Abstract
Understanding how the genomic instability that accompanies tumour development arises has been an important question for more than a century. One potential cause of such instability is defective chromosome segregation during mitosis. A cause of mitotic defects may lie in the acquisition of multiple mitotic spindle poles, through an increase in the number of centrosomes. Cancer cells frequently possess multiple centrosomes. DNA damaging treatments, or mutations in key DNA repair genes, also lead to centrosome amplification. Here, we review current models for how cells may lose the normal controls on centrosome duplication and acquire more than the normal number of these organelles. We also discuss how genotoxic stresses may contribute to the dysregulation of centrosome duplication and how this process may be a contributory factor in cellular transformation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ionise Radiation
- Spindle Assembly Checkpoint
- Centrosome Amplification
- Centrosome Duplication
- Chromosome Missegregation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Mechanisms of Aneuploidy
Aneuploidy has been described as the most common characteristic of cancer cells (Weaver and Cleveland 2006). Numerous genetic alterations have been observed in neoplastic cells, including chromosome and gene deletions, amplification and translocation. However, the presence of these alterations does not necessarily indicate that the tumour is genetically unstable. It was observed that in some haematological cancers, malignant cells were stably aneuploid, following chromosomal redistribution earlier during tumorigenesis. More often, though, aneuploid cancer cells derive from an increase in the rate of gain or loss of whole chromosomes, a condition known as chromosome instability (CIN) (Kops et al. 2005; Lengauer et al. 1998). Although aneuploidy has been often suggested as the driving force behind tumorigenesis, the rate at which chromosomes are gained or lost can cause different outcomes. While moderate levels of CIN facilitate tumour formation and development, massive changes in chromosome content can be intolerable to cancer cells (reviewed by Godinho et al. 2009). A number of studies have shown that high levels of chromosome missegregation and aneuploidy reduce cell viability in cancer cells by affecting a broad number of cellular processes (Kops et al. 2004; Thompson and Compton 2008; Williams et al. 2008). Thus, under normal circumstances, high levels of genetic instability impair cell growth, unless the mutations introduced provide a selective pressure for the accumulation of further changes, allowing cells to survive the adverse effects of aneuploidy (Holland and Cleveland 2009).
Aneuploidy or CIN can arise from defects in chromosome segregation during mitosis. Cells may gain or lose chromosomes as a result of defects in the mitotic checkpoint or in sister chromatid cohesion, of microtubule misattachments and of aberrant mitotic division (reviewed by Kops et al. 2005). The major cell cycle checkpoint ensuring the correct segregation of chromosomes between daughter cells is the spindle assembly checkpoint (SAC), which prevents metaphase–anaphase transition until all kinetochores have established a correct bi-orientation on the spindle (Musacchio and Salmon 2007). In mammalian cells, the complete inactivation of the mitotic spindle checkpoint results in cell death and early embryonic lethality due to massive chromosome missegregation (Kalitsis et al. 2000; Kops et al. 2004). However, altered expression or mutations in genes coding for components of the SAC have been observed in aneuploid human cancers (Cahill et al. 1998; Dai et al. 2004; Li et al. 2003). In these cells, the mitotic checkpoint is impaired and anaphase can begin even in the presence of unattached or misattached kinetochores, leading to chromosome missegregation and aneuploidy (Hanks et al. 2004; Sotillo et al. 2007).
Chromosome missegregation events may also occur following the generation of incorrect kinetochore–microtubule attachments. When one kinetochore interacts with microtubules coming from both spindle poles (merotelic attachment), the chromosome is attached and under tension, so that the SAC is not activated and cells can exit mitosis without any significant delay (Cimini et al. 2004; Khodjakov et al. 1997). Merotelic attachments are usually corrected before anaphase onset, although occasionally sister chromatids with merotelic attachment can missegregate, failing to move in either direction and yielding a lagging chromosome (reviewed by Salmon et al. 2005). At the end of mitosis, the lagging chromatid will be pushed into either one of the daughter cells, and upon nuclear envelope reassembly, will form a separate micronucleus (Cimini et al. 2002). A further source of CIN arises when cells enter mitosis with more than two centrosomes (Holland and Cleveland 2009). Centrosomes play a fundamental role in the organisation of the mitotic spindle. In the presence of supernumerary centrosomes, multipolar spindles may form and contribute to aneuploidy, although how such aneuploidy arises is not yet fully understood.
2 Centrosome Abnormalities and Tumorigenesis
In 1902, Theodor Boveri first described the detrimental effects on organism and cell physiology of an abnormal chromosome number. Several years earlier, the pathologist David Hansemann had observed the presence of aberrant chromosome segregation during mitosis in cancer cells. These findings led Boveri to propose that aneuploidy might promote tumorigenesis. In 1914, following his studies on sea urchin embryos, Boveri observed that cells forced to undergo multipolar mitosis produced progeny with an aberrant chromosome number. The prevalence of chromosome aberrations in cancer cells led Boveri to suggest that they were the result of multipolar mitoses in cells with supernumerary centrosomes (reviewed by Boveri 2008; Godinho et al. 2009; Holland and Cleveland 2009).
Since 1914, several studies have shown that supernumerary centrosomes are common to almost all types of solid and haematological malignancies, including breast, brain, lung, colon, ovary, liver, prostate, bone, gall bladder, head and neck cancers as well as lymphoma and leukaemia (Gustafson et al. 2000; Kramer et al. 2003; Kuo et al. 2000; Lingle et al. 1998; Nitta et al. 2006; Pihan et al. 1998; Pihan et al. 2001; Sato et al. 1999; Weber et al. 1998). Furthermore, in cancer cells, aberrations in centrosome number are often associated with structural irregularities such as increased centrosome size and alterations in the expression and phosphorylation status of PCM components (Lingle et al. 2002). Aberrant centrosomes often exhibit aberrant recruitment of gamma-TuRCs and defects in microtubule nucleation, which, in turn, affect the cellular architecture (Lingle et al. 2002; Lingle and Salisbury 2001). Furthermore, it has been shown that centrosome abnormalities correlate with increased levels of multipolar mitosis and aneuploidy in cancer cells (Ghadimi et al. 2000; Gisselsson et al. 2004). Several studies showed that in highly invasive cancers and in situ carcinoma, centrosomal defects are often associated with chromosomal aberrations, which occur at a later stage in tumor progression (Gisselsson et al. 2004; Lingle et al. 2002; Pihan et al. 2001). However, centrosome defects were also identified in cancers at an early stage in animal models and were shown to become more severe with tumour progression (D’Assoro et al. 2002a; Duensing et al. 2001; Goepfert et al. 2002; Shono et al. 2001). Although extra centrosomes failed to generate large-scale genome instability in Drosophila, likely due to the low proliferative index of mature Drosophila cells, serial transplantation in the abdomen of adult flies of larval brain cells carrying mutations in genes that encode centrosomal regulators and showing centrosome amplification, generated both benign and malignant hyperplasia, demonstrating that centrosome amplification can initiate tumorigenesis in flies (Castellanos et al. 2008). While these observations supported the theory that amplified centrosomes represent a cause of aneuploidy, they did not establish how the two phenomena are related or whether centrosome amplification was a cause or a consequence of cancer progression (reviewed by D’Assoro et al. 2002b; Nigg 2002).
3 Mechanisms of Aneuploidy that Involve Centrosome Amplification
Conceptually, the simplest mechanism of aneuploidy to arise from centrosome aberrations is that multipolar mitoses occur through the formation of multiple spindle poles and cause aneuploidy through unequal distribution of chromosomes between daughter cells (Fukasawa 2005). However, recent time-lapse video microscopy studies demonstrated that cultured human cells containing amplified centrosomes efficiently cluster their extra centrosomes and divide in a bipolar fashion. Only a small fraction of cells with extra centrosomes underwent multipolar division and the progeny originating from such divisions was mostly non-viable (Ganem et al. 2009), consistent with the view that massive aneuploidy induced by multipolar cell division is lethal. Similarly, analysis of Drosophila lines in which around 60 % of the cells possessed supernumerary centrosomes revealed a delay in mitosis due to the formation of a transient multipolar intermediate, but the cells ultimately divided in a bipolar fashion (Basto et al. 2008).
Centrosome clustering appears to be the major strategy that human cells employ to minimise the impact of multiple centrosomes (Quintyne et al. 2005), although there exist several other approaches, such as inactivation or sequestration of extra centrosomes (Gergely and Basto 2008; Godinho et al. 2009). However, even though centrosome clustering prevents lethality caused by multipolar division, centrosome amplification, nevertheless, leads to chromosome missegregation and instability (Ganem et al. 2009; Silkworth et al. 2009). A recent study suggested a novel potential mechanism for how supernumerary centrosomes cause chromosome aberrations and aneuploidy. Pellman and colleagues showed that cells with amplified centrosomes go through a transient multipolar state during spindle formation, before clustering their centrosomes (Ganem et al. 2009). This intermediate state predisposes cells to develop aberrant merotelic attachments with high frequency. Unresolved merotelic attachments impair chromosome segregation by causing lagging chromosomes during anaphase (Cimini et al. 2001; Gregan et al. 2011), so that this model provides an explanation for how multiple centrosomes can lead to chromosome abnormalities, without causing multipolar divisions. Therefore, how cells acquire multiple centrosomes is an important question in understanding how genome stability is normally maintained.
4 Centrosome Pathways
There are two key pathways by which centrosomes can arise: the normal, templated pathway in which the pre-existing centrioles serve as the scaffolding for new centriole formation during S-phase, and a de novo pathway (Loncarek and Khodjakov 2009). However, these are not distinct in terms of the controlling activities, but differ in the sense that the existing mother centrioles serve as regulators of the ‘templated’ process (Rodrigues-Martins et al. 2007b).
In experiments where centrosomes were removed from monkey kidney cells by micromanipulation (Hinchcliffe et al. 2001; Maniotis and Schliwa 1991) or laser microsurgery (Khodjakov et al. 2000; Khodjakov and Rieder 2001), the centrosomes did not regenerate. However, subsequent work that examined what happened when the centrosomes were removed from S-phase arrested CHO cells by laser ablation (Khodjakov et al. 2002), or from Chlamydomonas cells by a mutation that causes a fraction of the daughter cells to have no centrioles (Marshall et al. 2001), demonstrated that cells can form centrosomes de novo. These observations were further supported by the finding of de novo centriole assembly in transformed (La Terra et al. 2005) and normal (Uetake et al. 2007) human cells. A p53-dependent cell cycle arrest in late G1 phase is caused by the loss or damage of centrosomes (Mikule et al. 2007; Srsen et al. 2006), which suggests a reason why the potentiation of de novo centrosome formation was only observed when cells were treated after this point. The formation of the de novo structures and the maturation of these centrioles require passage through an entire cycle (Khodjakov et al. 2002; La Terra et al. 2005). Once activated, this de novo pathway allows cells to produce multiple centrosomes, suggesting that numerical control of the centrosome resides in the existing centrosomes (Khodjakov et al. 2002; La Terra et al. 2005). Together, these findings indicate a general pathway of de novo centrosome formation that is normally inhibited by the presence of existing centrioles (La Terra et al. 2005) but which, upon activation or loss of inhibition, can generate large numbers of centrioles.
An evolutionarily conserved series of proteins govern the process by which centrioles normally duplicate (Carvalho-Santos et al. 2010). A key polo box-containing kinase, PLK4 in human (Habedanck et al. 2005; Kleylein-Sohn et al. 2007), SAK in Drosophila melanogaster (Bettencourt-Dias et al. 2005) is recruited to the centrosome by the coiled-coil protein SPD2/CEP192, which also directs the recruitment of the pericentriolar material (PCM) to the nascent centriole (Kemp et al. 2004; Pelletier et al. 2004; Zhu et al. 2008). PLK4/SAK is required for the recruitment of the coiled-coil proteins, SAS-4 (CPAP/CENP-J in human cells) and SAS-6, which specify the base of the forming centriole, direct the elongation of its microtubules and are required for centriole duplication (Kirkham et al. 2003; Leidel et al. 2005; Leidel and Gonczy 2003; Pelletier et al. 2006; Rodrigues-Martins et al. 2007a; Strnad et al. 2007). A further coiled-coil component of the Caenorhabditis elegans centriole regulatory apparatus, SAS-5 (Ana2 in Drosophila), is also required for centriole duplication (Pelletier et al. 2006; Stevens et al. 2010). ZYG-1 plays a role similar to SAK/PLK4 in C. elegans (O’Connell et al. 2001) and is required for the localisation of SAS-4, SAS-5 and SAS-6 (Pelletier et al. 2006). Asterless/CEP152 has recently been described as a Plk4-interactor that is required for centriole duplication, being required for SAS-6 localisation to centrioles (Dzhindzhev et al. 2010; Guernsey et al. 2010; Hatch et al. 2010; Varmark et al. 2007).
Overexpression of SAK/PLK4, SAS-4 or SAS-6 causes centriole overduplication (Bettencourt-Dias et al. 2005; Habedanck et al. 2005; Kleylein-Sohn et al. 2007; Peel et al. 2007; Strnad et al. 2007), although the structure of centrioles formed through SAS-4 and SAS-6 overexpression may be abnormal (Kohlmaier et al. 2009; Rodrigues-Martins et al. 2007a). Notably, the centriole overduplication induced by overexpression of these key regulators involves the formation of multiple daughters around a single mother in a distinctive ‘rosette’ arrangement, rather than the general initiation of de novo centrosome assembly (Kleylein-Sohn et al. 2007; Strnad et al. 2007). However, in cells where there are no centrosomes, such as unfertilised Drosophila eggs, such overexpression does lead to de novo centriole assembly (Peel et al. 2007). These data indicate a limitation of centriole number that is imposed by a pre-existing mother.
Another element involved in the control of centriole number is the PCM. Establishment of a PCM cloud is a relatively early event in the de novo centriole duplication process, after which centrioles arise within the cloud (Khodjakov et al. 2002). Induction of an expanded PCM in cells with centrioles by overexpression of pericentrin led to the appearance of multiple daughter centrioles independently of any spatial or numerical control from the mother centrioles (Loncarek et al. 2008). This observation prompted the hypothesis that the mother centriole’s principal role in centriole assembly is the regulation and specification of a PCM scaffold, rather than the provision of a template (Loncarek et al. 2008). In either case, the control of centriole duplication resides in the extant structure.
5 Centrosome Amplification
Changes in the coordination of the chromosome and centrosome cycles lead to centrosome amplification, which has been noted when key cell cycle regulators or regulatory components of the centrosome are aberrantly expressed or suppressed (Hergovich et al. 2007; Hochegger et al. 2007; Leidel et al. 2005; McDermott et al. 2006; Mussman et al. 2000; Swanton et al. 2007; Tachibana et al. 2005). The altered expression of cell cycle regulators is a frequently observed phenomenon in human cancers, so this may represent one source of centrosome abnormalities. Alternatively, the dysregulation of such regulatory genes may occur as a consequence of ongoing genome instability during tumour development.
A further activity that disconnects the chromosome and centrosome cycles appears to be a controlled response to genotoxic stress. Abnormal amplification of centrosomes has also been observed following DNA damage induced by irradiation (Dodson et al. 2007; Sato et al. 2000a, b) or DNA replication stress (Balczon et al. 1995; Meraldi et al. 2002). Amplification of the centrosome occurs in cells that carry mutations in DNA repair or checkpoint genes (Bertrand et al. 2003; Dodson et al. 2004; Fukasawa et al. 1996; Griffin et al. 2000; Kraakman-van der Zwet et al. 2002; Mantel et al. 1999; Tutt et al. 2002; Yamaguchi-Iwai et al. 1999), express mutant forms of telomerase (Guiducci et al. 2001) or express viral oncogenes (Duensing et al. 2006; Duensing et al. 2000; Duensing and Munger 2003; Watanabe et al. 2000). Although these examples cover a broad range of genotoxic insults, it is clear that centrosome amplification is a potential consequence of DNA damage.
6 Mechanisms that Permit Centrosome Amplification
Multiple centrosomes can be observed in cells that suffer failure in cytokinesis due to altered expression of cell cycle and checkpoint regulators such as p53, BRCA2 and Aurora A (Daniels et al. 2004; Meraldi et al. 2002). In general, DNA-damaging treatments do not lead to tetraploidisation, so cytokinesis failure is not sufficient to explain how centrosome amplification occurs after genotoxic stress. Additional models are required, which we consider below.
Given the importance of ensuring the right number of centrosomes, normal centrosome duplication occurs in a manner that is strictly co-ordinated with the cell cycle (Delattre and Gonczy 2004; Hinchcliffe and Sluder 2001; Nigg 2007). This coordination is ensured by at least two controls:
-
i.
A requirement for cyclin-dependent kinase activity in centrosome duplication. A specific link between the chromosome and centrosome cycles is CDK2, which requires heterodimerisation with cyclin A or cyclin E for activity and which is necessary for the centrosome duplication that occurs during extended S-phase arrest in mammalian cells (Hinchcliffe et al. 1999; Lacey et al. 1999; Matsumoto et al. 1999; Meraldi et al. 1999), but not in chicken DT40 cells (Bourke et al. 2010). Cdk2 is also necessary for the centriole overduplication that is induced by expression of the human papillomavirus (HPV) type 16 E7 oncoprotein or by proteasome inhibition (Duensing et al. 2007; Duensing et al. 2006). However, Cdk2 is not required in mouse or chicken cells for normal centrosome duplication and it is likely that other kinases can compensate for its absence in the cell cycle (Adon et al. 2010; Duensing et al. 2006; Hochegger et al. 2007).
-
ii.
A ‘licensing’ of centrosome duplication through centriole disengagement, which is mediated by Polo-like kinase 1 and separase, a protease that is activated through anaphase promoting complex/cyclosome activity at the metaphase–anaphase transition (Tsou and Stearns 2006; Tsou et al. 2009). This licencing requirement normally limits when cells can duplicate their centrosomes, even within a cytoplasm that contains the requisite Cdk activity (Wong and Stearns 2003).
Therefore, for a cell to acquire multiple centrosomes, the following conditions must be fulfilled. Centrosomes must acquire a license for reduplication and the cell cycle regulators that drive centrosome duplication must be activated. As the generation of a centriole takes time, an additional condition may be added: the cell must not divide for a sufficient period to allow centriole duplication.
Taking one particular example of where centrosome amplification is induced experimentally, all these conditions are fulfilled. Extended S-phase arrest of many mammalian cells by hydroxyurea (HU) treatment allows the appearance of multiple centrosomes (Balczon et al. 1995; Prosser et al. 2009). The acquisition of multiple centrosomes during this arrest is dependent on Cdk activity (Prosser et al. 2009), and numerous reports have implicated Cdk2 as the particular kinase involved, acting predominantly with cyclin E (Hinchcliffe et al. 1999; Lacey et al. 1999; Matsumoto et al. 1999; Meraldi et al. 1999). Centrosomes are licensed in this cell cycle stage, Cdk2 is activated and cells do not progress through mitosis.
High levels of centrosome amplification are observed in p53-deficient mice and cells (Fukasawa et al. 1996). This is believed to arise from the dysregulation of Cdk2 activity when p53 is absent and p53-independent cell cycle arrest to provide sufficient time for amplification (Fukasawa 2008). Upregulation of Cdk2 activity through overexpression of cyclin E led to centrosome amplification in p53-deficient mouse cells, but little impact was seen in wild-type rat or mouse fibroblasts (Mussman et al. 2000; Spruck et al. 1999). Similarly, in human tumour cells, cyclin E overexpression induced centrosome amplification, but only in the absence of p53 function (Kawamura et al. 2004). Control of cyclin E levels has been cited as a mechanism by which Krüppel-like factor 4 influences centrosome amplification after irradiation (Yoon et al. 2005). HPV oncoprotein-induced centrosome amplification requires both the disabling of p53 and the loss of normal CDK2 regulation (Duensing and Munger 2002). The key downstream targets of CDK2 in centrosome duplication described to date include nucleophosmin (B23) (Okuda et al. 2000), Mps1 kinase (Fisk and Winey 2001) and CP110 (Chen et al. 2002). Interestingly, overactivation of a centrosomal nucleophosmin interactor, ROCK II kinase, has recently been shown to drive centrosome amplification in CDK2-deficient cells (Hanashiro et al. 2011), indicating a possible effector of CDK2 signalling in centrosome control.
7 DNA Damage and Centrosome Amplification
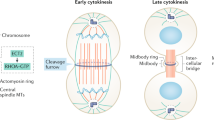
Early studies conducted on mouse cells showed that ionising radiation (IR) causes the amplification of microtubule-organising centres (MTOCs). Electron microscopy analysis of these MTOCs revealed structures which did not contain the paired centrioles and PCM typical of normal centrosomes (Sato et al. 1983). In Chinese hamster ovary (CHO) cells, incomplete DNA replication due to HU treatment caused mitotic centrosome fragmentation (Hut et al. 2003). A similar finding was made in Drosophila embryos, where it was shown that damaged DNA caused centrosome fragmentation, along with errors in chromosome segregation and cell death (Sibon et al. 2000). Premature centriole splitting has also been observed after IR in various human cell types (Saladino et al. 2009). Such splitting may indicate the disengagement of centrioles, providing licensed templates for centrosome reduplication (Tsou and Stearns 2006; Tsou et al. 2009). It should be noted that IR actually blocks the separation of duplicated centrosomes that accompanies normal entry into M phase, through an ATM-dependent, Plk1-mediated inhibition of the Nek2 kinase (Fletcher et al. 2004; Zhang et al. 2005), so that the precise impact of IR on the centriole cohesion machinery is not yet clear. Furthermore, although cell fusion experiments have indicated that irradiation is required for G2 phase centrosomes to acquire a licence for duplication (Inanc et al. 2010), it is not known what effect IR has on the principal licencing activity, separase, or its centrosomal target(s) (Tsou et al. 2009). DNA damage signalling actually inhibits the other known licencing signal, that of Plk1 activation (Smits et al. 2000; van Vugt et al. 2001; Zhang et al. 2005). In any case, as individual centrioles can organise spindle poles (Keryer et al. 1984; Ring et al. 1982; Sluder and Rieder 1985), aberrantly disengaged centrioles still retain their ability to nucleate microtubules and may contribute to multipolar spindle formation (Fig. 13.1).
Current models for centrosome amplification pathways: multiple centrosomes may be generated a during a prolonged cell cycle arrest, by templated centrosome duplication; b upon loss of existing centrioles, by a de novo pathway; c following DNA-damaging treatment by centrosome fragmentation; and d through the formation of multiple daughters from a single mother. Parental centrioles are schematically represented by rectangles coloured light blue for the mother, and dark blue for daughter centrioles. Red spots indicate centrobin association with centrioles during the cell cycle (Zou et al. 2005) and green spots indicate Cep170 localisation at the mature centriole (Guarguaglini et al. 2005; Saladino et al. 2009)
Although the process of DNA damage-induced licencing of centrosome duplication is not yet understood, additional time sufficient for duplication is provided by the cell cycle delays that arise as part of the DNA damage response. The MTOC amplification seen in human cells after irradiation (Sato et al. 2000a, b) was confirmed by light and electron microscopy as being due to centrosome amplification in a wide range of transformed and non-transformed cell lines from mammals and chickens (Bourke et al. 2007; Dodson et al. 2004; Saladino et al. 2009). Other forms of DNA-damaging treatment also induced centrosome amplification (Robinson et al. 2007; Saladino et al. 2009). Importantly, the principal signalling components of the DNA damage response that blocks cell cycle progress after genotoxic stress are required to permit centrosome overduplication. Loss of the apical DNA damage-responsive kinase, ATM, greatly impedes centrosome amplification after IR or DNA damage resulting from the absence of the Rad51 recombinase (Dodson et al. 2004), and IR-induced centrosome amplification is entirely abrogated by loss of the downstream Chk1 kinase (Bourke et al. 2007). IR-induced centrosome amplification occurs independently of p53 status (Dodson et al. 2007), even though the extent of G2-to-M arrest occasioned by IR is an important factor that has implicated p53 in some studies (Kawamura et al. 2006), suggesting that the process is not governed by the same mechanisms that alter centrosome numbers during extended S-phase arrest. Furthermore, the model proposed for how S-phase arrested cells overduplicate their centrosomes, in which multiple, immature daughter centrioles assemble around a single mother (Duensing et al. 2007; Guarguaglini et al. 2005), is not sufficient to explain what happens after IR, when amplification leads to centrosome splitting and/or the duplication of single daughters per mother and the majority of centrosomes carry the maturation marker CEP170 (Bourke et al. 2007; Saladino et al. 2009). The recent demonstration that IR-induced centrosome amplification can occur outside S phase (Inanc et al. 2010) provides further evidence that an arrest in G2 phase after IR is permissive for the overduplication of the centrosome, as we have proposed (Dodson et al. 2004).
Although an extended G2 phase delay is necessary for DNA damage-induced centrosome overduplication, a question that remains is whether such an arrest is sufficient. Chk1 localises to the centrosome, along with many other elements of the DNA damage response (Loffler et al. 2006; Oricchio et al. 2006), so that it has been technically challenging to address this issue. Inhibition of CDK1 by pharmacological means or by the use of an analogue-sensitive mutant causes a robust cell cycle arrest at the transition to mitosis, without any DNA damage signal, which is accompanied by high levels of centrosome amplification (Hochegger et al. 2007). However, CDK1 inhibition also elevates the activity of CDK2 (Bourke et al. 2010). Notably, IR also causes the activation of CDK2 activity in a subset of cell types (Bourke et al. 2010), so that of the conditions for centrosome amplification that we have outlined, DNA damage leads to the fulfilment of several at once.
In several instances given above (Bettencourt-Dias et al. 2005; Habedanck et al. 2005; Kleylein-Sohn et al. 2007; Peel et al. 2007; Strnad et al. 2007), overexpression of the key upstream regulators of centriole duplication causes the formation of multiple daughter centrioles. However, in a preliminary study on a subset of centrosomal candidates, we found no evidence for significant upregulation of centrosome protein-coding genes after irradiation of non-transformed human cells (Saladino 2010), suggesting that increasing the levels of the structural components of new centrioles is not how IR drives centrosome amplification. Another question that arises is how additional centrioles assemble after irradiation, once the conditions that allow their overduplication have been met. Time-lapse microscopy of CHO cells during extended S-phase arrest has indicated that multiple centrosomes can assemble around the pre-existing mother (Guarguaglini et al. 2005; Kuriyama et al. 2007), or from nuclear aggregates of centrin (Prosser et al. 2009). Multiple daughters are also induced by peptide vinyl sulfone proteasome inhibitor Z-L(3)VS treatment (Duensing et al. 2007) or by the HPV16 E7 oncoprotein (Duensing et al. 2006). However, apart from centriole splitting and fragmentation, which may reflect initial steps in centrosome reduplication, it appears that IR induces the duplication of the entire centrosome in the form of paired mother–daughter centrioles (Bourke et al. 2007; Dodson et al. 2007).
8 IR Impact on the Cell Cycle and on Cells
IR and other forms of DNA damage kill cells through caspase-dependent apoptosis or mitotic catastrophe (Blagosklonny 2007; Jonathan et al. 1999; Okada and Mak 2004; Roninson et al. 2001). Mitotic catastrophe is a consequence of a mitotic delay in which cells with incompletely replicated genomes or unrepaired DNA damage enter mitosis and undergo apoptosis during M phase (reviewed by Vakifahmetoglu et al. 2008). While the G2 checkpoint normally averts mitotic entry under such circumstances, problems with this checkpoint can allow cells to initiate premature mitosis, suffer mitotic delay through activation of the SAC and ultimately, die (Johnson et al. 1999; Mikhailov et al. 2002; Nitta et al. 2004; Shin et al. 2003; Vogel et al. 2005). Centrosome amplification, a response to DNA damage that occurs during a checkpoint-mediated delay, will also cause a mitotic delay and compromise cell viability during such a delay (Ganem et al. 2009; Inanc et al. 2010; Loffler et al. 2006). In support of the notion that centrosome amplification contributes to the death of cells with DNA damage, live-cell imaging analysis of human tumour cells demonstrated that the vast majority of irradiated cells with multiple centrosomes fail in mitosis, but also that >60 % of cells undergoing mitotic catastrophe have multiple centrosomes (Dodson et al. 2007).
As noted in a recent review of how aneuploidy arises, it is not yet clear whether centrosome amplification is a cause or a consequence of genome instability, or both (Chandhok and Pellman 2009). It is clear that a deficiency in the DNA damage response is likely to lead toward cancer. Recent data have demonstrated the activation of the DNA damage response in pre-cancerous lesions in a range of human tissues (Bartkova et al. 2005; Gorgoulis et al. 2005). This activation of the DNA damage response constrains tumourigenesis by inducing cell cycle delay, cell death or senescence, so that cells that no longer respond normally to DNA damage signals have a selective advantage in tumour development (Bartkova et al. 2005, 2006; Braig et al. 2005; Gorgoulis et al. 2005). However, the potential contribution of centrosome amplification to aneuploidy might make it a rather hazardous component of the normal DNA damage response or mechanism of cell death. Nevertheless, it is interesting to speculate that inducing centrosome amplification might be a means by which the killing effects of DNA damaging treatments could be potentiated.
References
Adon AM, Zeng X, Harrison MK, Sannem S, Kiyokawa H, Kaldis P, Saavedra HI (2010) Cdk2 and Cdk4 regulate the centrosome cycle and are critical mediators of centrosome amplification in p53-null cells. Mol Cell Biol 30(3):694–710
Balczon R, Bao L, Zimmer WE, Brown K, Zinkowski RP, Brinkley BR (1995) Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J Cell Biol 130(1):105–115
Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J (2005) DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434(7035):864–870
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG (2006) Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444(7119):633–637
Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, Raff JW (2008) Centrosome amplification can initiate tumorigenesis in flies. Cell 133(6):1032–1042
Bertrand P, Lambert S, Joubert C, Lopez BS (2003) Overexpression of mammalian Rad51 does not stimulate tumorigenesis while a dominant-negative Rad51 affects centrosome fragmentation, ploidy and stimulates tumorigenesis, in p53-defective CHO cells. Oncogene 22(48):7587–7592
Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM (2005) SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol 15(24):2199–2207
Blagosklonny MV (2007) Mitotic arrest and cell fate. Cell Cycle 6(1):e1–e5
Bourke E, Brown JA, Takeda S, Hochegger H, Morrison CG (2010) DNA damage induces Chk1-dependent threonine-160 phosphorylation and activation of Cdk2. Oncogene 29(4):616–624
Bourke E, Dodson H, Merdes A, Cuffe L, Zachos G, Walker M, Gillespie D, Morrison CG (2007) DNA damage induces Chk1-dependent centrosome amplification. EMBO Report 8(6):603–609
Boveri T (2008) Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J Cell Sci 121(Suppl 1):1–84
Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA (2005) Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436(7051):660–665
Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B (1998) Mutations of mitotic checkpoint genes in human cancers. Nature 392(6673):300–303
Carvalho-Santos Z, Machado P, Branco P, Tavares-Cadete F, Rodrigues-Martins A, Pereira-Leal JB, Bettencourt-Dias M (2010) Stepwise evolution of the centriole-assembly pathway. J Cell Sci 123(Pt 9):1414–1426
Castellanos E, Dominguez P, Gonzalez C (2008) Centrosome dysfunction in Drosophila neural stem cells causes tumors that are not due to genome instability. Curr Biol 18(16):1209–1214
Chandhok NS, Pellman D (2009) A little CIN may cost a lot: revisiting aneuploidy and cancer. Curr Opin Genet Dev 19(1):74–81
Chen Z, Indjeian VB, McManus M, Wang L, Dynlacht BD (2002) CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev Cell 3(3):339–350
Cimini D, Cameron LA, Salmon ED (2004) Anaphase spindle mechanics prevent mis-segregation of merotelically oriented chromosomes. Curr Biol 14(23):2149–2155
Cimini D, Fioravanti D, Salmon ED, Degrassi F (2002) Merotelic kinetochore orientation versus chromosome mono-orientation in the origin of lagging chromosomes in human primary cells. J Cell Sci 115(Pt 3):507–515
Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED (2001) Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol 153(3):517–527
D’Assoro AB, Barrett SL, Folk C, Negron VC, Boeneman K, Busby R, Whitehead C, Stivala F, Lingle WL, Salisbury JL (2002a) Amplified centrosomes in breast cancer: a potential indicator of tumor aggressiveness. Breast Cancer Res Treat 75(1):25–34
D’Assoro AB, Lingle WL, Salisbury JL (2002b) Centrosome amplification and the development of cancer. Oncogene 21(40):6146–6153
Dai W, Wang Q, Liu T, Swamy M, Fang Y, Xie S, Mahmood R, Yang YM, Xu M, Rao CV (2004) Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res 64(2):440–445
Daniels MJ, Wang Y, Lee M, Venkitaraman AR (2004) Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science 306(5697):876–879
Delattre M, Gonczy P (2004) The arithmetic of centrosome biogenesis. J Cell Sci 117(Pt 9):1619–1630
Dodson H, Bourke E, Jeffers LJ, Vagnarelli P, Sonoda E, Takeda S, Earnshaw WC, Merdes A, Morrison C (2004) Centrosome amplification induced by DNA damage occurs during a prolonged G2 phase and involves ATM. EMBO J 23(19):3864–3873
Dodson H, Wheatley SP, Morrison CG (2007) Involvement of centrosome amplification in radiation-induced mitotic catastrophe. Cell Cycle 6(3):364–370
Duensing A, Liu Y, Perdreau SA, Kleylein-Sohn J, Nigg EA, Duensing S (2007) Centriole overduplication through the concurrent formation of multiple daughter centrioles at single maternal templates. Oncogene 26(43):6280–6288
Duensing A, Liu Y, Tseng M, Malumbres M, Barbacid M, Duensing S (2006) Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene 25(20):2943–2949
Duensing S, Duensing A, Crum CP, Munger K (2001) Human papillomavirus type 16 E7 oncoprotein-induced abnormal centrosome synthesis is an early event in the evolving malignant phenotype. Cancer Res 61(6):2356–2360
Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum CP, Munger K (2000) The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 97(18):10002–10007
Duensing S, Munger K (2002) The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res 62(23):7075–7082
Duensing S, Munger K (2003) Human papillomavirus type 16 E7 oncoprotein can induce abnormal centrosome duplication through a mechanism independent of inactivation of retinoblastoma protein family members. J Virol 77(22):12331–12335
Dzhindzhev NS, Yu QD, Weiskopf K, Tzolovsky G, Cunha-Ferreira I, Riparbelli M, Rodrigues-Martins A, Bettencourt-Dias M, Callaini G, Glover DM (2010) Asterless is a scaffold for the onset of centriole assembly. Nature 467(7316):714–718
Fisk HA, Winey M (2001) The mouse Mps1p-like kinase regulates centrosome duplication. Cell 106(1):95–104
Fletcher L, Cerniglia GJ, Nigg EA, Yend TJ, Muschel RJ (2004) Inhibition of centrosome separation after DNA damage: a role for Nek2. Radiat Res 162(2):128–135
Fukasawa K (2005) Centrosome amplification, chromosome instability and cancer development. Cancer Lett 230(1):6–19
Fukasawa K (2008) P53, cyclin-dependent kinase and abnormal amplification of centrosomes. Biochim Biophys Acta 1786(1):15–23
Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF (1996) Abnormal centrosome amplification in the absence of p53. Science 271(5256):1744–1747
Ganem NJ, Godinho SA, Pellman D (2009) A mechanism linking extra centrosomes to chromosomal instability. Nature 460(7252):278–282
Gergely F, Basto R (2008) Multiple centrosomes: together they stand, divided they fall. Genes Dev 22(17):2291–2296
Ghadimi BM, Sackett DL, Difilippantonio MJ, Schrock E, Neumann T, Jauho A, Auer G, Ried T (2000) Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations. Genes Chromosom Cancer 27(2):183–190
Gisselsson D, Palsson E, Yu C, Mertens F, Mandahl N (2004) Mitotic instability associated with late genomic changes in bone and soft tissue tumours. Cancer Lett 206(1):69–76
Godinho SA, Kwon M, Pellman D (2009) Centrosomes and cancer: how cancer cells divide with too many centrosomes. Cancer Metastasis Rev 28(1–2):85–98
Goepfert TM, Adigun YE, Zhong L, Gay J, Medina D, Brinkley WR (2002) Centrosome amplification and overexpression of aurora A are early events in rat mammary carcinogenesis. Cancer Res 62(14):4115–4122
Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA Jr, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD (2005) Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434(7035):907–913
Gregan J, Polakova S, Zhang L, Tolić-Nørrelykke IM, Cimini D (2011) Merotelic kinetochore attachment: causes and effects. Trends Cell Biol 21(6):374–381
Griffin CS, Simpson PJ, Wilson CR, Thacker J (2000) Mammalian recombination-repair genes XRCC2 and XRCC3 promote correct chromosome segregation. Nat Cell Biol 2(10):757–761
Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, Nigg EA (2005) The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol Biol Cell 16(3):1095–1107
Guernsey DL, Jiang H, Hussin J, Arnold M, Bouyakdan K, Perry S, Babineau-Sturk T, Beis J, Dumas N, Evans SC, Ferguson M, Matsuoka M, Macgillivray C, Nightingale M, Patry L, Rideout AL, Thomas A, Orr A, Hoffmann I, Michaud JL, Awadalla P, Meek DC, Ludman M, Samuels ME (2010) Mutations in centrosomal protein CEP152 in primary microcephaly families linked to MCPH4. Am J Hum Genet 87(1):40–51
Guiducci C, Cerone MA, Bacchetti S (2001) Expression of mutant telomerase in immortal telomerase-negative human cells results in cell cycle deregulation, nuclear and chromosomal abnormalities and rapid loss of viability. Oncogene 20(6):714–725
Gustafson LM, Gleich LL, Fukasawa K, Chadwell J, Miller MA, Stambrook PJ, Gluckman JL (2000) Centrosome hyperamplification in head and neck squamous cell carcinoma: a potential phenotypic marker of tumor aggressiveness. Laryngoscope 110(11):1798–1801
Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA (2005) The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol 7(11):1140–1146
Hanashiro K, Brancaccio M, Fukasawa K (2011) Activated ROCK II by-passes the requirement of the CDK2 activity for centrosome duplication and amplification. Oncogene 30(19):2188–2197
Hanks S, Coleman K, Reid S, Plaja A, Firth H, Fitzpatrick D, Kidd A, Mehes K, Nash R, Robin N, Shannon N, Tolmie J, Swansbury J, Irrthum A, Douglas J, Rahman N (2004) Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat Genet 36(11):1159–1161
Hatch EM, Kulukian A, Holland AJ, Cleveland DW, Stearns T (2010) Cep152 interacts with Plk4 and is required for centriole duplication. J Cell Biol 191(4):721–729
Hergovich A, Lamla S, Nigg EA, Hemmings BA (2007) Centrosome-associated NDR kinase regulates centrosome duplication. Mol Cell 25(4):625–634
Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G (1999) Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science 283(5403):851–854
Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G (2001) Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 291(5508):1547–1550
Hinchcliffe EH, Sluder G (2001) “It takes two to tango”: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev 15(10):1167–1181
Hochegger H, Dejsuphong D, Sonoda E, Saberi A, Rajendra E, Kirk J, Hunt T, Takeda S (2007) An essential role for Cdk1 in S phase control is revealed via chemical genetics in vertebrate cells. J Cell Biol 178(2):257–268
Holland AJ, Cleveland DW (2009) Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol 10(7):478–487
Hut HM, Lemstra W, Blaauw EH, Van Cappellen GW, Kampinga HH, Sibon OC (2003) Centrosomes split in the presence of impaired DNA integrity during mitosis. Mol Biol Cell 14(5):1993–2004
Inanc B, Dodson H, Morrison CG (2010) A centrosome-autonomous signal that involves centriole disengagement permits centrosome duplication in G2 phase after DNA damage. Mol Biol Cell 21(22):3866–3877
Johnson PA, Clements P, Hudson K, Caldecott KW (1999) A mitotic spindle requirement for DNA damage-induced apoptosis in Chinese hamster ovary cells. Cancer Res 59(11):2696–2700
Jonathan EC, Bernhard EJ, McKenna WG (1999) How does radiation kill cells? Curr Opin Chem Biol 3(1):77–83
Kalitsis P, Earle E, Fowler KJ, Choo KH (2000) Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes Dev 14(18):2277–2282
Kawamura K, Izumi H, Ma Z, Ikeda R, Moriyama M, Tanaka T, Nojima T, Levin LS, Fujikawa-Yamamoto K, Suzuki K, Fukasawa K (2004) Induction of centrosome amplification and chromosome instability in human bladder cancer cells by p53 mutation and cyclin E overexpression. Cancer Res 64(14):4800–4809
Kawamura K, Morita N, Domiki C, Fujikawa-Yamamoto K, Hashimoto M, Iwabuchi K, Suzuki K (2006) Induction of centrosome amplification in p53 siRNA-treated human fibroblast cells by radiation exposure. Cancer Sci 97(4):252–258
Kemp CA, Kopish KR, Zipperlen P, Ahringer J, O’Connell KF (2004) Centrosome maturation and duplication in C. elegans require the coiled-coil protein SPD-2. Dev Cell 6(4):511–523
Keryer G, Ris H, Borisy GG (1984) Centriole distribution during tripolar mitosis in Chinese hamster ovary cells. J Cell Biol 98(6):2222–2229
Khodjakov A, Cole RW, McEwen BF, Buttle KF, Rieder CL (1997) Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J Cell Biol 136(2):229–240
Khodjakov A, Cole RW, Oakley BR, Rieder CL (2000) Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol 10(2):59–67
Khodjakov A, Rieder CL (2001) Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J Cell Biol 153(1):237–242
Khodjakov A, Rieder CL, Sluder G, Cassels G, Sibon O, Wang CL (2002) De novo formation of centrosomes in vertebrate cells arrested during S phase. J Cell Biol 158(7):1171–1181
Kirkham M, Muller-Reichert T, Oegema K, Grill S, Hyman AA (2003) SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell 112(4):575–587
Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA (2007) Plk4-induced centriole biogenesis in human cells. Dev Cell 13(2):190–202
Kohlmaier G, Loncarek J, Meng X, McEwen BF, Mogensen MM, Spektor A, Dynlacht BD, Khodjakov A, Gonczy P (2009) Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr Biol 19(12):1012–1018
Kops GJ, Foltz DR, Cleveland DW (2004) Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc Natl Acad Sci U S A 101(23):8699–8704
Kops GJ, Weaver BA, Cleveland DW (2005) On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer 5(10):773–785
Kraakman-van der Zwet M, Overkamp WJ, van Lange RE, Essers J, van Duijn-Goedhart A, Wiggers I, Swaminathan S, van Buul PP, Errami A, Tan RT, Jaspers NG, Sharan SK, Kanaar R, Zdzienicka MZ (2002) Brca2 (XRCC11) deficiency results in radioresistant DNA synthesis and a higher frequency of spontaneous deletions. Mol Cell Biol 22(2):669–679
Kramer A, Schweizer S, Neben K, Giesecke C, Kalla J, Katzenberger T, Benner A, Muller-Hermelink HK, Ho AD, Ott G (2003) Centrosome aberrations as a possible mechanism for chromosomal instability in non-Hodgkin’s lymphoma. Leukemia 17(11):2207–2213
Kuo KK, Sato N, Mizumoto K, Maehara N, Yonemasu H, Ker CG, Sheen PC, Tanaka M (2000) Centrosome abnormalities in human carcinomas of the gallbladder and intrahepatic and extrahepatic bile ducts. Hepatology 31(1):59–64
Kuriyama R, Terada Y, Lee KS, Wang CL (2007) Centrosome replication in hydroxyurea-arrested CHO cells expressing GFP-tagged centrin2. J Cell Sci 120(Pt 14):2444–2453
La Terra S, English CN, Hergert P, McEwen BF, Sluder G, Khodjakov A (2005) The de novo centriole assembly pathway in HeLa cells: cell cycle progression and centriole assembly/maturation. J Cell Biol 168(5):713–722
Lacey KR, Jackson PK, Stearns T (1999) Cyclin-dependent kinase control of centrosome duplication. Proc Natl Acad Sci U S A 96(6):2817–2822
Leidel S, Delattre M, Cerutti L, Baumer K, Gonczy P (2005) SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat Cell Biol 7(2):115–125
Leidel S, Gonczy P (2003) SAS-4 is essential for centrosome duplication in C elegans and is recruited to daughter centrioles once per cell cycle. Dev Cell 4(3):431–439
Lengauer C, Kinzler KW, Vogelstein B (1998) Genetic instabilities in human cancers. Nature 396(6712):643–649
Li GQ, Li H, Zhang HF (2003) Mad2 and p53 expression profiles in colorectal cancer and its clinical significance. World J Gastroenterol 9(9):1972–1975
Lingle WL, Barrett SL, Negron VC, D’Assoro AB, Boeneman K, Liu W, Whitehead CM, Reynolds C, Salisbury JL (2002) Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci U S A 99(4):1978–1983
Lingle WL, Lutz WH, Ingle JN, Maihle NJ, Salisbury JL (1998) Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci U S A 95(6):2950–2955
Lingle WL, Salisbury JL (2001) Methods for the analysis of centrosome reproduction in cancer cells. Methods Cell Biol 67:325–336
Loffler H, Lukas J, Bartek J, Kramer A (2006) Structure meets function–centrosomes, genome maintenance and the DNA damage response. Exp Cell Res 312(14):2633–2640
Loncarek J, Hergert P, Magidson V, Khodjakov A (2008) Control of daughter centriole formation by the pericentriolar material. Nat Cell Biol 10(3):322–328
Loncarek J, Khodjakov A (2009) Ab ovo or de novo? Mechanisms of centriole duplication. Mol Cells 27(2):135–142
Maniotis A, Schliwa M (1991) Microsurgical removal of centrosomes blocks cell reproduction and centriole generation in BSC-1 cells. Cell 67(3):495–504
Mantel C, Braun SE, Reid S, Henegariu O, Liu L, Hangoc G, Broxmeyer HE (1999) p21(cip-1/waf-1) deficiency causes deformed nuclear architecture, centriole overduplication, polyploidy, and relaxed microtubule damage checkpoints in human hematopoietic cells. Blood 93(4):1390–1398
Marshall WF, Vucica Y, Rosenbaum JL (2001) Kinetics and regulation of de novo centriole assembly. Implications for the mechanism of centriole duplication. Curr Biol 11(5):308–317
Matsumoto Y, Hayashi K, Nishida E (1999) Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr Biol 9(8):429–432
McDermott KM, Zhang J, Holst CR, Kozakiewicz BK, Singla V, Tlsty TD (2006) p16(INK4a) prevents centrosome dysfunction and genomic instability in primary cells. PLoS Biol 4(3):e51
Meraldi P, Honda R, Nigg EA (2002) Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J 21(4):483–492
Meraldi P, Lukas J, Fry AM, Bartek J, Nigg EA (1999) Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat Cell Biol 1(2):88–93
Mikhailov A, Cole RW, Rieder CL (2002) DNA damage during mitosis in human cells delays the metaphase/anaphase transition via the spindle-assembly checkpoint. Curr Biol 12(21):1797–1806
Mikule K, Delaval B, Kaldis P, Jurcyzk A, Hergert P, Doxsey S (2007) Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat Cell Biol 9(2):160–170
Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8(5):379–393
Mussman JG, Horn HF, Carroll PE, Okuda M, Tarapore P, Donehower LA, Fukasawa K (2000) Synergistic induction of centrosome hyperamplification by loss of p53 and cyclin E overexpression. Oncogene 19(13):1635–1646
Nigg EA (2002) Centrosome aberrations: cause or consequence of cancer progression? Nat Rev Cancer 2(11):815–825
Nigg EA (2007) Centrosome duplication: of rules and licenses. Trends Cell Biol 17(5):215–221
Nitta M, Kobayashi O, Honda S, Hirota T, Kuninaka S, Marumoto T, Ushio Y, Saya H (2004) Spindle checkpoint function is required for mitotic catastrophe induced by DNA-damaging agents. Oncogene 23(39):6548–6558
Nitta T, Kanai M, Sugihara E, Tanaka M, Sun B, Nagasawa T, Sonoda S, Saya H, Miwa M (2006) Centrosome amplification in adult T-cell leukemia and human T-cell leukemia virus type 1 Tax-induced human T cells. Cancer Sci 97(9):836–841
O’Connell KF, Caron C, Kopish KR, Hurd DD, Kemphues KJ, Li Y, White JG (2001) The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell 105(4):547–558
Okada H, Mak TW (2004) Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer 4(8):592–603
Okuda M, Horn HF, Tarapore P, Tokuyama Y, Smulian AG, Chan PK, Knudsen ES, Hofmann IA, Snyder JD, Bove KE, Fukasawa K (2000) Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 103(1):127–140
Oricchio E, Saladino C, Iacovelli S, Soddu S, Cundari E (2006) ATM is activated by default in mitosis, localizes at centrosomes and monitors mitotic spindle integrity. Cell Cycle 5(1):88–92
Peel N, Stevens NR, Basto R, Raff JW (2007) Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr Biol 17(10):834–843
Pelletier L, O’Toole E, Schwager A, Hyman AA, Muller-Reichert T (2006) Centriole assembly in Caenorhabditis elegans. Nature 444(7119):619–623
Pelletier L, Ozlu N, Hannak E, Cowan C, Habermann B, Ruer M, Muller-Reichert T, Hyman AA (2004) The Caenorhabditis elegans centrosomal protein SPD-2 is required for both pericentriolar material recruitment and centriole duplication. Curr Biol 14(10):863–873
Pihan GA, Purohit A, Wallace J, Knecht H, Woda B, Quesenberry P, Doxsey SJ (1998) Centrosome defects and genetic instability in malignant tumors. Cancer Res 58(17):3974–3985
Pihan GA, Purohit A, Wallace J, Malhotra R, Liotta L, Doxsey SJ (2001) Centrosome defects can account for cellular and genetic changes that characterize prostate cancer progression. Cancer Res 61(5):2212–2219
Prosser SL, Straatman KR, Fry AM (2009) Molecular dissection of the centrosome overduplication pathway in S-phase arrested cells. Mol Cell Biol&&&AQ
Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS (2005) Spindle multipolarity is prevented by centrosomal clustering. Science 307(5706):127–129
Ring D, Hubble R, Kirschner M (1982) Mitosis in a cell with multiple centrioles. J Cell Biol 94(3):549–556
Robinson HM, Bratlie-Thoresen S, Brown R, Gillespie DA (2007) Chk1 is required for G2/M checkpoint response induced by the catalytic topoisomerase II inhibitor ICRF-193. Cell Cycle 6(10):1265–1267
Rodrigues-Martins A, Bettencourt-Dias M, Riparbelli M, Ferreira C, Ferreira I, Callaini G, Glover DM (2007a) DSAS-6 organizes a tube-like centriole precursor, and its absence suggests modularity in centriole assembly. Curr Biol 17(17):1465–1472
Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M (2007b) Revisiting the role of the mother centriole in centriole biogenesis. Science 316(5827):1046–1050
Roninson IB, Broude EV, Chang BD (2001) If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat 4(5):303–313
Saladino C (2010) The impact of DNA damage on centrosomes. PhD Thesis, Biochemistry, National University of Ireland Galway, Galway
Saladino C, Bourke E, Conroy PC, Morrison CG (2009) Centriole separation in DNA damage-induced centrosome amplification. Environ Mol Mutagen 50(8):725–732
Salmon ED, Cimini D, Cameron LA, DeLuca JG (2005) Merotelic kinetochores in mammalian tissue cells. Philos Trans R Soc Lond B Biol Sci 360(1455):553–568
Sato C, Kuriyama R, Nishizawa K (1983) Microtubule-organizing centers abnormal in number, structure, and nucleating activity in X-irradiated mammalian cells. J Cell Biol 96(3):776–782
Sato N, Mizumoto K, Nakamura M, Nakamura K, Kusumoto M, Niiyama H, Ogawa T, Tanaka M (1999) Centrosome abnormalities in pancreatic ductal carcinoma. Clin Cancer Res 5(5):963–970
Sato N, Mizumoto K, Nakamura M, Tanaka M (2000a) Radiation-induced centrosome overduplication and multiple mitotic spindles in human tumor cells. Exp Cell Res 255(2):321–326
Sato N, Mizumoto K, Nakamura M, Ueno H, Minamishima YA, Farber JL, Tanaka M (2000b) A possible role for centrosome overduplication in radiation-induced cell death. Oncogene 19(46):5281–5290
Shin HJ, Baek KH, Jeon AH, Park MT, Lee SJ, Kang CM, Lee HS, Yoo SH, Chung DH, Sung YC, McKeon F, Lee CW (2003) Dual roles of human BubR1, a mitotic checkpoint kinase, in the monitoring of chromosomal instability. Cancer Cell 4(6):483–497
Shono M, Sato N, Mizumoto K, Maehara N, Nakamura M, Nagai E, Tanaka M (2001) Stepwise progression of centrosome defects associated with local tumor growth and metastatic process of human pancreatic carcinoma cells transplanted orthotopically into nude mice. Lab Invest 81(7):945–952
Sibon OC, Kelkar A, Lemstra W, Theurkauf WE (2000) DNA-replication/DNA-damage-dependent centrosome inactivation in Drosophila embryos. Nat Cell Biol 2(2):90–95
Silkworth WT, Nardi IK, Scholl LM, Cimini D (2009) Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE 4(8):e6564
Sluder G, Rieder CL (1985) Centriole number and the reproductive capacity of spindle poles. J Cell Biol 100(3):887–896
Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH (2000) Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol 2(9):672–676
Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, Benezra R (2007) Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 11(1):9–23
Spruck CH, Won KA, Reed SI (1999) Deregulated cyclin E induces chromosome instability. Nature 401(6750):297–300
Srsen V, Gnadt N, Dammermann A, Merdes A (2006) Inhibition of centrosome protein assembly leads to p53-dependent exit from the cell cycle. J Cell Biol 174(5):625–630
Stevens NR, Dobbelaere J, Brunk K, Franz A, Raff JW (2010) Drosophila Ana2 is a conserved centriole duplication factor. J Cell Biol 188(3):313–323
Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gonczy P (2007) Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev Cell 13(2):203–213
Swanton C, Marani M, Pardo O, Warne PH, Kelly G, Sahai E, Elustondo F, Chang J, Temple J, Ahmed AA, Brenton JD, Downward J, Nicke B (2007) Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell 11(6):498–512
Tachibana KE, Gonzalez MA, Guarguaglini G, Nigg EA, Laskey RA (2005) Depletion of licensing inhibitor geminin causes centrosome overduplication and mitotic defects. EMBO Report 6(11):1052–1057
Thompson SL, Compton DA (2008) Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol 180(4):665–672
Tsou MF, Stearns T (2006) Mechanism limiting centrosome duplication to once per cell cycle. Nature 442(7105):947–951
Tsou MF, Wang WJ, George KA, Uryu K, Stearns T, Jallepalli PV (2009) Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev Cell 17(3):344–354
Tutt AN, van Oostrom CT, Ross GM, van Steeg H, Ashworth A (2002) Disruption of Brca2 increases the spontaneous mutation rate in vivo: synergism with ionizing radiation. EMBO Report 3(3):255–260
Uetake Y, Loncarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G (2007) Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol 176(2):173–182
Vakifahmetoglu H, Olsson M, Zhivotovsky B (2008) Death through a tragedy: mitotic catastrophe. Cell Death Differ 15(7):1153–1162
van Vugt MA, Smits VA, Klompmaker R, Medema RH (2001) Inhibition of Polo-like kinase-1 by DNA damage occurs in an ATM- or ATR-dependent fashion. J Biol Chem 276(45):41656–41660
Varmark H, Llamazares S, Rebollo E, Lange B, Reina J, Schwarz H, Gonzalez C (2007) Asterless is a centriolar protein required for centrosome function and embryo development in Drosophila. Curr Biol 17(20):1735–1745
Vogel C, Kienitz A, Muller R, Bastians H (2005) The mitotic spindle checkpoint is a critical determinant for topoisomerase-based chemotherapy. J Biol Chem 280(6):4025–4028
Watanabe N, Yamaguchi T, Akimoto Y, Rattner JB, Hirano H, Nakauchi H (2000) Induction of M-phase arrest and apoptosis after HIV-1 Vpr expression through uncoupling of nuclear and centrosomal cycle in HeLa cells. Exp Cell Res 258(2):261–269
Weaver BA, Cleveland DW (2006) Does aneuploidy cause cancer? Curr Opin Cell Biol 18(6):658–667
Weber RG, Bridger JM, Benner A, Weisenberger D, Ehemann V, Reifenberger G, Lichter P (1998) Centrosome amplification as a possible mechanism for numerical chromosome aberrations in cerebral primitive neuroectodermal tumors with TP53 mutations. Cytogenet Cell Genet 83(3–4):266–269
Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, Amon A (2008) Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science 322(5902):703–709
Wong C, Stearns T (2003) Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat Cell Biol 5(6):539–544
Yamaguchi-Iwai Y, Sonoda E, Sasaki MS, Morrison C, Haraguchi T, Hiraoka Y, Yamashita YM, Yagi T, Takata M, Price C, Kakazu N, Takeda S (1999) Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J 18(23):6619–6629
Yoon HS, Ghaleb AM, Nandan MO, Hisamuddin IM, Dalton WB, Yang VW (2005) Kruppel-like factor 4 prevents centrosome amplification following gamma-irradiation-induced DNA damage. Oncogene 24(25):4017–4025
Zhang W, Fletcher L, Muschel RJ (2005) The role of Polo-like kinase 1 in the inhibition of centrosome separation after ionizing radiation. J Biol Chem 280(52):42994–42999
Zhu F, Lawo S, Bird A, Pinchev D, Ralph A, Richter C, Muller-Reichert T, Kittler R, Hyman AA, Pelletier L (2008) The mammalian SPD-2 ortholog Cep192 regulates centrosome biogenesis. Curr Biol 18(2):136–141
Zou C, Li J, Bai Y, Gunning WT, Wazer DE, Band V, Gao Q (2005) Centrobin: a novel daughter centriole-associated protein that is required for centriole duplication. J Cell Biol 171(3):437–445
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Humana Press, a part of Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Saladino, C., Bourke, E., Morrison, C.G. (2012). Centrosomes, DNA Damage and Aneuploidy. In: Schatten, H. (eds) The Centrosome. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-035-9_13
Download citation
DOI: https://doi.org/10.1007/978-1-62703-035-9_13
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-034-2
Online ISBN: 978-1-62703-035-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)