Abstract
Hemorrhagic cystitis is a common and potentially devastating urological disorder. Among the risk factors associated with the disease, previous pelvic irradiation and chemotherapeutic drugs such as cyclophosphamide are the most common etiologies of HC. Clinically, HC presents as hematuria often with progression to clot retention. Histologically, chronic fibrosis and progressive endarteritis are seen. Treatment for HC varies with the degree of hematuria. Treatment may include: urinary drainage, intravesical irrigation with topical astringents, and direct fulguration. Ultimately, the most durable and effective therapy for HC may be systemic hyperbaric oxygen therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hyperbaric Oxygen
- Necrotizing Fasciitis
- Interstitial Cystitis
- Hyperbaric Oxygen Therapy
- Hemorrhagic Cystitis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Hemorrhagic cystitis (HC) is a common urological disorder, presenting in 6.5 % of patients following pelvic radiation therapy and up to 25 % of patients receiving alkylating chemotherapeutic agents. HC can be devastating, with high morbidity and mortality despite aggressive interventions [1]. Massive urothelial hemorrhage may involve both upper and lower urinary tracts [2], leading to acute renal failure that requires emergent urological interventions.
HC is classified by five grades [3]:
-
Grade 1: Single minor bleeding
-
Grade 2: Repeated minor bleeding
-
Grade 3: Inpatient medical treatment needed
-
Grade 4: Inpatient surgical treatment needed
-
Grade 5: Death
HC often does not proceed in a stepwise fashion through the grading scale. Although most patients initially present with minor bleeding episodes, the initial presentation may be massive macroscopic hematuria with clot retention that may require clot evacuation and blood transfusion.
For clinical trials data analysis, the Radiation Therapy Oncology Group (RTOG) based in the United States and the European Organization for Research and Treatment of Cancer (EORTC) based in Belgium have classified radiation treatment-related genitourinary morbidity. Table 14.1 summarizes the RTOG morbidity scoring scheme for acute radiation genitourinary morbidity and RTOG/EORTC morbidity scoring scheme for late radiation bladder complications. Acute morbidity criteria are used to grade radiation treatment-related toxicity from the first day of radiation therapy for a total of 90 days. Any radiation treatment-related morbidity that occurs after the initial 90 days will be scored using the RTOG/EORTC criteria.
Etiology and Risk Factors
Among the risk factors for HC presented in Table 14.2, previous pelvic irradiation and chemotherapeutic drugs like cyclophosphamide are the most common causes of HC. In a randomized Danish study, up to 25 % of 118 patients with early ovarian cancer developed HC after external beam radiation and cyclophosphamide treatment [4].
Radiation-Induced HC
Radiation therapy induces chronic fibrosis and progressive endarteritis. When the urothelium is within the radiation field, the end result of such chronic scarring is urothelial sloughing and bleeding. In the treatment of prostatic, bladder, or cervical cancer, the bladder mucosa is primarily at risk. The onset of radiation effects may appear more than 10 years following pelvic irradiation [5]. Patients who are at high risk include those with wide radiation fields and a higher total radiation dose. There is no reliable predictor as to which patient will be affected or when the episodes of HC will occur. Genetic work suggests that some individuals may be more susceptible to radiation-associated bleeding due to differing gene expression profiles [6].
With the acceptance of contemporary conformal radiation delivery and interstitial therapy, the incidence of radiation-induced HC is expected to decline.
Chemotherapeutic Agent-Induced HC
HC is commonly associated with the use of chemotherapeutic agents in both cancer and nonneoplastic diseases. Most published literature on HC and its prevention focuses on bone marrow transplantation. The most commonly implicated agents are the alkylating oxazaphosphorine agents, which include cyclophosphamide [7–9] and ifosfamide [10, 11]. Bladder mucosal hemorrhage is caused by acrolein, the toxic metabolite of the alkylating agents. Acrolein toxicity may result in life-threatening exsanguination and disseminated intravascular coagulopathy [12].
A retrospective review of complications in 447 bone marrow transplant patients demonstrated that bleeding episodes are associated with prolonged thrombocytopenia, graft-vs.-host disease, and cyclophosphamide regimes [13]. The effects of these agents are dose-dependent but independent of patients’ age [11, 14].
Other cyclophosphamide-induced episodes of HC have been reported in the treatment of Wegener’s granulomatosis [15, 16], Ewing’s sarcoma [17], advanced nonsmall cell lung cancer [18], paratesticular rhabdomyosarcoma [19], and malignant brain tumors [20].
Contemporary chemotherapeutic agents such as busulfan [21] and temozolomide [22] have a lower incidence of HC.
Viral Infection-Induced HC
Superimposed infections often complicate clinical outcome in immunocompromised hosts after bone marrow or solid organ transplantations. Although viral-associated HC was uncommon in the past, the emerging frequency of viral infections from BK virus [23–25], cytomegalovirus [26], and adenovirus [27, 28] is a cause for concern.
BK virus is a human polyomavirus related to the papovavirus family. After primary infection, the BK virus stays dormant in renal parenchyma until reactivation in immunocompromised hosts [29]. This is one of the most common causes of viral-associated HC after bone marrow and renal transplantations. Using real-time quantitative polymerase chain reaction, BK viruria is related to the occurrence and severity of HC after bone marrow transplantation, without detectable increase in BK viremia [30]. Italian researchers using deoxyribonucleic acid (DNA) hybridization assay and polymerase chain reaction analysis also found concurrent urinary shedding of BK virus in prospective cases [31] of HC. This indicates a possible adverse role of direct viral contact on the urothelium with resultant HC.
Adenoviral infection (subtypes 11 and 35), although less common than BK infection, can occur in postrenal transplant recipients as well [32]. Adenoviral-associated HC usually occurs 6 weeks after transplantation, is self-limiting, and resolves with adequate hydration within 1–2 weeks. The viral load in the posttransplant is not predictive of subsequent HC development [33].
BK virus-associated HC has also been reported in nontransplant patients with human immunodeficiency virus [34].
Miscellaneous Causes of HC
Esoteric causes of HC include penicillin G [35], ischemic necrosis from bladder overdistension in neurogenic bladder [36], and Boon’s disease with massive apoptosis and exfoliation of urothelium caused by hypovolemia [37].
Pathology
In HC, mucosal hyperemia is associated with neutrophilic infiltration of the lamina propria, endarteritis, and fibroblastic reactions seen in chronic fibrosis (Fig. 14.1). Nonneoplastic variants may include squamous metaplasia (without cellular atypia or keratinization), cystitis cystica (with eosinophilic liquefaction of benign urothelium in lamina propria, almost similar to von Brunn’s nests), cystitis follicularis (with submucosal lymphoid follicles reacting to chronic bacterial infection), or inverted papilloma [38].
Because severe dysplasia, carcinoma in situ, and transitional or squamous cell carcinoma may present with macroscopic hematuria, it is important to exclude malignancies when diagnosing HC. In addition to causing HC, both external beam radiation and cyclophosphamide are associated with an increased risk of bladder cancer development [39].
Clinical Presentation
HC in adult and pediatric patients most commonly occurs in immunosuppressed, oncological, and autoimmune patients. Among transplants, bone marrow transplantation [40, 41] is the most significant contributor. An initial herald bleed may signify mucosal hemorrhage and warrants further investigations with upper tract imaging and cystoscopy to exclude common causes of hematuria. Quantity and frequency of hematuria are unpredictable. Independent of age, most patients present with a single-episode or recurrent minor bleeding. Less commonly, patients may present with chronic fatigue, syncope, or unexplained anemia.
Patients develop clot retention caused by either continuous profuse bleeding or prior bladder outlet obstruction. In elderly males with prostatic enlargement, higher urinary outflow resistance contributes to reduced clot clearance, resulting in the vicious cycle of increased intravesical blood clot formation. Rarely, hypotension and hypovolemic shock may occur with uncontrolled hematuria.
Investigations
HC presents with macroscopic hematuria, and thus several more common causes of hematuria must be excluded. These include urinary tract infection with inflammatory cystitis, urolithiasis, benign prostatic hypertrophy, transitional cell carcinoma, and aspirin or coumadin ingestion. First-line investigations to be done are urinary bacterial cultures, hematocrit level, and coagulation profile if indicated.
Cystoscopy
Cystoscopy under anesthesia is indicated if the patient has uncontrollable bleeding requiring blood transfusion, persistent clot retention despite bladder irrigation, or repeated episodes of HC (Fig. 14.2). Although it is a minimally invasive procedure, bladder perforation is a potential complication caused by friable bladder tissues. This is especially true in a scarred, contracted irradiated bladder with little compliance to saline distension and instrument manipulation.
Cystoscopy is used as a diagnostic and therapeutic tool for clot evacuation, bladder cauterization, and bladder biopsy to rule out transitional cell carcinoma. Even in patients who receive excessive anticoagulants, up to 18 % of patients in one series were diagnosed with concurrent urinary malignancies [42], justifying the procedure in such cases. After cystoscopic clot evacuation, continuous bladder irrigation is maintained to prevent development of new clots (see below for details of proper technique).
Radiological Imaging
Imaging studies concentrate on exclusion of upper urinary tract malignancies. Cross-sectional imaging with intravenous contrast that include the pyelographic phase, such as CT-IVP, is performed to exclude urothelial tumors. In patients with renal insufficiency, noncontrast imaging modalities such as MRI [43] should be utilized. An additional advantage of imaging studies is the ability to assess the bladder for clot volume. If identified clots cannot be fully evacuated at the bedside, operative cystoscopic evacuation is indicated.
A report of 12 patients used ultrasonographic features to describe three different types of bladder abnormalities in HC patients after bone marrow transplantation. These types are circumscribed thickening of bladder wall, diffuse thickening of the bladder wall, and intraluminal lobulated bulky mass [44]. Median bleeding duration was longer in patients with intraluminal lobulated bulky mass at 90 days. Although premature, this technique may be a useful noninvasive imaging modality to screen patients for potential future bleeding episodes.
Prophylaxis
Prevention of hemorrhagic episodes in high-risk patients is the most important strategy. Common methods used include use of mesna (sodium-2-mercaptoethansulfonate), intravenous hyperhydration, continuous bladder irrigation, or modification of the chemotherapeutic conditioning regime.
Mesna is a sulfhydryl compound that binds to acrolein metabolites in the urinary tract. It is administered through intravenous or subcutaneous routes and initially had raised interest for widespread applications [45–48]. However, mesna and its metabolites may cause significant vasculitic side effects such as erythroderma, bullous skin lesions, myalgia, fever, and perimyocarditis [49]. Hence, its routine use is superceded by the primary prevention strategies of forced diuresis and bladder irrigation.
During high-dose cyclophosphamide treatment in 303 patients, aggressive hyperhydration with intravenous fluid to maintain urine output above 200 mL/h, coupled with continuous bladder irrigation, showed impressive results with no macroscopic hematuria [50]. Using less-stringent requirements for forced diuresis, hyperhydration and mesna were equally effective in a randomized trial [51].
The most important factor in preventive measures is the dilution or rapid excretion of the chemotherapeutic toxic metabolites. Hyperhydration with intravenous crystalloids and furosemide to maintain hourly urine output of more than 150 mL is recommended as efficacious, cost-effective, and well tolerated [52].
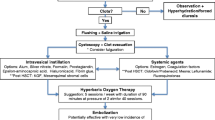
Management
Management of patients depends on the clinical presentation, severity of bleeding, and medical resources available. It is uncommon to see an actively bleeding patient with hemorrhagic shock who requires emergency surgical intervention. Be wary, however, of patients presenting with syncope and clot retention, which may reflect significant hemorrhage and hemodynamic compromise. The volume of blood contained in a distended bladder is often underestimated by less-experienced physicians. Most patients with mild symptoms are managed with the following first-line and second-line therapies.
First-Line Therapy
Minor bleeding usually resolves spontaneously. No medical treatment is needed other than investigations to exclude common causes of hematuria. If bleeding is persistent with clot formation, causing patient distress with urinary retention, immediate manual clot evacuation and bladder irrigation is done through a large-bore transurethral urinary catheter. The success of the bladder irrigation is highly dependent on a thorough and complete manual clot evacuation, using a piston syringe and repeated instillation and aspiration of 0.9 normal saline (NS). The manual clot evacuation is followed by continuous bladder irrigation using 0.9 NS, a large-bore irrigation tubing with drip chamber with a large-bore (e.g., 22–24 Fr) three-way urinary catheter for at least 24 h to ensure removal of residual clot fragments and termination of hematuria.
If bleeding persists despite conservative management, cystoscopy, clot evacuation, and bladder cauterization are performed. Bladder biopsies should be done concurrently in postradiation patients and in cases of recurrent hematuria of unknown etiology.
Second-Line Therapy
Second-line therapies are indicated for patients with recalcitrant HC without life-threatening hematuria. Several options are discussed and used depending on resource availability, including hyperbaric oxygen therapy, intravesical, and oral agents.
Hyperbaric Oxygen Therapy
With more than 200 monoplace and 20 multiplace hyperbaric chambers throughout North America [53], hyperbaric oxygen (HBO2) therapy is emerging as an important option for early intervention of HC. By definition, a patient who receives hyperbaric oxygen therapy must receive oxygen within an enclosed chamber with pressurization of 1.4 atm absolute (atm abs) or higher. Currently, established clinical uses of HBO2 include air embolism, carbon monoxide poisoning, decompression sickness, diabetic foot ulcers, postradiation tissue injuries, and soft tissue necrotizing fasciitis [54].
When placed in an enclosed chamber (Fig. 14.3), pressurized oxygen delivery results in the plasma hypersaturation of dissolved oxygen. Excess plasma oxygenation improves local and regional tissue oxygen supply in tissues with poor oxygenation caused by previous radiation, mechanical, or chemical injuries [55]. This is achieved by the creation of a steep oxygen gradient between the end arterioles and capillaries and the hypoxic tissues that require treatment.
Dissolved oxygen in the plasma diffuses across the capillary bed to improve local tissue oxygenation. Adequate tissue oxygenation will ensure efficient production of adenosine triphosphates (ATPs), as well as other components essential for normal cellular function, resulting in primary angiogenesis. Neovascularization occurs with capillary ingrowth into hypoxic tissues.
Compared to normobaric oxygen, hyperbaric oxygen provides an eight- to ninefold increase in vascular density in an irradiated rabbit model [56]. In mice models, neovascularization can be seen 5 days after initiation of HBO2 therapy, even in tissue that is typically nonvascularized (fat) [57]. In irradiated human oral tissues treated for mandibular osteoradionecrosis, HBO2 therapy resulted in significant increase of transmucosal oxygen tension after only five treatments [58]. It is the synergistic effect of greater molecular oxygen supply and increased vascular density in the hypoxic tissue that allows adequate collagen synthesis and wound repair to occur.
Hyperbaric oxygen therapy successfully resolves hematuria in most postradiation HC [59–64]. HBO2 is also the only form of therapy that promotes tissue healing. Most hyperbaric oxygen therapy sessions are 90 min. A typical treatment course requires at least 30 daily sessions at 2.36 atm abs pressure. Contraindications for hyperbaric therapy include: emphysema, middle ear dysfunction, congestive heart failure, untreated pneumothorax, and concurrent treatment with cisplatinum, doxorubicin, bleomycin, disulfiram, and mafenide acetate [65].
Potential complications to HBO2 include claustrophobia, otalgia, barotraumas, and seizures from central nervous system toxicity. To reduce central nervous system toxicity, at least three to four “air breaks” are given during each session. Air breaks are 5–10 min each of normal air breathing. If seizures occur during treatment, conversion to normal air breathing typically resolves the symptom.
Traditionally, hyperbaric oxygen therapy has been employed after failure to respond to other treatments. However, a recent meta-analysis of hyperbaric oxygen use in managing 190 patients with HC reported that 76.3 % of patients had improvement or resolution of hematuria [66]. Corman and associates reported on 57 patients treated with HBO2 between 1988 and 2001 at our institution, and 86 % of patients had complete resolution or marked reduction in hematuria episodes [67]. Despite these encouraging results from high volume institutions, reports from other centers have suggested lower response rates [68, 69].
Although hyperbaric oxygen has traditionally been used to treat HC secondary to radiotherapy, recent case reports suggest that it also may have a role in the treatment of cyclophosphamide-induced HC that is refractory to first-line management [70].
We strongly recommend hyperbaric oxygen as an early intervention to manage nonlife-threatening HC.
Intravesical Therapies
Most second-line therapies are intravesical instillations with variable efficacy and side effect profiles. They generally cause mucosal fibrosis and scarring as a form of chemical coagulation.
Alum
Intravesical 1 % alum solution (10 g aluminum potassium sulfate in 1 L of distilled water) has been used with reasonable efficacy and minimal side effects [71]. Alum functions as a chemical astringent causing protein precipitation over bleeding surfaces. It is of paramount importance to evacuate all clots from the bladder before administering Alum to prevent the development of giant bladder clots. It is usually well tolerated by continuous bladder instillation up to 300 cc/h and may take several days to induce hemostasis. It can be administered to patients with no risk of bladder scarring and can be used in patients with reflux [72]. Potential side effects include suprapubic pain, aluminum toxicity, and renal dysfunction. Moreover, toxicity can occur even in patients with normal kidney function [73]. Isolated reports of treating alum toxicity include using intravenous feroxamine [74].
Formalin
Intravesical 1 % formalin (with 0.37 % formaldehyde gas in solution) induces bladder mucosal fibrosis. While bedside instillation of formalin can be performed at dilute concentrations (<0.1 %) in critically ill patients, typical formalin therapy is performed cystoscopically under general or regional anesthesia. Prior to instillation a cystogram must be performed in order to rule out the presence of vesicoureteral reflux. If reflux is noted, embolization catheters should be placed in the affected ureter to prevent formalin reflux into the upper tracts.
One percent formalin is instilled for 30 min before the bladder is reinspected cystoscopically. If bleeding persists, repeat instillation of up to 4 % formalin may be performed (30 min intravesical dwell time per increased concentration) in order to effect cessation of bleeding. While mucosal fibrosis is typically observed histologically and is associated with resolution of the hematuria, bladder fibrosis and contracture are commonly seen following longer formalin dwell times and higher therapy concentrations. Such fibrosis and associated edema may result in profound bladder symptoms, pain, and dysfunction [75].
Miscellaneous Intravesical and Oral Treatment Options
Other intravesical options include ε-aminocaproic acid. This agent acts by inhibiting formation of stable plasmin. Fibrin degradation cannot occur effectively, and stable blood clots are formed to stop further bleeding. Short-term results appear favorable [76], but widespread use is limited due to its acceleration of clot formation, which can cause renal unit loss with bleeding from an upper tract source [77].
Intravesical prostaglandins E1, E2, and F2α (Carboprost®) have been tried with varying success [78–80]. Silver nitrate is another intravesical option, but reports of intraureteral salt precipitation and subsequent urinary obstruction temper its widespread use [81].
Cidofovir is a cytidine nucleoside analog that has activity against polyomavirus and adenovirus [82] and has been used intravesically in a small group of patients with BK adenovirus-associated HC with reported success [83]. Its clinical utility awaits testing in a formal clinical trial setting.
Pentosanpolysulphate (PPS) is a synthetic glycosaminoglycan molecule that is used in patients with interstitial cystitis [84] and is thought to replace damaged bladder wall mucosa and prevent absorption of noxious chemicals in these patients. Small case series in adult patients with HC have demonstrated success with its use [85, 86]. Its theoretical disadvantages include very low intravesical drug concentration (1–3 %) requiring a number of weeks of treatment before clinical response is achieved and an unknown toxicity profile in pediatric patients.
Emergency Options
Temporary Urinary Diversion
When uncontrolled bleeding is apparent, temporary urinary diversion may be performed to reduce distress from clot retention. Moreover, urokinase, which is present in urine is a potent thrombolytic that activates plasminogen and prevents hemostasis. Thus, urinary diversion helps promote hemostasis at the metabolic level in addition to its mechanical function. Suprapubic cystostomy or bilateral percutaneous nephrostomy tube placement [1] can slow bleeding while more invasive surgery is contemplated.
Specifically in the pediatric population, the small size of the urethra can limit catheter size (10–14 Fr), and thus impede the effective evacuation of large clots. In this instance, suprapubic cystostomy can be performed to aid in clot evacuation and associated pain, particularly in palliative pediatric cancer patients with intractable bleeding [87].
Embolization or Surgical Ligation of Internal Iliac Vessels
Selective transfemoral hypogastric artery embolization has been used to treat intractable hematuria due to tumor and trauma [88]. Similarly, embolization has been reported in patients with refractory HC after stem cell transplant, demonstrating full resolution in 8 of 10 patients [89].
Open Vesicostomy/Subtotal or Total Cystectomy
Exploratory laparotomy is the most aggressive and last resort. Attempts at ligating internal iliac arteries may stop the hemorrhage, and open vesicostomy may control bleeding.
In uncontrolled bleeding, subtotal cystectomy with two-layer repair using absorbable sutures may be adequate for single or clusters of persistent bleeding spots in proximity. However, this treatment is not appropriate therapy for trigonal bleeding, for which total cystectomy [90] is usually indicated.
Potential Future Therapies
Antiviral Therapies
Intravenous ribavirin [91] is an antiviral agent that can suppress infections of adenovirus subtypes 11 and 35, thereby reducing infections in individuals immunocompromised from bone marrow or renal transplantations. Another option is intravenous or intramuscular vidarabine [92].
Miscellaneous
Other reported treatment options include use of oral conjugated estrogens [93], intravenous factor XIII concentrate [94], recombinant activated factor VII [95], and intravesical recombinant human granulocyte-macrophage colony-stimulating factor [96].
Summary
Moderate to severe hemorrhagic cystitis (HC) has been reported in up to 5 % of patients following radiotherapy for prostate cancer and may occur up to 10 years following treatment. HC is caused by chronic fibrosis and progressive endarteritis of the bladder mucosa. The diagnosis is based upon clinical presentation, histologic assessment, and the absence of other sources of bleeding (e.g., malignancy).
The primary treatment modality for HC is bladder irrigation. Oral and intravenous agents such as aminocaproic acid, estrogens, and sodium pentosan polysulfate have been used with limited success. Intravesical treatments with alum, silver nitrate, and formalin are employed if bleeding is recalcitrant. Finally, selective embolization of the hypogastric arteries, urinary diversion, and cystectomy may be used in severe cases. In recent years, HBO2 has emerged as a primary option in the management of this challenging condition.
References
Cheng C, Foo KT. Management of severe chronic radiation cystitis. Ann Acad Med Singapore. 1992; 21(3):368–71.
Wong TM, Yeo W, Chan LW, Mok TS. Hemorrhagic pyelitis, ureteritis and cystitis secondary to cyclophosphamide: case report and review of literature. Gynecol Oncol. 2000;76(2):223–5.
Levenback C, Eifel PJ, Burke TW, Morris M, Gershenson DM. Hemorrhagic cystitis following radiotherapy for stage Ib cancer of the cervix. Gynecol Oncol. 1994;55(2):206–10.
Sell A, Bertelsen K, Andersen JE, Stroyer I, Panduro J. Randomized study of whole-abdomen irradiation vs pelvic irradiation plus cyclophosphamide in treatment of early ovarian cancer. Gynecol Oncol. 1990; 37(3):367–73.
de Vries CR, Freiha FS. Hemorrhagic cystitis: a review. J Urol. 1990;143(1):1–9.
Rieger KE, Hong WJ, Tusher VJ, et al. Toxicity from radiation therapy associated with abnormal transcriptional responses to DNA damage. Proc Natl Acad Sci U S A. 2004;101(17):6635–40.
Sencer SF, Haake RJ, Weisdorf DJ. Hemorrhagic cystitis after bone marrow transplantation. Risk factors and complications. Transplantation. 1993;56(4):875–9.
Stillwell TJ, Benson Jr RC. Cyclophosphamide-induced hemorrhagic cystitis. A review of 100 patients. Cancer. 1988;61(3):451–7.
Brugieres L, Hartmann O, Travagli JP, et al. Hemorrhagic cystitis following high-dose chemotherapy and bone marrow transplantation in children with malignancies: incidence, clinical course, and outcome. J Clin Oncol. 1989;7(2):194–9.
Mahjoubi M, Azab M, Ghosn M, Theodore C, Droz JP. Phase II trial of ifosfamide in the treatment of metastatic hormone-refractory patients with prostatic cancer. Cancer Invest. 1990;8(5):477–81.
Sarosy G. Ifosfamide—pharmacologic overview. Semin Oncol. 1989;16(1 Suppl 3):2–8.
Shanholtz C. Acute life-threatening toxicity of cancer treatment. Crit Care Clin. 2001;17(3):483–502.
Pihusch R, Salat C, Schmidt E, et al. Hemostatic complications in bone marrow transplantation: a retrospective analysis of 447 patients. Transplantation. 2002;74(9):1303–9.
Hong WK, Nicaise C, Lawson R, et al. Etoposide combined with cyclophosphamide plus vincristine compared with doxorubicin plus cyclophosphamide plus vincristine and with high-dose cyclophosphamide plus vincristine in the treatment of small-cell carcinoma of the lung: a randomized trial of the Bristol Lung Cancer Study Group. J Clin Oncol. 1989;7(4):450–6.
Stillwell TJ, Benson Jr RC, DeRemee RA, McDonald TJ, Weiland LH. Cyclophosphamide-induced bladder toxicity in Wegener’s granulomatosis. Arthritis Rheum. 1988;31(4):465–70.
Hu RQ, Mehter H, Nadasdy T, et al. Severe hemorrhagic cystitis associated with prolonged oral cyclophosphamide therapy: case report and literature review. Rheumatol Int. 2008;28:1161–4.
Stillwell TJ, Benson Jr RC, Burgert Jr EO. Cyclophosphamide-induced hemorrhagic cystitis in Ewing’s sarcoma. J Clin Oncol. 1988;6(1):76–82.
Williams SF, Bitran JD, Hoffman PC, et al. High-dose, multi-alkylator chemotherapy with autologous bone marrow reinfusion in patients with advanced non-small cell lung cancer. Cancer. 1989;63(2):238–42.
Heyn R, Raney Jr RB, Hays DM, Tefft M, Gehan E, Webber B, et al. Late effects of therapy in patients with paratesticular rhabdomyosarcoma. Intergroup Rhabdomyosarcoma Study Committee. J Clin Oncol. 1992;10(4):614–23.
Allen JC. Complications of chemotherapy in patients with brain and spinal cord tumors. Pediatr Neurosurg. 1991;17(4):218–24.
Crilley P, Topolsky D, Bulova S, Bigler R, Brodsky I. Bone marrow transplantation following busulfan and cyclophosphamide for acute myelogenous leukemia. Bone Marrow Transplant. 1990;5(3):187–91.
Islam R, Isaacson BJ, Zickerman PM, Ratanawong C, Tipping SJ. Hemorrhagic cystitis as an unexpected adverse reaction to temozolomide: case report. Am J Clin Oncol. 2002;25(5):513–4.
Mylonakis E, Goes N, Rubin RH, Cosimi AB, Colvin RB, Fishman JA. BK virus in solid organ transplant recipients: an emerging syndrome. Transplantation. 2001;72(10):1587–92.
Reploeg MD, Storch GA, Clifford DB. BK virus: a clinical review. Clin Infect Dis. 2001;33(2):191–202.
Iwamoto S, Azuma E, Hori H, et al. BK virus-associated fatal renal failure following late-onset hemorrhagic cystitis in an unrelated bone marrow transplantation. Pediatr Hematol Oncol. 2002; 19(4):255–61.
Bielorai B, Shulman LM, Rechavi G, Toren A. CMV reactivation induced BK virus-associated late onset hemorrhagic cystitis after peripheral blood stem cell transplantation. Bone Marrow Transplant. 2001; 28(6):613–4.
Akiyama H, Kurosu T, Sakashita C, et al. Adenovirus is a key pathogen in hemorrhagic cystitis associated with bone marrow transplantation. Clin Infect Dis. 2001;32(9):1325–30.
Echavarria MS, Ray SC, Ambinder R, Dumler JS, Charache P. PCR detection of adenovirus in a bone marrow transplant recipient: hemorrhagic cystitis as a presenting manifestation of disseminated disease. J Clin Microbiol. 1999;37(3):686–9.
Boubenider S, Hiesse C, Marchand S, Hafi A, Kriaa F, Charpentier B. Post-transplantation polyomavirus infections. J Nephrol. 1999;12(1):24–9.
Leung AY, Suen CK, Lie AK, Liang RH, Yuen KY, Kwong YL. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood. 2001;98(6):1971–8.
Azzi A, Fanci R, Bosi A, et al. Monitoring of polyomavirus BK viruria in bone marrow transplantation patients by DNA hybridization assay and by polymerase chain reaction: an approach to assess the relationship between BK viruria and hemorrhagic cystitis. Bone Marrow Transplant. 1994;14(2):235–40.
Londergan TA, Walzak MP. Hemorrhagic cystitis due to adenovirus infection following bone marrow transplantation. J Urol. 1994;151(4):1013–4.
Bil-Lula I, Ussowicz M, Rybka B, et al. Hematuria due to adenoviral infection in bone marrow transplant patients. Transplant Proc. 2010;42:3729–34.
Barouch DH, Faquin WC, Chen Y, Koralnik IK, Robbins GK, Davis BT. BK virus-associated hemorrhagic cystitis in a human immunodeficiency virus-infected patient. Clin Infect Dis. 2002;35(3):326–9.
Toma Y, Ishiki T, Nagahama K, et al. Penicillin-G induced hemorrhagic cystitis with hydronephrosis. Intern Med. 2009;48:1667–9.
Lopez AE, Rodriguez S, Flores I. Management of ischemic hemorrhagic cystitis with hyperbaric oxygen therapy. Undersea Hyperb Med. 2001; 28(1):35–6.
Koh LP. Boon’s disease: hemorrhagic cystitis in conjunction with massive exfoliation of degenerated urothelial cells (apoptosis?) during intercontinental flights in an otherwise healthy person. Diagn Cytopathol. 2001;25(6):361–4.
Messing EM, Catalona W. Urothelial tumors of the urinary tract. In: Walsh PC, Retik AB, Vaughan Jr ED, Wein AJ, editors. Campbell’s urology, vol. 3. 7th ed. Philadelphia: Saunders; 1998. p. 2327–410.
Pedersen-Bjergaard J, Ersboll J, Hansen VL, et al. Carcinoma of the urinary bladder after treatment with cyclophosphamide for non-Hodgkin’s lymphoma. N Engl J Med. 1988;318(16):1028–32.
Kondo M, Kojima S, Kato K, Matsuyama T. Late-onset hemorrhagic cystitis after hemotopoietic stem cell transplantation in children. Bone Marrow Transplant. 1998;22(10):995–8.
Nevo S, Swan V, Enger C, et al. Acute bleeding after bone marrow transplantation (BMT)—incidence and effect on survival. A quantitative analysis in 1402 patients. Blood. 1998;91(4):1469–77.
Avidor Y, Nadu A, Matzkin H. Clinical significance of gross hematuria and its evaluation in patients receiving anticoagulant and aspirin treatment. Urology. 2000;55(1):22–4.
Worawattanakul S, Semelka RC, Kelekis NL. Post radiation hemorrhagic cystitis: MR findings. Magn Reson Imaging. 1997;15(9):1103–6.
Cartoni C, Arcese W, Avvisati G, Corinto L, Capua A, Meloni G. Role of ultrasonography in the diagnosis and follow-up of hemorrhagic cystitis after bone marrow transplantation. Bone Marrow Transplant. 1993;12(5):463–7.
Katz A, Epelman S, Anelli A, et al. A prospective randomized evaluation of three schedules of mesna administration in patients receiving an ifosfamide-containing chemotherapy regime: sustained efficiency and simplified administration. J Cancer Res Clin Oncol. 1995;121(2):128–31.
Meisenberg B, Lassiter M, Hussein A, Ross M, Vredenburgh JJ, Peters WP. Prevention of hemorrhagic cystitis after high-dose alkylating agent chemotherapy and autologous bone marrow support. Bone Marrow Transplant. 1994;14(2):287–91.
Luce JK, Simons JA. Efficacy of mesna in preventing further cyclophosphamide-induced hemorrhagic cystitis. Med Pediatr Oncol. 1988;16(6):372–4.
Haselberger MB, Schwinghammer TL. Efficacy of mesna for prevention of hemorrhagic cystitis after high-dose cyclophosphamide therapy. Ann Pharmacother. 1995;29(9):918–21.
Reinhold-Keller E, Mohr J, Christophers E, Nordmann K, Gross WL. Mesna side effects which imitate vasculitis. Clin Investig. 1992;70(8):698–704.
Vose JM, Reed EC, Pippert GC, et al. Mesna compared with continuous bladder irrigation as uroprotection during high-dose chemotherapy and transplantation: a randomized trial. J Clin Oncol. 1993;11(7):1306–10.
Shepherd JD, Pringle LE, Barnett MJ, Klingemann HG, Reece DE, Phillips GL. Mesna vs hyperhydration for the prevention of cyclophosphamide-induced hemorrhagic cystitis in bone marrow transplantation. J Clin Oncol. 1991;9(11):2016–20.
Ballen KK, Becker P, Levebvre K, et al. Safety and cost of hyperhydration for the prevention of hemorrhagic cystitis in bone marrow transplant recipients. Oncology. 1999;57(4):287–92.
Moon RE, Camporesi EM. Hyperbaric oxygen therapy: from the 19th to the 21st century. Respir Care Clin N Am. 1999;5(1):1–5.
Hampson NB, editor. Hyperbaric oxygen therapy: 1999 Committee report. Kensington, MD: Undersea and Hyperbaric Medical Society; 1999.
Robertson PW, Hart BB. Assessment of tissue oxygenation. Respir Care Clin N Am. 1999;5(2):221–63.
Marx RE, Ehler WJ, Tayapongsak P, et al. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg. 1990;160(5):519–24.
Shoshani O, Shupak A, Ullmann Y, et al. The effect of hyperbaric oxygenation on the viability of human fat injected into nude mice. Plast Reconstr Surg. 2000; 106(6):1390–6.
Thorn JJ, Kallehave F, Westergaard P, et al. The effect of hyperbaric oxygen on irradiated oral tissues: transmucosal oxygen tension measurements. J Oral Maxillofac Surg. 1997;55(10):1103–7.
Matthew R, Rajan N, Josefson L, et al. Hyperbaric oxygen therapy for radiation induced hemorrhagic cystitis. J Urol. 1999;161(2):435–7.
Bevers RF, Bakker DJ, Kurth KH. Hyperbaric oxygen treatment for hemorrhagic radiation cystitis. Lancet. 1995;346(8978):803–5.
Norkool DM, Hampson NB, Gibbons RP, et al. Hyperbaric oxygen therapy for radiation-induced hemorrhagic cystitis. J Urol. 1993;150(2 Pt 1):332–4.
Weiss JP, Mattei DM, Neville EC, et al. Primary treatment of radiation-induced cystitis with hyperbaric oxygen: 10-year experience. J Urol. 1994; 151(6):1514–7.
Crew JP, Jephcott CR, Reynard JM. Radiation-induced hemorrhagic cystitis. Eur Urol. 2002;40(2):111–23.
Ennis RD. Hyperbaric oxygen for the treatment of radiation cystitis and proctitis. Curr Urol Rep. 2000;3(3):229–31.
O’Reilly KJ, Hampson NB, Corman JM. Hyperbaric oxygen in urology, AUA update series lesson 4, vol. 21. Houston: American Urological Association; 2002. p. 26–31.
Feldmeier JJ, Hampson NB. A systematic review of the literature reporting the application of hyperbaric oxygen prevention and treatment of delayed radiation injuries: an evidence based approach. Undersea Hyperb Med. 2002;29(1):4–30.
Corman JM, McClure RD, Pritchett TR, Kozlowski P, Hampson NB. Treatment of radiation-induced hemorrhagic cystitis with hyperbaric oxygen. J Urol. 2003;169:2200–2.
Al-Ali BM, Trummer H, Shamloul R, et al. Is treatment of hemorrhagic radiation cystitis with hyperbaric oxygen effective? Urol Int. 2010;84:467–70.
Yoshida T, Kawashima A, Ujike T, et al. Hyperbaric oxygen therapy for radiation-induced hemorrhagic cystitis. Int J Urol. 2008;15:639–41.
Jou YC, Lien FC, Cheng MC, et al. Hyperbaric oxygen therapy for cyclophosphamide-induced intractable refractory hemorrhagic cystitis in a systemic lupus erythematous patient. J Chin Med Assoc. 2008; 71:218–20.
Gol AK, Rao MS, Bhagwat AG, et al. Intravesical irrigation with alum for the control of massive bladder hemorrhage. J Urol. 1985;133:956–7.
Gattegno B, Guillemenot F, Fiatte P, et al. Treatment of HC caused by cyclophosphamide using intravesical installation of potassium alum: a propos of 5 cases. Ann Urol. 1990;24:190–2.
Bogris S, Johal NS, Musgtaq I. Commentary to “pediatric hemorrhagic cystitis”. J Pediatr Urol. 2010;6:98.
Kanwar VS, Jenkins JJ, Mandrell BN. Aluminium toxicity following intravesical alum irrigation for hemorrhagic cystitis. Med Pediatr Oncol. 1996; 27(1):64–7.
Sarnak MJ, Long J, King AJ. Intravesicular formaldehyde instillation and renal complications. Clin Nephrol. 1999;51(2):122–5.
Stefanini M, English HA, Taylor AE. Safe and effective, prolonged administration of epsilon-aminocaproic acid in bleeding from the urinary tract. J Urol. 1990;143(3):559–61.
Pitts TO, Spero JA, Bontempo FA, et al. Acute renal failure due to high grade obstruction following therapy with epsilon-aminocaproic acid. Am J Kidney Dis. 1986;8:441–4.
Ippoliti C, Przepiorka D, Mehra R, et al. Intravesical carboprost for the treatment of hemorrhagic cystitis after marrow transplantation. Urology. 1995; 46(6):811–5.
Laszlo D, Bosi A, Guidi S, et al. Prostaglandin E2 bladder instillation for the treatment of hemorrhagic cystitis after allogenic bone marrow transplantation. Haematologica. 1995;80(5):421–5.
Trigg ME, O’Reilly J, Rumelhart S, Morgan D, Holida M, de Alarcon P. Prostaglandin E1 bladder instillations to control severe hemorrhagic cystitis. J Urol. 1990;143(1):92–4.
Raghavaiah NV, Soloway MS. Anuria following silver nitrate irrigation for intractable bladder hemorrhage. J Urol. 1977;118:681–2.
Andrei G, Snoeck R, Vandeputte M, et al. Activities of various compounds against murine and primate polyomaviruses. Antimicrob Agents Chemother. 1997;41:587–93.
Bridges B, Donegan S, Badros A. Cidofovir instillation for the treatment of BK hemorrhagic cystitis after allogenic stem cell transplantation. Am J Hematol. 2006;81:535–7.
Mullholland SG, Hanno P, Parsons CL, et al. Pentosan polysulfate sodium for therapy of interstitial cystitis. Urology. 1990;35:552–8.
Hampson SJ, Woodhouse CR. Sodium pentosan polysulfate in the management of hemorrhagic cystitis: experience with 14 patients. Eur Urol. 1994;25:40–2.
Toren PJ, Norman RW. Cyclophosphamide-induced hemorrhagic cystitis successfully treated with pentosanpolysulfate. J Urol. 2005;173:103.
Ritch CR, Poon SA, Sulis ML, et al. Cutaneous vesicostomy for palliative management of hemorrhagic cystitis and urinary clot retention. J Urol. 2010;76:166–8.
Lang EK. Transcatheter embolization of pelvic vessels for control of intractable hemorrhage. Radiology. 1981;140:331–9.
Han Y, Wu D, Sun A, et al. Selective embolization of the internal iliac arteries for the treatment of severe hemorrhagic cystitis following hematopoietic SCT. Bone Marrow Transplant. 2008;41:881–6.
Koc S, Hagglund H, Ireton RC, Perez-Simon JA, Collins SJ, Appelbaum FR. Successful treatment of severe hemorrhagic cystitis with cystectomy following matched donor allogenic hematopoietic cell transplantation. Bone Marrow Transplant. 2000;26(8):899–901.
Miyamura K, Hamaguchi M, Taji H, et al. Successful ribavirin therapy for severe adenovirus hemorrhagic cystitis after allogenic marrow transplant from close HLA donors rather than distant donors. Bone Marrow Transplant. 2000;25(5):545–8.
Seabra C, Perez-Simon JA, Sierra M, et al. Intramuscular vidarabine therapy for polyomavirus-associated hemorrhagic cystitis following allogenic hemopoietic stem cell transplantation. Bone Marrow Transplant. 2000;26(11):1229–30.
Miller J, Burfield GD, Moretti KL. Oral conjugated estrogen therapy for treatment of hemorrhagic cystitis. J Urol. 1994;151(5):1348–50.
Demesmay K, Tissot E, Bulabois CE, et al. Factor XIII replacement in stem-cell transplant recipients with severe hemorrhagic cystitis: a report of four cases. Transplantation. 2002;74(8):1190–2.
Connolly SS, D’Arcy FT, Corcoran MO. Recombinant activated factor VII to control life-threatening hemorrhagic radiation cystitis. Ir J Med Sci. 2010;179:431–3.
Vela-Ojeda J, Tripp-Villanueva F, Sanchez-Cortes E, et al. Intravesical rhGM-CSF for the treatment of late onset hemorrhagic cystitis after bone marrow transplant. Bone Marrow Transplant. 1999;24(12):1307–10.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Lewinshtein, D.J., Chong, K.T., Corman, J.M. (2013). Hemorrhagic Cystitis. In: Wessells, H. (eds) Urological Emergencies. Current Clinical Urology. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-423-4_14
Download citation
DOI: https://doi.org/10.1007/978-1-62703-423-4_14
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-422-7
Online ISBN: 978-1-62703-423-4
eBook Packages: MedicineMedicine (R0)