Abstract
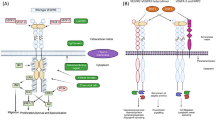
Angiogenesis, the formation of blood vessels, is a complex process involving numerous pathways and receptors that are essential for tumor growth and vascular metastasis. Tumors release a spectrum of proangiogenic cytokines, driven by metabolic and acidic environmental effects and hypoxia. Of them vascular endothelial growth factors (VEGFs) are essential regulators of tumor angiogenesis (Fig. 24.1) (Hicklin and Ellis 2005). The VEGF family consists of VEGF-A to VEGF-E and placental growth factors 1 and 2 which bind to three structurally related receptor tyrosine kinases, VEGFR-1, VEGFR-2, and VEGFR-3 (Fig. 24.2) (Takahashi and Shibuya 2005).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Angiogenesis, the formation of blood vessels, is a complex process involving numerous pathways and receptors that are essential for tumor growth and vascular metastasis. Tumors release a spectrum of proangiogenic cytokines, driven by metabolic and acidic environmental effects and hypoxia. Of them vascular endothelial growth factors (VEGFs) are essential regulators of tumor angiogenesis (Fig. 24.1) (Hicklin and Ellis 2005) (PlGF). The VEGF family consists of VEGF-A to VEGF-E and placental growth factors 1 and 2 which bind to three structurally related receptor tyrosine kinases, VEGFR-1, VEGFR-2, and VEGFR-3 (Fig. 24.2) (Takahashi and Shibuya 2005).
The role of VEGF/VEGFR in tumor angiogenesis (Source: Reprinted with permission from Hicklin and Ellis 2005) EPC endothelial progenitor cell, VEGF vascular endothelial growth factor
Downstream signaling of the VEGF receptors (Source: Takahashi and Shibuya 2005). The VEGF family of ligands and their receptor-binding patterns are shown at the top. Downstream VEGFR signaling pathways focusing on VEGFR-2 are shown at the bottom. Tyr1175 (Y1175) and Tyr1214 (Y1214) are the two major autophosphorylation sites in VEGFR-2. PLC-g binds to Y1175, leading to the phosphorylation and activation of this protein. Y1214 appears to be required to trigger the sequential activation of Cdc42 and p38 MAPK. Many proteins are activated by VEGFR-2 through an unknown mechanism, including FAK, PI3K, and Src. The activation of downstream signal transduction molecules leads to several different endothelial cell functions such as migration, vascular permeability, survival, and proliferation. NRP neuropilin, VEGF vascular endothelial growth factor, PlGF placenta growth factor, PLC phospholipase C, FAK focal adhesion kinase, ERK extracellular signal-regulated kinase, Akt cytosolic protein kinase, eNOS endothelial nitric oxide synthase
The best characterized member of the VEGF ligand family is VEGF-A. Several isoforms of VEGF-A exist; they differ chiefly according to the presence or absence of heparan sulfate (HS)-binding domains. In larger isoforms (e.g., VEGF-A165 and VEGF-A189), the HS-binding domains engage HS in the extracellular matrix (Krilleke et al. 2009; Poltorak et al. 2000). However, lower molecular weight VEGF-A, such as VEGF-A110 (a plasmin cleavage fragment of longer isoforms) and the VEGF-A121 isoform, lack this motif and are freely soluble. Extracellular matrix-bound and soluble VEGF-A isoforms have differing effects on vascular morphogenesis: (Lee et al. 2005) soluble VEGF-A is associated with large, tortuous, unbranched vessels, whereas matrix-bound VEGF-A is associated with thinner, more branched vessels.
Several anti-angiogenesis strategies have been developed and shown preclinical promises, and some have translated into clinical success. Of these, the development of inhibitors such as the monoclonal anti-VEGF antibody bevacizumab has progressed the furthest by demonstrating significantly improved efficacy when combined with standard therapy compared to standard therapy alone across a range of tumor types: in advanced colorectal, breast, non-small cell lung, renal, gastric, pancreatic, and ovarian cancers in terms of progression-free survival (PFS) (Hurwitz et al. 2004; Saltz et al. 2008; Giantonio et al. 2007; Arnold et al. 2012; Miller et al. 2007; Miles et al. 2010; Robert et al. 2011; Brufsky et al. 2011; Reck et al. 2009; Sandler et al. 2006; Escudier et al. 2007; Escudier et al. 2010; Rini et al. 2008; Ohtsu et al. 2011; Van Cutsem et al. 2009; Burger et al. 2011; Perren et al. 2011; Aghajanian et al. 2012; Pujade-Lauraine et al. 2012) and, in some cases, overall survival (OS) (Hurwitz et al. 2004; Giantonio et al. 2007; Arnold et al. 2012; Sandler et al. 2006) (Table 24.1). Subgroup analyses in these trials suggested that bevacizumab provides a significant but relatively modest benefit in almost all clinically the identification of who subsets of patients. Who will obtain the greater benefit from this therapy or for how long they should be administered in the treatment algorithm are major open questions for clinicians and challenges for present and future research.
To date, validated predictive markers to select patients who will obtain the greater benefit from this therapy are still lacking. The value of VEGF as prognostic and predictive marker across tumor types for the anti-VEGF agent bevacizumab has been examined.
2 Biomarker: Definition of Prognostic Versus Predictive Marker
The NCI define a biomarker as “a biological molecule found in blood, other body fluids, or tissues that is a sign of normal or abnormal process, or of a condition or disease. A biomarker may be used to see how well the body responds to a treatment for a disease or condition” (http://www.cancer.gov/dictionary?cdrid=45618). Biomarkers that are associated with the risk of developing a disease, the risk of spread, aggressiveness, or survival rates independent of treatment are termed “prognostic,” while those that predict the rate of response to a particular therapy are termed “predictive.” For example, estrogen and progesterone are weak prognostic biomarkers in breast cancer but are strong predictive factors for response to hormonal therapy. Oncological biomarkers are biological substances whose concentration or level of expression can be measured in the blood or other biospecimens such as tumor tissue. Although concentrations or expression levels of a biomarker are continuous variables, analysis of their association with disease is often easier if they are transformed into binary variables. This entails setting a threshold level and grouping patient data as high (above the threshold) or low (below it). Other markers, such as gene mutations, are binary variables, since a mutation is either present or not present in a given patient. Biomarkers are only valuable if the information they provide supplements or improves that already available from other measurable factors. It should be demonstrated that biomarker-“positive” patient populations derive clinically meaningful benefit from specific treatment compared to those who are biomarker “negative” (Working Group 2009). The potential for biomarker use should be validated in controlled, phase III clinical trials. Ideally, biomarkers should be measurable in an easily obtainable sample, and the method for quantifying them should be reliable and reproducible, have a high specificity and selectivity, and be widely available (Cummings et al. 2010).
3 Prognostic Value of VEGF Across Tumor Types
A number of studies have shown that VEGF tumor expression is associated with poor prognosis across various tumor types.
Farhat et al. (2012) reported findings from 11 studies showing a consistent negative prognostic effect of VEGF tumor expression in non-small cell lung cancer (NSCLC) patients. Another systematic literature review from 11 studies (total 767 patients) supports that tumor expression of VEGF represents a significant and reproducible marker of adverse prognosis in resected pancreatic cancer (PaC) (Smith et al. 2011). A meta-analysis (Des Guetz et al. 2006) of 18 studies with 2,050 colorectal cancer (CRC) patients reported that VEGF expression significantly predicted poor relapse-free survival (RR = 2.84; 95 % CI 1.95–4.16) and OS (RR = 1.65; 95 % CI 1.27–2.14). Similarly, Liu et al. (2012) reported from a meta-analysis on 44 published studies with 4,794 resected gastric cancer patients that positive expression of tissue VEGF, VEGF-C, VEGF-D, and circulating VEGF was all associated with poor prognosis in resected gastric cancer. However, authors hypothesized that circulating VEGF may be better than tissue VEGF in predicting prognosis.
A lesser number of studies than those assessing tumor VEGF expression have investigated the role of VEGF circulating levels as prognostic marker of patient outcomes in gastric and other tumor types.
In the AVAGAST study (bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study), retrospective analysis of pretreatment plasma VEGF-A levels has also been shown to be prognostic for PFS and OS patients. In the placebo group with high baseline plasma, VEGF-A levels had a shorter median overall survival (8.3 months) than patients with low levels (12.9 months) (Van Cutsem et al. 2012).
In patients with previously untreated NSCLC, who receive regimens without anti-VEGF therapy, significant correlation between high pretreatment serum VEGF levels of isoform 189VEGF-A and poor survival (p = 0.0002) has been observed (Yuan et al. 2001). In the AVAiL study (randomized, controlled study of bevacizumab in combination with platinum-based chemotherapy in NSCLC), retrospective analyses of pretreatment plasma VEGF-A levels have been shown to be prognostic for PFS: pVEGF-A low HR = 0.96 versus pVEGF-A high HR = 0.76, p = 0.13 (Fig. 24.3a) (Jayson et al. 2011); however, the prognostic value of VEGF-A was not observed for overall survival in E4599 in advanced non-squamous NSCLC patients randomized to chemotherapy +/− bevacizumab (Dowlati et al. 2008).
(a) Hazard ratios and survival data showing prognostic value of VEGF-A levels in CRC, NSCLC, and RCC. (b) Hazard ratios and survival data showing prognostic and predictive value of VEGF-A levels in PaC, Ga, and mBC (Source: Jayson et al. 2011). BEV bevacizumab, CI confidence interval, cis cisplatin, doc docetaxel, erlot erlotinib, gem gemcitabine, H trastuzumab, HR hazard ratio, IFL irinotecan + 5-fluorouracil + leucovorin, IFN interferon, OS overall survival, mBC metastatic breast cancer, PLA placebo, PFS progression-free survival, VEGF vascular endothelial growth factor
In patients with mCRC, baseline VEGF levels were treatment-independent prognostic biomarkers for PFS and OS in two randomized phase III studies HORIZON II (n = 860; FOLFOX/XELOX plus cediranib 20 mg (n = 502) or placebo (n = 358)) and HORIZON III (n = 1,422; mFOLFOX6 plus cediranib 20 mg (n = 709) or bevacizumab (n = 713)) (Jürgensmeier et al. 2013). The prognostic effect of circulating VEGF-A in CRC is consistent with the observation of Hurwitz et al. (2004) from the AVF2107 trial.
In AVITA phase III randomized study of bevacizumab with gemcitabine-erlotinib in patients with mPaC, pretreatment plasma concentration of VEGF-A showed prognostic effect. Patients in the control (non-bevacizumab-treated) groups who had high plasma VEGF-A concentrations had shorter PFS and OS than patients with low concentrations (Table 24.2). Similar prognostic value for baseline plasma VEGF-A has been observed from other randomized Ph3 trials of bevacizumab in breast cancer (AVADO, AVEREL) and renal cell carcinoma (AVOREN).
In summary, most research supports the clinical prognostic value of VEGF tumor expression in chemotherapy and anti-VEGF-naïve patients across tumor types. In most of these studies, VEGF overexpression was associated with poor prognosis. Although the data on the prognostic implication of circulating VEGF in blood samples of patients across tumor types is more heterogeneous, the evidence is persuasive but needs further investigation and validation. Different assays and storage techniques could explain the inconsistent results seen as these factors are known to affect the reliability of biomarker measurement.
4 Predictive Value of VEGF Across Tumor Types
The assessment of plasma and serum levels of VEGF as potential predictive marker of anti-VEGF therapies has been reported in a number of studies (Jayson et al. 2011).
Low baseline plasma VEGF levels were also associated with superior PFS in studies of vandetanib (a multi-kinase inhibitor) versus gefitinib (HR 0.55 [95 % CI 0.35–0.86], p = 0.01) and in docetaxel plus vandetanib or placebo (HR 0.25 [95 % CI 0.09–0.68], p = 0.01) (Hanrahan et al. 2009). High plasma VEGF-A levels were also shown to be predictive of increased response to bevacizumab plus carboplatin/paclitaxel (BCP) compared with carboplatin/paclitaxel alone (CP), p = 0.004 in advanced non-squamous NSCLC patients in a study by Dowlati et al. (2008).
Plasma VEGF-A levels have shown potential predictive value in trials of bevacizumab in gastric, pancreatic, and breast cancer (Jayson et al. 2011). High baseline plasma VEGF-A concentrations correlated with greater PFS benefit and, in some cases, OS benefit in patients receiving bevacizumab-containing therapy compared with those treated without bevacizumab. Results from the AVEREL trial in HER2-positive metastatic breast cancer corroborated these findings despite a limited sample size (Gianni et al. 2011).
Consequently an attempt to investigate and hopefully replicate these results in NSCLC, RCC, and CRC was undertaken. Plasma samples from trials in mCRC, NSCLC, and RCC were reanalyzed using a novel ELISA-based assay which has a better sensitivity for short VEGF-A isoforms that might be diverse in different tumor types (Fig. 24.4) (Jayson et al. 2011). However, the predictive value of plasma VEGF-A levels was not reproduced in the AVF2107g (mCRC), AVOREN (metastatic RCC), and AVAiL (NSCLC) trials (Table 24.2) (Jayson et al. 2011).
VEGF-A ELISA assay better sensitivity for short isoforms (Source: Jayson et al. 2011)
Interpretation of these apparently differing results is complex. Confounding factors such as variations of pre-analytical and analytical could have contributed to the conflicting intertrial findings, although true negative results in colorectal, renal, and non-small-cell lung cancers cannot be excluded; thus, VEGF-A may be predictive for bevacizumab efficacy in some but not all tumor types. There was also a suggestion of potential predictive value for VEGFR-2, at least for PFS in breast cancer in which high VEGFR-2 concentrations were associated with a greater bevacizumab effect, indicating potential predictive value (Carmeliet et al. 2012) (beatrice, AVADO, AVEREL).
Although a pan-tumor effect of VEGF-A was not confirmed in the collective data from these bevacizumab trials in six tumor types, the demonstration of predictive potential of both VEGF-A and VEGFR-2 in two trials in breast cancer, supported by a suggestion of a predictive effect of VEGFR-2 in a third trial, is noteworthy.
5 Issues with Interpreting Results of VEGF as a Predictive Biomarker Across Tumor Types
There is controversy regarding whether plasma, serum, or whole blood will provide the best reflection of the situation at the tumor site (Webb et al. 1998; Banks et al. 1998; Vermeulen et al. 1999). From the trials mentioned above, it has been hypothesized that differences in the way samples were handled, known to influence plasma VEGF-A levels, could affect the results. Significant amounts of VEGF can be released from platelets and leukocytes during sampling and handling. The choice of anticoagulant is of importance. Serum VEGF levels may reflect blood platelet counts rather than VEGF synthesis in peripheral tissues. Some authors advise the use of a citrate rather than an EDTA buffer in AVF2107g, AVOREN, and AVAiL could theoretically have changed the observed, measured levels of VEGF-A. Some authors advise the use of plasma (citrated, EDTA treated, or heparinized) in glass tubes for this reason. It has also been demonstrated that VEGF levels further increase with clotting duration and temperature. The biosamples were stored for prolonged periods in AVF2107g and AVOREN, and there were more than two freeze/thaw cycles in a subset of samples in AVF2107g, AVOREN, and AVAiL that could all contribute to the inconsistent predictive values observed between studies.
Another possibility for the apparent disparities is that a median baseline biomarker cutoff value might not be a uniformly appropriate cutoff value across tumor types. The median plasma concentration of VEGF-A appears to be a reasonable cutoff in the AVADO (breast), AVAGAST (gastric), and AViTA (pancreatic) trials; for example, in the AVADO trial (7.5 mg/kg arm), a median cutoff gives the greater hazard ratio difference when comparing the “low” plasma VEGF-A cohort (i.e., no treatment effect) and the high plasma VEGF-A cohort, representing one of the most substantial treatment effects when comparing different cutoffs. The threshold for defining high versus low baseline plasma VEGF-A concentrations was lower in the AVF2107g and AVAiL trials than in the other trials.
Perhaps the most obvious explanation for the apparent discrepancies between the biomarker results of the different trials is that the potential predictive value of pretreatment plasma VEGF-A is tumor specific. Thus, VEGF-A may be predictive for bevacizumab efficacy in some but not all tumor types. The presence of shorter isoforms (VEGF-A121 and VEGF-A110), which are detected with greater sensitivity than longer isoforms by the novel ELISA, may vary between tumor types and contribute to heterogeneity of predictive value across tumor type. It has been reported by Oshika et al. that VEGF-A isoform VEGF189 was more frequently expressed in NSCLC (90.5 %) than in extraneoplastic lung tissue (57.6 %, p = 0.00004) (Oshika et al. 1998).
It would seem to be important for the research community to reach a consensus with regard to the preferred biospecimen, optimal collection, handling, analytical method, and storage, as well as the most appropriate approach to define cutoff, in order to facilitate data interpretation and cross-trial comparisons.
6 Summary and Conclusion
Cancer is a genetic disease that involves a number of biological pathways at each step of its progression. The nature of the tumor microenvironment is complex and transient, which can lead to challenges in certain areas of oncology biomarker discovery. In the era of personalized medicine, there is a growing interest in novel screening tools and a more individualized approach to the treatment of cancer depending on the tumor type. VEGF expression has been observed across multiple and various tumor types. Presently, there is evidence that high blood and tumor levels of VEGF across tumor types are negative prognostic indicators for survival. While VEGF-A has been potentially shown as a predictive marker in NSCLC and other tumor types for bevacizumab efficacy, it has yet to be validated. Thus, further large prospective studies are still needed to define the role of VEGF and other markers in different tumor types and to define their utility.
References
Aghajanian C, Blank SV, Goff BA et al (2012) OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 30:2039–2045
Arnold D, Andre T, Bennouna J, et al (2012) Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first progression in patients with metastatic colorectal cancer (mCRC) previously treated with BEV plus CT: results of a randomized phase III intergroup study (TML study). J Clin Oncol 30(suppl):abstr CRA3503
Banks R, Forbes M, Kinsey S et al (1998) Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer 77:956–964
Brufsky AM, Hurvitz S, Perez E et al (2011) RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29:4286–4293
Burger RA, Brady MF, Bookman MA, et al, Gynecologic Oncology Group (2011) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473–2483
Carmeliet P, Pallaud C, Deurloo RJ et al (2012) Plasma (p) VEGF-A and VEGFR-2 biomarker (BM) results from the BEATRICE phase III trial of bevacizumab (BEV) in triple-negative early breast cancer (BC). Cancer Res 72:321 s, suppl; abstr P3-06-34
Cummings J, Ward T, Dive C (2010) Fit-for-purpose biomarker method validation in anticancer drug development. Drug Discov Today 15:816–825
Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY (2006) Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 94(12):1823–1832
Dowlati A, Gray R, Sandler A (2008) Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumaban Eastern Cooperative Oncology Group Study. Clin Cancer Res 14:1407–1412
Escudier B, Pluzanska A, Koralewski P, AVOREN Trial investigators et al (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370:2103–2111
Escudier B, Bellmunt J, Négrier S et al (2010) Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 28:2144–2150
Farhat F, Tfayli A, Fakhruddin N et al (2012) Expression, prognostic and predictive impact of VEGF and bFGF in non-small cell lung cancer. Crit Rev Oncol Hematol 84(2):149–160
Gianni L, Romieu G, Lichinitser M et al (2011) First results of AVEREL, a randomized phase III trial to evaluate bevacizumab (BEV) in combination with trastuzumab (H) + docetaxel (DOC) as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer (LR/mBC). Cancer Res 71(24 suppl):109 s (abstr S4–8)
Giantonio BJ, Catalano PJ, Meropol NJ et al (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–1544
Hanrahan E, Ryan A, Mann H (2009) Baseline vascular endothelial growth factor concentration as a potential predictive marker of benefit from vandetanib in non-small cell lung cancer. Clin Cancer Res 15:3600–3609
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23:1011–1027
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Jayson G, de Haas S, Delmar P et al. (2011) Evaluation of plasma VEGFA as a potential predictive pantumour biomarker for Bevacizumab. Eur J Cancer 47(suppl 1):S96 (Abstract 804)
Jürgensmeier JM, Schmoll HJ, Robertson JD, Brooks L, Taboada M, Morgan SR, Wilson D, Hoff PM (2013) Prognostic and predictive value of VEGF, sVEGFR-2 and CEA in mCRC studies comparing cediranib, bevacizumab and chemotherapy. Br J Cancer 108(6):1316–1323
Krilleke D, Ng YS, Shima DT (2009) The heparin-binding domain confers diverse functions of VEGF-A in development and disease: a structure-function study. Biochem Soc Trans 37:1201–1206
Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML (2005) Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol 169:681–691
Liu L, Ma XL, Xiao ZL, Li M, Cheng SH, Wei YQ (2012) Prognostic value of vascular endothelial growth factor expression in resected gastric cancer. Asian Pac J Cancer Prev 13(7):3089–3097
Miles DW, Chan A, Dirix LY et al (2010) Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28:3239–3247
Miller K, Wang M, Gralow J et al (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676
Ohtsu A, Shah MA, Van Cutsem E et al (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29:3968–3976
Oshika Y, Nakamura M, Tokunaga T et al (1998) Expression of cell-associated isoform of vascular endothelial growth factor 189 and its prognostic relevance in non-small cell lung cancer. Int J Oncol 12(3):541–544
Perren TJ, Swart AM, Pfisterer J, et al, ICON7 Investigators (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484–2496
Poltorak Z, Cohen T, Neufeld G (2000) The VEGF splice variants: properties, receptors, and usage for the treatment of ischemic diseases. Herz 25:126–129
Pujade-Lauraine E, Hilpert F, Weber B et al (2012) AURELIA: a randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC). J Clin Oncol 30(suppl):abstr LBA5002
Reck M, von Pawel J, Zatloukal P et al (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small cell lung cancer: AVAiL. J Clin Oncol 27:1227–1234
Rini BI, Halabi S, Rosenberg JE et al (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 26:5422–5428
Robert NJ, Diéras V, Glaspy J et al (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29:1252–1260
Saltz LB, Clarke S, Díaz-Rubio E et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Smith RA, Tang J, Tudur-Smith C, Neoptolemos JP, Ghaneh P (2011) Meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Br J Cancer 104(9):1440–1451
Takahashi H, Shibuya M (2005) The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 109:227–241
Van Cutsem E, Vervenne WL, Bennouna J et al (2009) Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 27:2231–2237
Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ, Shah MA (2012) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 30(17):2119–2127
Vermeulen P, Salven P, Benoy I et al (1999) Blood platelets and serum VEGF in cancer patients. Br J Cancer 79:370–373
Webb N, Bottomley MJ, Watson CJ et al (1998) Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci (Lond) 94:395–404
Working Group (2009) Evaluation of Genomic Applications in Practice and Prevention (EGAPP). Genet Med 11:3–14
Yuan A, Yu C-J, Kuo S-H (2001) Vascular endothelial growth factor 189 mRNA isoform expression specially correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J Clin Oncol 19:432–441
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag France
About this chapter
Cite this chapter
Pallaud, C. (2014). The Prognostic and Predictive Value of VEGF Across Various Tumor Types. In: Feige, JJ., Pagès, G., Soncin, F. (eds) Molecular Mechanisms of Angiogenesis. Springer, Paris. https://doi.org/10.1007/978-2-8178-0466-8_24
Download citation
DOI: https://doi.org/10.1007/978-2-8178-0466-8_24
Published:
Publisher Name: Springer, Paris
Print ISBN: 978-2-8178-0465-1
Online ISBN: 978-2-8178-0466-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)