coagulation status should be reviewed before removal of the drain, as described prior to drain insertion. In the event a patient develops sepsis in the postoperative period, the risk-benefit balance of removing the CSFD should be carefully considered, the CSFD not only being a potential source of infection but also a receptacle for colonization. In the event of delayed neurological deterioration after removal of the CSFD that does not improve with blood pressure support, the need to reinsert the CFSD will need to be considered.
While there is limited evidence to validate the optimal technique for CSFD placement and management, evidence substantiates that there should be an agreed standard operating procedure developed at an institutional level [68]. All staff involved in the care of patient’s having TAAA surgery should be orientated to this, and any deviations from care be discussed at a multidisciplinary level.
Renal Protection Strategies
Renal dysfunction is a common complication after TAAA surgery, and while a much smaller proportion proceed to require permanent dialysis, it is a consistent independent predictor of major morbidity and mortality [158, 159]. The ability to assess the incidence, risk factors, and effective therapeutic interventions to prevent renal dysfunction continues to be hampered by the lack of consistency in the criteria to define acute kidney failure [159, 160]. Additionally, the mechanisms for renal injury are diverse, and the contribution variable between patients, including [85, 161, 162]:
-
Nephrotoxic insult, namely, with contrast media preoperatively
-
Ischemia related to the aortic cross-clamp, hypotension, and anemia
-
Atheroembolic phenomenon
-
Reperfusion tubular injury, mediated by various chemical immunomodulating agents
Preoperative renal dysfunction (creatinine >1.5 mg/dL) is consistently found to be an important predictor of postoperative renal dysfunction [85, 158, 159, 161, 162]. Complex surgery with prolonged ischemic times (clamp time > 100 min), greater intraoperative hemodynamic instability, increased bleeding, and the need to return to operating room is understandably associated with worse renal outcomes [158, 159].
In the absence of any effective interventions to treat renal failure, prevention of injury is critical. Despite an extensive body of research, there is limited evidence to support any pharmacological agents as being renoprotective [163]. Furosemide, mannitol, and dopamine [164,165,166,167] have been shown to be ineffective and potentially even harmful [4, 168,169,170], while the utility of corticosteroids, fenoldopam, and dopexamine remains unproven [85, 158].
Considering the potential for preoperative optimization is important if possible. Identifying the “at-risk” patient provides an opportunity to institute additional intraoperative support. Contrast-induced nephropathy should be allowed to resolve with hydration support and N-acetylcysteine [171, 172] or avoid contrast altogether by using alternative imaging techniques such as magnetic resonance angiography [162]. Limited evidence from animal studies also support the concept of post-conditioning, where a gradual increase in blood flow on unclamping reduces the degree of reperfusion injury [173].
In addition to maintaining euvolemia and supporting an adequate cardiac output and blood pressure perioperatively, renal perfusion can be selectively supported. This involves inserting perfusion catheters into the renal ostia and infusing a rapid 250–300 ml bolus, followed by ~20 ml/min or ~1000 ml/h of a cold crystalloid perfusion solution into each kidney. Although technically simple, the specific indications for use and techniques are controversial. Randomized data supports the use of cold (4 °C) crystalloid (Ringer’s lactate) as superior to normothermic or cold blood [174, 175]; however, the specifics of the type of crystalloid or the utility of adding mannitol or steroids to the perfusate is unclear. American guidelines recommend considering cold (4 °C) crystalloid or blood perfusion [4], while the European guidelines specify utility of cold renal perfusion when the ischemia time is greater than 30 min [5].
Postoperative Care
Part XII of this textbook comprehensively covers the postoperative requirements and potential complications following thoracic surgery. Chapters 46 and 47 specifically cover strategies for managing significant postoperative pain and preventing chronic post-thoracotomy pain.
Specific to TAAA surgery, preservation of spinal cord and end-organ function continues to be the primary concern in the immediate postoperative period. As in the intraoperative period, tight hemodynamic control, euvolemia, normal coagulation function, close protocolized CSFD management, and one hourly neurological assessment are crucial to optimal long-term recovery outcomes.
In our institute patients are transferred to the intensive care sedated and ventilation. Once the patient is hemodynamically stable, the sedation is temporarily held to assess neurological function by regular clinical examination. Sedation is weaned, and the patient is usually extubated in the first 12–24 h postoperatively. Our routine postoperative analgesia regimen comprises of a continuous local anesthetic infusion (ropivacaine 0.2% 5 cc/h) through surgically placed paravertebral catheters started on arrival to the ICU and opioid (hydromorphone/morphine) patient-controlled analgesia (PCA) boluses once the patient is conscious.
Summary
Cambria said it best – “absolutes in TAAA surgery are usually proven to be wrong” [176]. This not only recognizes the many limitations in the quality of current literature but the multifaceted complexity of the pathology, with no individual case being predictable.
Despite the limitations in knowledge and specifics in practice standards, fundamentals in improving outcomes are being established, including the development of protocols/packages of care, establishing institutional familiarity and experience, proactive measures to protect sensitive neural and renal tissue from ischemia, and the early identification of compromise in order to minimize the severity of long-term injury.
References
Acher C, Wynn M. Outcomes in open repair of the thoracic and thoracoabdominal aorta. J Vasc Surg. 2010;52(4 Suppl):3S–9S.
Cowan JA Jr, Dimick JB, Henke PK, Huber TS, Stanley JC, Upchurch GR Jr. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg. 2003;37(6):1169–74.
Schermerhorn ML, Giles KA, Hamdan AD, Dalhberg SE, Hagberg R, Pomposelli F. Population-based outcomes of open descending thoracic aortic aneurysm repair. J Vasc Surg. 2008;48(4):821–7.
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Anesth Analg. 2010;111(2):279–315.
Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873–926.
Adams HD, Van Geertruyden HH. Neurologic complications of aortic surgery. Ann Surg. 1956;144(4):574–610.
Panthee N, Ono M. Spinal cord injury following thoracic and thoracoabdominal aortic repairs. Asian Cardiovasc Thorac Ann. 2015;23(2):235–46.
Cina CS, Abouzahr L, Arena GO, Lagana A, Devereaux PJ, Farrokhyar F. Cerebrospinal fluid drainage to prevent paraplegia during thoracic and thoracoabdominal aortic aneurysm surgery: a systematic review and meta-analysis. J Vasc Surg. 2004;40(1):36–44.
Etz CD, Kari FA, Mueller CS, Silovitz D, Brenner RM, Lin HM, et al. The collateral network concept: a reassessment of the anatomy of spinal cord perfusion. J Thorac Cardiovasc Surg. 2011;141(4):1020–8.
Coselli JS, LeMaire SA, Preventza O, de la Cruz KI, Cooley DA, Price MD, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg. 2016;151(5):1323–37.
Mehmedagic I, Jorgensen S, Acosta S. Mid-term follow-up of patients with permanent sequel due to spinal cord ischemia after advanced endovascular therapy for extensive aortic disease. Spinal Cord. 2015;53(3):232.
Bisdas T, Panuccio G, Sugimoto M, Torsello G, Austermann M. Risk factors for spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2015;61(6):1408–16.
Buth J, Harris PL, Hobo R, van Eps R, Cuypers P, Duijm L, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: incidence and risk factors. A study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg. 2007;46(6):1103–10. discussion 10-1.
Conrad MF, Ye JY, Chung TK, Davison JK, Cambria RP. Spinal cord complications after thoracic aortic surgery: long-term survival and functional status varies with deficit severity. J Vasc Surg. 2008;48(1):47–53.
Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17(2):357–68. discussion 68-70
Eagleton MJ, Shah S, Petkosevek D, Mastracci TM, Greenberg RK. Hypogastric and subclavian artery patency affects onset and recovery of spinal cord ischemia associated with aortic endografting. J Vasc Surg. 2014;59(1):89–94.
Becker DA, McGarvey ML, Rojvirat C, Bavaria JE, Messe SR. Predictors of outcome in patients with spinal cord ischemia after open aortic repair. Neurocrit Care. 2013;18(1):70–4.
Keith CJ Jr, Passman MA, Carignan MJ, Parmar GM, Nagre SB, Patterson MA, et al. Protocol implementation of selective postoperative lumbar spinal drainage after thoracic aortic endograft. J Vasc Surg. 2012;55(1):1–8. discussion 8.
Yan TD, Tian DH, LeMaire SA, Misfeld M, Elefteriades JA, Chen EP, et al. The ARCH projects: design and rationale (IAASSG 001). Eur J Cardiothorac Surg. 2014;45(1):10–6.
The Society of Thoracic Surgeons. STS National Database, Data Managers 2017. Available from: http://www.sts.org/sts-national-database/data-managers.
Boening A, Karck M, Conzelmann LO, Easo J, Krüger T, Rylski B, et al. German registry for acute aortic dissection type A: structure, results, and future perspectives. Thorac Cardiovasc Surg. 2017;65(2):77–84.
Beck AW, Sedrakyan A, Mao J, Venermo M, Faizer R, Debus S, et al. Variations in abdominal aortic aneurysm care: a report from the international consortium of vascular registries. Circulation. 2016;134(24):1948–58.
Zammert M, Gelman S. The pathophysiology of aortic cross-clamping. Best Pract Res Clin Anaesthesiol. 2016;30(3):257–69.
Crawford ES, Crawford JL, Safi HJ, Coselli JS, Hess KR, Brooks B, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg. 1986;3(3):389–404.
Gelman S, Khazaeli MB, Orr R, Henderson T. Blood volume redistribution during cross-clamping of the descending aorta. Anesth Analg. 1994;78(2):219–24.
Stokland O, Miller MM, Ilebekk A, Kiil F. Mechanism of hemodynamic responses to occlusion of the descending thoracic aorta. Am J Phys. 1980;238(4):H423–9.
Roizen MF, Beaupre PN, Alpert RA, Kremer P, Cahalan MK, Shiller N, et al. Monitoring with two-dimensional transesophageal echocardiography. Comparison of myocardial function in patients undergoing supraceliac, suprarenal-infraceliac, or infrarenal aortic occlusion. J Vasc Surg. 1984;1(2):300–5.
Acher CW, Wynn M. A modern theory of paraplegia in the treatment of aneurysms of the thoracoabdominal aorta: an analysis of technique specific observed/expected ratios for paralysis. J Vasc Surg. 2009;49(5):1117–24. discussion 24.
Melissano G, Bertoglio L, Rinaldi E, Leopardi M, Chiesa R. An anatomical review of spinal cord blood supply. J Cardiovasc Surg. 2015;56(5):699–706.
Connolly JE. Hume memorial lecture. Prevention of spinal cord complications in aortic surgery. Am J Surg. 1998;176(2):92–101.
Khan SN, Stansby G. Cerebrospinal fluid drainage for thoracic and thoracoabdominal aortic aneurysm surgery. Cochrane Database Syst Rev. 2012;10:CD003635.
Jacobs MJ, de Mol BA, Elenbaas T, Mess WH, Kalkman CJ, Schurink GW, et al. Spinal cord blood supply in patients with thoracoabdominal aortic aneurysms. J Vasc Surg. 2002;35(1):30–7.
Griepp RB, Griepp EB. Spinal cord protection in surgical and endovascular repair of thoracoabdominal aortic disease. J Thorac Cardiovasc Surg. 2015;149(2 Suppl):S86–90.
Etz CD, Zoli S, Bischoff MS, Bodian C, Di Luozzo G, Griepp RB. Measuring the collateral network pressure to minimize paraplegia risk in thoracoabdominal aneurysm resection. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S125–30. discussion S42-S46.
Backes WH, Nijenhuis RJ, Mess WH, Wilmink FA, Schurink GW, Jacobs MJ. Magnetic resonance angiography of collateral blood supply to spinal cord in thoracic and thoracoabdominal aortic aneurysm patients. J Vasc Surg. 2008;48(2):261–71.
Svensson LG, Crawford ES. Cardiovascular and vascular disease of the aorta. Philadelphia: W.B. Saunders Company; 1997.
Katz NM, Blackstone EH, Kirklin JW, Karp RB. Incremental risk factors for spinal cord injury following operation for acute traumatic aortic transection. J Thorac Cardiovasc Surg. 1981;81(5):669–74.
O’Callaghan A, Mastracci TM, Eagleton MJ. Staged endovascular repair of thoracoabdominal aortic aneurysms limits incidence and severity of spinal cord ischemia. J Vasc Surg. 2015;61(2):347–54.e1.
Davidovic L, Ilic N, Koncar I. Differences between immediate and late onset of spinal cord ischemia after open and endovascular aortic interventions. J Cardiovasc Surg. 2015;56(5):737–44.
Etz CD, Homann TM, Plestis KA, Zhang N, Luehr M, Weisz DJ, et al. Spinal cord perfusion after extensive segmental artery sacrifice: can paraplegia be prevented? Eur J Cardiothorac Surg. 2007;31(4):643–8.
Maurel B, Delclaux N, Sobocinski J, Hertault A, Martin-Gonzalez T, Moussa M, et al. The impact of early pelvic and lower limb reperfusion and attentive peri-operative management on the incidence of spinal cord ischemia during thoracoabdominal aortic aneurysm endovascular repair. Eur J Vasc Endovasc Surg. 2015;49(3):248–54.
Etz CD, Weigang E, Hartert M, Lonn L, Mestres CA, Di Bartolomeo R, et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European Association for Cardio-Thoracic Surgery dagger. Eur J Cardiothorac Surg. 2015;47(6):943–57.
Marini CP, Levison J, Caliendo F, Nathan IM, Cohen JR. Control of proximal hypertension during aortic cross-clamping: its effect on cerebrospinal fluid dynamics and spinal cord perfusion pressure. Semin Thorac Cardiovasc Surg. 1998;10(1):51–6.
Jacobs MJ, Mess W, Mochtar B, Nijenhuis RJ, Statius van Eps RG, Schurink GW. The value of motor evoked potentials in reducing paraplegia during thoracoabdominal aneurysm repair. J Vasc Surg. 2006;43(2):239–46.
Safi HJ, Miller CC 3rd, Azizzadeh A, Iliopoulos DC. Observations on delayed neurologic deficit after thoracoabdominal aortic aneurysm repair. J Vasc Surg. 1997;26(4):616–22.
Acher CW, Wynn MM, Mell MW, Tefera G, Hoch JR. A quantitative assessment of the impact of intercostal artery reimplantation on paralysis risk in thoracoabdominal aortic aneurysm repair. Ann Surg. 2008;248(4):529–40.
Estrera AL, Miller CC, Chen EP, Meada R, Torres RH, Porat EE, et al. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann Thorac Surg. 2005;80(4):1290–6. discussion 6.
Minatoya K, Ogino H, Matsuda H, Sasaki H, Yagihara T, Kitamura S. Replacement of the descending aorta: recent outcomes of open surgery performed with partial cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2008;136(2):431–5.
Kulik A, Castner CF, Kouchoukos NT. Replacement of the descending thoracic aorta: contemporary outcomes using hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2010;139(2):249–55.
Di Luozzo G, Griepp RB. Experimental basis and clinical studies of brain protection in aortic arch surgery. In: Bosner RS, Pagano D, Haverich A, editors. Brain protection in cardiac surgery. London: Springer; 2011. p. 219–28.
Di Mauro M, Iaco AL, Di Lorenzo C, Gagliardi M, Varone E, Al Amri H, et al. Cold reperfusion before rewarming reduces neurological events after deep hypothermic circulatory arrest. Eur J Cardiothorac Surg. 2013;43(1):168–73.
Svensson LG, Crawford ES, Hess KR, Coselli JS, Raskin S, Shenaq SA, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg. 1993;106(1):19–28; discussion -31.
Ziganshin BA, Rajbanshi BG, Tranquilli M, Fang H, Rizzo JA, Elefteriades JA. Straight deep hypothermic circulatory arrest for cerebral protection during aortic arch surgery: safe and effective. J Thorac Cardiovasc Surg. 2014;148(3):888–98; discussion 98-900.
Tian DH, Wan B, Bannon PG, Misfeld M, LeMaire SA, Kazui T, et al. A meta-analysis of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest with selective antegrade cerebral perfusion. Ann Cardiothorac Surg. 2013;2(2):148–58.
De Paulis R, Czerny M, Weltert L, Bavaria J, Borger MA, Carrel TP, et al. Current trends in cannulation and neuroprotection during surgery of the aortic arch in Europe. Eur J Cardiothorac Surg. 2015;47(5):917–23.
Kamiya H, Hagl C, Kropivnitskaya I, Bothig D, Kallenbach K, Khaladj N, et al. The safety of moderate hypothermic lower body circulatory arrest with selective cerebral perfusion: a propensity score analysis. J Thorac Cardiovasc Surg. 2007;133(2):501–9.
Zierer A, El-Sayed Ahmad A, Papadopoulos N, Moritz A, Diegeler A, Urbanski PP. Selective antegrade cerebral perfusion and mild (28–30 °C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg. 2012;144(5):1042–9.
Lindsay H, Srinivas C, Djaiani G. Neuroprotection during aortic surgery. Best Pract Res Clin Anaesthesiol. 2016;30(3):283–303.
Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S, et al. Canadian cardiovascular society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17–32.
Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130(24):2215–45.
Roshanov PS, Rochwerg B, Patel A, Salehian O, Duceppe E, Belley-Côté EP, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patIents cOhort evaluatioN prospective cohort. Anesthesiology. 2017;126(1):16–27.
Coselli JS, LeMaire SA, Miller CC 3rd, Schmittling ZC, Koksoy C, Pagan J, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg. 2000;69(2):409–14.
Safi HJ, Estrera AL, Miller CC, Huynh TT, Porat EE, Azizzadeh A, et al. Evolution of risk for neurologic deficit after descending and thoracoabdominal aortic repair. Ann Thorac Surg. 2005;80(6):2173–9; discussion 9
Greenberg RK, Lu Q, Roselli EE, Svensson LG, Moon MC, Hernandez AV, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation. 2008;118(8):808–17.
Schlosser FJ, Mojibian H, Verhagen HJ, Moll FL, Muhs BE. Open thoracic or thoracoabdominal aortic aneurysm repair after previous abdominal aortic aneurysm surgery. J Vasc Surg. 2008;48(3):761–8.
American Society of Anesthesiologists. 10.28.15 Standards for Basic Anesthetic Monitoring Amended 2010, Affirmed 2015.
Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348(1):5–14.
Dias NV, Sonesson B, Kristmundsson T, Holm H, Resch T. Short-term outcome of spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2015;49(4):403–9.
Meylaerts SA, Jacobs MJ, van Iterson V, De Haan P, Kalkman CJ. Comparison of transcranial motor evoked potentials and somatosensory evoked potentials during thoracoabdominal aortic aneurysm repair. Ann Surg. 1999;230(6):742–9.
Coselli JS, Tsai PI. Motor evoked potentials in thoracoabdominal aortic surgery: CON. Cardiol Clin. 2010;28(2):361–8.
Etz CD, von Aspern K, Gudehus S, Luehr M, Girrbach FF, Ender J, et al. Near-infrared spectroscopy monitoring of the collateral network prior to, during, and after thoracoabdominal aortic repair: a pilot study. Eur J Vasc Endovasc Surg. 2013;46(6):651–6.
Boezeman RP, van Dongen EP, Morshuis WJ, Sonker U, Boezeman EH, Waanders FG, et al. Spinal near-infrared spectroscopy measurements during and after thoracoabdominal aortic aneurysm repair: a pilot study. Ann Thorac Surg. 2015;99(4):1267–74.
Urbanski PP, Lenos A, Kolowca M, Bougioukakis P, Keller G, Zacher M, et al. Near-infrared spectroscopy for neuromonitoring of unilateral cerebral perfusion. Eur J Cardiothorac Surg. 2013;43(6):1140–4.
Zheng F, Sheinberg R, Yee MS, Ono M, Zheng Y, Hogue CW. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: a systematic review. Anesth Analg. 2013;116(3):663–76.
Arrowsmith JE, Ganugapenta MSSR. Intraoperative brain monitoring in cardiac surgery. In: Bosner RS, Pagano D, Haverich A, editors. Brain protection in cardiac surgery. London: Spinger; 2011. p. 83–111.
Douds MT, Straub EJ, Kent AC, Bistrick CH, Sistino JJ. A systematic review of cerebral oxygenation-monitoring devices in cardiac surgery. Perfusion. 2014;29(6):545–52.
Fedorow C, Grocott HP. Cerebral monitoring to optimize outcomes after cardiac surgery. Curr Opin Anaesthesiol. 2010;23(1):89–94.
Mohandas BS, Jagadeesh AM, Vikram SB. Impact of monitoring cerebral oxygen saturation on the outcome of patients undergoing open heart surgery. Ann Cardiac Anesth. 2013;16(2):102–6.
Deschamps A, Hall R, Grocott H, Mazer CD, Choi PT, Turgeon AF, et al. Cerebral oximetry monitoring to maintain Normal Cerebral Oxygen Saturation during high-risk cardiac surgery: a randomized controlled feasibility trial. Anesthesiology. 2016;124(4):826–36.
Khoyratty SI, Gajendragadkar PR, Polisetty K, Ward S, Skinner T. Flow rates through intravenous access devices: an in vitro study. J Clin Anesth. 2016;31:101–5.
van der Zee EN, Egal M, Gommers D, Groeneveld AB. Targeting urine output and 30-day mortality in goal-directed therapy: a systematic review with meta-analysis and meta-regression. BMC Anesthesiol. 2017;17(1):22.
Egal M, de Geus HR, van Bommel J, Groeneveld AB. Targeting oliguria reversal in perioperative restrictive fluid management does not influence the occurrence of renal dysfunction: a systematic review and meta-analysis. Eur J Anaesthesiol. 2016;33(6):425–35.
Alpert RA, Roizen MF, Hamilton WK, Stoney RJ, Ehrenfeld WK, Poler SM, et al. Intraoperative urinary output does not predict postoperative renal function in patients undergoing abdominal aortic revascularization. Surgery. 1984;95(6):707–11.
Kheterpal S, Tremper KK, Englesbe MJ, O’Reilly M, Shanks AM, Fetterman DM, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107(6):892–902.
Sear JW. Kidney dysfunction in the postoperative period. Br J Anaesth. 2005;95(1):20–32.
Shine T, Nugent M. Sodium nitroprusside decreases spinal cord perfusion pressure during descending thoracic aortic cross-clamping in the dog. J Cardiothorac Anesth. 1990;4(2):185–93.
Flaherty JT, Magee PA, Gardner TL, Potter A, MacAllister NP. Comparison of intravenous nitroglycerin and sodium nitroprusside for treatment of acute hypertension developing after coronary artery bypass surgery. Circulation. 1982;65(6):1072–7.
Gelman S, Reves JG, Fowler K, Samuelson PN, Lell WA, Smith LR. Regional blood flow during cross-clamping of the thoracic aorta and infusion of sodium nitroprusside. J Thorac Cardiovasc Surg. 1983;85(2):287–91.
National Blood Authority. Patient Blood Management Guidelines: Module 1- Critical Bleeding/Massive Transfusion. Canberra, Australia 2011.
Management ASoATFoPB. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology. 2015;122(2):241–75.
Karkouti K, Callum J, Wijeysundera DN, Rao V, Crowther M, Grocott HP, et al. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation. 2016;134(16):1152–62.
Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376(2):136–48.
Cheung AT, Weiss SJ, McGarvey ML, Stecker MM, Hogan MS, Escherich A, et al. Interventions for reversing delayed-onset postoperative paraplegia after thoracic aortic reconstruction. Ann Thorac Surg. 2002;74(2):413–9; discussion 20-1.
Coselli JS, LeMaire SA, Koksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002;35(4):631–9.
Ullery BW, Cheung AT, Fairman RM, Jackson BM, Woo EY, Bavaria J, et al. Risk factors, outcomes, and clinical manifestations of spinal cord ischemia following thoracic endovascular aortic repair. J Vasc Surg. 2011;54(3):677–84.
Wong CS, Healy D, Canning C, Coffey JC, Boyle JR, Walsh SR. A systematic review of spinal cord injury and cerebrospinal fluid drainage after thoracic aortic endografting. J Vasc Surg. 2012;56(5):1438–47.
Dardik A, Perler BA, Roseborough GS, Williams GM. Subdural hematoma after thoracoabdominal aortic aneurysm repair: an underreported complication of spinal fluid drainage? J Vasc Surg. 2002;36(1):47–50.
Estrera AL, Sheinbaum R, Miller CC, Azizzadeh A, Walkes JC, Lee TY, et al. Cerebrospinal fluid drainage during thoracic aortic repair: safety and current management. Ann Thorac Surg. 2009;88(1):9–15; discussion 15.
Hanna JM, Andersen ND, Aziz H, Shah AA, McCann RL, Hughes GC. Results with selective preoperative lumbar drain placement for thoracic endovascular aortic repair. Ann Thorac Surg. 2013;95(6):1968–74; discussion 74-5.
McHardy FE, Bayly PJ, Wyatt MG. Fatal subdural hemorrhage following lumbar spinal drainage during repair of thoraco-abdominal aneurysm. Anaesthesia. 2001;56(2):168–70.
Verhoeven EL, Katsargyris A, Bekkema F, Oikonomou K, Zeebregts CJ, Ritter W, et al. Editor’s choice-ten-year experience with endovascular repair of thoracoabdominal aortic aneurysms: results from 166 consecutive patients. Eur J Vasc Endovasc Surg. 2015;49(5):524–31.
Wynn MM, Sebranek J, Marks E, Engelbert T, Acher CW. Complications of spinal fluid drainage in thoracic and thoracoabdominal aortic aneurysm surgery in 724 patients treated from 1987 to 2013. J Cardiothorac Vasc Anesth. 2015;29(2):342–50.
Youngblood SC, Tolpin DA, LeMaire SA, Coselli JS, Lee VV, Cooper JR Jr. Complications of cerebrospinal fluid drainage after thoracic aortic surgery: a review of 504 patients over 5 years. J Thorac Cardiovasc Surg. 2013;146(1):166–71.
Weaver KD, Wiseman DB, Farber M, Ewend MG, Marston W, Keagy BA. Complications of lumbar drainage after thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2001;34(4):623–7.
Bobadilla JL, Wynn M, Tefera G, Acher CW. Low incidence of paraplegia after thoracic endovascular aneurysm repair with proactive spinal cord protective protocols. J Vasc Surg. 2013;57(6):1537–42.
Augoustides JG, Stone ME, Drenger B. Novel approaches to spinal cord protection during thoracoabdominal aortic interventions. Curr Opin Anaesthesiol. 2014;27(1):98–105.
Fedorow CA, Moon MC, Mutch WA, Grocott HP. Lumbar cerebrospinal fluid drainage for thoracoabdominal aortic surgery: rationale and practical considerations for management. Anesth Analg. 2010;111(1):46–58.
Field M, Doolan J, Safar M, Kuduvalli M, Oo A, Mills K, et al. The safe use of spinal drains in thoracic aortic surgery. Interact Cardiovasc Thorac Surg. 2011;13(6):557–65.
Cheung AT, Pochettino A, McGarvey ML, Appoo JJ, Fairman RM, Carpenter JP, et al. Strategies to manage paraplegia risk after endovascular stent repair of descending thoracic aortic aneurysms. Ann Thorac Surg. 2005;80(4):1280–8; discussion 8-9.
Rizvi AZ, Sullivan TM. Incidence, prevention, and management in spinal cord protection during TEVAR. J Vasc Surg. 2010;52(4 Suppl):86s–90s.
Kise Y, Kuniyoshi Y, Inafuku H, Nagano T, Hirayasu T, Yamashiro S. Directly measuring spinal cord blood flow and spinal cord perfusion pressure via the collateral network: correlations with changes in systemic blood pressure. J Thorac Cardiovasc Surg. 2015;149(1):360–6.
Chiesa R, Melissano G, Marrocco-Trischitta MM, Civilini E, Setacci F. Spinal cord ischemia after elective stent-graft repair of the thoracic aorta. J Vasc Surg. 2005;42(1):11–7.
Crawford ES, Svensson LG, Hess KR, Shenaq SS, Coselli JS, Safi HJ, et al. A prospective randomized study of cerebrospinal fluid drainage to prevent paraplegia after high-risk surgery on the thoracoabdominal aorta. J Vasc Surg. 1991;13(1):36–45; discussion -6.
Chang CK, Chuter TA, Reilly LM, Ota MK, Furtado A, Bucci M, et al. Spinal arterial anatomy and risk factors for lower extremity weakness following endovascular thoracoabdominal aortic aneurysm repair with branched stent-grafts. J Endovasc Ther. 2008;15(3):356–62.
Coselli JS, LeMaire SA. Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 1999;67(6):1931–4; discussion 53−8.
Nijenhuis RJ, Jacobs MJ, Schurink GW, Kessels AG, van Engelshoven JM, Backes WH. Magnetic resonance angiography and neuromonitoring to assess spinal cord blood supply in thoracic and thoracoabdominal aortic aneurysm surgery. J Vasc Surg. 2007;45(1):71–7; discussion 7–8
Cambria RP, Clouse WD, Davison JK, Dunn PF, Corey M, Dorer D. Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg. 2002;236(4):471–9; discussion 9.
Cambria RP, Davison JK, Carter C, Brewster DC, Chang Y, Clark KA, et al. Epidural cooling for spinal cord protection during thoracoabdominal aneurysm repair: a five-year experience. J Vasc Surg. 2000;31(6):1093–102.
Svensson LG, Hess KR, Coselli JS, Safi HJ. Influence of segmental arteries, extent, and atriofemoral bypass on postoperative paraplegia after thoracoabdominal aortic operations. J Vasc Surg. 1994;20(2):255–62.
Safi HJ, Miller CC 3rd, Carr C, Iliopoulos DC, Dorsay DA, Baldwin JC. Importance of intercostal artery reattachment during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 1998;27(1):58–66; discussion -8.
Etz CD, Halstead JC, Spielvogel D, Shahani R, Lazala R, Homann TM, et al. Thoracic and thoracoabdominal aneurysm repair: is reimplantation of spinal cord arteries a waste of time? Ann Thorac Surg. 2006;82(5):1670–7.
Griepp RB, Ergin MA, Galla JD, Lansman S, Khan N, Quintana C, et al. Looking for the artery of Adamkiewicz: a quest to minimize paraplegia after operations for aneurysms of the descending thoracic and thoracoabdominal aorta. J Thorac Cardiovasc Surg. 1996;112(5):1202–13; discussion 13–5.
Czerny M, Eggebrecht H, Sodeck G, Verzini F, Cao P, Maritati G, et al. Mechanisms of symptomatic spinal cord ischemia after TEVAR: insights from the European Registry of Endovascular Aortic Repair Complications (EuREC). J Endovasc Ther. 2012;19(1):37–43.
Amabile P, Grisoli D, Giorgi R, Bartoli JM, Piquet P. Incidence and determinants of spinal cord ischemia in stent-graft repair of the thoracic aorta. Eur J Vasc Endovasc Surg. 2008;35(4):455–61.
Drinkwater SL, Goebells A, Haydar A, Bourke P, Brown L, Hamady M, et al. The incidence of spinal cord ischemia following thoracic and thoracoabdominal aortic endovascular intervention. Eur J Vasc Endovasc Surg. 2010;40(6):729–35.
Rizvi AZ, Murad MH, Fairman RM, Erwin PJ, Montori VM. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: a systematic review and meta-analysis. J Vasc Surg. 2009;50(5):1159–69.
Patterson BO, Holt PJ, Nienaber C, Fairman RM, Heijmen RH, Thompson MM. Management of the left subclavian artery and neurologic complications after thoracic endovascular aortic repair. J Vasc Surg. 2014;60(6):1491–7.e1.
Sepehripour AH, Ahmed K, Vecht JA, Anagnostakou V, Suliman A, Ashrafian H, et al. Management of the left subclavian artery during endovascular stent grafting for traumatic aortic injury - a systematic review. Eur J Vasc Endovasc Surg. 2011;41(6):758–69.
Weigang E, Parker JA, Czerny M, Lonn L, Bonser RS, Carrel TP, et al. Should intentional endovascular stent-graft coverage of the left subclavian artery be preceded by prophylactic revascularization? Eur J Cardiothorac Surg. 2011;40(4):858–68.
De Bakey ME, Cooley DA, Creech O Jr. Resection of the aorta for aneurysms and occlusive disease with particular reference to the use of hypothermia; analysis of 240 cases. Trans Am Coll Cardiol. 1955;5:153–7.
Conrad MF, Crawford RS, Davison JK, Cambria RP. Thoracoabdominal aneurysm repair: a 20-year perspective. The Annals of thoracic surgery. 2007;83(2):S856–61; discussion S90–2.
Kouchoukos NT, Masetti P, Murphy SF. Hypothermic cardiopulmonary bypass and circulatory arrest in the management of extensive thoracic and thoracoabdominal aortic aneurysms. Semin Thorac Cardiovasc Surg. 2003;15(4):333–9.
Kulik A, Castner CF, Kouchoukos NT. Outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2011;141(4):953–60.
Frank SM, Parker SD, Rock P, Gorman RB, Kelly S, Beattie C, et al. Moderate hypothermia, with partial bypass and segmental sequential repair for thoracoabdominal aortic aneurysm. J Vasc Surg. 1994;19(4):687–97.
Svensson LG, Hess KR, D’Agostino RS, Entrup MH, Hreib K, Kimmel WA, et al. Reduction of neurologic injury after high-risk thoracoabdominal aortic operation. Ann Thorac Surg. 1998;66(1):132–8.
Griepp RB, Di Luozzo G. Hypothermia for aortic surgery. J Thorac Cardiovasc Surg. 2013;145(3 Suppl):S56–8.
Bush HL Jr, Hydo LJ, Fischer E, Fantini GA, Silane MF, Barie PS. Hypothermia during elective abdominal aortic aneurysm repair: the high price of avoidable morbidity. J Vasc Surg. 1995;21(3):392–400; discussion -2.
Safi HJ, Miller CC 3rd, Subramaniam MH, Campbell MP, Iliopoulos DC, O’Donnell JJ, et al. Thoracic and thoracoabdominal aortic aneurysm repair using cardiopulmonary bypass, profound hypothermia, and circulatory arrest via left side of the chest incision. J Vasc Surg. 1998;28(4):591–8.
Acher CW, Wynn MM, Hoch JR, Popic P, Archibald J, Turnipseed WD. Combined use of cerebral spinal fluid drainage and naloxone reduces the risk of paraplegia in thoracoabdominal aneurysm repair. J Vasc Surg. 1994;19(2):236–46; discussion 47–8.
Kunihara T, Matsuzaki K, Shiiya N, Saijo Y, Yasuda K. Naloxone lowers cerebrospinal fluid levels of excitatory amino acids after thoracoabdominal aortic surgery. J Vasc Surg. 2004;40(4):681–90.
Lima B, Nowicki ER, Blackstone EH, Williams SJ, Roselli EE, Sabik JF 3rd, et al. Spinal cord protective strategies during descending and thoracoabdominal aortic aneurysm repair in the modern era: the role of intrathecal papaverine. J Thorac Cardiovasc Surg. 2012;143(4):945–52.e1.
Nylander WA Jr, Plunkett RJ, Hammon JW Jr, Oldfield EH, Meacham WF. Thiopental modification of ischemic spinal cord injury in the dog. Ann Thorac Surg. 1982;33(1):64–8.
Fowl RJ, Patterson RB, Gewirtz RJ, Anderson DK. Protection against postischemic spinal cord injury using a new 21-aminosteroid. J Surg Res. 1990;48(6):597–600.
Apaydin AZ, Buket S. Regional lidocaine infusion reduces postischemic spinal cord injury in rabbits. Tex Heart Inst J. 2001;28(3):172–6.
Wu GJ, Chen WF, Sung CS, Jean YH, Shih CM, Shyu CY, et al. Preventive effects of intrathecal methylprednisolone administration on spinal cord ischemia in rats: the role of excitatory amino acid metabolizing systems. Neuroscience. 2007;147(2):294–303.
Qayumi AK, Janusz MT, Jamieson WR, Lyster DM. Pharmacologic interventions for prevention of spinal cord injury caused by aortic crossclamping. J Thorac Cardiovasc Surg. 1992;104(2):256–61.
Lim KH, Connolly M, Rose D, Siegman F, Jacobowitz I, Acinapura A, et al. Prevention of reperfusion injury of the ischemic spinal cord: use of recombinant superoxide dismutase. Ann Thorac Surg. 1986;42(3):282–6.
Smith PD, Bell MT, Puskas F, Meng X, Cleveland JC Jr, Weyant MJ, et al. Preservation of motor function after spinal cord ischemia and reperfusion injury through microglial inhibition. Ann Thorac Surg. 2013;95(5):1647–53.
Mares JM, Foley LS, Bell MT, Bennett DT, Freeman KA, Meng X, et al. Erythropoietin activates the phosporylated cAMP [adenosine 3’5’ cyclic monophosphate] response element-binding protein pathway and attenuates delayed paraplegia after ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2015;149(3):920–4.
Gurer B, Kertmen H, Kasim E, Yilmaz ER, Kanat BH, Sargon MF, et al. Neuroprotective effects of testosterone on ischemia/reperfusion injury of the rabbit spinal cord. Injury. 2015;46(2):240–8.
Freeman KA, Fullerton DA, Foley LS, Bell MT, Cleveland JC Jr, Weyant MJ, et al. Spinal cord protection via alpha-2 agonist-mediated increase in glial cell-line-derived neurotrophic factor. J Thorac Cardiovasc Surg. 2015;149(2):578–84; discussion 84–6.
Horlocker TT, Wedel DJ, Rowlingson JC, Enneking FK, Kopp SL, Benzon HT, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (third edition). Reg Anesth Pain Med. 2010;35(1):64–101.
Wynn MM, Mell MW, Tefera G, Hoch JR, Acher CW. Complications of spinal fluid drainage in thoracoabdominal aortic aneurysm repair: a report of 486 patients treated from 1987 to 2008. J Vasc Surg. 2009;49(1):29–34; discussion -5.
American Society of Anesthesiologists. Practice advisory for the prevention, diagnosis, and management of infectious complications associated with neuraxial techniques: an updated report by the American Society of Anesthesiologists Task Force on infectious complications associated with neuraxial techniques and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2017;126(4):585–601.
Australia New Zealand College of Anaesthetists Guidelines for the Management of Major Regional Analgesia. Australia New Zealand College of Anaesthetists; 2011. p. 6.
Obstetric Anaesthetists’ Association, Campbell JP, Plaat F, Checketts MR, Bogod D, Tighe S, et al. Safety guideline: skin antisepsis for central neuraxial blockade. Anaesthesia. 2014;69(11):1279–86.
Tshomba Y, Leopardi M, Mascia D, Kahlberg A, Carozzo A, Magrin S, et al. Automated pressure-controlled cerebrospinal fluid drainage during open thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2017;66:37.
Wynn MM, Acher C, Marks E, Engelbert T, Acher CW. Postoperative renal failure in thoracoabdominal aortic aneurysm repair with simple cross-clamp technique and 4°C renal perfusion. J Vasc Surg. 2015;61(3):611–22.
Kashyap VS, Cambria RP, Davison JK, L’Italien GJ. Renal failure after thoracoabdominal aortic surgery. J Vasc Surg. 1997;26(6):949–55; discussion 55–7.
Dariane C, Coscas R, Boulitrop C, Javerliat I, Vilaine E, Goeau-Brissonniere O, et al. Acute kidney injury after open repair of intact abdominal aortic aneurysms. Ann Vasc Surg. 2017;39:294–300.
Yeung KK, Groeneveld M, Lu JJ, van Diemen P, Jongkind V, Wisselink W. Organ protection during aortic cross-clamping. Best Pract Res Clin Anaesthesiol. 2016;30(3):305–15.
Swaminathan M, Stafford-Smith M. Renal dysfunction after vascular surgery. Curr Opin Anaesthesiol. 2003;16(1):45–51.
Zacharias M, Mugawar M, Herbison GP, Walker RJ, Hovhannisyan K, Sivalingam P, et al. Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev. 2013;9:CD003590.
Marik PE. Low-dose dopamine: a systematic review. Intensive Care Med. 2002;28(7):877–83.
Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomized trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356(9248):2139–43.
Kellum JA, M Decker J. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001;29(8):1526–31.
Friedrich JO, Adhikari N, Herridge MS, Beyene J. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142(7):510–24.
Doi K, Ogawa N, Suzuki E, Noiri E, Fujita T. Mannitol-induced acute renal failure. Am J Med. 2003;115(7):593–4.
Perdue PW, Balser JR, Lipsett PA, Breslow MJ. "Renal dose" dopamine in surgical patients: dogma or science? Ann Surg. 1998;227(4):470–3.
Visweswaran P, Massin EK, Dubose TD. Mannitol-induced acute renal failure. J Am Soc Nephrol. 1997;8(6):1028–33.
Subramaniam RM, Suarez-Cuervo C, Wilson RF, Turban S, Zhang A, Sherrod C, et al. Effectiveness of prevention strategies for contrast-induced nephropathy: a systematic review and meta-analysis. Ann Intern Med. 2016;164(6):406–16.
Xu R, Tao A, Bai Y, Deng Y, Chen G. Effectiveness of N-Acetylcysteine for the prevention of contrast-induced nephropathy: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5(9):e003968.
Durrani NK, Yavuzer R, Mittal V, Bradford MM, Lobocki C, Silberberg B. The effect of gradually increased blood flow on ischemia-reperfusion injury in rat kidney. Am J Surg. 2006;191(3):334–7.
Köksoy C, LeMaire SA, Curling PE, Raskin SA, Schmittling ZC, Conklin LD, et al. Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg. 2002;73(3):730–8.
Lemaire SA, Jones MM, Conklin LD, Carter SA, Criddell MD, Wang XL, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2009;49(1):11–9; discussion 9.
Cambria R. Commentary. Thoracic and thoracoabdominal aneurysm repair: is reimplantation of spinal cord arteries a waste of time? Perspect Vasc Surg Endovasc Ther. 2008;20(2):221–3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Clinical Case Discussion
Clinical Case Discussion
A 40-year- old lady with Marfan syndrome has a Crawford type II TAAA which measures 6.4 cm. She is an ex-smoker with no other significant comorbidities.
-
Question 1. What are the major perioperative concerns?
-
Question 2: There is a suitable proximal clamp site just distal to the left subclavian artery. What are the options for perfusion management for this case?
-
Question 3: The patient develops weakness in both legs in ICU on the first postoperative day. How would you manage this situation?
-
Answer 1: For a complete discussion of these issues, see the section “Intraoperative Management” (from page 14)
-
(a)
Massive bleeding and coagulopathy
-
(b)
Spinal cord injury
-
(c)
Acute kidney injury
-
(d)
Acute lung injury due to prolonged OLV, surgical manipulation, and massive transfusion
-
(a)
-
Answer 2: For a complete discussion, see section “Surgical Technique” (from page 6)
-
(a)
Left heart bypass – preferred
-
(b)
Partial cardiopulmonary bypass
-
(c)
Deep hypothermic circulatory arrest
-
(a)
-
Answer 3: For a complete discussion, see sections “Spinal Cord Protection Strategies” and “Technical Specifics of Cerebrospinal Fluid Drainage” (specifically page 21)
-
(a)
Increase MAP to 90–100 mmHg
-
(b)
Increase CSF drainage to maintain ICP less than 10 mmHg
-
(c)
Raise the hemoglobin to over 90–100 g/dl
-
(d)
MRI/CT scan to rule out epidural hematoma
-
(a)
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lindsay, H.A., Srinivas, C., Ouzounian, M. (2019). Open Thoracoabdominal Aortic Aneurysm Repair. In: Slinger, P. (eds) Principles and Practice of Anesthesia for Thoracic Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-00859-8_41
Download citation
DOI: https://doi.org/10.1007/978-3-030-00859-8_41
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-00858-1
Online ISBN: 978-3-030-00859-8
eBook Packages: MedicineMedicine (R0)