Abstract
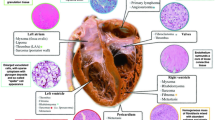
Although the term tumor generally recalls the idea of “cancer,” at cardiac level most of primary tumors are biologically benign and malignancy is mostly hemodynamic, due to obstruction of the blood flow because of intracavitary growth and embolism following neoplastic fragmentation with ischemic damage of several organs. The first book on cardiac tumors was published in 1945 by Ivan Mahaim, Professor at the University of Lausanne and was a collection of postmortem observations and a thorough review of the literature. While treating atrial myxoma (“Le polype du coeur”), the most frequent cardiac tumor (nearly two-thirds of primary heart neoplasms), he said “…surgical resection of atrial polyp encounters apparently insurmountable difficulties. However, we should not give up because of this feeling. In any field of science, with technological progress, the impossible is just a moment during the evolution of our powers. As Mummery said about alpinism, the inaccessible peak becomes an easy route for ladies…”. In fact, some years later the era of “surgical pathology” started with the advent of cardiac imaging and open heart surgery in the 1960s, when cardiac neoplasms were diagnosed during life and not only in the autopsy room and became surgically resectable with excellent long-term survival. The historical watershed in the diagnosis and treatment came in the 1980s, with the advent of non-invasive imaging, i.e., echocardiography, which, together with computed tomography and cardiac magnetic resonance, substantially improved non-invasive diagnosis and subsequent treatment. It is possible to easily visualize cardiac tumors at the first onset of symptoms or even incidentally, during routine diagnostic procedures, and send the patient promptly to the surgeon for resection with a nearly 100 % success in the benign forms. Nowadays, the pathologist is on call to achieve the in vivo diagnosis on endomyocardial or surgical biopsies, by establishing the nature (benign, malignant, or non-neoplastic, usually thrombi or vegetations) and the histotype, and to make the differential diagnosis with secondary tumors. In this chapter we will come across single case observations that witness the advances in the clinical diagnosis and therapy of cardiac tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
In the popular mind, the term “tumor” recalls the concept of “cancer,” i.e., a highly aggressive biological process eventually leading to body consumption due to malignant infiltration and metastasis.
This is not the case at the heart level, since malignant primary cardiac tumors are rare (about 10% of all primary cardiac tumors). Malignancy is hemodynamic rather than biological, due to obstruction of the blood circulation because of intracavitary growth and embolism of neoplastic fragments with potentially devasting ischemic damage to several organs.
Before the advent of cardiac imaging and of open heart surgery, cardiac neoplasms were not diagnosed in vivo and were mostly fatal due to complications along their natural history and, as such, diagnosed by the pathologist only at postmortem.
Noteworthy, the first description of cardiac tumors was made by Matteo Realdo Colombo in 1559 in his book De Re Anatomica [1]. While performing the autopsy of the body of Cardinal Gambaro from Brescia, since he was the archiater in Rome at that time, he discovered a left ventricular mass described as follows in Latin “In Cardinali Gambara Brixiano tumorem praedurum, et ad ovi magnitudinem in sinistro cordis ventricolo Romae vidi, ubi illum in affinium gratiam dissecarem” (“in Rome I saw a solid tumour, large like an egg, in the left ventricle of Cardinal Gambaro of whom I was committed by the Pope to make autopsy”) (Fig. 1.1). Most likely it was a post-infarction endocavitary apical mural thrombus rather than a true neoplasm.
In the famous article entitled Tumors of the heart: review of the subject and report of one hundred and fifty cases, dated 1951 [2], Richard Prichard wrote “…the most common cardiac tumour (cardiac myxoma) has never been diagnosed antemortem…”. Noteworthy, in the Mayo Clinic experience published in 1980, 23% of surgically resected cardiac myxomas were referred to surgery on the basis of auscultatory signs and symptoms suggestive of rheumatic mitral valve stenosis [3].
The first book on cardiac tumors was published by Ivan Mahaim in 1945 (Fig. 1.2) with the title Les tumeurs et les polypes du coeur: etude anatomo-clinique [4].
At the Institute of Pathological Anatomy of the peaceful Lausanne, spared by the second world war due to the neutral position of Switzerland, the young cardiac pathologist Mahaim wrote an extraordinary book, that was a collection of personal observations at postmortem as well as of literature review, and represented a milestone publication on the topic of cardiac tumors for several generations of pathologists and physicians involved in the field. From him we learned that cardiac myxoma (called in French “le polype” due to its resemblance to a pedunculated gastro-enteric polyp), can have a clinical presentation with syncope or dyspnea due to atrio-ventricular valve orifice occlusion (functional mitral stenosis), with peripheral artery occlusion due to embolic phenomena, or can be totally asymptomatic being an incidental finding (“les polypes silencieux”). Although these were all autopsy cases, Mahaim was optimist on the perspectives of Medicine. While treating atrial myxoma (“Le polype du coeur”), the most frequent cardiac tumor (nearly two-thirds of primary heart neoplasms), he said “…surgical resection of atrial polyp encounters apparently unsurmountable difficulties. However, we should not give up because of this feeling. In any field of science, with technological progress, the impossible is just a moment during the evolution of our powers. As Mummery said about alpinism, the inaccessible peak becomes an easy route for ladies…” [4].
On the contrary, in 1951 Prichard manifested a pessimistic attitude by saying “…of the surgical treatment of these tumours we never heard” [2]. Surprisingly, in the same year 1951 Goldberg et al. [5] for the first time successfully made a clinical diagnosis of left atrial myxoma using angiography and in 1954 Crafoord resected a myxoma using extracorporeal circulation [6].
Thus, the era of “surgical pathology” started with clinical diagnosis mostly based upon angiography (Fig. 1.3) and the pathologist on call to establish in vivo the nature and histotype of the neoplasm, as in any other field of oncology.
Angiographic diagnosis of right ventricular myxoma in a 21- year-old male (from Bortolotti et al. [20] modified). Reprinted with permission of the Italian Society of Cardiology. (a) Angiographic image showing a contrast medium defect at the level of the right outflow tract. (b) At surgery, a bi-lobated myxoma was found
However, the historical watershed in the diagnosis and treatment came in the 1980s, with the advent of non-invasive imaging, i.e., echocardiography (Fig. 1.4) which, together with computed tomography and cardiac magnetic resonance (MRI), substantially improved diagnosis and subsequent treatment. It is possible to easily visualize cardiac tumors at the first onset of symptoms or even incidentally, during routine diagnostic procedures, and send the patient promptly to the surgeon for resection with a nearly 100% success in the benign forms. Before the 1980s cardiac myxomas were a postmortem finding, thereafter they became almost exclusively a clinical and surgical observation.
The role of the pathologists is now to establish the nature of the resected mass (non-neoplastic, benign, or malignant neoplasms) and, last but not least, to make the differential diagnosis with secondary neoplasms. The advent of immunohistochemistry to characterize the antigenic markers, by using monoclonal and polyclonal antibodies, leads to major advances in the diagnosis of primary and secondary cardiac tumors as in other fields of oncology, but particularly of malignant primary sarcomas, where the tumor cell of origin can be the endothelial cell, fibroblast, cardiomyocyte, smooth muscle cell, adipocyte, etc. [7].
We report some anecdotal cases collected along the last 35 years at our center in Padua, which are emblematic of the historical evolution in the field of cardiac oncology.
In 1976 a 61 -year-old man died during angiographic examination performed due to acute pulmonary edema and peripheral embolisms. The need to wait for angiography before referring the patient to surgery resulted to be fatal. A giant villous left atrial myxoma occupied the mitral atrio-ventricular orifice, herniating and protruding into the left ventricle (Fig.1.5).
A 61-year-old man who died due to acute pulmonary edema in 1976. (a) A giant left atrial, cluster-shaped myxoma was found occupying the left atrio-ventricular valve orifice. (b) A similar drawing is available in the book by Mahaim (observation by Ferrari E. Sui tumori poliposi dell’endocardio atriale. Arch Sc Mediche 1941;72:75). Reprinted with permission of the Italian Society of Cardiology
In 1979 a 45- year-old man suffered a sudden left leg ischemia. An embolus was retrieved by Fogarty, and histology showed the a thrombotic composition. Two days later the patient had an embolic “storm,” including the cerebral arteries and died. At autopsy a smooth left atrial myxoma, covered by a stratified thrombus as the probable source of multiple embolisms, was found (Fig. 1.6). Since then, we learned that the true nature of the embolic source cannot be derived with certainty from the histological examination of emboli fragments alone. An echocardiographic examination should have been performed to look at the heart and save the life of the patient by emergency operation to resect the embolic source.
A 45- year-old man who died in 1979 after several episodes of peripheral embolism. (a) A left atrial myxoma was found at autopsy (“polyp”) with the endocardial surface covered by thrombus. Histological examination of peripheral embolisms retrieved during life was negative for myxoma (only thrombus). (b) Drawing of a left atrial myxoma in the book by Mahaim, herein described as a “pedunculated polyp” accounting for functional mitral stenosis. Note the similarity between the two cases. Reprinted with permission of the Italian Society of Cardiology
In 1978, a 15-year-old boy had a diagnosis of myocarditis due to chest pain with cardiac enzymes release and ST segment elevation on 12 lead ECG. Discharged at home 20 days later, he suffered an embolic stroke while playing soccer and died suddenly. Autopsy examination revealed a bi-atrial myxoma (Fig. 1.7a, b) with myxomatous embolisms of the cerebral and right coronary artery, the latter being the cause of the previously misdiagnosed inferior myocardial infarction (Fig. 1.7c). Again, echocardiography examination should have identified the cardiac mass, with prompt indication to surgical resection thus preventing the second fatal embolic episode.
A 15-year-old boy who died due to cerebral embolism after a “myocarditis-like” presentation (from Valente and Montaguti [24] modified). Reprinted with permission of the Italian Society of Cardiology. (a) Villous left atrial myxoma; (b) villous right atrial myxoma, covered by thrombi; and (c) right coronary artery occluded by myxomatous embolism (Alcian PAS ×15)
In 1979, a 43- year-old man, brother of a distinguished surgeon at the University of Padua, complained of malaise and leg arthralgias. Although the brother reassured him by saying that he was only stressed, the patient asked a chiromancer who, by massaging his legs, told that his problems came from far away, i.e., from the heart. Few days later, he suffered bi-lateral leg ischemia and the infra-renal abdominal aorta appeared occluded at angiography. At emergency vascular surgery, a big white–gray embolus, of gelatinous consistency in keeping with myxomatous material at histology, was retrieved from the aortic carrefour. At angiography, a left atrial mass was found and cardiac surgery was immediately performed with resection of a huge villous myxoma from the left atrial cavity (Fig. 1.8).
A 43-year-old man with villous left atrial myxoma accounting for leg ischemia with abdominal aortic carrefour occlusion. The vascular surgeon removed a sausage-like huge mass, which resulted to be an embolus detached from the left atrial myxoma, as identified by cardiac angiography and then surgically resected (from Ballotta et al. [18] modified). Reprinted with permission of the Italian Society of Cardiology
Some anecdotal cases belong also to the so-called echocardiographic era.
A 6- year-old boy suffered a stroke with hemiplegia and echocardiographic examination detected a left atrial mass. The surgically resected mass revealed to be at histology a villous, gelatinous, and friable myxoma (Fig. 1.9). During the follow-up, the boy well recovered without neurological sequelae. Since then, echocardiography is always performed in any patient, even in the pediatric age, with neurological or syncopal episodes, to exclude a cardiac source.
A 47- year-old woman coming from the South of Italy to Padua as devotee of Saint Antony, went to the Church in Padua with malaise and arthralgias; while kneeling to Saint Antony, she had loss of consciousness and fainted. Promptly transferred to the Intensive Care Unit in Padua, echocardiography disclosed a left atrial mass wedging into the mitral valve orifice (Fig. 1.10). She had emergency operation and survived without any complication: a joint venture between the Patavian Medical School and the thaumaturgical power of the Saint!
A 47-year-old woman with syncopal episodes. The prompt echocardiographic examination (a, b) detected a polypous left atrial mass, impinging during diastole into the mitral valve orifice. The successfully resected mass revealed to be a smooth myxoma (“polyp”) (c). Reprinted with permission of the Italian Society of Cardiology
After the implementation of non-invasive cardiac imaging and with the exponential increase of in vivo diagnosis and “surgical pathology” cases, we did not observe anymore autopsy deaths due to cardiac myxomas along the natural history. The rare postmortem observations were incidental findings of tumors of small size or of myxoma with calcific involution, a condition for whom we coined the term “lithomyxoma” [8] (Fig. 1.11).
Incidental autopsy finding of left atrial myxoma with calcific involution (lithomyxoma) in a 72-year-old woman who died due to ischemic heart disease (from Basso et al. [8] modified). Reprinted with permission of the Italian Society of Cardiology. (a) gross view; (b) ex vivo X-Ray
On the contrary, the availability of echocardiography performed as a routine/screening test in several conditions due to its non-invasiveness, led to the frequent casual identification of many small benign endocavitary tumors, particularly fibroelastomas (or fibroelastic papillomas). When localized in the left chambers, surgical resection is always indicated, for primary prevention of embolic phenomena. On the contrary, right-sided papillomas can be followed up.
Malignant cardiac tumors represent a different clinico-pathologic challenge.
Secondary metastatic tumors can involve the pericardium, myocardium, and endocardium. In the latter instance, endocardial metastases may be also observed of such a size to create endocavitary obstruction like primary cardiac tumors (Fig. 1.12). Noteworthy, the diagnosis of secondary cardiac mass is no so rarely achieved only by histological examination of the resected cardiac mass that reveals a histotype different from that of primary malignant cardiac tumors. For instance, we recall a case of a right atrial endocavitary villous tumor, labeled as myxoma at naked eye observation and then diagnosed as testis corion carcinoma after histological and immunohistochemical investigation; as well as the case of a right atrial mass located at the orifice of the inferior caval vein, subsequently diagnosed as clear cell renal carcinoma (Fig. 1.13). In both instances, a total body imaging (echo, CT) has been then performed with discovery of the primary malignant tumors and orchiectomy and nephrectomy were carried out, respectively, with adjuvant chemotherapy.
Right atrial mass with pre-operative diagnosis of cardiac myxoma in a 42- year-old woman. Reprinted with permission of the Italian Society of Cardiology. (a) Gross view of the surgically resected mass with a smooth surface. (b) At histology, clear cells with round nuclei and vacuolated cytoplasm, aggregated in cordon-like structures are visible (hematoxylin–eosin). (c) At immunohistochemistry, a few cells are positive for vimentin. A final diagnosis of cardiac metastasis of clear cell renal carcinoma was done
Primary malignant cardiac tumors are mostly infiltrating masses with inherent difficulties in achieving a complete surgical resection and as such with a poor prognosis. An exception is represented by the rare primary malignant cardiac tumors with an endocavitary growth and a clinical presentation mimicking myxoma due to obstructive symptoms and signs. The patient can even present with cardiogenic shock, when the intracardiac mass occupies the atrio-ventricular orifices or the pulmonary artery, mimicking pulmonary embolism. Histological examination of the resected “embolus” is mandatory, to assess the benign or malignant nature of the process and characterize the histotype [7].
In the last two decades, there have been many steps forward in the clinical diagnosis and treatment of cardiac tumors:
-
1.
Endomyocardial biopsy. Tissue sampling to assess the neoplastic nature of the mass during life can be achieved through either surgical thoracotomy or endomyocardial biopsy. With the latter technique, tissue samples may be taken with the bioptome introduced antegrade in the right cardiac chambers either through femoral or jugular veins or, more rarely, retrograde in the left chambers through femoral arteries [9]. The procedure is under echocardiographic guidance and avoids thoracotomy for diagnosis, allowing therapeutic planning, including cardiac transplantation, in cases of malignant neoplasm without extra-cardiac metastasis. Moreover, endomyocardial biopsy can be useful in the setting of tumors which are unresectable or require histological characterization before chemotherapy is started, such as lymphomas. An adequate number and size of samples (4–5 pieces, 1–2 mm each) is usually enough for a thorough histological investigation, including immuno-histochemistry, to achieve a precise diagnosis (Fig. 1.14).
Fig. 1.14 Histologic diagnosis of cardiac metastasis by T-cell lymphoma through endomyocardial biopsy in a 36- year-old woman (from Testolin et al. [40] modified). Reprinted with permission of the Italian Society of Cardiology. (a) Trans-esophageal echocardiography, four-chamber view: a mass on both side of the interatrial septum is visible (arrows). (b) Right ventricular endomyocardial biopsy shows a lympho-proliferative lesion infiltrating the myocardium. (c) At immunohistochemistry, the cells are positive for T lymphocytes (CD3) (AML anterior mitral leaflet, CVC central venous catheter, RA right atrium, RV right ventricle, LA left atrium, LV left ventricle)
-
2.
Prenatal echocardiographic diagnosis. Nowadays, prenatal echography is a routine examination during pregnancy and the request for fetal echocardiography is increasing. Many congenital heart diseases are diagnosed before birth, including cardiac tumors [10]. They consist mostly of teratomas, fibromas, rhabdomyomas either intramural or endocavitary, and more frequent indications include arrhythmias, hydrops, retarded intrauterine growth, and familiarity of tuberous sclerosis. Prenatal diagnosis is essential to plan the surgical intervention soon after delivery.
-
3.
Cardiac transplantation. Organ replacement with orthotopic cardiac transplantation is an extreme therapeutic option which is indicated in the setting of non-resectable benign cardiac tumors, or in cases of infiltrating primary malignant neoplasms without extra-cardiac metastasis. The ideal situation is typically represented by cardiac fibroma, which is usually located within the interventricular septum (Fig. 1.15) or the left ventricular free wall [10]; total heart removal with ex vivo repair on the bench, followed by auto-transplant, can be also carried out. A different situation is represented by infiltrating primary malignant cardiac tumors, where cardiac transplantation is considered only in the absence of metastases. However, the cardiac surgeon has always to take into consideration the low number of donors and the high number of patients in the waiting list with a potentially better long-term prognosis.
Fig. 1.15 A 40-year-old woman, with echocardiographic diagnosis of asymmetric hypertrophic cardiomyopathy, underwent cardiac transplantation due to refractory congestive heart failure (from Valente et al. [10]). Reprinted with permission of the Italian Society of Cardiology. (a) Long axis section of the native heart showing a huge oval shape whitish mass occupying the interventricular septum and accounting for subaortic bulging/obstruction. (b) At histology, a cardiac fibroma was diagnosed (Heidenhain trichrome)
-
4.
Cardiac magnetic resonance imaging (MRI) and computed tomography (CT). MRI is the best available non-invasive procedure for cardiac tumor diagnosis in terms of site, morphology, dimensions, extension, topographic relations, and infiltration of surrounding structures. It may also help for tissue characterization (adipose tissue, necrosis, hemorrhage, vascularization, calcification), although the specificity is still low [11]. A “probabilistic” histopathological diagnosis is more reliable and the term “mass” should be employed, leaving the final answer to histology. In case of lipoma, with hyper-intensity signal in T1 imaging, the precise histotype may be established by MRI, with a very high diagnostic probability. Use of contrast medium may be of help to detect highly vascularized tumors, like myxomas, angiomas, and angiosarcomas. In case of fibroma, delayed contrast enhancement shows homogeneous uptake of gadolinium, indicating fibrous tissue.
Multi-slice CT has the advantage of a better spatial resolution in case of possible lung, pleural, and mediastinal involvement. Moreover, calcification is easily detected within the mass and may point to fibroma, in case of mural mass, myxoma in case of an intracavitary atrial mass in the elderly, or teratoma in case of pericardial mass in infancy. MRI and CT can also differentiate serous from hemorrhagic pericardial effusion.
However, multi-slice CT does not allow to fully investigate involvement of cardiac valves, and presents the limitation of high dosage radiation, so as to preclude its employment in the follow-up of young subjects. Fast heart rates and arrhythmias may jeopardize the quality of imaging of both MRI and multi-slice CT.
Obviously, two-dimensional echocardiography remains the first diagnostic approach for detection of cardiac masses, whereas MRI and CT may be complementary tools, with their own advantages and limitations. Due to their non-invasiveness and lack of radiation, two-dimensional echo and MRI are also the gold standard for follow-up studies.
Non-neoplastic Cardiac Masses
Only histological examination may establish whether a cardiac mass is neoplastic in nature, either benign or malignant. When seen by clinical imaging, the use of term “mass” is advisable, since it may have several explanations other than cardiac tumors.
-
1.
Thrombi. An isolated mass inside an atrial appendage is almost exclusively a thrombus. In the left atrium, free floating masses like “ball thrombus” should point to a mitral valve pathology, usually rheumatic in origin (Fig. 1.16). However, differential diagnosis with left atrial myxoma is always needed (Fig. 1.17). Small mural thrombi may be observed at the apex of the left ventricle, even in the setting of a preserved contractility, and misinterpreted clinically as left ventricular myxomas (Fig. 1.18). Non-bacterial thrombotic endocarditis may mimic valve papillary fibroelastoma. Valvular and non-valvular endocardial thrombosis may be observed in antiphospholipid syndrome [12]. Histological investigation is always needed to achieve the final diagnosis [13] (Fig. 1.19).
Fig. 1.16 (a) Rheumatic mitral valve stenosis with left atrial ball-thrombus, mimicking a myxoma (a 72- year-old woman with history of peripheral embolism who underwent mitral valve replacement). (b) Similar drawing in the original book by Mahaim. Reprinted with permission of the Italian Society of Cardiology
Fig. 1.17 A 61- year-old man with pulmonary edema. Reprinted with permission of the Italian Society of Cardiology. (a) Two-dimensional echocardiography, four-chamber view shows a mass occupying the mitral valve orifice. (b) At surgery, a myxoma with strangulation corresponding to the sphincter contraction of the mitral valve anulus is found
Fig. 1.18 A 33 -year-old woman with recurrent left ventricular apex thrombosis, mimicking a endocardial tumor. Reprinted with permission of the Italian Society of Cardiology. (a) Two-dimensional echocardiography, four-chamber view (LA left atrium, LV left ventricle, RA right atrium, RV right ventricle). (b) At histology, the resected mass consists of thrombotic material (Heidenhain trichrome)
Fig. 1.19 A 20-year-old patient with a small left ventricular apical mass (from Basso et al. [12] modified). Reprinted with permission of the Italian Society of Cardiology. (a) Two-dimensional echocardiography, apical four-chamber view shows a round mass at the left ventricular apex (arrow) (LA left atrium, LV left ventricle, RA right atrium, RV right ventricle). (b) Gross view of the surgically resected mass: note the smooth surface and the pink color. (c, d) At histology and immunohistochemistry, the benign proliferation shows a myxoid background with vessel proliferation in keeping with myxomatous angioma (hematoxylin–eosin and CD31)
-
2.
Cardiomyopathies. Endocardial fibroplastic Loeffler endocarditis is a restrictive (obliterative) cardiomyopathy, usually associated with eosinophilia, hypersensitivity, or eosinophilic leukemia, consisting of a mural thrombus filling the apical and inflow portions of the ventricles to such an extent to entrap the mitral and/or tricuspid valves apparatus. Cardiac imaging may mis-diagnose an apical form of hypertrophic cardiomyopathy or an endocardial tumor. Endomyocardial biopsy with tissue characterization may be of help for differential diagnosis (Fig. 1.20).
Fig. 1.20 A 61- year-old man affected by Loeffler fibroplastic endocarditis (from Basso et al. [36] modified). Reprinted with permission of the Italian Society of Cardiology. (a) Left ventricular angiography showing a contrast medium defect at the apex corresponding to a round oval mass. (b) Four samples retrieved by endomyocardial biopsy, two of which consist of thrombotic material (hematoxylin–eosin). (c) One of the endomyocardial biopsy samples shows endocardial fibrous thickening (Heidenhain trichrome). (d) At higher magnification, eosinophilic infiltrates are visible (hematoxylin–eosin)
-
3.
Infections. Infective vegetations of cardiac valves may mimic papillary fibroelastoma, and sometimes at the mitral level they may be so huge as to simulate embolizing atrial myxoma. Free floating masses may not necessarily be thrombotic, but even septic (fungal in immunosuppressed patients) (Fig. 1.21). Pericardial or myocardial cysts may be hydatid cysts by echinococcosis, to be treated cautiously during the surgery to avoid rupture and spread of infection (Fig. 1.22).
Fig. 1.21 A 23-year-old man under chemotherapic therapy due to acute myeloid leukemia (from Vida et al. [14] modified). Reprinted with permission of the Italian Society of Cardiology. (a, b) at two-dimensional echocardiography, a free-floating round mass is visible within the left ventricular cavity (A aspergilloma, Ao aorta, LA left atrium, LV left ventricle); (c) the resected whitish mass shows a small pedicle and a rough surface; (d) at histology, fungal hyphas are detected (Alcian PAS)
-
4.
Calcium. Calcium stones, intramural or intra-cavitary, may occur and not necessarily are the outcome of dystrophic calcification of previous infections/tumors, as in the case of lithomyxoma. The phenomenon may be so massive to transform the heart into a sort of a stone quarry. Dystrophic calcification of the mitral valve annulus can also account for misdiagnosis of an atrio-ventricular mass by echocardiography. Endocavitary calcific masses have been reported even in the pediatric age as a consequence of calcific involution of thrombi upon central venous catheters.
The single center experience collected at the University of Padua since 1970, when the first surgical intervention for resection of a cardiac myxoma was accomplished [15], is witnessed by the numerous publications on the topic, available in the literature [16–55].
References
Columbus MR. De Re Anatomica, Libri XV. Venice: N Bevilacque,1559, p 269.
Prichard RW. Tumors of the heart: review of the subject and report of 150 cases. Arch Pathol. 1951;51:98–128.
Wold LE, Lie JT. Cardiac myxomas: a clinicopathologic profile. Am J Pathol. 1980;101:219–40.
Mahaim I. Le tumeurs et les polypes du coeur: etude anatomoclinique. Paris: Masson; 1945.
Goldberg HP, Glenn F, Dotter CT, Steinberg I. Myxoma of the left atrium: diagnosis made during life with operative and post-mortem findings. Circulation. 1952;6:762–7.
Crafoord C. Panel discussion on late results of mitral commissurotomy. In: Lam CR, editor. International symposium on cardiovascular surgery. Philadelphia, PA: Saunders; 1955. p. 161–78
Basso C, Valente M, Poletti A, Casarotto D, Thiene G. Surgical pathology of primary cardiac and pericardial tumors. Eur J Cardiothorac Surg. 1997;12:730–7.
Basso C, Valente M, Casarotto D, Thiene G. Cardiac lithomyxoma. Am J Cardiol. 1997;80:1249–51.
Poletti A, Cocco P, Valente M, Fasoli G, Chioin R, Thiene G. In vivo diagnosis of cardiac angiosarcoma by endomyocardial biopsy. Cardiovasc Pathol. 1993;2:89–91.
Valente M, Cocco P, Thiene G, Casula R, Poletti A, Milanesi O, Fasoli G, Livi U. Cardiac fibroma and heart transplantation. J Thorac Cardiovasc Surg. 1993;106:1208–12.
Hoffmann U, Globits S, Schima W, Loewe C, Puig S, Oberhuber G, Frank H. Usefulness of magnetic resonance imaging of cardiac and paracardiac masses. Am J Cardiol. 2003;92:890–5.
Basso C, Fasoli G, Casarotto D, Thiene G. Unusual left ventricular mass. Circulation. 1998;98:1036–7.
Basso C, Bottio T, Rubino M, Ruffatti A, Pittarello D, Thiene G, Gerosa G. Antiphospholipid sindrome and right atrial mass. J Thorac Cardiovasc Surg. 2005;130:1462–3.
Vida V, Biffanti R, Thiene G, Stellin G, Milanesi O, Basso C. Left ventricular mass after treatment with chemotherapic drugs. Circulation. 2004;109:e300–1.
Casarotto D. Mixoma dell’atrio sinistro. Chirurgia Triveneta. 1971;11:279–87.
Thiene G, Miraglia G, Menghetti L, Nava A, Rossi L. Multiple lesions of the conduction system in a case of cardiac rhabdomyosarcoma with complex arrhythmias. An anatomic and clinical study. Chest. 1976;70:378–81.
Casarotto D, Bortolotti U, Russo D, Betti D, Schivazappa L, Thiene G. Surgical removal of a left atrial myxoma during pregnancy. Chest. 1979;75:390–2.
Ballotta E, Thiene G, Valente M, Deriu GP. Mixoma dell’atrio sinistro rivelato da un’occlusione acuta del carrefour aortico. G Ital Cardiol. 1979;9:627–30.
Stritoni P, Daliento L, Fasoli G, Schivazappa L, Chioin R, Fusaro A, Casarotto D, Bortolotti U, Valente M, Thiene G. Mixoma atriale. Esperienza clinica, diagnostico-strumentale, chirurgica e patologica in 13 casi. G Ital Cardiol. 1979;9:1001–16.
Gallucci V, Stritoni P, Fasoli G, Thiene G. Giant blood cyst of tricuspid valve. Successful excision in an infant. Br Heart J. 1976;38:990–2.
Bortolotti U, Mazzucco A, Valfrè C, Valente M, Pennelli N, Gallucci V. Right ventricular myxoma: review of the literature and report of two patients. Am Thorac Surg. 1982;33:277–84.
Stellin G, Bortolotti U, Valfré C, Mazzucco A, Thiene G, Cavarzerani A, Gallucci V. Mural papilloma of the left ventricle and floppy mitral valve: report of an unusual association. Texas Heart Inst J. 1983;10:89–92.
Valente M. Structural profile of cardiac myxoma. Appl Pathol. 1983;1:251–63.
Valente M, Montaguti A. Il mixoma cardiaco nell’adolescenza. Studio anatomo-patologico in tre pazienti. Pathologica. 1984;76:323–32.
Livi U, Bortolotti U, Milano A, Valente M, Prandi A, Frugoni C, De Mozzi P, Valfré C, Mazzucco A, Gallucci V. Cardiac myxomas: results of 14 years experience. Thorac Cardiovasc Surg. 1984;32:143–7.
Thiene G, Valente M. I tumori primitivi del cuore e del pericardio nell’esperienza dell’Istituto di Anatomia Patologica dell’Università di Padova (1972–1984). In: Pansini R, editors. Progressi Clinici: Medicina. Padova: Piccin Ed., 1985;2/2–3: p. 169–207.
Bortolotti U, Mazzucco A, Rubino M, Maraglino G, Sturaro M, Milano A, Stellin G, Thiene G, Gallucci V. I mixomi dell’atrio destro. Tecnica operatoria, risultati e follow-up a distanza. Chir Torac. 1989;42:371–2.
Mazzucco A, Bortolotti U, Thiene G, Dan M, Stritoni P, Scutari M, Stellin G. Left ventricular papillary fibroelastoma with coronary embolization. Eur J Cardio-Thorac Surg. 1989;3:471–3.
De Dominicis E, Frigiola A, Thiene G, Menicanti L, Bozzola L, Finocchi G. Subaortic stenosis by solitary rhabdomyoma. Successful excision in an infant following 2D echocardiogram and Doppler diagnosis. Chest. 1989;95:470–2.
Bortolotti U, Maraglino G, Rubino M, Santini F, Mazzucco A, Milano A, Fasoli G, Livi U, Thiene G, Gallucci V. Surgical excision of intracardiac myxomas: a 20-year follow-up. Ann Thorac Surg. 1990;49:449–53.
Bortolotti U, Faggian G, Mazzucco A, Milano A, Thiene G, Fasoli G, Gallucci V. Right atrial myxoma originating from the inferior vena cava. Ann Thorac Surg. 1990;49:1000–2.
Cocco P, Valente M, Thiene G. I tumori primitivi del cuore nell’era cardiochirurgica: dall’autopsia al trapianto cardiaco. Riv Anat Pat Oncol. 1991;49–50:135–72.
Mazzucco A, Faggian G, Bortolotti U, Bonato R, Pittarello D, Centonze G, Thiene G. Embolizing papillary fibroelastoma of the mitral valve. Texas Heart Inst J. 1991;18:62–6.
Valente M, Basso C, Thiene G, Bressan M, Stritoni P, Cocco P, Fasoli G. Fibroelastic papilloma: a not-so-benign cardiac tumor. Cardiovasc Pathol. 1992;1:161–6.
Basso C, Stefani A, Calabrese F, Fasoli G, Valente M. Primary right atrial fibrosarcoma diagnosed by endocardial biopsy. Am Heart J. 1996;131:399–402.
Basso C, Poletti A, Valente M, Thiene G. Diagnosi di tumore cardiaco tramite biopsia endomiocardica. In: Baroldi G, Thiene G, editors. Testo Atlante Biopsia Endomiocardica. Padova: Piccin Ed; 1996. p. 178–96.
Basso C, Valente M, Poletti A, Casarotto D, Thiene G. Surgical pathology of primary cardiac and pericardial tumors. Eur J Cardio-thorac Surg. 1997;12:730–8.
Scalia D, Basso C, Rizzoli G, Lupia M, Budano S, Thiene G, Venturini A. Should right-sided fibroelastomas be operated upon? J Heart Valve Dis. 1997;6:647–50.
Basso C, Barbazza R, Thiene G. Lipomatous hypertrophy of the atrial septum. Circulation. 1998;97:1423.
Testolin L, Basso C, Pittarello D, Casarotto D, Valente M. Cardiogenic shock due to metastatic cardiac lymphoma: still a diagnostic and therapeutic challenge. Eur J Cardiothorac Surg. 2001;19:365–8.
Bottio T, Basso C, Rizzoli G, Casarotto D, Thiene G. Case report: fibroelastoma of the papillary muscle of the mitral valve: diagnostic implications and review of the literature. J Heart Valve Dis. 2002;11:288–91.
Basso C, Valente M, Thiene G. Aspetti anatomo-clinici dei tumori del cuore e del pericardio. In: Crepaldi G, Baritussio A, editors. Trattato di Medicina Interna: Malattie del cuore e dei vasi. Padova: Piccin Editore; 2002. p. 757–68.
Padalino MA, Basso C, Thiene G, Stellin G. Images in cardiovascular medicine: Giant right ventricular fibroma in an infant. Circulation. 2002;106:386.
Padalino MA, Basso C, Moreolo GS, Thiene G, Stellin G. Left atrial myxoma in a child: case report and review of the literature. Cardiovasc Pathol. 2003;12:233–6.
Basso C, Bottio T, Valente M, Bonato R, Casarotto D, Thiene G. Primary cardiac valve tumours. Heart. 2003;89:1259–60.
Rizzoli G, Bottio T, Pittarello D, Napodano M, Thiene G, Basso C. Atrial septal mass: transesophageal echocardiographic assessment. J Thorac Cardiovasc Surg. 2004;128:767–9.
Bottio T, Pittarello D, Bonato R, Thiene G, Gerosa G, Casarotto D, Basso C. Echocardiographic diagnosis of aortic valve papillary fibroelastoma. Tex Heart Inst J. 2004;31:322–323
Vida V, Cerutti A, Thiene G, Stellin S, Milanesi O, Basso C. Calcified mass in the right atrium. Ann Thorac Surg. 2005;79:717.
Padalino M, Basso C, Milanesi O, Vida VL, Svaluto Moreolo G, Thiene G, Stellin G. Surgically treated primary cardiac tumors in early infancy and childhood. J Thorac Cardiovasc Surg. 2005;129:1358–63.
Mazzola A, Spano JP, Valente M, Gregorini R, Villani C, Di Eusanio M, Ciocca M, Minuti U, Giancola R, Basso C, Thiene G. Leiomyosarcoma of the left atrium mimicking a left atrial myxoma. J Thorac Cardiovasc Surg. 2006;131:224–6.
Thiene G, Valente M, Lombardi M, Basso C. Tumours of the heart. In: Camm JA, Luscher TF, Serruys PV, editors. ESC textbook of cardiovascular medicine. Oxford: Oxford University Press; 2009.
Rickelt S, Rizzo S, Doerflinger Y, Zentgraf H, Basso C, Gerosa G, Thiene G, Moll R, Franke WW. A novel kind of tumor type-characteristic junction: plakophilin-2 as a major protein of adherens junctions in cardiac myxomata. Mod Pathol. 2010;23:1429–37.
Padalino MA, Vida VL, Bhattarai A, Reffo E, Milanesi O, Thiene G, Stellin G, Basso C. Giant intramural left ventricular rhabdomyoma in a newborn. Circulation. 2011;124:2275–7.
Perazzolo M, Thiene G, De Lazzari M, Calabrese F, Lacognata C, Rizzo S, Cacciavillani L, Tona F, Corbetti F, Iliceto S, Basso C. Concealed metastatic lung carcinoma presenting as acute coronary syndrome with progressive conduction abnormalities. Circulation. 2012;125:e499–502.
Padalino MA, Vida VL, Boccuzzo G, Tonello M, Sarris GE, Berggren H, Comas JV, Di Carlo D, Di Donato RM, Ebels T, Hraska V, Jacobs JP, Gaynor JW, Metras D, Pretre R, Pozzi M, Rubay J, Sairanen H, Schreiber C, Maruszewski B, Basso C, Stellin G. Surgery for primary cardiac tumors in children: early and late results in a multicenter European congenital heart surgeons association study. Circulation. 2012;126:22–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Thiene, G., Valente, M., Basso, C. (2013). Cardiac Tumors: From Autoptic Observations to Surgical Pathology in the Era of Advanced Cardiac Imaging. In: Basso, C., Valente, M., Thiene, G. (eds) Cardiac Tumor Pathology. Current Clinical Pathology. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-143-1_1
Download citation
DOI: https://doi.org/10.1007/978-1-62703-143-1_1
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-142-4
Online ISBN: 978-1-62703-143-1
eBook Packages: MedicineMedicine (R0)