Abstract
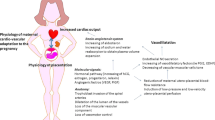
People with fetal growth restriction (FGR) and a low birth weight possess a more limited nephron mass (in proportion to their body size), a reduced renal volume, and a smaller quantity of glomeruli. From a physiopathological standpoint, FGR due to placental insufficiency is a mainly vascular disorder caused by the chronic vasoconstriction suffered by tertiary villi owing to inadequate trophoblastic invasion of the maternal spiral arteries. The resulting hypoxia affects sodium and potassium channels, and the consequent adaptive response leads to the onset of a chronic vasoconstriction. In the initial stages of this pathological condition, the fetus reacts by reducing its growth rate and increasing its oxygen extraction capacity. In the long term, however, hypoxemia sets in and may persist for weeks, with a subsequent activation of specific chemoreceptors and cardiovascular modifications designed to preserve the delivery of oxygen to the major organs, for example the heart, brain and adrenal glands. Recent ultrasound studies have revealed significant differences in fetuses with FGR based on comparisons with normal fetuses, especially as concerns diastolic function. This cardiac dysfunction would seem to be a constitutive characteristic of growth restriction, which would begin early, remain in a subclinical stage (demonstrated by a normal cardiac output), and then gradually deteriorate. For the time being, however, the exact pathophysiology of hypoxic damage in fetuses with FGR and its influence on the development of the cardiocirculatory system in adults remain the topic of lively scientific debate.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Fetal growth restriction (FGR) lacks a widely agreed upon definition in the literature. The most commonly used definition includes the presence of a fetus that does not reach its maximum growth potential owing to several pathological insults.
The causes of FGR may be related to the following conditions: (1) maternal problems: infections, chronic hypertension, diabetes mellitus, cardiovascular disease, or substance abuse (as smoke); (2) placental problems: inadequate vascular supply, chorioangioma, infarct, circumscribed placenta, confined placental mosaicism, or obliterative placental vasculopathy; (3) fetal problems: infections, chromosome disease, or genetic disease; (4) idiopathologies.
The etiology commonly understood to be the most frequent appears to be placental insufficiency [1,2,3,4,5,6]. There is, thus, vascular compromise, which inevitably leads to an increase in vascular resistance in the umbilical artery, limiting blood flow and, therefore, nourishment to the fetus [2, 4, 5]. The fetus implements compensation mechanisms that allow delivery of most nutrients to the major organs, i.e. adrenal glands, heart, and brain [7]. Cardiac output is also redistributed in favor of the left ventricle. This hemodynamic adaptation leads to a doubling of oxygen delivery from the umbilical vein to the myocardium and fetal brain [8]. A further mechanism of control and local compensation is activated at the cerebral level. In fact, by dilating the vessels that supply the nuclei of the brain base with blood, there is preferential flow toward these structures and away from the cerebral cortex. The reduction of cardiac flow to certain organs places them in a suboptimal condition. When the liver, the main producer of fetal proteins , receives 30% less blood than usual it becomes limited in its operation, which results in a low fetal body weight from FGR. From an ultrasound point of view, the evaluation of maternal vessels (uterine arteries) plays a predominantly diagnostic role and has a high negative predictive value [9, 10]. These vessels are generally assessed during the first and second trimesters of pregnancy. Normal pulsatility index values preclude, with high probability, the emergence of pre-eclampsia or FGR [9, 10]. With regard to fetal vessels, the first Doppler abnormality corresponds to an increase in the pulsatility index in the umbilical artery (PIUA).
In normal fetuses, in fact, a large percentage of cardiac output supply the vascular bed, one third during II trimester and one fifth near term [11]. In FGR fetuses, a lower quantity of blood is directed to the placenta while normal cardiac output is maintained. The purpose of this is so that umbilical blood can recirculate widely in the fetal body to obtain a more efficient extraction of oxygen and nutrients. A reactive polycythemia is often present as a further compensation method [8, 11]. At this stage the fetus enters a phase of compensation. Specifically, the compensation state allows a satisfactory fetal condition that could continue for a long time. As long the fetus in in this condition, there are only Doppler changes. With the progression of the pathology, and therefore of the obstruction of vascular flow, absent end diastolic flow (AEDF) and reverse end diastolic flow (REDF) develop [12]. The cerebral compensation is manifested itself by changes in the Doppler reading, such as a vasodilatation of the middle cerebral artery [12]. When the metabolic stress becomes more intensive, the fetus is no longer able to meets the demands of single organs, which leads to a state of decompensation and fetal suffering. Absolutely late sign and acute cardiac compromise index is represented by the absent or the reverse at the level of the venous duct, which leads to a state of hypercarbia and cardiac compromize resembled by absent or reverse A wave in Doctus venosus [13].
Following a morphofunctional alteration of the placental bed, in fact, the fetus is not allowed to reach its (genetically predetermined) maximum development and lacks in fact the environmental and metabolic requirements for such development.
The gestational age in which the pathology develops is also fundamental in the diagnostic, therapeutic, and prognostic framework. The development of the disease below 32 weeks (early FGR) greatly increases the risk of rapid progression of the disease itself, thus having a more precocious and more severe prognosis than late FGR [6]. The fetal response to placental dysfunction evolves from early compensatory reactions to late multiple organ failure disorders. This response contributes to fetal intrauterine programming and, as a result, short- and long-term morbidity [6]. The consequence is a smaller fetus than expected. Fundamental, however, both in terms of management and prognosis, is the correct classification of a small fetus, specifically a correct differentiation between the small for gestational age (SGA) and a true FGR.
Recently the Delphi definition , formulated by expert consensus, both for early and late FGR [14], has emerged as the most widely used classification. It divides early and late FGR as follows:
-
Early FGR (< 32 weeks)
-
Solitary parameters
-
1.
Abdominal circumference (AC) < 3(rd) centile
-
2.
Estimated fetal weight (EFW) < 3(rd) centile
-
3.
AEDF in the umbilical artery (UA)
-
1.
-
Contributory parameters: AC or EFW < 10(th) centile combined with a pulsatility index (PI) > 95(th) centile in either the UA or uterine artert
-
-
Late FGR (≥ 32 weeks)
-
Solitary parameters
-
1.
AC < 3(rd) centile or
-
2.
EFW < 3(rd) centile
-
1.
-
Contributory parameters:
-
1.
EFW or AC < 10(th) centile
-
2.
AC or EFW crossing centiles by > two quartiles on growth charts
-
3.
Cerebroplacental ratio < 5(th) centile or UA-PI > 95(th) centile
-
1.
-
Furthermore, a series of functional morphological modifications is made. From a hemodynamic point of view, there is a tendency to increase pressure. The main determinants of this condition are vascular and renal factors. The whole vascular bed is in fact characterized by the presence of forces that constantly act on the endothelium in a varied and turbulent manner, changing in intensity, direction, and frequency. Thus we have both forces acting perpendicular to the vessel and forces acting tangentially [15]. Thus, an increase in the average arterial pressure causes greater trauma to the vessel, leading to unavoidable cellular modifications, including cell proliferation, apoptosis, and matrix modification, with its destruction and new synthesis [15].
A condition of chronic hypoxemia , when decompensated, can negatively involve different organs. At the heart level, hypoxemia can result in hypoxia. Fibrocells furthest from the vessel are the first to undergo cell death. This results in the formation of scars that further limit the contractile capacity of the heart. The remaining myocytes undergo compensatory hypertrophy in an attempt to meet the increased peripheral and cardiac muscle demands. This situation puts the heart in a suboptimal condition already in the fetal phase of life.
Thus, as an adult, a person who experienced FGR is more likely to have difficulties meeting increased cardiac and peripheral muscle demands in the course of physical exertion, making the heart more fragile and, therefore, prone to damage or acute myocardial infarction [16, 17].
Developments in molecular biology have allowed for the creation of microarrays that facilitate the evaluation of gene expression during stressful fetal conditions [18]. They might also lead to a deepening of the molecular mechanisms of this phenomenon from a therapeutic point of view, making it possible to regressively remodel cardiac muscle (and therefore help to develop a more normal heart) already in utero.
Another very important organ in the context of FGR is the kidney. In fact, fetuses suffering from FGR, which typically shows up as a vitamin A deficiency [19], see a reduction in the number of glomeruli and nephrons, followed by renal hyperfiltration with the result of glomerulosclerosis and an increased risk of developing hypertension in adulthood [20]. However, this is a risk factor for renal damage too, so it can trigger a self-perpetuating cycle of renal and hemodynamic damage. Some studies in the literature have shown that these fetuses undergo genetic reprogramming with an increase in apical sodium transporters in the nephrons [21, 22]. This could increase the risk of salt-dependent hypertension in adulthood.
In fact, it seems that already during intrauterine life there is a genetic programming of the fetus to help it cope with the difficulties of living in its environment. But this also changes fetal hemodynamics , unavoidably affecting adult life. This consideration was already hypothesized in 1997 in a very large cohort study of nearly 150,000 adolescents in Sweden that demonstrated that systolic blood pressure was significantly higher in young men with the lowest birth weight [23].
Endothelial dysfunction is thought to be an innate trait in individuals with FGR and that this innate predisposition inevitably also affects neonatal and adult life. Useful methods for assessing endothelial dysfunction may be aorta intima-media thickness (aIMT ), carotid intima-media thickness (cIMT ), carotid stiffness, central pulse wave velocity, brachial artery flow-mediated dilation, endothelium-dependent microvascular vasodilatation, and echocardiographic evaluation [24,25,26,27,28,29,30]. Recent studies have shown that prenatal programming leads to an increase in apical sodium transporters in multiple nephron segments that could lead to salt-sensitive hypertension, as described in models of developmental hypertension and glucocorticoids and placental dysfunction [21].
Barker theory considers what may happen to a fetus in a condition of maternal malnutrition. In the case of nutritional deprivation, endocrine-metabolic changes occur in the unborn child. The ultimate aim is to strengthen the fetus, make it less needy in terms of nutrients for development, which represents a prophylactic mechanism in case of subsequent food shortages. This may certainly be very useful in acute conditions, and perhaps also from an evolutionary point of view if the conditions and the environment in which the newborn and then the adult will live are difficult to sustain. In the case of subsequent abundance of food, in contrast, typically this intrauterine imprint inevitably predisposes the fetus to developing diseases such as metabolic syndrome or diabetes. And this is generally what happens later in life. The lack of nourishment is not due to an actual lack of food but to a placental dysfunction.
Unfortunately, the fetus cannot understand the difference, so it is “forged” in a counterproductive way. In fact FGR will occur, but the child will be predisposed to rapid weight gain in the first years of life, as well as to adolescent obesity and increased risk of cardiovascular disease (CVD), stroke, glucose intolerance, and type II diabetes in adulthood [31,32,33]. Thus, FGR leads to intrinsic vascular damage, which contributes to hemodynamic alterations. However, additional factors damage a vessel that has already been structurally altered. In fact, we have seen that in this type of fetus suffers from an increase in sympathetic tone and an alteration of the lipid condition, leading to dyslipidemia [22, 34]. Thus, multiple actors and conditions arise, including nutritional and metabolic ones, that contribute to the formation of atherosclerotic plaques. Endothelial cells, smooth muscle cells, and cells of the immune system are definitely involved, and later, calcium crystals are also deposited [35, 36].
In fact, first of all, various types of cells migrate and move in the intimal space, contributing to its thickening. The cells present are leukocytes, monocytes, macrophages (identified by the specific marker CD68), smooth muscle cells, and quiescent (identified by the specific marker CD31) or activated (identified by the specific E-selectin marker) endothelial cells , the latter two typical of preinjury atherosclerosis [37]. The condition is further exacerbated by the deposition of interstitial glycosaminoglycans, always at the level of this layer [38].
Some authors [24,25,26] have confirmed that ultrasound-based measurement of aIMT was inversely proportional, both in fetuses and in newborns, to EFW, hypothesizing how various Doppler anomalies of UA and low birth weight (LBW) could be correlated with an abnormal vascular structure and endothelial damage. It is hypothesized that such histological changes cause greater arterial stiffness and that this correlates with an increase in the aortic PI [25]. This fact is not to be underestimated, because it represents, from a cardiovascular point of view, a higher risk factor, similar to hypertension [39]. This applies to both single pregnancies and twin pregnancies [40] . When twins have both biometric and flowmeter alterations (thus falling within the definition of FGR placenta dependent), it has been shown that they also have a greater thickness of the aorta in the studied ultrasound, leading to hemodynamic changes in the vascular tree. This happens regardless of gender or chorionicity.
Unfortunately these lesions do not have only a histological significance, but they manifest themselves clinically and, depending on the degree of development of atherosclerotic plaques, can also lead to massive compromise, with the final result being death. Some studies report autopsies on children (aged between 2 and 15 years) in which a possible cardiovascular cause has been excluded, showing the presence of lipid striae and fibro-atherosclerotic lesions on the wall of the aorta [41]. Specifically, anatomical pathological analysis of these lesions showed that they actually generated a thickening of the intima, thus confirming the ultrasound data. Immunohistochemical investigations have revealed the presence of condensed elastic fibers to form a strongly defined and marked internal elastic membrane [37]. Therefore, in conclusion, the plaques that involve marked functional morphological alterations of the vessels develop progressively over time, starting from an intrinsically pathological vascular structure, eventually leading to very unfavorable prognosis.
The importance of differentiating between an adequate gestational age (AGA) fetus and an FGR one was mentioned previously. In addition to the biometric and flowmetry parameters, a valid aid may also be provided by metabolomics, based on a different distribution of the essential amino acids [42,43,44]. Specifically, we have seen how sphingosine 1-phosphate, a molecule expressed in the cardiovascular system, is involved in the pathophysiology of diseases associated with endothelial dysfunction [45]. This molecule has as its precursor the ceramide, which, if increased, can induce an endothelium-dependent release of thromboxane A2, involved in hypertension and inflammation and has a vasoactive effect [45].
Therefore, during intrauterine life, these changes must also be considered in the large pathophysiological picture that determines the vascular bed in a pathological way, predisposing such fetuses to pathologies such as hypertension and nephropathy, especially glomerulonephritis, with a decrease in the number of nephrons in proportion to body weight at birth [24, 34, 46]. Even the heart muscle suffers from this systemic remodeling. A prospective cohort study (FGR children aged 3–6 years) showed that the prevalence of globular hearts and impaired myocardial relaxation increased. This leads to an inevitable increase in postload and compromise of cardiac compliance [21].
Numerous studies show an increase in aIMT and microalbuminuria in FGR fetuses compared to AGA fetuses, both in single and twin pregnancies, probably contributing also to an early and pathological stiffening of the arterial vascular tree. It is important to underline that intrauterine fetal remodeling does not occur only in severe FGR, typically early FGR, but can also occur in late FGR. Some studies on SGA infants have shown a correlation between weight recovery after birth (therefore substantial fat accumulation) and increase in blood pressure in subsequent ages, regardless of birth weight [34, 47,48,49]. Not all studies agree on this, but very likely the diversity of results also depends on the different statistical methods of analysis used and the different sample groups considered [50].
Other studies showed that infants with the lowest birth weight and who had a strong nutritional reward in the postbirth period developed a higher cIMT in adulthood compared to those adults who had been normal size fetuses and with normal postnatal growth [47, 51]. It is clear that the rapid accumulation of fat in the postnatal period should be avoided in order to limit the risk of developing increased blood pressure in adulthood. Further research is needed to understand more thoroughly whether FGR with postnatal weight recovery also carries a greater risk of developing cardiovascular adverse events and obesity in adulthood [52].
Prospective Studies
Baker’s hypothesis was corroborated by numerous in vivo prospective studies, which related FGR patients with the development of cardiovascular, metabolic, and blood diseases in adulthood [53, 54]. Until recently, most studies focused on the phenotype resulting from changes in maternal nutrition . Today there is a greater interest in the study of the mechanisms through which this phenotype is created. In this context it is fundamental to investigate the role of overnutrition and undernutrition at the prenatal age with experimental and epidemiological methods, thereby allowing for a balancing of the link between genotype and saver phenotype.
The deepening of the molecular and epigenetic pathways involved in this field might make it possible to reveal the mechanisms underlying CVD and renal diseases in adulthood. In this sense, in vitro research is fundamental, mainly through animal models. The fundamentals of research for the future will be represented by the medicine of reproduction, nutrition, and the study of the vascular system, as well as metabolomics. Evaluation of these aspects will allow researchers to reach new therapeutic targets with important implications for the wellbeing of the general population [55].
In this context it is important to note the role of FGR diagnosis and in particular follow-up during adulthood. To date, pediatric guidelines do not include FGR as a risk factor for childhood diseases, but it would be desirable for it to be considered. This would make it possible to monitor on a large scale the cardiovascular, hypertensive, and metabolic problems in these children and to intervene with primary and secondary prevention. It has been shown that lifestyle interventions, such as physical activity promotion, passive smoking protection, and weight control, greatly improve cardiovascular wellbeing of these children [56]. It is interesting to note, then, recent evidence showing that high doses of omega-3 fatty acids help to reduce cases of hypertension and can slow down the progression of atherosclerosis in FGR children [57, 58] and play a preventive role in the thickening of the arterial part that occurs in these children after birth [59].
In conclusion, such evidence suggests the desirability for postnatal monitoring of FGR fetuses to become routine, allowing for important interventions to improve the quality of life of patient using strategies that include lifestyle changes and possibly pharmacological or nutraceutical interventions.
References
American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 134: fetal growth restriction. Obstet Gynecol. 2013;121:1122–33.
Unterscheider J, Daly S, Geary MP, Kennelly MM, McAuliffe FM, O’Donoghue K, et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am J Obstet Gynecol. 2013;208:290.e1–6.
Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol. 1995;6:168–74.
Royal College of Obstetricians and Gynecologists. The investigation and management of the small-for-gestational-age fetus (guideline no. 31). London: Royal College of Obstetricians and Gynecologists; 2002.
Society for Maternal-Fetal Medicine Publications Committee, Berkley E, Chauhan SP, Abuhamad A. Doppler assessment of the fetus with intrauterine growth restriction. Am J Obstet Gynecol. 2012;206:300–8.
Baschat AA, Cosmi E, Bilardo CM, Wolf H, Berg C, Rigano S, et al. Predictors of neonatal outcome in early-onset placental dysfunction. Obstet Gynecol. 2007;109:253–61.
Garg M, Thamotharan M, Dai Y, Lagishetty V, Matveyenko AV, Lee WN, et al. Glucose intolerance and lipid metabolic adaptations in response to intrauterine and postnatal calorie restriction in male adult rats. Endocrinology. 2013;154:102–13.
Al-Ghazali W, Chita SK, Chapman MG, Allan LD. Evidence of redistribution of cardiac output in asymmetrical growth retardation. Br J Obstet Gynaecol. 1989;96:697–704.
Słowakiewicz K, Perenc M, Sieroszewski P. Biochemical prenatal tests and uterine artery Doppler examination in prediction of PIH and IUGR in the third trimester of pregnancy. Ginekol Pol. 2010;81:352–7.
Valensise H, Romanini C. Uterine Doppler in the identification of patients at risk for hypertension and IUGR. J Perinat Med. 1994;22(Suppl 1):69–72.
Rizzo G, Capponi A, Cavicchioni O, Vendola M, Arduini D. Low cardiac output to the placenta: an early hemodynamic adaptive mechanism in intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32:155–9.
Jang DG, Jo YS, Lee SJ, Kim N, Lee GS. Perinatal outcomes and maternal clinical characteristics in IUGR with absent or reversed end-diastolic flow velocity in the umbilical artery. Arch Gynecol Obstet. 2011;284:73–8.
Picconi JL, Hanif F, Drennan K, Mari G. The transitional phase of ductus venosus reversed flow in severely premature IUGR fetuses. Am J Perinatol. 2008;25:199–203.
Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48:333–9.
White CR, Haidekker M, Bao X, Frangos JA. Temporal gradients in shear, but not spatial gradients, stimulate endothelial cell proliferation. Circulation. 2001;103:2508–13.
McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633.
Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10:265–74.
Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, et al. Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol. 2003;81:177–99.
Lelievre-Pegorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, et al. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 1998;54:1455–62.
Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–47.
Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol. 2010;298:F235–47.
Mizuno M, Siddique K, Baum M, Smith S. Prenatal programming of hypertension induces sympathetic over activity in response to physical stress. Hypertension. 2013;61:180–6.
Nilsson PM, Ostergren PO, Nyberg P, Söderström M, Allebeck P. Low birth weight is associated with elevated systolic blood pressure in adolescence: a prospective study of a birth cohort of 149,378 Swedish boys. J Hypertens. 1997;15:1627–31.
Skilton MR, Evans N, Griffiths KA, Harmer JA, Celermajer DS. Aortic wall thickness in newborns with intrauterine growth restriction. Lancet. 2005;365:1484–6.
Cosmi E, Visentin S, Fanelli T, Mautone AJ, Zanardo V. Aortic intima media thickness in fetuses and children with intrauterine growth restriction. Obstet Gynecol. 2009;114:1109–14.
Järvisalo MJ, Jartti L, Näntö-Salonen K, Irjala K, Rönnemaa T, Hartiala JJ, et al. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104:2943–7.
Comas M, Crispi F, Cruz-Martinez R, Figueras F, Gratacos E. Tissue Doppler echocardiographic markers of cardiac dysfunction in small-for-gestational age fetuses. Am J Obstet Gynecol. 2011;205:57.e1–6.
Ley D, Stale H, Marsal K. Aortic vessel wall characteristics and blood pressure in children with intrauterine growth retardation and abnormal fetal aortic blood flow. Acta Paediatr. 1997;86:299–305.
Norman M, Martin H. Preterm birth attenuates association between low birth weight and endothelial dysfunction. Circulation. 2003;108:996–1001.
Leeson CP, Whincup PH, Cook DG, Donald AE, Papacosta O, Lucas A, et al. Flow-mediated dilation in 9- to 11-year-old children: the influence of intrauterine and childhood factors. Circulation. 1997;96:2233–8.
Barker DJ. The developmental origins of well-being. Philos Trans R Soc Lond Ser B Biol Sci. 2004;359:1359–66.
Rich-Edwards JW, Kleinman K, Michels KB, Stampfer MJ, Manson JE, Rexrode KM, et al. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. BMJ. 2005;330:1115.
Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, et al. Arterial stiffness and the development of hypertension: the ARIC study. Hypertension. 1999;34:201–6.
Koklu E, Kurtoglu S, Akcakus M, Koklu S, Buyukkayhan D, Gumus H, et al. Increased aortic intima-media thickness is related to lipid profile in newborns with intrauterine growth restriction. Horm Res. 2006;65:269–75.
Minshall RD, Tiruppathi C, Vogel SM, Malik AB. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem Cell Biol. 2002;117:105–12.
Furchgott RF, Zawadzki JV. The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6.
Lo Vasco VR, Salmaso R, Zanardo V, Businaro R, Visentin S, Trevisanuto D, et al. Fetal aorta wall inflammation in ultrasound detected aortic intima/media thickness and growth retardation. J Reprod Immunol. 2011;91:103–7.
Meyer WW, Lind J, Yao AC, Kauffman SL. Early arterial lesions in infancy and childhood and ways of prevention. Paediatrician. 1982;11:136–56.
Zanardo V, Fanelli T, Weiner G, Fanos V, Zaninotto M, Visentin S, et al. Intrauterine growth restriction is associated with persistent aortic wall thickening and glomerular proteinuria during infancy. Kidney Int. 2011;80:119–23.
Visentin S, Grisan E, Zanardo V, Bertin M, Veronese E, Cavallin F, et al. Developmental programming of cardiovascular risk in intrauterine growth-restricted twin fetuses according to aortic intima thickness. J Ultrasound Med. 2013;32:279–84.
McGill HC Jr, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72:1307S–15S.
Cecconi D, Lonardoni F, Favretto D, Cosmi E, Tucci M, Visentin S, et al. Changes in amniotic fluid and umbilical cord serum proteomic profiles of fetuses with intrauterine growth retardation. Electrophoresis. 2011;32:3630–7.
Favretto D, Cosmi E, Ragazzi E, Visentin S, Tucci M, Fais P, et al. Cord blood metabolomic profiling in intrauterine growth restriction. Anal Bioanal Chem. 2012;402:1109–21.
Cosmi E, Visentin S, Favretto D, Tucci M, Ragazzi E, Viel G, et al. Selective intrauterine growth restriction in monochorionic twin pregnancies: markers of endothelial damage and metabolomic profile. Twin Res Hum Genet. 2013;16:816–26.
Spijkers LJ, van den Akker RF, Janssen BJ, Debets JJ, De Mey JG, Stroes ES, et al. Hypertension is associated with marked alterations in sphingolipid biology: a potential role for ceramide. PLoS One. 2011;6:e21817.
Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–7.
Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega AC. Effect of birth size and catch-up growth on adult blood pressure and carotid intima-media thickness. Horm Res Paediatr. 2012;77:394–401.
Uiterwaal CS, Anthony S, Launer LJ, Witteman JC, Trouwborst AM, Hofman A, et al. Birth weight, growth, and blood pressure: an annual follow-up study of children aged 5 through 21 years. Hypertension. 1997;30:267–71.
Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension. 2003;41:451–6.
Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birth weight and subsequent blood pressure? Lancet. 2002;360:659–65.
Oren A, Vos LE, Uiterwaal CS, Gorissen WH, Grobbee DE, Bots ML. Birth weight and carotid intima-media thickness: new perspectives from the atherosclerosis risk in young adults (ARYA) study. Ann Epidemiol. 2004;14:8–16.
Santos MS, Joles JA. Early determinants of cardiovascular disease. Best Pract Res Clin Endocrinol Metab. 2012;26:581–97.
Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–4.
Tintu A, Rouwet E, Verlohren S, Brinkmann J, Ahmad S, Crispi F, et al. Hypoxia induces dilated ardiomyopathy in the chick embryo: mechanism, intervention, and long-term consequences. PLoS One. 2009;4:e5155.
Skilton MR, Mikkila V, Wurtz P, Ala-Korpela M, Sim KA, Soininen P, et al. Fetal growth, omega-3 (ω-3) fatty acids, and progression of subclinical atherosclerosis: preventing fetal origins of disease? The Cardiovascular Risk in Young Finns Study. Am J Clin Nutr. 2013;97:58–65.
Williams CL, Hayman LL, Daniels SR, Robinson TN, Steinberger J, Paridon S, et al. Cardiovascular health in childhood: a statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2002;106:143–60.
Skilton MR, Raitakari OT, Celermajer DS. High intake of dietary long-chain ω-3 fatty acids is associated with lower blood pressure in children born with low birth weight: NHANES 2003–2008. Hypertension. 2013;61:972–6.
Skilton MR, Mikkila V, Wurtz P, Ala-Korpela M, Sim KA, Soininen P, et al. Fetal growth, omega-3 (ω-3) fatty acids, and progression of subclinicalatherosclerosis: preventing fetal origins of disease? The Cardiovascular Risk in Young Finns Study. Am J Clin Nutr. 2013;97:58–65.
Skilton MR, Ayer JG, Harmer JA, Webb K, Leeder SR, Marks GB, et al. Impaired fetal growth and arterial wall thickening: a randomized trial of ω-3 supplementation. Pediatrics. 2012;129:e698–703.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cosmi, E., Andolfatto, M., Arata, M., Calanducci, M., Visentin, S. (2019). Postnatal Prognosis. In: Nardozza, L., Araujo Júnior, E., Rizzo, G., Deter, R. (eds) Fetal Growth Restriction. Springer, Cham. https://doi.org/10.1007/978-3-030-00051-6_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-00051-6_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-00050-9
Online ISBN: 978-3-030-00051-6
eBook Packages: MedicineMedicine (R0)