Abstract
Angioimmunoblastic T-cell lymphoma (AITL), the second most common peripheral T-cell lymphoma (PTCL) entity, encompasses distinct clinical and pathological features and often manifests with symptoms reflective of immune dysfunction. The pathological spectrum of AITL is broad; the tumor cells are typically outnumbered by a reactive cellular infiltrate comprising non-neoplastic small lymphocytes, Epstein–Barr virus (EBV)-infected large B cells, eosinophils, plasma cells, histiocytes, arborizing vessels, and follicular dendritic cells (FDCs). The recent identification of follicular helper T (TFH) cell as the cell of origin of this neoplasm represents a major step in our understanding of the pathobiological characteristics of the disease. Several markers of normal TFH cells have been recently validated for use in diagnostic pathology practice as they represent useful adjuncts to AITL identification. Yet the genetic alterations underlying AITL pathogenesis are still poorly understood. Deciphering the pathogenesis of the disease is needed to identify targets for new therapies that are expected to improve the poor outcome of AITL patients, when treated with conventional chemotherapy regimens. In this respect, efforts will be needed to evaluate promising innovative therapies in prospective clinical trials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vascular Endothelial Growth Factor
- Extranodal Site
- Follicular Helper
- Denileukin Diftitox
- Polyclonal Hypergammaglobulinemia
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction: Historical Perspective
Angioimmunoblastic T-cell lymphoma (AITL) was originally described in the 1970s as “angioimmunoblastic lymphadenopathy (AILD) with dysproteinemia” by Frizzera et al. [1] and subsequently as “immunoblastic lymphadenopathy” [2] or “lymphogranulomatosis X” [3]. The disease was initially reported as a non-neoplastic lymphoproliferative disorder and believed to represent an abnormal “hyperimmune” reaction of the B-cell system or an atypical lymphoid process, despite a clinical course characterized by multiple relapses, the development of malignant lymphoma in some cases, and a fatal outcome in the majority of patients [2]. Subsequently, Shimoyama and colleagues reported morphologic features of malignancy in cases with clinical and histological features otherwise similar to those of AILD, for which they proposed the designation of “immunoblastic T-cell lymphoma,” and that they suggested to consider as a variant of PTCL [4]. In addition, later in the 1980s, the identification of clonal cytogenetic abnormalities and the demonstration of clonal T-cell receptor (TCR) gene rearrangements definitively established the neoplastic nature of the disease [5–8].
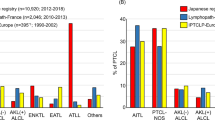
Thereafter, AITL was recognized as one of the most common forms of PTCL in the REAL and subsequent WHO classifications of hematologic malignancies [9, 10]. According to a recent international survey, AITL represents the second most common form of mature T/NK-cell malignancies, accounting for 18.5% of the cases worldwide [11]. Interestingly, there are important geographic variations, and the disease is more common in Europe (representing 29% of the cases) than in North America or Asia, where its prevalence is estimated to 16% and 18% of the cases, respectively [12]. The reasons for this heterogeneous distribution across different countries are not known. Part of it might be explained by the overall low prevalence of T-cell neoplasms in Western countries and a relative overrepresentation of other NK/T-cell lymphoma types in Asia, but true differences might exist. Yet no risk factors or etiologic agent(s) have been identified, and no racial predisposition is recognized.
Pathological Spectrum of Angioimmunoblastic T-Cell Lymphoma
Architectural Patterns
Lymph nodes almost constantly involved in AITL represent the tissue most frequently harvested for pathologic analysis and diagnosis (Table 4.1). AITL displays distinctive pathological features. According to Attygalle and colleagues, lymph node involvement in AITL can be classified according to three overlapping architectural patterns [13–16]. In pattern I (AITL with hyperplastic follicles) seen least frequently, the lymph node has a partially preserved architecture and contains hyperplastic follicles with numerous tingible body macrophages, poorly developed mantles, and ill-defined borders, merging into the paracortex expanded by a polymorphous infiltrate comprising often inconspicuous neoplastic cells, which tend to distribute in the vicinity of the hyperplastic follicles. In pattern II (AITL with depleted follicles), occasional depleted follicles are present. The pattern III (AITL without follicles), by far the most frequent, is characterized by complete loss of architecture, absence of residual B-cell follicles, and prominent irregular proliferation of FDCs. Conversely, FDCs are normal or minimally increased in patterns I and II. These three patterns, reported to comprise increasing numbers of neoplastic cells and to evolve toward a more diffuse distribution, are thought to reflect morphologic evolution of the disease rather than clinical progression [13–16].
Usual Morphologic Features
In pattern III most commonly encountered, the architecture of the lymph node is effaced, often with capsular and perinodal infiltration sparing the peripheral cortical sinus. The lymph node is occupied by a diffuse polymorphous infiltrate including variable proportions of medium-sized neoplastic T cells, admixed with small lymphocytes, histiocytes or epithelioid cells, immunoblasts, eosinophils, and plasma cells, and associated with prominent arborizing blood vessels and a perivascular proliferation of FDCs (Fig. 4.1a, b). Typically, the lymphoma cells are medium-sized cells with round or slightly irregular nuclei and abundant clear cytoplasm and distinct cell membranes (Fig. 4.1c). They are often less abundant than the reactive background and may even comprise only a minority of the T-cell compartment [17, 18]. This neoplastic component, which may be difficult to readily identify by morphology alone and is better highlighted by immunohistochemistry for T-cell markers, tends to form small clusters around high endothelial venules. In a subset of cases, the neoplastic cells are smaller with only slight pleomorphism and atypia, and without striking clear cell component. The disease is accompanied by a variable number of scattered large B-blasts often infected by the EBV, which may morphologically mimic Reed–Sternberg cells.
Typical histopathologic features of angioimmunoblastic T-cell lymphoma. (a) Lymph node involvement characterized by a diffuse lymphoproliferation associated with arborizing blood vessels (×100); (b) follicular dendritic cell (FDC) proliferation in angioimmunoblastic T-cell lymphoma highlighted by CD21 immunostaining (×25); (c) high magnification showing medium-sized neoplastic lymphoid cells with clear cytoplasm (×200); (d) CXCL13 usually produces intense cytoplasmic staining of most tumor cells (×200); (e) PD1 is positive in most neoplastic cells (×200)
Morphologic Variants According to Cell Content
A subset of cases contain a high proportion of large B-cell blasts (>25%) (B-cell-rich AITL), which are usually but not always infected by EBV; the importance of the large B-cell component does not seem to impact the clinical outcome [18, 19]. Although the neoplastic infiltrate in disease tissues is often subtle, a subset of AITL cases (less than 10%) comprise an overt lymphomatous proliferation of sheets of “clear” neoplastic cells [20]. Such cases, referred to as “clear cell-rich AITL,” do not appear to differ from usual cases in terms of clinical outcome [18]. The epithelioid variant of AITL, characterized by a high content of epithelioid cells, raises differential diagnosis issues as it can suggest a granulomatous disease or be mistaken for other histiocyte-rich lymphomas such as the lymphoepithelioid variant of PTCL, NOS (Lennert’s lymphoma), mixed cellularity Hodgkin lymphoma, or even with B-cell lymphomas (lymphoplasmacytic lymphoma or T-cell/histiocyte-rich large B-cell lymphoma in cases rich in large B cells) [21].
Immunophenotype
In involved tissues, the majority of infiltrating lymphocytes are T cells comprising an admixture of CD4+ and CD8+ cells, among which the tumor cell population may be difficult to identify [22, 23]. Nevertheless, it is established that in the majority of cases, the neoplastic cells of AITL consist of mature αβ CD4+ CD8− T cells [17, 23–25]. Aberrancies in the pattern of expression of pan-T-cell antigens are frequently observed—although this may be difficult to assess due to the low content in neoplastic cells—most commonly loss or reduced expression of CD7 or surface CD3, sometimes low or heterogeneous CD4 expression. Conversely, the expression of CD2 and CD5 is usually intact. Partial expression of CD30 by the neoplastic cells has been documented in up to one-third of the cases [23].
Aberrant CD10 antigen expression first reported in 2002 [13] is now validated by immunohistochemistry and/or flow cytometry as a sensitive marker for AITL, observed in around 80% of the cases [14, 18, 23, 26–28]. In many cases, the expression of CD10 is heterogeneous (detected on an often minor subset of the tumor cells and of variable intensity). Since aberrant CD10 expression is maintained in most involved extranodal sites, it is a useful marker for identification of AITL dissemination [26]. As detailed below, it has been recently shown that neoplastic T cells can be recognized by the expression of several markers of the follicular helper T cells (TFH), such as CXCL13, PD1, inducible costimulator (ICOS), CD200 [29], or BCL6 or c-MAF (Fig. 4.1d, e). Immunohistochemical criteria also include the demonstration of a variable proportion of scattered CD20+ B blasts, often expressing CD30 and EBV-infected, and of a proliferation of FDCs—highlighted by using classical FDC markers CD21, CD23, CNA.42, and/or CD35—typically in association with expanded high endothelial venules.
Extranodal Organs
Extranodal sites, especially bone marrow and skin, are also frequently involved in AITL patients. Bone marrow involvement has been reported to occur in up to 70% of the cases [30]. In the bone marrow, AITL infiltrates are often subtle, appearing as often small single or multiple nodular or interstitial foci of infiltration in a paratrabecular or non-paratrabecular distribution, which are often difficult to distinguish from reactive lymphocytic infiltrates. Accordingly, despite the absence of peripheral blood leukocytosis with lymphocytosis in most patients, low numbers of circulating tumor cells may be demonstrated due to an aberrant immunophenotype (most commonly CD10+ and/or sCD3− or dim) and/or clonal TCR gene rearrangement [30–32].
Exceptionally, AITL may lead to complete replacement of hematopoietic tissues [33]. In addition, secondary changes of the hematopoietic tissue are frequently observed, including bone marrow hypercellularity, eosinophilia, myelofibrosis, hematophagocytosis, or reactive polyclonal plasmacytosis, which may obscure the lymphoma infiltrate [30, 34].
Different histopathologic aspects can be seen in skin biopsies, ranging from subtle nonspecific mild perivascular lymphocytic infiltrate to, more rarely, an overtly lymphomatous diffuse infiltration containing numerous atypical T cells [35, 36]. The distribution of the atypical lymphoid infiltrates in other organs is less well characterized. Splenomegaly and tonsillar swelling are common in AITL, but spleen and Waldeyer’s ring are rarely the site of diagnosis [37]. The multiple extranodal sites of involvement can be paralleled with the common detection of tumoral cells in peripheral blood in AITL patients at diagnosis.
Clonality Analysis in AITL
Several large studies have reported clonality analysis of the TCR and immunoglobulin (IG) genes in AITL [8, 14, 17, 38–42]. In the most recent publications using sensitive PCR techniques, the detection of monoclonal or oligoclonal rearrangement of the TCR is found in the majority of cases—although not all—(up to 95% in the series reported by the Biomed-2 consortium when multiplex strategies targeting the β, γ, and δ TCR loci). In one recent study, sequence analysis of the rearranged TCRB genes showed overrepresentation of the BV17S1 family compared to the use of other Vβ segments [43].
In addition to TCR rearrangement, a clonal or oligoclonal rearrangement of the IG gene(s) is also found in up to one-third of patients. B-cell clonality tends to be evidenced in cases comprising an increased number of B-cell blasts, which may or may not be associated with EBV infection [41, 44]. Most EBV-infected B cells show ongoing mutational activity and carry hypermutated IG genes with destructive mutations, suggesting that in AITL alternative pathways operate to allow the survival of these mutating “forbidden” (Ig-deficient) B cells [44].
Genetic Alterations in AITL
By conventional cytogenetic analysis, clonal aberrations are detected in up to 90% of the cases (reviewed in ref. [45]). The most common recurrent abnormalities are trisomies of chromosomes 3, 5, and 21, gain of X, and loss of 6q [6, 46, 47]. Specific chromosomal abnormalities do not seem to be associated with survival, but complex karyotypes adversely impact the clinical outcome [46, 48]. Genetic heterogeneity manifested by unrelated clonal and non-clonal abnormalities in the same patient has been mentioned in cytogenetic reports, possibly reflective of genetic instability [48], and also perhaps linked to genetic alterations in the EBV-positive B-cell blasts [47]. Intriguingly, whereas a high frequency of chromosomal imbalances was confirmed by matrix-based CGH in a series of 39 AITL, with gains identified more commonly than losses, there was little overlap with classical cytogenetic data: trisomies 3 and 5 were infrequent and the most frequent gains were of 22q, 19, and 11p11-q14, and losses of 13q [49].
Chromosomal breakpoints affecting the TCR gene loci appear to be extremely rare: only one of 54 AITLs analyzed in two recent FISH-based studies was found to have a break, involving the TCR A/B locus [50, 51]. The molecular alterations underlying the neoplastic transformation remain unknown. Mutations of p53 are infrequent, and mutations in the 5′ region of BCL6 have not been detected [52]. A role for the c-MAF transcription factor has been suggested, because its overexpression in transgenic mice induces the development of T-cell lymphomas, and high levels of c-MAF have been detected in human AITL tissues [53]. However, c-MAF rearrangements are not evidenced in AITL, and hence the presence of c-MAF protein in AITL neoplastic cells represents an additional witness of their ontogenic derivation from TFH cells rather than the reflection of an oncogenic event [54]. Recently, novel recurrent mutations have been reported in AITL, involving the Ten Eleven Ten 2 (TET2) and isocitrate dehydrogenase 2 (IDH2) genes in approximately 35% and 20% of the cases, respectively [55, 56].
Molecular Signature Provides Further Evidence That AITL Derives from Follicular Helper T Cells
Critical insights into the understanding of AITL have been gained from molecular profiling analyses. In our study, we firstly defined the global molecular signature of 18 AITL cases in comparison to that of 16 PTCL, NOS samples, and subsequently, given the availability of sorted tumor cell suspensions for profiling, distinguished the respective contributions of the tumor cells and the nontumor cells to the AITL signature [57]. Somewhat in accordance with the known pathological features and diagnostic criteria, the AITL molecular profile was dominated by a strong microenvironment imprint, including overexpression of B-cell- and FDC-related genes, chemokines, and chemokine receptors, and genes related to extracellular matrix and vascular biology. Interestingly, the signature contributed by the neoplastic cells, albeit quantitatively minor, was enriched in genes normally expressed by TFH cells (Fig. 4.2). TFH cells constitute a minor subset of effector T cells with a specific microanatomic distribution in the apical zone of reactive germinal centers, and distinct gene signature and functions separable from the other known Th1, Th2, Th17 effector subsets [58, 59]. The demonstration of molecular similarities between AITL tumor cells and TFH cells at a genome-wide level definitively established the cellular derivation of AITL from TFH cells, initially suspected on the basis of the expression of single TFH markers in AITL tumor cells, in particular the CXCL13 chemokine [62]. The molecular link between TFH cells and AITL has been confirmed in other gene expression profiling datasets by independent investigators [60, 61].
Molecular signatures of AITL and PTCL, NOS by gene expression profiling analysis. Expression data from two normal TFH cell populations, 2 AITL tumor cell suspension samples (arrows), 17 AITL tissue samples, and 16 PTCL, NOS samples are shown. The genes represented in this heatmap include a set of 42 core genes representative of normal TFH cells, overexpressed in AITL (by comparison to PTCL, NOS) and overrepresented in sorted AITL tumor cells (arrows) in comparison to AITL tissues (as demonstrated by gene set enrichment analyses). Standardized expression ranges from −2.0 (blue) to 2.0 (red) (adapted from de Leval et al. [57], with permission from John Wiley & Sons, Inc.)
This peculiar gene expression signature translates into the expression of TFH markers that represent novel diagnostic markers of the disease. Strikingly, cytoplasmic expression of the CXCL13 chemokine is found in the neoplastic cells in most AITL cases [62, 63]. Additional markers of normal TFH cells, including the cell surface molecules CXCR5, CD154, programmed death-1 (PD-1) (a member of the CD28 costimulatory receptor family resulting in negative regulation of T-cell activity), and ICOS (a CD28 homologue with costimulatory function in T-cell activation and expansion), have been demonstrated in AITL by immunohistochemistry [16, 27, 64–68]. Another cytoplasmic protein, SAP (SLAM-associated protein), has been recently validated as a novel marker of both normal germinal center T cells and AITL [66]. Nuclear expressions of the BCL6 and c-MAF transcription factors, which are characteristic of the TFH subset of CD4+ cells, represent other phenotypic traits of AITL tumor cells [15, 28, 54]. On tissue sections, the distribution and the intensity of the immunostainings observed for the different TFH markers tend to correlate and, similar to CD10, to show a relationship to the FDC meshwork. For diagnostic purposes, CXCL13, PD1, and BCL6 currently represent the most useful and robust TFH markers amenable to routine paraffin section immunohistochemistry. In terms of specificity, CXCL13 is probably superior to PD1, ICOS, and BCL6, which can also be expressed in a subset of extrafollicular T cells. It has also been reported that CXCL13 is a more helpful marker than CD10 to recognize the neoplastic nature of subtle infiltrates in extranodal sites, for example, the skin [36].
The cellular derivation of AITL from TFH cells provides a rational model to explain several of the peculiar pathological and biological features inherent to this disease, i.e., the expansion of B cells, the intimate association with germinal centers in early disease stages, and the striking proliferation of FDCs. Among the molecular mediators of TFH cells, CXCL13 likely plays a key role. New future insights in the precise functions of the network of mediators involved in the generation of TFH cells and their role in the immune system will certainly contribute to the better understanding of the pathogenesis of AITL. The reason why this peculiarly small subset of T cells gives rise to one of the most frequent T-cell lymphoma entity is unexplained and the molecular alterations targeting neoplastic TFH cells and their role in lymphomagenesis remain to be deciphered. Moreover, these recently accumulated data suggest that the morphological spectrum of AITL may be larger than previously defined. Indeed, a TFH imprint has been demonstrated in a subset of cases morphologically classified as PTCL, NOS that may represent evolution from AITL, and in rare forms of PTCL with a follicular growth pattern [57, 69, 70].
Role of the Tumor Microenvironment
Non-neoplastic cells typically represent a quantitatively major component of AITL and, clinically, the manifestations of the disease mostly reflect a deregulated immune and/or inflammatory response rather than direct complications of tumor growth [18] supporting the concept of a paraneoplastic immunological dysfunction (Fig. 4.3). Moreover, AITL patients have defective T-cell responses, linked to both quantitative and qualitative perturbations of T-cell subsets [32, 71]. A depletion in Treg cells and an accumulation of Th17 cells have been recently reported in AITL tissues [72, 73]. Interestingly, normal TFH cells suppress T-cell responses by inhibiting the proliferation and function of conventional CD4 T cells, especially through TGF-β and IL-10 production [74]. The complex pathways and networks and the mediators linking the various cellular non-neoplastic and neoplastic components are only partly deciphered. Lymphotoxin beta demonstrated in AITL tumor cells [75] and potentially released by B cells under CXCL13 stimulation might be involved in inducing FDC proliferation.
Pathogenetic model of angioimmunoblastic T-cell lymphoma. In AITL, a complex network of interactions take place between the tumor cells and the various cellular components of the reactive microenvironment, the molecular mediators of which are partly deciphered. Different factors released by TFH cells are involved in B-cell recruitment, activation, and differentiation (CXCL13), in the modulation of other T-cell subsets (IL21, IL10, TFGβ), or in promoting vascular proliferation (VEGF, angiopoietin), and may also act as autocrine factors. CXCL13 may also attract mast cells (MC), which are a source of IL6 promoting Th17 differentiation. EBV reactivation occurs in the context of a deregulated immune response, which also favors the expansion of both TFH cells and B cells. TGFβ is a mediator of FDC differentiation and proliferation, and FDC in turn are a source of CXCL13 and VEGF. B B-cell; FDC FDC; HEV high endothelial venule; MC mast cell; PC plasma cell
Upregulation of several angiogenic mediators has been demonstrated in AITL. Vascular endothelial growth factor (VEGF) is overexpressed in AITL and probably acts as a key mediator of the prominent vascularization observed in the disease [61, 69]. By immunostaining, neoplastic cells and endothelial cells are positive for both VEGF and its receptor, suggesting the possibility of some paracrine and/or autocrine loop [61, 76]. Moreover, FDCs represent another source of VEGF. The angiopoietin system may also play an important role as angiopoietin 1, which is expressed by AITL neoplastic cells and FDCs [61, 76].
Overall, the importance of the microenvironment and its potential role in tumor growth may have clinical implications for the use of novel targeted therapies.
Infectious Agents in AITL
The etiology of AITL remains unknown, and there are no consistent risk factors for the disease. The original descriptions of AILD emphasized that in many patients the disease was preceded by allergic reactions, infections, and/or administration of drugs, especially antibiotics, which led to the speculation that AITL occurred as a result of abnormal immune reaction. Several reports also mentioned an association with various bacterial or fungal infections. It is now believed that these associations likely reflect the consequences of immune deregulation in AITL patients and do not bear a causal relationship.
EBV-positive cells are detected in most cases of AITL, and it is now established that these EBV-infected cells are B cells, indicating that the virus is unlikely to play a primary role in lymphomagenesis. Zhou et al. recently found that higher EBV viral loads in biopsies correlated with progression of histological patterns and with B-cell clonality [77]. In the latter study, PCR showed the presence of HHV6B in almost half of the cases. The involvement of other herpesviruses, in particular HHV8, appears very unlikely [77]. Although viral infection/reactivation likely occurs as a consequence of the underlying immune dysfunction, EBV and potentially also HHV6B may, through the modulation of cytokines, chemokines, and membrane receptors, play a role in the development of the tumor microenvironment, ultimately favoring disease progression. Interestingly, HHV6B also has immunosuppressive properties.
Clinical Features and Treatment
The median age of presentation for AITL is 59–65 years. A slight or marked male predominance is repeatedly reported. AITL may present as a subacute or acute systemic illness and may be associated with infections or other disorder of immune deregulation. Generalized lymphadenopathy and constitutional symptoms such as fever and weight loss are common features. A high proportion of patients have hepatomegaly and/or splenomegaly. Bone marrow involvement has been reported in up to 70% of the cases and tends to correlate with a higher frequency of B symptoms, hepatosplenomegaly, laboratory abnormalities, and the presence of circulating tumor cells [30]. Up to half of the patients have skin rash—either generalized or a predominantly truncal maculopapular eruption mimicking an inflammatory dermatosis—and/or pruritus, prior to or concurrent with the diagnosis of lymphoma, or at relapse. Nodular lesions, plaques, purpura, and urticarial lesions can also be seen [78]. Overall, most patients have concomitant extranodal disease, and the disease is stage III or IV in more than 80% of cases.
Laboratory tests often disclose a variety of hematological, biochemical, and/or immunological abnormalities. Anemia, polyclonal hypergammaglobulinemia, and hypereosinophilia are the most common alterations seen at diagnosis. Other common findings include lymphopenia, thrombocytopenia, and the presence of various autoantibodies (rheumatoid factor, antinuclear factor, anti-smooth muscle), cryoglobulins, or cold agglutinins.
The prognostic significance of various other clinicobiological and pathological features was evaluated in a retrospective series of 157 AITL patients retrieved from the Goupe d’ Etude des Lymphomes de l’ Adulte (GELA) LNH87-LNH93 randomized trials [18]. In multivariate analysis, only male sex, mediastinal lymphadenopathy, and anemia adversely affected overall survival.
Clinical Approaches for AITL
The optimal therapeutic approach for AITL has not been determined. Steroids have been used as a single-agent approach, especially in elderly patients. Most patients receive combination chemotherapeutic regimens, such as cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP); and cyclophosphamide, vincristine, prednisolone, bleomycin, doxorubicin, procarbazine, ifosfamide, methotrexate, and etoposide (COPBLAM/IMVP-16), but none of these has increased the long-term survival rate to more than 30%. A number of single-agent therapies have also been used, including methotrexate together with steroids, fludarabine or 2-chlorodeoxyadenosine, and cyclosporine A [79–84]. These reports are often anecdotal or correspond to limited phase II trials with response rates averaging 30%, and there is no consensus whether any of these agents improve outcomes more than conventional treatment, even in combination.
Is More Intensive Treatment with Transplantation Better?
The possible benefit of high-dose chemotherapy with ASCT (HDT-ASCT) for AITL patients was demonstrated in a retrospective multicenter study of the European Group for Blood and Marrow Transplantation, which reported 146 AITL patients who received ASCT in different situations (relapse, refractory, first complete remission) [85]. After a median follow-up of 31 months, the actuarial overall survival (OS) was 67% at 24 months and 59% at 48 months. The estimated progression-free survival (PFS) rates were 70% and 56% at 24 and 48 months, respectively, for patients who received their transplants in CR; 42% and 30% for patients with chemotherapy-sensitive disease; and 23% at both time points for patients with chemotherapy-refractory disease.
There are now emerging data on allogeneic transplantation in small cohorts of patients with PFS and OS rates of 66% and 64% at 3 years, respectively, with a relapse rate at 3 years estimated at 20%. However, whether allogeneic transplantation offers additional benefit over autologous transplantation to young patients remains to be determined.
Newer Agents for AITL
The most commonly used treatment for PTCL is CHOP or variant regimens. However, the results with CHOP are inadequate, and new approaches are needed. The activities of new drugs are being described in studies designed for PTCL patients, and attempts at novel combinations are emerging. Alemtuzumab, a monoclonal anti-CD52 antibody, has recently been added to CHOP for the treatment of PTCL. Twenty-four consecutive patients with newly diagnosed PTCL, including six AITL, enrolled in a prospective multicenter trial received a combination of alemtuzumab with CHOP [86]. There was CR in 17 of 24 (71%) patients. At a median follow-up of 16 months, 13/24 patients (54%) were disease-free with an estimated 2-year OS and failure-free survival of 53% and 48% respectively. Several Phase II trials with alemtuzumab alone or combined with chemotherapy gave encouraging results for first-line treatment, with manageable toxicities. A phase II study of CHOP with denileukin diftitox for 37 untreated PTCL, including 10 AITL, showed clinical activity with an 86% response rate (76% CR) and a 2-year PFS estimate of 41%, and only little added toxicity over CHOP [87]. The addition of rituximab to target CD20-positive B-cells along with CHOP was explored as first-line therapy for AITL, but results were similar to CHOP alone.
Therapies targeting VEGF mediators have been proposed in relation to the expression of both VEGF and its receptor by neoplastic cells of AITL. Bevacizumab has shown anecdotal activity in PTCL, particularly AITL, and has been added to CHOP in an ongoing Eastern Cooperative Oncology Group trial for newly diagnosed patients with PTCL. This study has been associated with enhanced cardiac toxicity [88].
Pralatrexate, a novel antifolic drug, demonstrated activity in PTCL in a study of 111 patients with relapsed and refractory PTCL including 13 AITL [89]. The overall response rate was 27, including 11 CR. Median duration of response was 9 months, and the dose-limiting toxicities were thrombocytopenia and stomatitis. Romidepsin, a novel histone deacetylase inhibitor (HDACi), showed a 35% response rate in relapsed/refractory PTCL, including in several patients with AITL [90].
In summary, despite the use of aggressive regimens with anthracycline-based chemotherapy, AITL is largely incurable without a stem cell transplant. Prognostic factors associated with response to various modalities have not been elucidated. Novel agents are being explored with promising results in small numbers of patients.
References
Frizzera G, Moran E, Rappaport H. Angio-immunoblastic lymphadenopathy with dysproteinemia. Lancet. 1974;1:1070–3.
Lukes RJ, Tindle BH. Immunoblastic lymphadenopathy. A hyperimmune entity resembling Hodgkin’s disease. N Engl J Med. 1975;292:1–8.
Lennert K. [Nature, prognosis and nomenclature of angioimmunoblastic lymphadenopathy (lymphogranulomatosis X or T-zone lymphoma)]. Dtsch Med Wochenschr. 1979;104:1246–7.
Shimoyama M, Minato K. [Clinical, cytological and immunological analysis of T-cell type lymphoid malignancies: a classification of T-cell type lymphoid malignancy (author’s transl)]. Rinsho Ketsueki. 1979;20:1056–69.
Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549–56.
Kaneko Y, Maseki N, Homma C, et al. Chromosome translocations involving band 7q35 or 7p15 in childhood T-cell leukemia/lymphoma. Blood. 1988;72:534–8.
Tobinai K, Minato K, Ohtsu T, et al. Clinicopathologic, immunophenotypic, and immunogenotypic analyses of immunoblastic lymphadenopathy-like T-cell lymphoma. Blood. 1988;72:1000–6.
Weiss LM, Strickler JG, Dorfman RF, Horning SJ, Warnke RA, Sklar J. Clonal T-cell populations in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Am J Pathol. 1986;122:392–7.
Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–92.
Jaffe ES, Krenacs L, Raffeld M. Classification of cytotoxic T-cell and natural killer cell lymphomas. Semin Hematol. 2003;40:175–84.
Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–30.
Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 2002;13:140–9.
Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627–33.
Attygalle AD, Kyriakou C, Dupuis J, et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression. Am J Surg Pathol. 2007;31:1077–88.
Ree HJ, Kadin ME, Kikuchi M, et al. Angioimmunoblastic lymphoma (AILD-type T-cell lymphoma) with hyperplastic germinal centers. Am J Surg Pathol. 1998;22:643–55.
Rodriguez-Justo M, Attygalle AD, Munson P, Roncador G, Marafioti T, Piris MA. Angioimmunoblastic T-cell lymphoma with hyperplastic germinal centres: a neoplasia with origin in the outer zone of the germinal centre? Clinicopathological and immunohistochemical study of 10 cases with follicular T-cell markers. Mod Pathol. 2009;22:753–61.
Willenbrock K, Renne C, Gaulard P, Hansmann ML. In angioimmunoblastic T-cell lymphoma, neoplastic T cells may be a minor cell population. A molecular single-cell and immunohistochemical study. Virchows Arch. 2005;446:15–20.
Mourad N, Mounier N, Briere J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463–70.
Lome-Maldonado C, Canioni D, Hermine O, et al. Angio-immunoblastic T cell lymphoma (AILD-TL) rich in large B cells and associated with Epstein-Barr virus infection. A different subtype of AILD-TL? Leukemia. 2002;16:2134–41.
Merchant SH, Amin MB, Viswanatha DS. Morphologic and immunophenotypic analysis of angioimmunoblastic T-cell lymphoma: emphasis on phenotypic aberrancies for early diagnosis. Am J Clin Pathol. 2006;126:29–38.
Patsouris E, Noel H, Lennert K. Angioimmunoblastic lymphadenopathy—type of T-cell lymphoma with a high content of epithelioid cells. Histopathology and comparison with lymphoepithelioid cell lymphoma. Am J Surg Pathol. 1989;13:262–75.
Chen W, Kesler MV, Karandikar NJ, McKenna RW, Kroft SH. Flow cytometric features of angioimmunoblastic T-cell lymphoma. Cytometry B Clin Cytom. 2006;70:142–8.
Karube K, Aoki R, Nomura Y, et al. Usefulness of flow cytometry for differential diagnosis of precursor and peripheral T-cell and NK-cell lymphomas: analysis of 490 cases. Pathol Int. 2008;58:89–97.
Lee PS, Lin CN, Chuang SS. Immunophenotyping of angioimmunoblastic T-cell lymphomas by multiparameter flow cytometry. Pathol Res Pract. 2003;199:539–45.
Stacchini A, Demurtas A, Aliberti S, et al. The usefulness of flow cytometric CD10 detection in the differential diagnosis of peripheral T-cell lymphomas. Am J Clin Pathol. 2007;128:854–64.
Attygalle AD, Diss TC, Munson P, Isaacson PG, Du MQ, Dogan A. CD10 expression in extranodal dissemination of angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2004;28:54–61.
Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30:802–10.
Yuan CM, Vergilio JA, Zhao XF, Smith TK, Harris NL, Bagg A. CD10 and BCL6 expression in the diagnosis of angioimmunoblastic T-cell lymphoma: utility of detecting CD10+ T cells by flow cytometry. Hum Pathol. 2005;36:784–91.
Dorfman D, Shahsafaei A. CD200 (OX-2 membrane glycoprotein) is expressed by follicular helper T cells and in angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2011;35(1):76–83.
Cho YU, Chi HS, Park CJ, Jang S, Seo EJ, Huh J. Distinct features of angioimmunoblastic T-cell lymphoma with bone marrow involvement. Am J Clin Pathol. 2009;131:640–6.
Baseggio L, Berger F, Morel D, et al. Identification of circulating CD10 positive T cells in angioimmunoblastic T-cell lymphoma. Leukemia. 2006;20:296–303.
Delfau-Larue MH, de Leval L, Joly B, et al. Targeting intratumoral B-cells with Rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma.A clinicobiological study of the GELA. Haematologica. 2012 Feb 27. [Epub ahead of print]
Sakai H, Tanaka H, Katsurada T, Yoshida Y, Okamoto E, Ohno H. Angioimmunoblastic T-cell lymphoma initially presenting with replacement of bone marrow and peripheral plasmacytosis. Intern Med. 2007;46:419–24.
Grogg KL, Morice WG, Macon WR. Spectrum of bone marrow findings in patients with angioimmunoblastic T-cell lymphoma. Br J Haematol. 2007;137:416–22.
Martel P, Laroche L, Courville P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol. 2000;136:881–6.
Ortonne N, Dupuis J, Plonquet A, et al. Characterization of CXCL13+ neoplastic t cells in cutaneous lesions of angioimmunoblastic T-cell lymphoma (AITL). Am J Surg Pathol. 2007;31:1068–76.
Frizzera G, Moran EM, Rappaport H. Angio-immunoblastic lymphadenopathy. Diagnosis and clinical course. Am J Med. 1975;59:803–18.
Attygalle AD, Chuang SS, Diss TC, Du MQ, Isaacson PG, Dogan A. Distinguishing angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified, using morphology, immunophenotype and molecular genetics. Histopathology. 2007;50:498–508.
Bruggemann M, White H, Gaulard P, et al. Powerful strategy for polymerase chain reaction-based clonality assessment in T-cell malignancies. Report of the BIOMED-2 Concerted Action BHM4 CT98-3936. Leukemia. 2007;21:215–21.
Kawano R, Ohshima K, Wakamatsu S, Suzumiya J, Kikuchi M, Tamura K. Epstein-Barr virus genome level, T-cell clonality and the prognosis of angioimmunoblastic T-cell lymphoma. Haematologica. 2005;90:1192–6.
Tan BT, Warnke RA, Arber DA. The frequency of B- and T-cell gene rearrangements and Epstein-Barr virus in T-cell lymphomas: a comparison between angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified with and without associated B-cell proliferations. J Mol Diagn. 2006;8:466–75; quiz 527.
Vrsalovic MM, Korac P, Dominis M, Ostojic S, Mannhalter C, Kusec R. T- and B-cell clonality and frequency of human herpes viruses-6, -8 and Epstein Barr virus in angioimmunoblastic T-cell lymphoma. Hematol Oncol. 2004;22:169–77.
Shah ZH, Harris S, Smith JL, Hodges E. Monoclonality and oligoclonality of T cell receptor beta gene in angioimmunoblastic T cell lymphoma. J Clin Pathol. 2009;62:177–81.
Brauninger A, Spieker T, Willenbrock K, et al. Survival and clonal expansion of mutating “forbidden” (immunoglobulin receptor-deficient) Epstein-Barr virus-infected b cells in angioimmunoblastic t cell lymphoma. J Exp Med. 2001;194:927–40.
de Leval L, Bisig B, Thielen C, Boniver J, Gaulard P. Molecular classification of T-cell lymphomas. Crit Rev Oncol Hematol. 2009;72(2):125–43.
Nelson M, Horsman DE, Weisenburger DD, et al. Cytogenetic abnormalities and clinical correlations in peripheral T-cell lymphoma. Br J Haematol. 2008;141:461–9.
Dogan A, Attygalle AD, Kyriakou C. Angioimmunoblastic T-cell lymphoma. Br J Haematol. 2003;121:681–91.
Schlegelberger B, Feller A, Godde W, Lennert K. Stepwise development of chromosomal abnormalities in angioimmunoblastic lymphadenopathy. Cancer Genet Cytogenet. 1990;50:15.
Thorns C, Bastian B, Pinkel D, et al. Chromosomal aberrations in angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma unspecified: a matrix-based CGH approach. Genes Chromosomes Cancer. 2007;46:37–44.
Gesk S, Martin-Subero JI, Harder L, et al. Molecular cytogenetic detection of chromosomal breakpoints in T-cell receptor gene loci. Leukemia. 2003;17:738–45.
Leich E, Haralambieva E, Zettl A, et al. Tissue microarray-based screening for chromosomal breakpoints affecting the T-cell receptor gene loci in mature T-cell lymphomas. J Pathol. 2007;213:99–105.
Kerl K, Vonlanthen R, Nagy M, et al. Alterations on the 5′ noncoding region of the BCL-6 gene are not correlated with BCL-6 protein expression in T cell non-Hodgkin lymphomas. Lab Invest. 2001;81:1693–702.
Murakami YI, Yatabe Y, Sakaguchi T, et al. c-Maf expression in angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2007;31:1695–702.
Bisig B, et al. c-Maf expression in angioimmunoblastic T-cell lymphoma reflects follicular helper T-cell derivation rather than oncogenesis. Histopathology. 2012;60:371–6.
Quivoron C, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20:25–38.
Cairns RA, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012;119:1901–3.
De Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br J Haematol. 2010;148(5):673–89.
Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–35.
Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–65.
Iqbal J, Weisenburger DD, Greiner TC, et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood. 2010;115:1026–36.
Piccaluga PP, Agostinelli C, Califano A, et al. Gene expression analysis of angioimmunoblastic lymphoma indicates derivation from T follicular helper cells and vascular endothelial growth factor deregulation. Cancer Res. 2007;67:10703–10.
Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am J Surg Pathol. 2006;30:490–4.
Grogg KL, Attygalle AD, Macon WR, Remstein ED, Kurtin PJ, Dogan A. Angioimmunoblastic T-cell lymphoma: a neoplasm of germinal-center T-helper cells? Blood. 2005;106:1501–2.
Krenacs L, Schaerli P, Kis G, Bagdi E. Phenotype of neoplastic cells in angioimmunoblastic T-cell lymphoma is consistent with activated follicular B helper T cells. Blood. 2006;108:1110–1.
Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432–9.
Roncador G, Garcia Verdes-Montenegro JF, Tedoldi S, et al. Expression of two markers of germinal center T cells (SAP and PD-1) in angioimmunoblastic T-cell lymphoma. Haematologica. 2007;92:1059–66.
Xerri L, Chetaille B, Serriari N, et al. Programmed death 1 is a marker of angioimmunoblastic T-cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Hum Pathol. 2008;39:1050–8.
Yu H, Shahsafaei A, Dorfman DM. Germinal-center T-helper-cell markers PD-1 and CXCL13 are both expressed by neoplastic cells in angioimmunoblastic T-cell lymphoma. Am J Clin Pathol. 2009;131:33–41.
de Leval L, Rickman DS, Thielen C, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–63.
Huang Y, Moreau A, Dupuis J, et al. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am J Surg Pathol. 2009;33:682–90.
Pizzolo G, Vinante F, Agostini C, et al. Immunologic abnormalities in angioimmunoblastic lymphadenopathy. Cancer. 1987;60:2412–8.
Bruneau J, Canioni D, Renand A, et al. Regulatory T-cell depletion in angioimmunoblastic T-cell lymphoma. Am J Pathol. 2010;177:570–4.
Tripodo C, Gri G, Piccaluga PP, et al. Mast cells and Th17 cells contribute to the lymphoma-associated pro-inflammatory microenvironment of angioimmunoblastic T-cell lymphoma. Am J Pathol. 2010;177:792–802.
Marinova E, Han S, Zheng B. Germinal center helper T cells are dual functional regulatory cells with suppressive activity to conventional CD4+ T cells. J Immunol. 2007;178:5010–7.
Foss HD, Anagnostopoulos I, Herbst H, et al. Patterns of cytokine gene expression in peripheral T-cell lymphoma of angioimmunoblastic lymphadenopathy type. Blood. 1995;85:2862–9.
Zhao WL, Mourah S, Mounier N, et al. Vascular endothelial growth factor-A is expressed both on lymphoma cells and endothelial cells in angioimmunoblastic T-cell lymphoma and related to lymphoma progression. Lab Invest. 2004;84:1512–9.
Zhou Y, Attygalle AD, Chuang SS, et al. Angioimmunoblastic T-cell lymphoma: histological progression associates with EBV and HHV6B viral load. Br J Haematol. 2007;138:44–53.
Yoon GS, Choi YK, Bak H, et al. Angioimmunoblastic T cell lymphomas: frequent cutaneous skin lesions and absence of human herpes viruses. Ann Dermatol. 2009;21:1–5.
Advani R, Warnke R, Sikic BI, Horning S. Treatment of angioimmunoblastic T-cell lymphoma with cyclosporine. Ann Oncol. 1997;8:601–3.
Bourgeois E, Auxenfants E, Jouffrey C, Dubest C, Mahieu M, Rose C. [Efficacy of fludarabine in the treatment of angioimmunoblastic lymphoma (AIL)]. Ann Med Interne (Paris). 2000;151:230–1.
Gerlando Q, Barbera V, Ammatuna E, Franco V, Florena AM, Mariani G. Successful treatment of angioimmunoblastic lymphadenopathy with dysproteinemia-type T-cell lymphoma by combined methotrexate and prednisone. Haematologica. 2000;85:880–1.
Murayama T, Imoto S, Takahashi T, Ito M, Matozaki S, Nakagawa T. Successful treatment of angioimmunoblastic lymphadenopathy with dysproteinemia with cyclosporin A. Cancer. 1992;69:2567–70.
Quintini G, Iannitto E, Barbera V, et al. Response to low-dose oral methotrexate and prednisone in two patients with angio-immunoblastic lymphadenopathy-type T-cell lymphoma. Hematol J. 2001;2:393–5.
Sallah AS, Bernard S. Treatment of angioimmunoblastic lymphadenopathy with dysproteinemia using 2-chlorodeoxyadenosine. Ann Hematol. 1996;73:295–6.
Kyriakou C, Canals C, Goldstone A, et al. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: complete remission at transplantation is the major determinant of Outcome-Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:218–24.
Gallamini A, Zaja F, Patti C, et al. Alemtuzumab (Campath-1 H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110:2316–23.
Foss FM, Sjak-Shie NN, Goy A, Advani R, Jacobsen ED. Phase II study of denileukin diftitox with CHOP chemotherapy in newly-diagnosed PTCL: CONCEPT trial [ASCO meeting abstracts]. J Clin Oncol. 2010;28:8045.
Advani RH, Hong F, Ganjoo KN, et al. Cardiac toxicity associated with the anti-VEGF monoclonal antibody bevacizumab (avastin) in combination with CHOP (A-CHOP) chemotherapy for peripheral T cell lymphoma (PTCL): the ECOG 2404 trial [ASH annual meeting abstracts]. Blood. 2009;114:1671.
O’Connor O, Pro B, Pinter-Brown L, et al. PROPEL: results of the pivotal, multicenter, phase II study of pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma (PTCL) [ASCO meeting abstracts]. J Clin Oncol. 2009;27:8561.
Coiffier B, Pro B, Prince HM, et al. Final results from a pivotal, multicenter, international, open-label, phase 2 Study of romidepsin in progressive or relapsed peripheral T-cell lymphoma (PTCL) following prior systemic therapy [ASH annual meeting abstracts]. Blood. 2010;116:114.
Acknowledgments
This study was supported in part by grants from FRM (Fondation pour la Recherche Médicale, équipe labellisée) and the Cancer Plan research Programme (Belgium, LdL).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
de Leval, L., Foss, F., Gaulard, P. (2013). Molecular and Clinical Aspects of Angioimmunoblastic T-Cell Lymphoma. In: Foss, F. (eds) T-Cell Lymphomas. Contemporary Hematology. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-170-7_4
Download citation
DOI: https://doi.org/10.1007/978-1-62703-170-7_4
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-169-1
Online ISBN: 978-1-62703-170-7
eBook Packages: MedicineMedicine (R0)